Abstract
Serum lipids are biomarkers of cardiometabolic disease risk, and understanding genomic factors contributing to their distribution is of interest. Studies of lipids in Africans are rare, though it is expected that such studies could identify novel loci. We conducted a GWAS of 4317 Africans enrolled from Nigeria, Ghana and Kenya. We evaluated linear mixed models of high-density lipoprotein cholesterol (HDLC), low-density lipoprotein cholesterol (LDLC), total cholesterol (CHOL), triglycerides (TG) and TG/HDLC. Replication was attempted in 9542 African Americans (AA).
In our main analysis, we identified 28 novel associations in Africans. Of the 18 of these that could be tested in AA, three associations replicated (GPNMB-TG, ENPP1-TG and SMARCA4-LDLC). Five additional novel loci were discovered upon meta-analysis with AA (rs138282551-TG, PGBD5-HDLC, CD80-TG/HDLC, SLC44A1-CHOL and TLL2-CHOL). Analyses considering only those with predominantly West African ancestry (Nigeria, Ghana and AA) yielded new insights: ORC5-LDLC and chr20:60973327-CHOL.
Among our novel findings are some loci with known connections to lipids pathways. For instance, rs147706369 (TLL2) alters a regulatory motif for sterol regulatory element-binding proteins, a family of transcription factors that control the expression of a range of enzymes involved in cholesterol, fatty acid and TG synthesis, and rs115749422 (SMARCA4), an independent association near the known LDLR locus that is rare or absent in populations without African ancestry. These findings demonstrate the utility of conducting genomic analyses in Africans for discovering novel loci and provide some preliminary evidence for caution against treating ‘African ancestry’ as a monolithic category.
Introduction
Serum lipids are an important biomarker of cardiometabolic disease risk. As such, understanding the genomic factors contributing to their distribution has been of considerable interest. Large meta-analyses have identified over 150 loci associated with the distribution of serum lipids (1–8); GWAS in Africa are rare (9). There are several reasons that this lack is concerning. First, as those of African ancestry have the greatest degree of genetic diversity in the world (10), unless these populations are sufficiently represented in genomic research, a great deal of that diversity is not being evaluated for an association with serum lipids, potentially limiting the molecular insights that can be gained in our understanding of these traits. Second, although genome-wide studies of African Americans (AA) have been conducted, the drastically different environments of AA and continental Africans (AF) may differentially impact genetic associations. For instance, gene–environment interactions with environmental factors that differ between AF and AA have been found to influence serum lipid distributions, which may affect the power to detect a genetic association across populations (11–18). As representation of those of African ancestry in genomic research has been relatively limited, it is common to analyze all those of African ancestry together to increase sample size; however, collapsing groups with such genomic and environmental diversity may obscure findings that are unique to populations within this broad category.
Here, we conducted a genome-wide association study (GWAS) of serum lipids in 4317 Africans from the Africa America Diabetes Mellitus (AADM) study, a case–control study of type 2 diabetes (T2D) with sites in Nigeria, Ghana and Kenya. Replication of GWAS-significant loci was sought in 9542 AA. We report 28 novel loci with P < 5 × 10−8 in Africans. Of the 18 of these that were available in the dataset of AA, three replicated. Meta-analyses of Africans and AA identified a further five associations. Novel loci were also identified when conducting analyses in West Africans (WA) separately, highlighting the importance of considering heterogeneity between African populations when conducting genomic analyses.
Results
The study population was heavier and had a worse lipid profile than would be expected of a general population sampled in these regions, consistent with the fact that this study was nested within a case–control study of T2D and approximately half of participants were T2D cases (Table 1). Additionally, all recruitment was conducted in urban areas, and urban location is associated with worse cardiometabolic outcomes in West Africa (19).
Table 1.
Descriptive statistics for participants included in discovery analyses
| WA | EA | All | |
|---|---|---|---|
| N | 3583 | 734 | 4317 |
| Age (years) | 50.6 (13.2) | 55.4 (10.2) | 51.4 (12.9) |
| BMI (kg/m2) | 26.6 (5.7) | 26.4 (5.4) | 26.6 (5.6) |
| T2D* | 50.7% | 50.7% | 50.7% |
| HDLC (mg/dl) | 45.3 (16.7) | 47.4 (16.8) | 45.6 (16.7) |
| LDLC (mg/dl) | 131.1 (46.6) | 130.0 (46.3) | 130.0 (46.3) |
| TG (mg/dl) | 98.3 (48.3) | 167.5 (91.9) | 110.0 (63.5) |
| CHOL (mg/dl) | 196.8 (52.9) | 206.2 (58.0) | 198.4 (53.9) |
| TG/HDLC | 2.63 (2.13) | 3.96 (2.77) | 2.85 (2.3) |
Mean (standard deviation) reported, except where indicated. *Proportion shown.
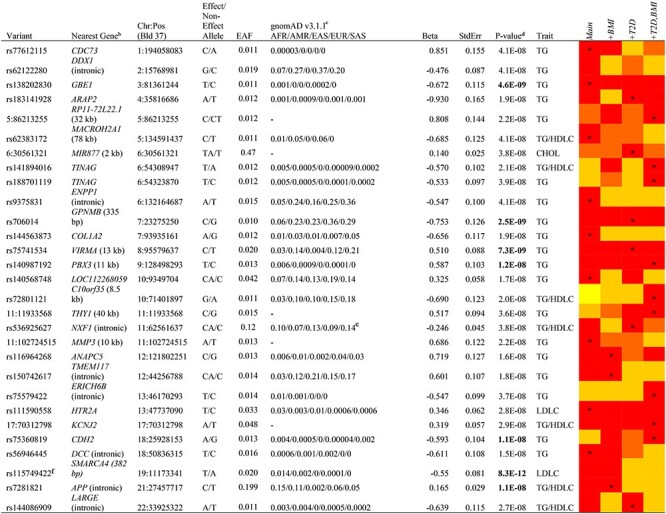
Our main GWAS analysis in Africans identified 28 novel lipids loci (34 variants) associated at P < 5 × 10−8[Table 2; Supplementary Material, Fig. S1 (Manhattan plots) and Supplementary Material, Fig. S2 (QQ plots)]. While all associations P < 5 × 10−8 are reported, associations that would have passed a stricter threshold for statistical significance (adjusted for number of analyses undertaken, P < 1.25 × 10−8) are indicated. Novelty was determined by conditional analyses where known loci for that trait are within 1 MB of an association. Notable among these findings were associations of variants with higher frequency among Africans compared with other worldwide populations, perhaps allowing for their detection in this analysis. For instance, the minor alleles for rs138202830 (GBE1), rs188701119 (TINAG), rs140987192 (PBX3), rs75360819 (CDH2), rs115749422 (SMARCA4), rs114213467 (QTRT1), rs111590558 (HTR2A) and rs7281821 (APP) are at their highest frequencies in the African/African-American (AFR) compared with other gnomAD populations (Table 2).
