ABSTRACT
Persistent methicillin-resistant Staphylococcus aureus (MRSA) endovascular infections represent a significant clinically challenging subset of invasive, life-threatening S. aureus infections. We have recently demonstrated that purine biosynthesis plays an important role in such persistent infections. Cyclic di-AMP (c-di-AMP) is an essential and ubiquitous second messenger that regulates many cellular pathways in bacteria. However, whether there is a regulatory connection between the purine biosynthesis pathway and c-di-AMP impacting persistent outcomes was not known. Here, we demonstrated that the purine biosynthesis mutant MRSA strain, the ΔpurF strain (compared to its isogenic parental strain), exhibited the following significant differences in vitro: (i) lower ADP, ATP, and c-di-AMP levels; (ii) less biofilm formation with decreased extracellular DNA (eDNA) levels and Triton X-100-induced autolysis paralleling enhanced expressions of the biofilm formation-related two-component regulatory system lytSR and its downstream gene lrgB; (iii) increased vancomycin (VAN)-binding and VAN-induced lysis; and (iv) decreased wall teichoic acid (WTA) levels and expression of the WTA biosynthesis-related gene, tarH. Substantiating these data, the dacA (encoding diadenylate cyclase enzyme required for c-di-AMP synthesis) mutant strain (dacAG206S strain versus its isogenic wild-type MRSA and dacA-complemented strains) showed significantly decreased c-di-AMP levels, similar in vitro effects as seen above for the purF mutant and hypersusceptible to VAN treatment in an experimental biofilm-related MRSA endovascular infection model. These results reveal an important intersection between purine biosynthesis and c-di-AMP that contributes to biofilm-associated persistence in MRSA endovascular infections. This signaling pathway represents a logical therapeutic target against persistent MRSA infections.
KEYWORDS: MRSA, c-di-AMP, purine biosynthesis, biofilm, vancomycin, persistence, endovascular infection
INTRODUCTION
Persistent methicillin-resistant Staphylococcus aureus (MRSA) bacteremia (PB) (defined as ≥7 days of positive blood cultures despite appropriate antibiotic therapy) represents up to 30% of S. aureus endovascular infections (1, 2). Importantly, many PB clinical isolates are susceptible in vitro to anti-MRSA agents, such as vancomycin (VAN) and daptomycin (DAP), by Clinical and Laboratory Standards Institute (CLSI) standards, yet they persist in vivo (2, 3). Therefore, persistent MRSA infections pose an unmet therapeutic challenge, and understanding the specific molecular mechanisms involved in these outcomes are essential for their ultimate successful treatment.
S. aureus strains employ a wide variety of small nucleotide-signaling molecules that allow them to adjust their cellular physiology to cope with unfavorable environmental conditions for survival (4, 5). Among these molecules, the recently discovered cyclic di-AMP (c-di-AMP) is an essential and ubiquitous second messenger in Gram-positive bacteria (6–8). In S. aureus, c-di-AMP is synthesized from two molecules of ATP via a complex pathway involving the diadenylate cyclase enzyme, DacA (encoded by dacA) and the phosphodiesterase enzyme, GdpP (encoded by gdpP) (6, 9). Although the role of c-di-AMP in bacterial cell physiology, biofilm formation, adaptation to environmental stresses, and virulence has been reported (6, 7, 9–12), little is known about its impact on the persistent outcomes in MRSA endovascular infections.
Recently, we discovered an important role of purine biosynthesis in staphylococcal cell growth, regulation of global regulators, and the stringent response, which contributes significantly to persistent outcomes in MRSA endovascular infections (13, 14). It is well known that purine biosynthesis is crucial for cell growth through DNA and RNA synthesis, as well as ATP energy supply (15). Interestingly, as a second messenger, c-di-AMP, synthesized from nucleotide ATP, is also critical for bacterial cell growth and has interconnections with the stringent response (6, 9). Hence, we hypothesized that purine biosynthesis and c-di-AMP regulation may positively bias toward persistent phenotypes (e.g., biofilm formation and persistence to cell wall-active anti-MRSA antibiotics [16, 17]). The current investigation was designed to explore this hypothesis relating purine biosynthesis, c-di-AMP, and the persistent outcomes in MRSA endovascular infections both in vitro and in an experimental endocarditis model.
RESULTS
purF and c-di-AMP participate in a coordinated network.
We recently demonstrated that clinical PB strains (versus resolving MRSA bacteremia [RB] strains, defined as isolates from patients with negative blood cultures 2 to 4 days after initiation of therapy, [1, 2]) showed significantly higher expression of purine biosynthesis pathway genes, including purF (18). In addition, purine biosynthesis produces critical substrates for c-di-AMP synthesis (i.e., ATP), thus raising the possibility of a correlation between these events (6). To assess the role of purine biosynthesis in cellular c-di-AMP production, MRSA parental strain JE2 and its isogenic purF mutant and purF-complemented strains were employed in this study. Decreased levels of ATP, ADP, and c-di-AMP were found in the purF mutant strain when compared with those of its parental and purF-complemented strains (Fig. 1A and B, respectively). In S. aureus, c-di-AMP is synthesized by DacA and hydrolyzed by GdpP (9). To examine whether the purine biosynthesis pathway coordinates the transcription of c-di-AMP-related genes, dacA and gdpP expressions were also determined. Significantly decreased dacA and increased gdpP expressions were found in the purF mutant versus those in its isogenic JE2 parental and purF-complemented strains (Fig. 1C). These results support a unique and coordinated network connecting purine biosynthesis and c-di-AMP production.
FIG 1.
The purF mutant had significantly lower intracellular ATP and ADP levels (A), impaired c-di-AMP levels (B), and a relative lower expression of dacA and higher expression of gdpP (C) versus its isogenic JE2 MRSA strain (WT) and purF-complemented strains (Compl.). Relative expression levels of dacA and gdpP were calculated by normalizing the expression level of each gene versus housekeeping gene gyrB. All experiments were performed independently at least twice with triplicates (n ≥ 6). Error bars represent standard deviations. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (purF mutant versus WT and purF-complemented strains).
c-di-AMP promotes purF-mediated biofilm formation, extracellular DNA levels, and autolysis activity.
The purF mutant strain exhibited significantly less biofilm formation versus its isogenic MRSA JE2 parental and purF-complemented strains (Fig. 2A). The impaired biofilm formation in the purF mutant could be fully restored by adding exogenous 0.01 μM c-di-AMP (Fig. 2A). In addition, it is known that released extracellular DNA (eDNA) in the biofilm matrix derived from genomic DNA promotes biofilm formation; a potential source for this eDNA derives from cell lysis (19–21). Supporting this concept, significantly decreased eDNA levels (∼7.0-fold) paralleling an ∼6.5-fold reduction of Triton X-100-induced cell lysis (at 24-h exposure) were observed within the purF mutant compared to those in its isogenic JE2 parental and purF-complemented strains (Fig. 2B and C, respectively).
FIG 2.
The purF mutant showed decreased biofilm formation (A), eDNA levels (B), and Triton X-100-induced autolysis (C) and increased expression of lytS (D), lytR (E), and lrgB (F) versus its MRSA JE2 parental (WT) and purF-complemented (Compl.) strains. The impact of purF on these profiles could be reversed by addition of c-di-AMP. The relative expression levels of lytS, lytR, and lrgB were calculated by normalizing the expression level of each gene versus housekeeping gene gyrB. All experiments were performed independently at least twice with triplicates (n ≥ 6). Error bars represent standard deviations. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (purF mutant versus WT and purF-complemented strains and groups with additional c-di-AMP exposure).
As it has been reported, one of the two-component signal transduction systems, lytSR, plays an important role in biofilm development (22) and regulates the expression of lrgB, which negatively correlates with eDNA levels, cell lysis, and biofilm formation in S. aureus (23). Therefore, we evaluated lytSR and lrgB expression to establish whether the observed effects in eDNA and autolysis as above were attributable to altered lytSR and lrgB transcriptions in the JE2 strain set. Indeed, there was a reciprocal relationship between the loss of purF function and gain of lytSR and lrgB expressions. The purF mutant exhibited significantly increased lytSR and lrgB expressions versus those of its JE2 parental and purF-complemented strains (Fig. 2D to F). Notably, the addition of exogenous c-di-AMP significantly increased eDNA levels and cell lysis activity (Fig. 2B and C) and decreased lytSR and lrgB expressions in the purF mutant strain in a concentration-dependent manner (Fig. 2D to F). Taken together, these results demonstrated that purine biosynthesis positively regulates biofilm formation, eDNA levels, and autolysis activity but negatively impacts lytSR and lrgB expressions through a mechanism that is modulated by c-di-AMP.
purF is involved in VAN-MRSA binding and ensuing lysis.
Our previous work showed that the JE2 parental, its isogenic purF mutant, and purF-complemented strains have identical VAN MICs (2 μg/ml) (13). However, the purF mutant exhibited significantly lower in vitro survival rates with VAN exposure under in vivo-like conditions than its JE2 parental and purF-complemented strains (13). Thus, we investigated the impact of purine biosynthesis on VAN-induced lysis of this JE2 strain set. In the presence of 10× MIC of VAN (20 μg/ml), the purF mutant showed a significantly higher percentage of lysis than the JE2 parental or purF-complemented strains at the 24-h time point (Fig. 3A).
FIG 3.

The purF mutant showed enhanced VAN-induced lysis (A) and VAN binding (B), lower WTA levels (C), and tarH expression (D) versus JE2 parental (WT) and purF-complemented (Compl.) strains. The impact of purF on these profiles could be reversed by addition of c-di-AMP. The relative expression level of tarH was calculated by normalizing the expression level of tarH versus housekeeping gene gyrB. All experiments were performed independently at least twice with triplicates (n ≥ 6). Error bars represent standard deviations. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (purF mutant versus WT and purF-complemented strains and groups with additional c-di-AMP exposure).
In S. aureus, VAN inhibits cell wall synthesis by binding to the terminal d-Ala-d-Ala of peptidoglycan (24). Therefore, we hypothesized that the enhanced susceptibility to VAN would correlate with VAN-MRSA binding. Indeed, a significantly higher percentage of VAN binding was observed in the purF mutant strain (54%) than in its isogenic JE2 parental (30%) and purF-complemented (40%) strains (Fig. 3B).
purF and c-di-AMP promote WTA and tarH-mediated integrity.
Next, we explored the hypothesis that purF and c-di-AMP influence cell wall composition and integrity. In S. aureus, cell wall teichoic acid (WTA) serves as an integral component of the cell wall and has been reported to alter VAN susceptibility in S. aureus (25, 26). Thus, we quantified the relationship between purF and c-di-AMP on WTA levels to assess the impact of purine biosynthesis and c-di-AMP regulation on WTA levels. Significantly reduced WTA levels were observed in the purF mutant versus its JE2 parental and purF-complemented strains (Fig. 3C). These data suggested that repression of purine biosynthesis can lead to a reduction of WTA levels. To further define the mechanisms underlying the WTA-related phenotypes, we focused on the expression of tarH in the study strain set, which is positively correlated with WTA biosynthesis (27). In the purF mutant, transcription of tarH was markedly diminished compared with that of its isogenic parental JE2 and purF-complemented strains (Fig. 3D). Importantly, exposure to exogenous c-di-AMP increased WTA levels and tarH expression in the purF mutant strain in a concentration-dependent manner (Fig. 3C and D).
Collectively, these data indicated that purine biosynthesis contributes to VAN persistence via cell binding and induced lysis, corresponding to WTA levels and tarH expression. Because exogenous c-di-AMP reverses these effects, these data also points to a novel mechanistic relationship among purine biosynthesis, c-di-AMP, and WTA levels, which may contribute to persistent outcomes.
The roles of c-di-AMP in the JE2 background are validated in a distinct MRSA background.
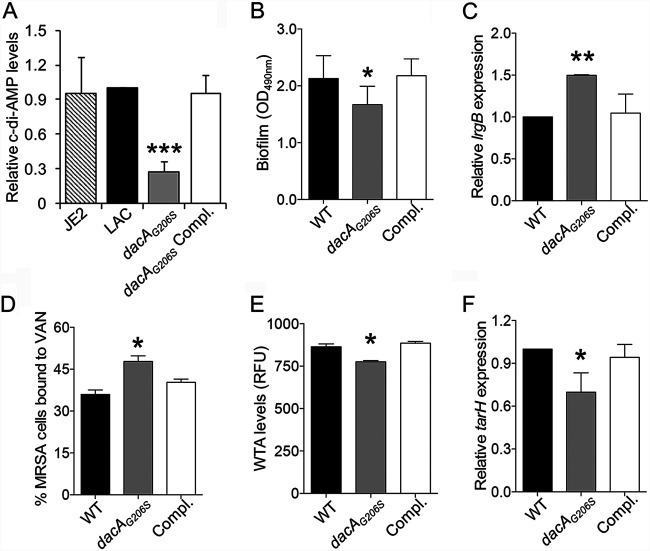
It is essential to demonstrate that the above findings are not restricted to one S. aureus genetic background; hence, MRSA parental strain LAC parental, its isogenic dacAG206S mutant, and dacA-complemented strains were employed. First, we demonstrated that there were no significant differences in c-di-AMP levels between the LAC parental strain and its plasmid-cured derivation strain JE2 parental, which has been used above (Fig. 4A). Consistent with previously published results (6), the dacAG206S mutant showed significantly lower c-di-AMP levels compared to those of its parental strain LAC and the dacA-complemented variant (Fig. 4A). Despite all of the MRSA isolates having a VAN-susceptible phenotype (MICs = 2 μg/ml), the dacAG206S mutant exhibited significantly (i) reduced biofilm formation paralleling increased lrgB expression (Fig. 4B and C, respectively), (ii) increased VAN binding (Fig. 4D), and (iii) decreased WTA levels with lower tarH expression (Fig. 4E and F, respectively). Of note, results observed in the dacAG206S mutant could be partially restored by the addition of exogenous c-di-AMP at the concentration of 0.01 μM or 0.1 μM (data not shown).