Table 2.
Novel genome-wide lipids associations P < 5E−08 among Africans (n = 4317)a
|
Notes: Red indicates P < 5E−8; orange 5E−8 < P ≤ 1E−8; gold 1E−8 < P ≤ 1E−7; yellow 1E−7 < P ≤ 1E−6; models indicated with an asterisk were the most statistically significant results and are shown in the table. EAF, effect allele frequency.
aTable includes novel associations in known loci (annotated with a footnote) as well as novel loci.
bNearest gene to variant is listed, with distance shown in parenthesis where <50 kb.
cgnomAD v3.1.1 populations: AFR; Latino/Admixed American (AMR); East Asian (EAS); EUR; South Asian (SAS).
dBolded P-values pass a stricter threshold for statistical significance, P < 1.25 × 10−8, with correction for multiple analyses (see methods).
eGlobal distribution estimates differed greatly in gnomAD and 1000 Genomes databases: showing 1000 Genomes data, as that is consistent with what was observed in our data.
fAssociation persisted after adjustment for known lipids locus within 1 MB (Supplementary Material, Table S3).
Given the potential impact of both body mass index (BMI) and T2D on associations with serum lipids, particularly in this dataset nested within a case–control study of T2D, we tested models with and without adjustment for these two covariates. In terms of statistical significance, adjustment for BMI and T2D had relatively minor effects. For 27 of the 28 novel loci, associations that were statistically significant in one model had P ≤ 10−7 for all other models (for the remaining locus, all models were P ≤ 10−6; Table 2).
To determine the degree to which these associations were also found among AA, we evaluated these loci in 9542 AA (Supplementary Material, Table S1). Of 34 variants in 28 loci investigated, 20 variants in 18 loci passed frequency and quality control criteria and were able to be evaluated (Supplementary Material, Table S2). Three of these loci were replicated (P < 0.05 with matching direction of association). The minor (G) allele of rs706014 (5′ of GPNMB, Glycoprotein Nonmetastatic Melanoma Protein B) was associated with lower triglycerides (TG) in both Africans (β −0.75 TG, P = 2.5 × 10−9) and in AA (β −0.084 TG, P = 0.0093). The minor (T) allele of rs9375831, intronic to ENPP1 (ectonucleotide pyrophosphatase/phosphodiesterase 1), was also associated with lower TG in Africans (β −0.55 TG, P = 4.1 × 10−8) and in AA (β −0.076 TG, P = 0.037). As variants in this gene have been previously associated with insulin resistance and related traits (20–25), we also investigated this relationship in our data. An association of the minor allele of rs9375831 with HOMA-IR in AF participants without T2D did not reach statistical significance (P = 0.2) but was directionally consistent: the minor allele was associated with decreased TG and decreased HOMA-IR. We also evaluated the ratio of TG to HDLC, a biomarker of insulin resistance: the minor allele was associated with decreased TG/HDLC (P = 0.0002), though this association may be largely driven by the association of this variant with TG. We also replicated a novel association near the known LDLR locus: the minor (T) allele for rs115749422, 382 bp downstream of SMARCA4, was associated with lower low-density lipoprotein cholesterol (LDLC) in both AF (β −0.55, P = 8.3 × 10−12) and AA (β −0.58, P = 5.8 × 10−16), associations that persisted after adjustment for rs73015024 in LDLR (Supplementary Material, Table S3). There were no further GWAS significant associations after adjustment for both of these variants. Notably, rs115749422 is absent or rare in non-AFR populations.
We conducted meta-analyses of Africans and AA, which yielded five additional novel GWAS-significant loci (Table 3). Notable among these findings are associations with variants that are more common or only found among African ancestry populations; rs147706369 (TLL2) and rs138282551 (intergenic). A similar magnitude of association was observed in Africans and AA for many of these associations: rs1468291761 (PGBD5, β 0.14 versus 0.12), rs148194085 (CD80, β 0.22 versus 0.18) and rs147706369 (TLL2, β 0.34 versus 0.28). We also conducted meta-analysis with MetaSoft, a tool that incorporates Han and Eskin’s random effects model, which may be more appropriate in the presence of differential effect sizes across diverse ancestry groups. The differences are negligible across all analyses between these results and those from meta-analysis using METAL both in terms of loci that reached statistical significance and in distribution of P-values and are not discussed further.
Table 3.