FIG 4.
The dacAG206S mutant had a similar impact on biofilm formation, lrgB expression, VAN binding, WTA levels, and tarH expression as the purF mutant strain. Intracellular c-di-AMP levels (A), biofilm formation (B), relative expression of lrgB (C), VAN binding (D), WTA levels (E), and relative expression of tarH (F) in the MRSA LAC parental strain (WT), its isogenic dacAG206S mutant strain (dacAG206S), and the dacA-complemented (Compl.) strain. The relative expression levels of lrgB and tarH were calculated by normalizing the expression level of each gene versus housekeeping gene gyrB. All experiments were performed independently at least twice with triplicates (n ≥ 6). Error bars represent standard deviations. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (dacAG206S mutant versus WT and dacA-complemented strain).
c-di-AMP contributes to VAN persistence in a rabbit model of endocarditis.
Our findings above demonstrated a functional relationship among purine biosynthesis, c-di-AMP, VAN-MRSA binding and lysis, and biofilm formation. Together, these observations strongly supported our hypothesis that the interaction of purine biosynthesis and c-di-AMP contributes to persistent outcomes during VAN therapy in vivo. Therefore, to assess the putative impact of c-di-AMP on the persistent outcomes in vivo, an experimental infective endocarditis model was used. At baseline (without VAN therapy), the dacAG206S mutant had similar MRSA counts in vegetations but lower counts in kidney and spleen compared to its parental LAC or dacA-complemented strains (Fig. 5). Importantly, animals infected by the dacAG206S mutant were hypersusceptible to VAN treatment, with significantly reduced MRSA densities (<3.5 log10 CFU/g tissue) in all target tissues compared to those of the parental or dacA-complemented strains (Fig. 5). These in vivo data affirmed that reduced c-di-AMP levels are associated with enhanced VAN responsiveness in the endocarditis model.
FIG 5.
The dacAG206S mutant significantly enhanced the efficacy of VAN in a rabbit endocarditis model. Densities of MRSA in target tissues in the endocarditis model due to 105 CFU challenges of the LAC parental strain, its isogenic dacAG206S mutant (dacAG206S), or dacA-complemented (Compl.) strain with/without VAN treatment. Each dot represents one animal. Horizontal black bars indicate means of MRSA densities.
DISCUSSION
The regulatory intersection between purine biosynthesis and c-di-AMP generation in persistent MRSA endovascular infections is not well studied. The present study assessed this interrelationship and its key downstream impacts on phenotypes relevant to persistent outcome in MRSA infections. Our study demonstrated that purine biosynthesis positively regulates c-di-AMP synthesis, which can ultimately modulate biofilm formation, WTA levels, VAN binding, and lysis and contribute to the persistent MRSA endovascular infections (Fig. 6).
FIG 6.
Model depicting the role of the second messenger c-di-AMP through purine biosynthesis in persistent MRSA endovascular infection. As a rate-limit enzyme, the ATase (PRPP→PRA)-encoding gene purF participates in turning 5-phosphoribosyl-1-pyrophosphate (PRPP) into IMP in the purine biosynthesis pathway (15). The branch-point intermediate IMP ultimately converts to essential nucleobases adenine including AMP, ADP, and ATP (55). c-di-AMP is synthesized from two molecules of ATP through the diadenylate cyclase enzyme DacA and hydrolyzed by the phosphodiesterase enzyme GdpP (6, 9). In the current study, purine biosynthesis was shown to contribute to the induction and repression of the expression of dacA and gdpP, respectively, and then subsequently elevated c-di-AMP levels. Increased c-di-AMP benefits persistent-related factors, such as (i) downregulation of lrgB expression, which leads to increased lysis, eDNA, and biofilm formation; and (ii) upregulation of tarH expression, which results in higher WTA levels, and subsequent decreased VAN-induced lysis and VAN-binding, thus ultimately facilitating persistent outcomes.
A positive regulatory correlation between purine biosynthesis and c-di-AMP was demonstrated in our study strain set. For instance, significantly decreased dacA (encoding c-di-AMP synthesis enzyme) and increased gdpP (encoding c-di-AMP hydrolysis enzyme) expression levels were found in the purF mutant versus its parental and purF-complemented strains. Consistent with our current findings, DeFrancesco et al. also reported a positive relationship between purine biosynthesis and c-di-AMP, showing that deletion of the purine biosynthesis repressor, PurR, was associated with increased c-di-AMP levels in S. aureus (19). Therefore, it appears that any conditions significantly affecting purine biosynthesis may consequently impact c-di-AMP levels.
Biofilm formation, a major virulence factor in medical device-related S. aureus endovascular infections, accelerates bacterial colonization in host tissues and promotes resistance to host immune responses and antimicrobial agents (16, 28). The role of c-di-AMP on biofilm formation has been studied in S. aureus (29) and Streptococcus mutans (10). In the current study, our data revealed that the biofilm formation significantly decreased in the purF mutant was restored by the addition of exogenous c-di-AMP. This finding indicates a dependent effect of purine biosynthesis on c-di-AMP in the biofilm formation. Similar to c-di-AMP, the second messenger c-di-GMP has also been shown to affect biofilm formation in bacteria (30). However, no quantifiable amounts of c-di-GMP could be detected in the JE2 strain set after multiple quantification attempts (data not shown). These results are consistent as previously reported by Corrigan et al. (29) and Holland et al. (31) showing that c-di-GMP levels are not detectable in the study S. aureus strains. Thus, c-di-GMP appears unlikely to be involved in biofilm formation in the S. aureus strains tested. Release of eDNA occurs through autolysis during programmed cell death (23, 32, 33). Dengler et al. reported that a dacA mutation decreased c-di-AMP levels, resulting in reduced autolysis (Triton X-100) in S. aureus (7). In line with this report, we demonstrated that lower c-di-AMP levels in the purF mutant correlated with decreased eDNA versus that of its parental and purF-complemented strains. In contrast, DeFrancesco et al. observed an opposite result, that disruption of GdpP yielded increased c-di-AMP but lower eDNA levels (19). Our results revealed that the reduced eDNA levels in the purF mutant were partially restored by exogenous c-di-AMP exposure at a concentration of 0.01 μM or 0.1 μM. Interestingly, however, a higher c-di-AMP concentration (1 μM) exposure led to an opposite effect in the purF mutant, corresponding with reduced eDNA (data not shown); thus, these contrasting findings may be due to an “overdose” of intracellular c-di-AMP. In parallel, the two-component regulatory system lytSR and its downstream lrgB operon are known to be involved in biofilm formation by controlling cell lysis and releasing eDNA in S. aureus (22, 34). To date, it has been established that the lytSR two-component regulatory system positively regulates lrgAB transcriptions (22, 35). In addition, Beltrame et al. reported a direct correlation between the expression of lrgB and eDNA levels (23). Interestingly, the current study demonstrated that the purF mutant had a significantly lower c-di-AMP level but increased lytSR and lrgB expressions. In turn, this effect led to the decreased cell lysis and eDNA release, corresponding to reduced biofilm formation (versus the parental and purF-complemented strains). Importantly, the isogenic strain defective in c-di-AMP synthesis (i.e., dacAG206S mutant) confirmed the correlation among c-di-AMP, lrgB expression, and biofilm formation. These results provide a plausible linkage among purine biosynthesis, c-di-AMP generation, lytSR and lrgB expressions, and biofilm formation. This novel relationship has not been previously identified and is likely to contribute to in vivo persistent outcomes in S. aureus infection.
VAN was selected in our study since it has remained the gold standard for the treatment of invasive MRSA infections. Peschel et al. reported that the level of WTA is inversely associated with VAN binding, cell lysis, and subsequently VAN susceptibility in S. aureus (26). We studied the potential impact of purine biosynthesis and c-di-AMP in this regard. Prior results support our current findings, demonstrating significantly lower WTA levels and higher VAN binding and VAN-induced lysis in the purF mutant versus its parental strain. The addition of c-di-AMP restored WTA levels that were decreased in the purF mutant strain, providing mechanistic confirmation of this phenotype. These results further support an important linkage among purine biosynthesis, c-di-AMP, and WTA synthesis, which influences VAN susceptibility related to persistence. Next, we explored the impact of tarH expression on WTA composition. TarH is part of the two-component ATP-binding cassette (ABC) transporter, TarGH, which is responsible for the translocation of WTA through the cell membrane (36). Thus, tarH expression directly affects WTA levels in S. aureus (37). A positive relationship between WTA levels and tarH transcription has been previously reported (27), consistent with our results showing a decreased tarH expression and WTA levels in the purF mutant versus those in its isogenic parental strain. Importantly, exogenous c-di-AMP reversed this effect, restoring tarH expression in the purF mutant strain. Unlike the expression of tarH, no significant differences in tarG expression were observed between the purF and dacAG206S mutant strains versus their respective parental and complementary strains (data not shown). These results are consistent with the previous observation by Wanner et al. (27) showing that, among the analyzed WTA biosynthesis genes (tarO, tarA, tarK, tarL, tarG, and tarH), only tarH transcription was significantly increased in WTA-elevated S. aureus. Therefore, these results suggest that the impact of c-di-AMP on WTA synthesis might be mainly through regulating tarH expression. Furthermore, the impacts of c-di-AMP on these in vitro phenotypic and genotypic profiles related to persistence (e.g., biofilm formation, VAN binding, and WTA synthesis) were confirmed in a genetically defined strain set, including the MRSA LAC parental strain and its isogenic dacAG206S mutant strain. These findings are supported by a previous study demonstrating that c-di-AMP is involved in cell envelope signaling and can influence cell wall-active antibiotic resistance in S. aureus (7). Collectively, these results uncovered the interactions among purine biosynthesis pathway, c-di-AMP, and persistence related profiles.
The impacts of c-di-AMP in bacterial pathogenesis have been noted in experimental murine lung and skin infection models due to Mycobacterium tuberculosis and Streptococcus pyogenes, respectively (38, 39). In our current investigations, animals infected with the dacAG206S mutant were significantly more susceptible to VAN treatment than those infected with the parental strain in experimental endocarditis. These outcomes suggest that the in vivo effect of c-di-AMP in VAN persistence in MRSA may be due, at least in part, to a combination of impacts on biofilm formation, WTA levels, VAN binding, and VAN-induced lysis.
We recognize that there were certain limitations in the current study. First, we only studied one MRSA genetic background strain set. However, validation of the primary findings in the JE2 strain set using a predominant clinical isolate MRSA strain set (LAC) supports the key concepts of the current study. Nonetheless, it is possible that the regulation of this important network may differ somewhat in other MRSA genetic background. Second, we understand that many other factors may also impact c-di-AMP generation and purine biosynthesis, which could contribute to persistent MRSA infections (e.g., other cell wall components, such as peptidoglycan [29], or host anti-inflammatory response, such as macrophage, etc. [11]). Such factors are certainly priorities for investigations beyond the scope of the current effort. Lastly, determining the detailed mechanisms of how c-di-AMP regulates autolysis and WTA are ongoing in our laboratories.
In summary, the present findings are the first to our knowledge to demonstrate the interaction between purine biosynthesis pathway and c-di-AMP favoring persistence-related in vitro phenotypes and persistence outcomes in VAN therapy of MRSA endovascular infections in vivo. This coordinated network offers novel therapeutic targets and strategies needed to address the growing threat of persistent MRSA infections and suggests the existence of a previously unknown adaptive genetic mechanism contributing to persistent MRSA infections.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth medium.
MRSA USA300 strain LAC and its derivative JE2 (cured of three plasmids) (40) were used as parental strains. The JE2 purF mutant from the Nebraska Transposon Mutant Library (NTML) and the LAC dacAG206S mutant in which the glycine at amino acid position 206 is replaced with a serine (6, 8) were also used. It has been reported that the dacA deletion mutant has a severe growth defect; thus, the dacA point mutant strain that grows robustly in rich medium (e.g., Trypticase soy broth [TSB]), while producing decreased c-di-AMP, was chosen for use in this study (6, 8). The purF and dacAG206S mutants were complemented by transforming plasmid pSK236::purF and pCL55::dacA, respectively, as described previously (6, 13). Unless otherwise stated, all S. aureus study strains were grown at 37°C in TSB (Difco) or on TSB agar plates.