Novel signals in meta-analysis of Africans and AA
| rsID | Chr:Posa | Eff/Non-Eff Alleles | gnomAD MAFb AFR/AMR/EAS/EUR/SAS | Traitc | Analysis | N | EAF | Beta | SE | P-valued |
|---|---|---|---|---|---|---|---|---|---|---|
| rs138282551 | 1:81649749 | T/C | 0.03/0.003/0/0.0002/0.0004 | TG | Africans | 4292 | 0.04 | 0.29 | 0.058 | 4.5E−07 |
| Intergenic | Afr. Americans | 8537 | 0.03 | 0.14 | 0.049 | 3.5E−03 | ||||
| Meta-analysis | 12 829 | 0.035 | 0.20 | 0.037 | 4.6E−08 | |||||
| rs1468291761e | 1:230471310 | C/G | TOPMed: 0.22f | HDLC | Africans | 4311 | 0.18 | 0.14 | 0.037 | 9.9E−05 |
| PGBD5 | GGVP: 0.10 | Afr. Americans | 8833 | 0.16 | 0.12 | 0.029 | 2.5E−05 | |||
| Intronic | Meta-analysis | 13 144 | 0.17 | 0.13 | 0.023 | 1.1E−08 | ||||
| rs148194085e | 3:119258568 | T/ | 0.04/0.02/0.0004/0.03/0.01 | TG/HDLC | Africans | 4269 | 0.04 | 0.22 | 0.057 | 1.1E−04 |
| CD80 | TGAATGTAGG | Afr. Americans | 8516 | 0.04 | 0.18 | 0.045 | 7.9E−05 | |||
| Intronic | Meta-analysis | 12 785 | 0.04 | 0.19 | 0.035 | 4.0E−08 | ||||
| rs79922971e,g | 9:107949663 | G/A | 0.04/0.08/0.04/0.12/0.14 | CHOL | Africans | 4284 | 0.02 | -0.31 | 0.084 | 2.4E−04 |
| SLC44A1 | Afr. Americans | 8838 | 0.04 | −0.17 | 0.04 | 1.3E−05 | ||||
| 57 kb upstream | Meta-analysis | 13 122 | 0.04 | −0.20 | 0.036 | 3.2E−08 | ||||
| rs147706369 | 10:98213172 | T/C | 0.02/0.0009/0/0/0 | CHOL | Africans | 4300 | 0.016 | 0.34 | 0.090 | 1.5E−04 |
| TLL2 | Afr. Americans | 9217 | 0.015 | 0.28 | 0.067 | 2.6E−05 | ||||
| Intronic | Meta-analysis | 13 517 | 0.016 | 0.30 | 0.054 | 1.7E−08 |
aBld. 37.
bgnomAD v3.1.1.
cAll traits inverse normal transformed.
dBolded P-values pass a stricter threshold for statistical significance, P < 1.25 × 10−8, with correction for multiple analyses (see Materials and Methods).
eAssociation persisted after adjustment for known lipids locus within 1 MB (Supplementary Material, Table S3).
fgnomAD and 1000 Genomes data unavailable for this variant. Providing instead MAF for TOPMed and the Gambian Genome Variation Project (GGVP).
gSimilar associations observed for rs72742531, rs7029574 and rs375690244.
Our main analyses of Africans included participants from both West Africa [Nigeria (n = 2207) and Ghana (n = 1376)] and East Africa [Kenya (n = 734)]. Given the genetic and environmental differences among the sampled populations, it is possible that a signal present in participants from only one of these geographic regions could be masked when the two were included together. Additionally, mean values for TG and closely related parameters [TG/high-density lipoprotein cholesterol (HDLC) and total cholesterol (CHOL)] differed dramatically in East Africans (EA) compared with WA (TG 167.5 versus 98.3 mg/dl, respectively; Supplementary Material, Table S4A). These differences do not appear to be driven by BMI (Supplementary Material, Table S4A) or by T2D severity or duration. The duration of T2D was similar in WA (mean 7.2 years) and EA (mean 6.8 years), and the vast majority of patients in both regions were being treated with oral medications and/or insulin (West: 94.8%; East: 93.0%; Supplementary Material, Table S4B). Based on these observations, we conducted a separate analysis of WA; a separate analysis of EA is not presented due to the relatively small sample size. There were no novel hits P < 5 × 10−8 when WA were considered on their own, but known signals that were not apparent in the full GWAS were observed in the analysis of WA only, including the known chromosome 11 locus BUD13 and, to a lesser extent, APOE/APOC1 on chromosome 19 (Fig. 1). As AA have predominantly WA ancestry (79.9% (26)), meta-analysis of WA and AA was also conducted, and two additional hits were identified (Table 4). The minor (C) allele of rs7797481 (ORC5) was associated with higher LDLC in both WA [β 0.25, standard error (SE) = 0.075, P = 8.4 × 10−4] and AA (β 0.16, SE = 0.035, P = 2.5 × 10−6). The association was quite different among the EA (β −0.38, SE = 0.20, P = 0.053; P for heterogeneity between WA and EA = 0.0028), and their inclusion in the meta-analysis reduced the statistical significance of the association (Fig. 2A). In contrast, for the association between the minor (T) allele of 20:60973327 [chromosome:position (build 37)] and higher TG in both WA (β 0.21, SE = 0.049, P = 2.2 × 10−5) and AA (β 0.13, SE = 0.035, P = 2.2 × 10−4), the effect size was similar among EA but with a large SE (β 0.13, SE = 0.11, P = 0.24), making the association above our threshold for statistical significance when they were included in the meta-analysis (Fig. 2B; P for heterogeneity between WA and EA = 0.50).
Figure 1 .
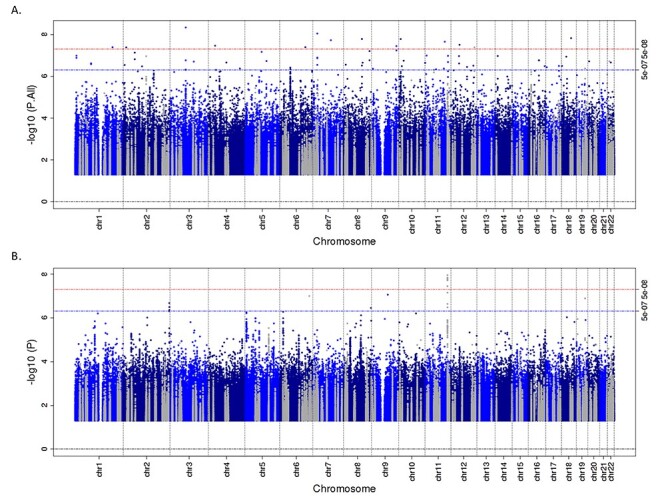
Manhattan plot for TG in all Africans (A) and in WA only (B). The grayed points represent variants within 1 MB of known lipids loci.