Determination of VAN MICs.
MICs of VAN on the study MRSA strains were determined by standard Etest method according to the manufacturer’s recommended protocols (bioMérieux, La Balme-les-Grottes, France).
ATP and ADP levels.
ATP and ADP levels of study strains from overnight cultures were quantified by using Promega BacTiter Glo kit and Promega ADP-Glo kinase kit (Promega, Madison, WI), according to the manufacturer’s instructions, respectively (41, 42). ATP and ADP levels were determined by measuring luminescence levels compared to ATP and ADP standard curves, respectively, and presented as the levels normalized to CFU.
Purification of c-di-AMP binding protein, CabP.
Escherichia coli strain ST2789, containing pET28a(+) with the cabP open reading frame (ORF) in E. coli strain BL21, was used to purify CabP protein according to the method described previously (43, 44). Briefly, the expression of CabP was induced by adding isopropyl-β-d-1-thiogalactopyranoside (IPTG) at 1 mM to the LB culture (30°C) at an optical density at 600 nm (OD600) of 0.5. Three hours after induction, E. coli cells were harvested and resuspended in lysis buffer (50 mM Tris-HCl [pH 7.5], 500 mM NaCl, 10 mM imidazole, and 10% glycerol). After sonication, bacteria debris was removed by centrifugation (20,000 × g) at 4°C. The CabP protein in the supernatant was purified by using a Capturem His-tagged purification miniprep kit (TaKaRa Bio USA, Ann Arbor, MI). The purity of the purified CabP protein was determined by SDS-PAGE. The concentration of the purified CabP protein was determined with a Pierce bicinchoninic acid (BCA) protein assay kit (Thermo Scientific).
Detection of c-di-AMP levels.
c-di-AMP levels in the study MRSA strains were detected using a competitive enzyme-linked immunosorbent assay (ELISA) method as published previously (44, 45). Prior to determining the MRSA intracellular c-di-AMP levels, a standard curve was generated to calibrate the ELISA by using serial samples containing 25 nM biotin-labeled c-di-AMP and 2-fold serially diluted artificial c-di-AMP (ranged from 250 nM down to 7.8 nM) in Tris-HCl (45). MRSA cells from 10-ml overnight cultures were adjusted at an OD600 of 1.0, harvested, and resuspended in 500 μl of 50 mM Tris-HCl (pH 8.0). Following sonication and boiling, bacterial debris was removed by centrifugation for 5 min at 20,000 × g, and the supernatant was used to detect c-di-AMP levels. A 96-well plate was coated with CabP protein at 10 μg/ml at 4°C for at least 14 h. After washing and blocking the plates with 1% bovine serum albumin (BSA) for 1 h, biotin-labeled c-di-AMP (25 nM; Biolog) was added and incubated for 2 h. Then, the plate was washed and incubated with horseradish peroxidase-conjugated streptavidin (Thermo Scientific) for 1 h. The peroxidase was detected with the substrate o-phenylenediamine dihydrochloride (OPD) (Sigma) and measured at OD492.
Biofilm formation.
Biofilm formation under static conditions with/without the addition of artificial c-di-AMP (InvivoGen, San Diego, CA) exposure was performed as previously described (28, 46). The adhering dye (0.1% safranin) was dissolved in 30% acetic acid, and absorption was measured as OD490 to quantify biofilm formation (28, 46).
Detection of eDNA levels.
eDNA levels of overnight cultured study MRSA cells were detected by using a SYTOX green nucleic acid stain (Thermo Scientific) (19, 47). Briefly, 100 μl of filtered supernatant from the overnight cultures was mixed with 100 μl of 2 μM SYTOX green nucleic acid stain. Fluorescence was measured by using a BioTek Synergy 2 plate reader (BioTek, Winooski, VT, USA) with excitation and emission wavelengths of 465 nm and 510 nm, respectively. eDNA levels were expressed as relative fluorescence units (RFU).
Lytic assays with Triton X-100 and VAN.
Lytic assays were performed as described elsewhere (48, 49). In brief, S. aureus cells from overnight cultures with/without c-di-AMP exposure were adjusted to an OD580 of 1.0, washed, and then exposed to 50 mM Tris-Cl (pH 7.2) containing 0.1% Triton X-100 or 20 μg/ml (10× MIC of VAN) and incubated at 30°C with agitation (200 rpm). Staphylococcal lysis was measured by the changes in OD580.
VAN binding to MRSA.
VAN binding to study MRSA strains was measured using a boron dipyrromethene difluoride-labeled VAN strategy (Bodipy FL VAN; Invitrogen Corp., Carlsbad, CA) (46). Briefly, overnight cultured S. aureus cells were adjusted to an OD600 of 1.0 and then exposed to Bodipy FL VAN at the concentration of 20 μg/ml (10× MIC of VAN) for 30 min at 37°C in cation-adjusted Mueller-Hinton broth (MHB). The binding of VAN was measured by quantitative flow cytometry (FACSCalibur; Becton, Dickinson [BD]) (46, 50). For each sample, 10,000 cells were acquired and analyzed. The results were expressed as the percentage of acquired cells exhibiting threshold levels of the fluorescence signal.
Detection of WTA in MRSA.
Staphylococcal cell wall GlcNAc, one of the important WTA structural components, was quantified by using a wheat germ agglutinin (WGA)-Alexa Fluor 594 conjugate (Invitrogen) (51, 52). Alexa Fluor 594 WGA selectively binds to GlcNAc substituents in WTA on the surface of S. aureus (53). Briefly, overnight cultured S. aureus cells with/without c-di-AMP exposure were adjusted to an OD600 of 1.0, washed, and resuspended within 1 ml phosphate-buffered saline with Tween 20 (PBST) buffer (120 mM NaCl, 50 mM phosphate, 0.1% Tween 20, pH 8.0). The 100-μl samples were mixed with 50 μl of Alexa Fluor 594 WGA solution (100 μg/ml) and incubated for 10 min at room temperature. After washing with a PBST buffer, fluorescence was measured by using a BioTek Synergy 2 plate reader (BioTek, Winooski, VT, USA) with excitation and emission wavelengths of 590 nm and 617 nm, respectively. GlcNAc levels were expressed as RFU.
RNA isolation and real-time quantitative reverse transcription-PCR.
Total RNA for dacA, gdpP, lytS, lytR, lrgB, and tarH expression was isolated from overnight cultured study MRSA strains (same incubation time as the experiments above for measuring ADP, ATP, and c-di-AMP levels) by using an RNeasy kit (Qiagen, Valencia, CA) (18). Then DNase-treated RNA was transcribed into complementary DNA. Real-time quantitative reverse transcription-PCR (RT-qPCR) was performed using an ABI Prism 7000 instrument (Applied Biosystems) and a SYBR green PCR master kit (Applied Biosystems). The primers for dacA, gdpP, lytS, and lytR amplification have been described previously (11, 54). The primers used to amplify lrgB and tarH were lrgB-F (5′-ACAATCTGTTTTGCGATTCCG-3′) and lrgB-R (5′-CTGTAGTTGCTGCTTGAGGT-3′) (23) and tarH-F (5′-TAACGAAGCGGGACTCATCG-3′) and tarH-R (5′-TGCTTGGATTGAAGGCGGAA-3′), respectively. gyrB was used to normalize the transcript quantification, and relative expression of the study genes was calculated by the ΔΔCT method (3).
Experimental endocarditis model in rabbits.
A well-characterized rabbit model of catheter-induced aortic valve endocarditis was used to study the composite metrics of virulence and responsiveness to VAN therapy among the MRSA LAC parental strain and its isogenic dacAG206S mutant and dacA-complemented strains (18, 28). The Institutional Animal Care and Use Committee of the Lundquist Institute at Harbor-UCLA Medical Center approved all animal study protocols. After 72 h of catheterization, animals were infected intravenously (i.v.) with the LAC parental strain, its dacAG206S mutant, or dacA-complemented strain at 105 CFU/animal, an 95% infective dose (ID95) as established previously (18, 28). At 24 h postinfection, animals were randomized to receive no therapy (controls) or VAN (3.75 mg/kg of body weight, i.v., twice daily for 3 days; this dose-regimen of VAN was shown to exert limited microbiologic clearance of the parental strain from any target tissue based on extensive pilot studies). Control animals were sacrificed at 24 h postinfection in order to determine MRSA density in target tissues at the beginning of VAN treatment. VAN-treated animals were sacrificed 24 h after the last treatment dose to avoid VAN carryover effect. At sacrifice, cardiac vegetation, kidney, and spleen were sterilely removed and quantitatively cultured (18, 28). MRSA counts in the target tissues were given as the mean log10 CFU per gram of tissue (± standard deviation [SD]).
Statistical analysis.
All in vitro experiments were performed in triplicate and repeated at least twice. Statistical significance values of in vitro and in vivo experiments were obtained by performing a two-tailed Student's t test and one-way analysis of variance (ANOVA) with Tukey’s multiple-comparison test (no adjustment), respectively. P values of <0.05 were considered statistically significant.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health research grant (1R01-AI139244 to Y.Q.X.).
We thank Angelika Gründling at Imperial College London, London, UK, for kindly providing us the dacAG206S mutant strain.
L.L., A.L.C., R.A.P., and Y.Q.X. designed the research; L.L., Y.L., F.Z., G.W., and Y.Q.X. performed the experiments; G.B. contributed new reagents/analytic tools; L.L., Y.L., F.Z., A.S.B., and Y.Q.X. analyzed data; L.L. and Y.Q.X. wrote the paper; and A.L.C., M.R.Y., and A.S.B., edited the paper.
Footnotes
Citation Li L, Li Y, Zhu F, Cheung AL, Wang G, Bai G, Proctor RA, Yeaman MR, Bayer AS, Xiong YQ. 2021. New mechanistic insights into purine biosynthesis with second messenger c-di-AMP in relation to biofilm-related persistent methicillin-resistant Staphylococcus aureus infections. mBio 12:e02081-21. https://doi.org/10.1128/mBio.02081-21.
Contributor Information
Yan Q. Xiong, Email: yxiong@lundquist.org.
Paul Dunman, University of Rochester.
REFERENCES
- 1.Fowler VG, Jr, Sakoulas G, McIntyre LM, Meka VG, Arbeit RD, Cabell CH, Stryjewski ME, Eliopoulos GM, Reller LB, Corey GR, Jones T, Lucindo N, Yeaman MR, Bayer AS. 2004. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J Infect Dis 190:1140–1149. doi: 10.1086/423145. [DOI] [PubMed] [Google Scholar]
- 2.Xiong YQ, Fowler VG, Yeaman MR, Perdreau-Remington F, Kreiswirth BN, Bayer AS. 2009. Phenotypic and genotypic characteristics of persistent methicillin-resistant Staphylococcus aureus bacteremia in vitro and in an experimental endocarditis model. J Infect Dis 199:201–208. doi: 10.1086/595738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seidl K, Chen L, Bayer AS, Hady WA, Kreiswirth BN, Xiong YQ. 2011. Relationship of agr expression and function with virulence and vancomycin treatment outcomes in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 55:5631–5639. doi: 10.1128/AAC.05251-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guerra FE, Borgogna TR, Patel DM, Sward EW, Voyich JM. 2017. Epic immune battles of history: neutrophils vs. Staphylococcus aureus. Front Cell Infect Microbiol 7:286. doi: 10.3389/fcimb.2017.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vidaillac C, Gardete S, Tewhey R, Sakoulas G, Kaatz GW, Rose WE, Tomasz A, Rybak MJ. 2013. Alternative mutational pathways to intermediate resistance to vancomycin in methicillin-resistant Staphylococcus aureus. J Infect Dis 208:67–74. doi: 10.1093/infdis/jit127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowman L, Zeden MS, Schuster CF, Kaever V, Grundling A. 2016. New insights into the cyclic di-adenosine monophosphate (c-di-AMP) degradation pathway and the requirement of the cyclic dinucleotide for acid stress resistance in Staphylococcus aureus. J Biol Chem 291:26970–26986. doi: 10.1074/jbc.M116.747709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dengler V, McCallum N, Kiefer P, Christen P, Patrignani A, Vorholt JA, Berger-Bachi B, Senn MM. 2013. Mutation in the c-di-AMP cyclase dacA affects fitness and resistance of methicillin resistant Staphylococcus aureus. PLoS One 8:e73512. doi: 10.1371/journal.pone.0073512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeden MS, Schuster CF, Bowman L, Zhong Q, Williams HD, Grundling A. 2018. Cyclic di-adenosine monophosphate (c-di-AMP) is required for osmotic regulation in Staphylococcus aureus but dispensable for viability in anaerobic conditions. J Biol Chem 293:3180–3200. doi: 10.1074/jbc.M117.818716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corrigan RM, Bowman L, Willis AR, Kaever V, Grundling A. 2015. Cross-talk between two nucleotide-signaling pathways in Staphylococcus aureus. J Biol Chem 290:5826–5839. doi: 10.1074/jbc.M114.598300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng X, Zhang Y, Bai G, Zhou X, Wu H. 2016. Cyclic di-AMP mediates biofilm formation. Mol Microbiol 99:945–959. doi: 10.1111/mmi.13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gries CM, Bruger EL, Moormeier DE, Scherr TD, Waters CM, Kielian T. 2016. Cyclic di-AMP released from Staphylococcus aureus biofilm induces a macrophage type I interferon response. Infect Immun 84:3564–3574. doi: 10.1128/IAI.00447-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romling U. 2008. Great times for small molecules: c-di-AMP, a second messenger candidate in Bacteria and Archaea. Sci Signal 1:pe39. doi: 10.1126/scisignal.133pe39. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Abdelhady W, Donegan NP, Seidl K, Cheung A, Zhou YF, Yeaman MR, Bayer AS, Xiong YQ. 2018. Role of purine biosynthesis in persistent methicillin-resistant Staphylococcus aureus infection. J Infect Dis 218:1367–1377. doi: 10.1093/infdis/jiy340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Bayer AS, Cheung A, Lu L, Abdelhady W, Donegan NP, Hong JI, Yeaman MR, Xiong YQ. 2020. The stringent response contributes to persistent methicillin-resistant Staphylococcus aureus endovascular infection through the purine biosynthetic pathway. J Infect Dis 222:1188–1198. doi: 10.1093/infdis/jiaa202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Morar M, Ealick SE. 2008. Structural biology of the purine biosynthetic pathway. Cell Mol Life Sci 65:3699–3724. doi: 10.1007/s00018-008-8295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seidl K, Bayer AS, Fowler VG, Jr, McKinnell JA, Abdel Hady W, Sakoulas G, Yeaman MR, Xiong YQ. 2011. Combinatorial phenotypic signatures distinguish persistent from resolving methicillin-resistant Staphylococcus aureus bacteremia isolates. Antimicrob Agents Chemother 55:575–582. doi: 10.1128/AAC.01028-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L, Wang G, Li Y, Francois P, Bayer AS, Chen L, Seidl K, Cheung A, Xiong YQ. 2020. Impact of the novel prophage ɸSA169 on persistent methicillin-resistant Staphylococcus aureus endovascular infection. mSystems 5:e00178-20. doi: 10.1128/mSystems.00178-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, Cheung A, Bayer AS, Chen L, Abdelhady W, Kreiswirth BN, Yeaman MR, Xiong YQ. 2016. The global regulon sarA regulates β-lactam antibiotic resistance in methicillin-resistant Staphylococcus aureus in vitro and in endovascular infections. J Infect Dis 214:1421–1429. doi: 10.1093/infdis/jiw386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeFrancesco AS, Masloboeva N, Syed AK, DeLoughery A, Bradshaw N, Li GW, Gilmore MS, Walker S, Losick R. 2017. Genome-wide screen for genes involved in eDNA release during biofilm formation by Staphylococcus aureus. Proc Natl Acad Sci USA 114:E5969–E5978. doi: 10.1073/pnas.1704544114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otto M. 2008. Staphylococcal biofilms. Curr Top Microbiol Immunol 322:207–228. doi: 10.1007/978-3-540-75418-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allesen-Holm M, Barken KB, Yang L, Klausen M, Webb JS, Kjelleberg S, Molin S, Givskov M, Tolker-Nielsen T. 2006. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol Microbiol 59:1114–1128. doi: 10.1111/j.1365-2958.2005.05008.x. [DOI] [PubMed] [Google Scholar]
- 22.Sharma-Kuinkel BK, Mann EE, Ahn JS, Kuechenmeister LJ, Dunman PM, Bayles KW. 2009. The Staphylococcus aureus LytSR two-component regulatory system affects biofilm formation. J Bacteriol 191:4767–4775. doi: 10.1128/JB.00348-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beltrame CO, Cortes MF, Bonelli RR, Correa AB, Botelho AM, Americo MA, Fracalanzza SE, Figueiredo AM. 2015. Inactivation of the autolysis-related genes lrgB and yycI in Staphylococcus aureus increases cell lysis-dependent eDNA release and enhances biofilm development in vitro and in vivo. PLoS One 10:e0138924. doi: 10.1371/journal.pone.0138924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romaniuk JA, Cegelski L. 2015. Bacterial cell wall composition and the influence of antibiotics by cell-wall and whole-cell NMR. Philos Trans R Soc Lond B Biol Sci 370:20150024. doi: 10.1098/rstb.2015.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang F, Zhou H, Olademehin OP, Kim SJ, Tao P. 2018. Insights into key interactions between vancomycin and bacterial cell wall structures. ACS Omega 3:37–45. doi: 10.1021/acsomega.7b01483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peschel A, Vuong C, Otto M, Gotz F. 2000. The D-alanine residues of Staphylococcus aureus teichoic acids alter the susceptibility to vancomycin and the activity of autolytic enzymes. Antimicrob Agents Chemother 44:2845–2847. doi: 10.1128/AAC.44.10.2845-2847.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wanner S, Schade J, Keinhorster D, Weller N, George SE, Kull L, Bauer J, Grau T, Winstel V, Stoy H, Kretschmer D, Kolata J, Wolz C, Broker BM, Weidenmaier C. 2017. Wall teichoic acids mediate increased virulence in Staphylococcus aureus. Nat Microbiol 2:16257. doi: 10.1038/nmicrobiol.2016.257. [DOI] [PubMed] [Google Scholar]
- 28.Abdelhady W, Bayer AS, Seidl K, Moormeier DE, Bayles KW, Cheung A, Yeaman MR, Xiong YQ. 2014. Impact of vancomycin on sarA-mediated biofilm formation: role in persistent endovascular infections due to methicillin-resistant Staphylococcus aureus. J Infect Dis 209:1231–1240. doi: 10.1093/infdis/jiu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corrigan RM, Abbott JC, Burhenne H, Kaever V, Grundling A. 2011. c-di-AMP is a new second messenger in Staphylococcus aureus with a role in controlling cell size and envelope stress. PLoS Pathog 7:e1002217. doi: 10.1371/journal.ppat.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valentini M, Filloux A. 2016. Biofilms and cyclic di-GMP (c-di-GMP) signaling: lessons from Pseudomonas aeruginosa and other bacteria. J Biol Chem 291:12547–12555. doi: 10.1074/jbc.R115.711507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holland LM, O'Donnell ST, Ryjenkov DA, Gomelsky L, Slater SR, Fey PD, Gomelsky M, O'Gara JP. 2008. A staphylococcal GGDEF domain protein regulates biofilm formation independently of cyclic dimeric GMP. J Bacteriol 190:5178–5189. doi: 10.1128/JB.00375-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mann EE, Rice KC, Boles BR, Endres JL, Ranjit D, Chandramohan L, Tsang LH, Smeltzer MS, Horswill AR, Bayles KW. 2009. Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS One 4:e5822. doi: 10.1371/journal.pone.0005822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cue D, Junecko JM, Lei MG, Blevins JS, Smeltzer MS, Lee CY. 2015. SaeRS-dependent inhibition of biofilm formation in Staphylococcus aureus Newman. PLoS One 10:e0123027. doi: 10.1371/journal.pone.0123027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ranjit DK, Endres JL, Bayles KW. 2011. Staphylococcus aureus CidA and LrgA proteins exhibit holin-like properties. J Bacteriol 193:2468–2476. doi: 10.1128/JB.01545-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunskill EW, Bayles KW. 1996. Identification of LytSR-regulated genes from Staphylococcus aureus. J Bacteriol 178:5810–5812. doi: 10.1128/jb.178.19.5810-5812.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schirner K, Stone LK, Walker S. 2011. ABC transporters required for export of wall teichoic acids do not discriminate between different main chain polymers. ACS Chem Biol 6:407–412. doi: 10.1021/cb100390w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown S, Santa Maria JP, Jr, Walker S. 2013. Wall teichoic acids of gram-positive bacteria. Annu Rev Microbiol 67:313–336. doi: 10.1146/annurev-micro-092412-155620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang J, Bai Y, Zhang Y, Gabrielle VD, Jin L, Bai G. 2014. Deletion of the cyclic di-AMP phosphodiesterase gene (cnpB) in Mycobacterium tuberculosis leads to reduced virulence in a mouse model of infection. Mol Microbiol 93:65–79. doi: 10.1111/mmi.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fahmi T, Faozia S, Port GC, Cho KH. 2019. The second messenger c-di-AMP regulates diverse cellular pathways involved in stress response, biofilm formation, cell wall homeostasis, SpeB expression, and virulence in Streptococcus pyogenes. Infect Immun 87:e00147-19. doi: 10.1128/IAI.00147-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spentzas T, Kudumula R, Acuna C, Talati AJ, Ingram KC, Savorgnan F, Meals EA, English BK. 2011. Role of bacterial components in macrophage activation by the LAC and MW2 strains of community-associated, methicillin-resistant Staphylococcus aureus. Cell Immunol 269:46–53. doi: 10.1016/j.cellimm.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Mempin R, Tran H, Chen C, Gong H, Kim Ho K, Lu S. 2013. Release of extracellular ATP by bacteria during growth. BMC Microbiol 13:301. doi: 10.1186/1471-2180-13-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zegzouti H, Zdanovskaia M, Hsiao K, Goueli SA. 2009. ADP-Glo: a bioluminescent and homogeneous ADP monitoring assay for kinases. Assay Drug Dev Technol 7:560–572. doi: 10.1089/adt.2009.0222. [DOI] [PubMed] [Google Scholar]
- 43.Bai Y, Yang J, Zarrella TM, Zhang Y, Metzger DW, Bai G. 2014. Cyclic di-AMP impairs potassium uptake mediated by a cyclic di-AMP binding protein in Streptococcus pneumoniae. J Bacteriol 196:614–623. doi: 10.1128/JB.01041-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bai Y, Yang J, Eisele LE, Underwood AJ, Koestler BJ, Waters CM, Metzger DW, Bai G. 2013. Two DHH subfamily 1 proteins in Streptococcus pneumoniae possess cyclic di-AMP phosphodiesterase activity and affect bacterial growth and virulence. J Bacteriol 195:5123–5132. doi: 10.1128/JB.00769-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Underwood AJ, Zhang Y, Metzger DW, Bai G. 2014. Detection of cyclic di-AMP using a competitive ELISA with a unique pneumococcal cyclic di-AMP binding protein. J Microbiol Methods 107:58–62. doi: 10.1016/j.mimet.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdelhady W, Bayer AS, Seidl K, Nast CC, Kiedrowski MR, Horswill AR, Yeaman MR, Xiong YQ. 2013. Reduced vancomycin susceptibility in an in vitro catheter-related biofilm model correlates with poor therapeutic outcomes in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 57:1447–1454. doi: 10.1128/AAC.02073-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iyer VS, Hancock LE. 2012. Deletion of sigma(54) (rpoN) alters the rate of autolysis and biofilm formation in Enterococcus faecalis. J Bacteriol 194:368–375. doi: 10.1128/JB.06046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brunskill EW, Bayles KW. 1996. Identification and molecular characterization of a putative regulatory locus that affects autolysis in Staphylococcus aureus. J Bacteriol 178:611–618. doi: 10.1128/jb.178.3.611-618.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manna AC, Ingavale SS, Maloney M, van Wamel W, Cheung AL. 2004. Identification of sarV (SA2062), a new transcriptional regulator, is repressed by SarA and MgrA (SA0641) and involved in the regulation of autolysis in Staphylococcus aureus. J Bacteriol 186:5267–5280. doi: 10.1128/JB.186.16.5267-5280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiong YQ, Van Wamel W, Nast CC, Yeaman MR, Cheung AL, Bayer AS. 2002. Activation and transcriptional interaction between agr RNAII and RNAIII in Staphylococcus aureus in vitro and in an experimental endocarditis model. J Infect Dis 186:668–677. doi: 10.1086/342046. [DOI] [PubMed] [Google Scholar]
- 51.Nir-Paz R, Eugster MR, Zeiman E, Loessner MJ, Calendar R. 2012. Listeria monocytogenes tyrosine phosphatases affect wall teichoic acid composition and phage resistance. FEMS Microbiol Lett 326:151–160. doi: 10.1111/j.1574-6968.2011.02445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winstel V, Sanchez-Carballo P, Holst O, Xia G, Peschel A. 2014. Biosynthesis of the unique wall teichoic acid of Staphylococcus aureus lineage ST395. mBio 5:e00869-14. doi: 10.1128/mBio.00869-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wright CS. 1984. Structural comparison of the two distinct sugar binding sites in wheat germ agglutinin isolectin II. J Mol Biol 178:91–104. doi: 10.1016/0022-2836(84)90232-8. [DOI] [PubMed] [Google Scholar]
- 54.Neumann Y, Ohlsen K, Donat S, Engelmann S, Kusch H, Albrecht D, Cartron M, Hurd A, Foster SJ. 2015. The effect of skin fatty acids on Staphylococcus aureus. Arch Microbiol 197:245–267. doi: 10.1007/s00203-014-1048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Voet DV, Charlotte JP. 2008. Fundamentals of biochemistry: life at the molecular level, 3rd ed. Wiley, Hoboken, NJ. [Google Scholar]