Table 4.
Additional novel signals in meta-analysis of WA and AA
| rsID | Chr:Posa | Eff/Non-Eff Alleles | gnomAD MAFb AFR/AMR/EAS/EUR/SAS | Traitc | Analysis | N | EAF | Beta | SE | P-value |
|---|---|---|---|---|---|---|---|---|---|---|
| rs7797481 | 7:103770056 | C/G | 0.05/0.15/0.001/0.18/0.09- | LDLC | WA | 3566 | 0.028 | 0.25 | 0.075 | 8.4E−04 |
| ORC5 | Afr. Americans | 8501 | 0.057 | 0.16 | 0.035 | 2.5E−06 | ||||
| intronic | Meta-analysis | 12 067 | 0.052 | 0.18 | 0.031 | 1.4E−08 | ||||
| – | 20:60973327 | T/C | 0.0002/0/0.002/0.0006/- | CHOL | WA | 3565 | 0.110 | 0.21 | 0.049 | 2.2E−05 |
| Afr. Americans | 8901 | 0.105 | 0.13 | 0.035 | 2.2E−04 | |||||
| Meta-analysis | 12 466 | 0.107 | 0.16 | 0.029 | 4.4E−08 |
aBld. 37
bgnomAD v3.1.1.
cAll traits inverse normal transformed.
Figure 2 .
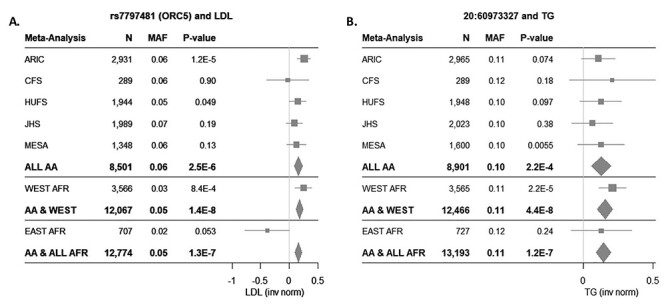
Novel associations in meta-analysis of WA and AA. Forest plots showing the associations of rs7797481 (ORC5) and LDLC (A) and 20:60973327 and TG (B) across included African Ancestry cohorts.
To assess transferability of our findings to populations of European ancestry, we conducted lookups of all novel associations in our analyses in a recent meta-analysis of serum lipids among up to 90 266 individuals of European ancestry (16). In total, 14 of the 35 loci had a minor allele frequency (MAF) < 0.01 among European, non-Finnish (EUR) in gnomAD and were not present in these data (analyses were filtered for MAF < 0.01). Of the remaining variants that were able to be evaluated, all associations were in the same direction as reported in our data, but none of these approached statistical significance (Supplementary Material, Table S5).
In addition to the novel associations described above, we also report significant associations in known lipids loci in the GWAS of Africans (n = 4: PCSK9, CETP, LDLR and APOE), meta-analysis of Africans and AA (n = 18) and the meta-analysis of WA and AA (n = 16; Supplementary Material, Table S6). For further exploration of known loci in our data, we sought replication for 1853 previously identified single-nucleotide polymorphism (SNP)–trait associations from large genome-wide studies of serum lipids (1–8). Of 1534 SNP–trait associations available to be tested in our data, 947 were in a consistent direction (62%) and 149 of those replicated (consistent direction and P < 0.05). This represents an exact replication rate of 9.7% (Supplementary Material, Table S7). Of the SNP–trait associations that did not replicate in the exact analysis, 702 had variants that were suitable for evaluation using ‘local’ replication [a linkage disequilibrium (LD)-based replication technique, see Materials and Methods]. An additional 27 SNP–trait associations in 24 loci were replicated using these criteria (Supplementary Material, Table S8). In total, we replicated 83 out of 303 loci (27%).
Discussion
In this GWAS of serum lipids in 4317 AFs, we identified 28 novel lipids loci. Of these, 18 loci were available to be tested in 9542 AA for replication and three of these associations replicated (GPNMB-TG, ENPP1-TG and SMARCA4-LDLC). Upon meta-analysis of the Africans and AA, five additional associations were found. Given genetic and phenotypic diversity between WA and EA, we also conducted analyses excluding EA. Two additional novel associations were identified with meta-analysis of WA and AA: ORC5-LDLC and chr20:60973327-CHOL.
Our focus on African populations facilitated discovery of novel TG findings based on both the understudied environmental and genomic context of these individuals. The majority of our findings were for TG. In a recent study assessing transferability of known lipids loci from European ancestry populations to Ugandans, ~70% of HDLC and LDLC loci were transferable to Ugandans, whereas only 10.5% of TG loci were transferable. The authors suggested that this difference may have resulted from differences in the environmental context (27). While environmental context is a likely contributor to variation in TG levels, differences in genetic context may also be important. Descriptive data from a recent serum lipids meta-analysis of nearly 100 cohorts (n = 387 272) under the aegis of the CHARGE consortium offer useful comparisons in terms of ancestry-level differences in TG (16): mean TG levels in CHARGE AA are relatively low compared with those of other ancestries [~100 mg/dl for all included AA studies, except Atherosclerosis Risk in Communities study (ARIC, 115 mg/dl)] and similar to the WA from AADM (mean 98.3 mg/dl), despite dramatically different environmental backgrounds. In contrast, the mean values for EA were markedly higher: mean 168 mg/dl. This distribution suggests that genomic factors underlie some of the variation across populations in TG levels.
As the numbers of studies and samples with African ancestry are low relative to European ancestry, studies including individuals of African ancestry generally consider them as a single group. However, this grouping may not be appropriate given the genetic diversity among those who could be categorized as having African ancestry and the diverse environmental backgrounds in which those of African ancestry reside. Given that our study population included individuals from both West and East Africa, who display genetic and dietary differences, we conducted analyses separately for WA. The exclusion of EA from the analyses, leaving the two groups with predominantly WA ancestry, allowed for the discovery of novel loci, presumably because of genetic similarities between the WA and AA, in contrast to the EA. The AA are still not an ideal replication sample for the WA; however, given both their admixture (with an average of 20% European ancestry) and the environmental background, which is quite distinct between those of African ancestry living in West Africa and in the USA. Thus, the differences in observed associations between these groups are likely to represent a combination of factors, including genetic differences, environmental context and false positives in unknown proportions.
Higher BMI and T2D are both associated with dyslipidemia, and Mendelian randomization studies suggest that BMI and T2D are causal for dyslipidemia (28,29). As such, we sought to establish whether associations were independent of an effect on either of these traits by conducting all analyses with and without adjustment for BMI and T2D. Given that this study is nested within a case–control study for T2D, and thus, participants had higher proportions of T2D and elevated BMI compared with what would be expected in a general population study, it was of particular importance to account for the potential impact of these factors on our analyses. In our experience, however, adjusting for either of these factors did not alter the findings considerably. For all of our main findings, statistical significance of the associations was quite similar across models, although some of the minor fluctuations meant that some associations in some models met our criteria for statistical significance, whereas others just missed. For our 28 loci with associations P < 5 × 10−8, all other models yielded associations with P ≤ 10−6, and for 27 of them, all other models yielded association with P ≤ 10−7. Based on these findings, while the presence or absence of BMI or T2D in the model may have made some signals easier to detect, their presence did not eliminate any associations, as would have been expected if an association with lipids was mediated by an effect of BMI or T2D.
The proportion of known lipids loci that replicated among Africans was 27%. Attempted replication of the previously reported index SNPs yielded a replication rate of only 9.8%. This was increased to 27% by using a LD-based ‘local’ replication method, which accounted for the generally lower LD among African ancestry individuals by evaluating all of the variants in LD with the index variant in the previously reported population (with correction for the number of variants tested). These proportions are similar to those reported for replication of known T2D loci in Africans, in which 15% replicated exactly and an additional 15% replicated using a local replication method (30), giving us confidence that these findings are in a reasonable range. While this relatively low proportion reflects in part the smaller sample size present for the Africans compared with the very large meta-analyses in which many of these loci were identified, there are other factors that may have contributed. For instance, there is evidence of differential associations across ancestry (31). Additionally, the environmental background of the Africans compared with those more well-represented populations could play a role. A recent analysis of the transferability of lipids loci to Ugandans reported that among major lipids loci (P < 10−100 in the largest European ancestry meta-analysis), 71% of those that were not transferable to Ugandans showed evidence of pleiotropy with BMI among European ancestry populations, whereas none of those that were transferable were also associated with BMI (these mostly represented well-established lipids pathways). The authors theorize that these discrepancies may stem from gene–environment interactions, with effects on lipids not shared across different environmental backgrounds. For these major known lipids loci, evidence for and against transferability to our WA and EA study populations matched very closely the results in the study in Ugandans (90% concordance) (27).
Functional annotation provides promising understanding of the biological mechanisms that may underlie some of the novel associations. rs115749422 (SMARCA4-LDLC) is 26 kb upstream of the well-known lipids locus LDLR, with evidence for the association of upstream variants with lipids (32,33). rs9375831 (ENPP1-TG) is in a gene known for a role in the development of insulin resistance (34,35), of which hypertriglyceridemia is a hallmark. ENPP1, also known as PC-1, directly interacts with the α-subunit of the insulin receptor, inhibiting insulin signaling. We also found some evidence for an association of rs9375831 with insulin resistance (TG/HDLC ratio) in our data, similar to associations of other ENPP1 variants (20–25), some of which also reported evidence for elevated TG (20,25). rs79922971 (SLC44A1-CHOL) alters a regulatory motif for C/EBP homologous protein, which has been shown to have a role in the transcriptional regulation of lipid metabolism (36). rs138282551 (intergenic-TG) alters motifs for CHD2, E2F and TCF4 (37). Studies of E2F1 knockout mice demonstrated that E2F1 is involved in global transcriptional regulation of de novo lipid synthesis and has been characterized as a major regulator of lipid metabolism (38). Deletion of E2f1 in cellular and mouse models leads to decreased Pcsk9 expression, increased LDLR expression and abnormal cholesterol accumulation in the liver (39). rs147706369 (TLL2-CHOL) alters a regulatory motif for sterol regulatory element-binding proteins (37), a family of transcription factors that control the expression of a range of enzymes involved in cholesterol, fatty acid and TG synthesis and have been called the ‘Master Regulators of Lipid Homeostasis’ (40). rs7797481 (ORC5-LDLC) alters regulatory motifs for FOXA and GR (37). Members of the FOXA family have been shown to alter lipid metabolism through regulation of multiple lipids pathways. Foxa1 inhibits the expression of many of the genes involved in TG synthesis and accumulation and in very low-density lipoprotein synthesis, including APOB (which encodes the major structural component of LDLC), in human hepatocytes (41).
Our conclusions should be evaluated in the context of our study’s strengths and weaknesses. One strength of this study is the large sample size for a study of AFs, who remain understudied in genomic analyses for serum lipids. Notably, all these individuals were recruited as part of one study, with identical questionnaires and assay methodology, to limit the potential for observed differences in EA versus WA being created by study methods. Differences among African ancestry individuals are explored by considering WA and EA in stratified analyses rather than treating ‘African ancestry’ as a monolithic category. The dataset of AA, all imputed together using the same reference panel, brought an additional large number of African ancestry samples to the analysis. We used both an exact and a local replication method for assessing transferability of previously identified loci to Africans thereby ensuring that we had accounted for the LD-based differences in the discovery and replication datasets. It would have been ideal to have replication cohorts that were a better match for the environmental and genomic background of the Africans in our study for replication. With the replication cohorts that we used, we were unable to determine whether association differences represented false positives, differences in environment or differences in genomic context. We look forward to future studies in which these types of analyses are possible.
In summary, we conducted a GWAS of serum lipids in Africans in Nigeria, Ghana and Kenya, with meta-analysis with and replication in AA. Among the overall study findings, several of the novel loci are important in TG or cholesterol synthesis, storage or turnover. While functional work will be useful to confirm and to understand the biological mechanisms underlying these associations, this study demonstrates the power of conducting large-scale genomic analyses in Africans.
Materials and Methods
Participants
Participants included in this study were drawn from the AADM study, which has been previously described (30,42,43). Briefly, AADM is a genetic epidemiology study of T2D, enrolling participants from university medical centers in Nigeria (Enugu, Lagos and Ibadan), Ghana (Accra and Kumasi) and Kenya (Eldoret). Participants underwent a clinical examination that included a medical history, clinical anthropometry, blood pressure measurements and blood sampling.
Serum measurements were made on fasting samples. Serum lipids (TG, CHOL, LDLC and HDLC) were determined enzymatically either with the COBAS Integra 400 Plus or Modular-E analyzers (Roche Diagnostics, Indianapolis, IN). Methods were standardized to in-house and other appropriate reference methods (e.g. CDC reference methods for HDLC, isotope dilution mass spectrometry for CHOL and TG). Missing LDLC measurements were calculated using the Friedewald equation (44). Ethical approval was obtained from the National Institutes of Health and from the ethical committees in each study site. All participants gave written informed consent before participation in the study.
Genotyping and imputation
Genotyping was performed using either the Affymetrix Axiom® PANAFR SNP array or the Illumina Consortium Multi-Ethnic Global Array (MEGA). Quality control was conducted separately for each of the resulting datasets. After technical quality control, sample-level genotype call rate was at least 0.95 for all subjects. Each SNP dataset was filtered for missingness (<0.05), Hardy–Weinberg equilibrium (P < 10−6) and MAF < 0.01). SNPs that passed quality control filters were used as the basis for imputation. Imputation of all samples was done using the African Genome Resources Haplotype Reference Panel using the Sanger Imputation Service (https://imputation.sanger.ac.uk/). Variants were included in the analysis if they had a MAF ≥ 0.01 and an info score ≥ 0.3.
Statistical analysis
Analyses were conducted using linear mixed models of the inverse normal transformations of lipids traits using EPACTS (version 3.2.6; http://genome.sph.umich.edu/wiki/EPACTS). All models were fitted using the dosage coding of variants. Models were adjusted for age, age2 and sex. Additionally, we included the first three principal components of the genotypes, based on prior work (30,45) (see figure S4 of (46)). Models were fitted with and without adjustment for BMI, as an effect on lipids can occur through and independent of body adiposity. As this study is nested within a case–control study of T2D, models were also fitted with and without adjustment for T2D to evaluate a potential effect of disease status. Use of lipid-lowering medication is not common in these regions and rare in our study population: 0.88% (1.0% among WA and 0.14% among EA). As such, no adjustment for lipids-lowering medication was included. All models included a genetic relationship matrix to account for the random effect due to relatedness, as related individuals were included in AADM. As we have conducted multiple analyses throughout this work (a GWAS in Africans, a GWAS limited to WA, a meta-analysis of Africans and AA and a meta-analysis of WA and AA), a strict Bonferroni-corrected P-value threshold would be P < 1.25 × 10−8. We have reported, however, all associations meeting a 5 × 10−8 given the exploratory nature of these analyses but indicated those that would have passed the stricter threshold in each of the results tables. The independence of associated variants within 1 MB of each other was determined using conditional analyses conducted in EPACTS.
As our study included participants from both West Africa [Nigeria (n = 2207)] and Ghana (n = 1376)] and East Africa [Kenya (n = 734)], we investigated whether the inclusion of EA may have obscured findings by conducting analyses in WA alone. EA were not considered separately because of their relatively small sample size. Heterogeneity between EA and WA was evaluated by with the P-value for heterogeneity using the ‘analyze heterogeneity’ option in METAL (https://genome.sph.umich.edu/wiki/METAL) (47).
Replication in AA
We sought replication for our genome-wide statistically significant associations in a combined dataset of AA (n = 9542). Included AA samples were drawn from five datasets: the Howard University Family Study (HUFS) (48) along with data available through dbGaP for the ARIC (phs000280.v2.p1, phs000090.v2.p1), the Cleveland Family Study (CFS phs000284.v1.p1), the Jackson Heart Study (phs000286.v4.p1, phs000499.v2.p1) and the Multi-Ethnic Study of Atherosclerosis (phs000209.v13.p1, phs000420.v6.p3). When participants had more than one study visit, the visit with the most complete lipid measurements was selected, and if there were multiple visits with complete measurements, the most recent was selected (CFS). All studies obtained ethical approval from the relevant institutions and written informed consent from each participant prior to participation. Genome-wide genotyping data for all studies were imputed using the methods described above. Meta-analysis of AA study results was conducted with the inverse-variance-weighted fixed-effect method as implemented in METAL, and replication was assessed based on results of meta-analyses (49).
To maximize sample size, we also conducted discovery analyses by meta-analyzing the results from Africans with AA using the inverse-variance-weighted fixed effect method in METAL. Given the genetic similarity between AA and WA, results from the WA analyses were separately meta-analyzed with AA data. To evaluate whether meta-analysis tool focused on handling ancestral heterogeneity would yield different results, we also conducted all meta-analyses with MetaSoft (http://genetics.cs.ucla.edu/meta/) (49) using the Han and Eskin’s random effects model (RE2) and compared the findings with our fixed effects analysis using METAL.
Assessing transferability to European ancestry individuals
We conducted lookups of all novel associations in our data in a recent meta-analysis of serum lipids of European ancestry individuals (16). Although the focus of this study was on gene-smoking interactions on serum lipids, the authors also conducted meta-analyses of standard GWAS models (without interactions) for comparison. We evaluated our loci in these meta-analyses of up to 90 266 European ancestry individuals.
Replication of known lipids loci
To evaluate the degree to which known lipids loci are also observed among Africans, we sought replication for 1853 previously identified SNP–trait associations from large genome-wide studies of serum lipids (1–8). First, we conducted ‘exact’ replication: evaluating the index variant reported in previous publications, with replication defined by consistent direction and P < 0.05. Additionally, to account for differences in LD between the initial reports (reflecting predominantly European ancestry) and our African samples, we conducted ‘local’ replication. Local replication is an LD-based replication technique, investigating all variants with r2 > 0.3 with the index variant among CEU (1000 Genomes samples of Northern Europeans from Utah). A locus was included in the local replication analysis if there was no exact replication of that locus in our data and if there were additional variants with r2 > 0.3 [CEU] with the index variant that passed filtering criteria and were available in our data. Associations were considered replicated locally with consistent direction (based on direction of association of minor allele for index and local variant) and P < 0.05/LD-based effective number of tests in the region (calculated as in (50)).
Ethics approval and consent to participate
All human participants included in the analyses of this manuscript provided written informed consent prior to enrollment. The AADM study was approved by the Institutional Review Board (IRB) at the National Institutes of Health. The HUFS study was approved by the IRB at Howard University. Our protocol for the controlled access to dbGaP datasets was approved by the IRB at the National Institutes of Health, and each of the dbGaP studies included (dbGaP Study Accession described in the Methods section) obtained ethical approvals from the relevant institutions.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the AADM Investigators.
Conflict of Interest statement. None declared.
Contributor Information
Amy R Bentley, Center for Research on Genomics and Global Health, National Human Genome Research Institute, NIH, Bethesda, MD 20892, USA.
Guanjie Chen, Center for Research on Genomics and Global Health, National Human Genome Research Institute, NIH, Bethesda, MD 20892, USA.
Ayo P Doumatey, Center for Research on Genomics and Global Health, National Human Genome Research Institute, NIH, Bethesda, MD 20892, USA.
Daniel Shriner, Center for Research on Genomics and Global Health, National Human Genome Research Institute, NIH, Bethesda, MD 20892, USA.
Karlijn A C Meeks, Center for Research on Genomics and Global Health, National Human Genome Research Institute, NIH, Bethesda, MD 20892, USA.
Mateus H Gouveia, Center for Research on Genomics and Global Health, National Human Genome Research Institute, NIH, Bethesda, MD 20892, USA.
Kenneth Ekoru, Center for Research on Genomics and Global Health, National Human Genome Research Institute, NIH, Bethesda, MD 20892, USA.
Jie Zhou, Center for Research on Genomics and Global Health, National Human Genome Research Institute, NIH, Bethesda, MD 20892, USA.
Adebowale Adeyemo, Center for Research on Genomics and Global Health, National Human Genome Research Institute, NIH, Bethesda, MD 20892, USA.
Charles N Rotimi, Center for Research on Genomics and Global Health, National Human Genome Research Institute, NIH, Bethesda, MD 20892, USA.
Funding
This project was largely supported by the Intramural Research Program of the National Human Genome Research Institute of the National Institutes of Health (NIH) through the Center for Research on Genomics and Global Health (CRGGH). The CRGGH is also supported by the National Institute of Diabetes and Digestive and Kidney Diseases and the Office of the Director at the NIH (Z01HG200362). Support for participant recruitment and initial genetic studies of the AADM study was provided by National Institutes of Health grant no. 3T37TW00041-03S2 from the Office of Research on Minority Health.
References
- 1. Liu, D.J., Peloso, G.M., Yu, H., Butterworth, A.S., Wang, X., Mahajan, A., Saleheen, D., Emdin, C., Alam, D., Alves, A.C. et al. (2017) Exome-wide association study of plasma lipids in >300,000 individuals. Nat. Genet., 49, 1758–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoffmann, T.J., Theusch, E., Haldar, T., Ranatunga, D.K., Jorgenson, E., Medina, M.W., Kvale, M.N., Kwok, P.Y., Schaefer, C., Krauss, R.M., Iribarren, C. and Risch, N. (2018) A large electronic-health-record-based genome-wide study of serum lipids. Nat. Genet., 50, 401–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klarin, D., Damrauer, S.M., Cho, K., Sun, Y.V., Teslovich, T.M., Honerlaw, J., Gagnon, D.R., DuVall, S.L., Li, J., Peloso, G.M. et al. (2018) Genetics of blood lipids among ~300,000 multi-ethnic participants of the Million Veteran Program. Nat. Genet., 50, 1514–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Do, R., Willer, C.J., Schmidt, E.M., Sengupta, S., Gao, C., Peloso, G.M., Gustafsson, S., Kanoni, S., Ganna, A., Chen, J. et al. (2013) Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat. Genet., 45, 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Teslovich, T.M., Musunuru, K., Smith, A.V., Edmondson, A.C., Stylianou, I.M., Koseki, M., Pirruccello, J.P., Ripatti, S., Chasman, D.I., Willer, C.J. et al. (2010) Biological, clinical and population relevance of 95 loci for blood lipids. Nature, 466, 707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Willer, C.J., Schmidt, E.M., Sengupta, S., Peloso, G.M., Gustafsson, S., Kanoni, S., Ganna, A., Chen, J., Buchkovich, M.L., Mora, S. et al. (2013) Discovery and refinement of loci associated with lipid levels. Nat. Genet., 45, 1274–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim, T., Park, A.Y., Baek, Y. and Cha, S. (2017) Genome-wide association study reveals four loci for lipid ratios in the Korean population and the constitutional subgroup. PLoS One, 12, e0168137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peloso, G.M., Auer, P.L., Bis, J.C., Voorman, A., Morrison, A.C., Stitziel, N.O., Brody, J.A., Khetarpal, S.A., Crosby, J.R., Fornage, M. et al. (2014) Association of low-frequency and rare coding-sequence variants with blood lipids and coronary heart disease in 56,000 whites and blacks. Am. J. Hum. Genet., 94, 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bentley, A.R., Callier, S. and Rotimi, C.N. (2017) Diversity and inclusion in genomic research: why the uneven progress? J Community Genet., 8, 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Auton, A., Abecasis, G.R., Altshuler, D.M., Durbin, R.M., Abecasis, G.R., Bentley, D.R., Chakravarti, A., Clark, A.G., Donnelly, P., Eichler, E.E. et al. (2015) A global reference for human genetic variation. Nature, 526, 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garske, K.M., Pan, D.Z., Miao, Z., Bhagat, Y.V., Comenho, C., Robles, C.R., Benhammou, J.N., Alvarez, M., Ko, A., Ye, C.J. et al. (2019) Reverse gene-environment interaction approach to identify variants influencing body-mass index in humans. Nat. Metab., 1, 630–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim, M., Yoo, H.J., Lee, H.J. and Lee, J.H. (2019) Longitudinal interaction between APOA5 -1131T>C and overweight in the acceleration of age-related increase in arterial stiffness through the regulation of circulating triglycerides. Hypertens. Res., 42, 241–248. [DOI] [PubMed] [Google Scholar]
- 13. Farook, V.S., Reddivari, L., Mummidi, S., Puppala, S., Arya, R., Lopez-Alvarenga, J.C., Fowler, S.P., Chittoor, G., Resendez, R.G., Kumar, B.M. et al. (2017) Genetics of serum carotenoid concentrations and their correlation with obesity-related traits in Mexican American children. Am. J. Clin. Nutr., 106, 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu, Y., Tian, Y., Wang, M., Wang, X., Wu, J., Wang, Z. and Hu, Y. (2020) Short-term exposure to air pollution and its interaction effects with two ABO SNPs on blood lipid levels in northern China: a family-based study. Chemosphere, 249, 126120. [DOI] [PubMed] [Google Scholar]
- 15. Khodarahmi, M., Kahroba, H., Jafarabadi, M.A., Mesgari-Abbasi, M. and Farhangi, M.A. (2020) Dietary quality indices modifies the effects of melanocortin-4 receptor (MC4R) rs17782313 polymorphism on cardio-metabolic risk factors and hypothalamic hormones in obese adults. BMC Cardiovasc. Disord., 20, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bentley, A.R., Sung, Y.J., Brown, M.R., Winkler, T.W., Kraja, A.T., Ntalla, I., Schwander, K., Chasman, D.I., Lim, E., Deng, X. et al. (2019) Multi-ancestry genome-wide gene-smoking interaction study of 387,272 individuals identifies new loci associated with serum lipids. Nat. Genet., 51, 636–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Vries, P.S., Brown, M.R., Bentley, A.R., Sung, Y.J., Winkler, T.W., Ntalla, I., Schwander, K., Kraja, A.T., Guo, X., Franceschini, N. et al. (2019) Multiancestry genome-wide association study of lipid levels incorporating gene-alcohol interactions. Am. J. Epidemiol., 188, 1033–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kilpeläinen, T.O., Bentley, A.R., Noordam, R., Sung, Y.J., Schwander, K., Winkler, T.W., Jakupović, H., Chasman, D.I., Manning, A., Ntalla, I. et al. (2019) Multi-ancestry study of blood lipid levels identifies four loci interacting with physical activity. Nat. Commun., 10, 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Agyemang, C., Meeks, K., Beune, E., Owusu-Dabo, E., Mockenhaupt, F.P., Addo, J., de Graft Aikins, A., Bahendeka, S., Danquah, I., Schulze, M.B. et al. (2016) Obesity and type 2 diabetes in sub-Saharan Africans–is the burden in today’s Africa similar to African migrants in Europe? The RODAM study. BMC Med., 14, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tasic, I., Milojkovic, M., Sunder-Plassmann, R., Lazarevic, G., Tasic, N.M. and Stefanovic, V. (2007) The association of PC-1 (ENPP1) K121Q polymorphism with metabolic syndrome in patients with coronary heart disease. Clin. Chim. Acta, 377, 237–242. [DOI] [PubMed] [Google Scholar]
- 21. Frittitta, L., Baratta, R., Spampinato, D., Di Paola, R., Pizzuti, A., Vigneri, R. and Trischitta, V. (2001) The Q121 PC-1 variant and obesity have additive and independent effects in causing insulin resistance. J. Clin. Endocrinol. Metab., 86, 5888–5891. [DOI] [PubMed] [Google Scholar]
- 22. Baratta, R., Di Paola, R., Spampinato, D., Fini, G., Marucci, A., Coco, A., Vigneri, R., Frittitta, L. and Trischitta, V. (2003) Evidence for genetic epistasis in human insulin resistance: the combined effect of PC-1 (K121Q) and PPARγ2 (P12A) polymorphisms. J. Mol. Med., 81, 718–723. [DOI] [PubMed] [Google Scholar]
- 23. Abate, N., Carulli, L., Cabo-Chan, A., Jr., Chandalia, M., Snell, P.G. and Grundy, S.M. (2003) Genetic polymorphism PC-1 K121Q and ethnic susceptibility to insulin resistance. J. Clin. Endocrinol. Metab., 88, 5927–5934. [DOI] [PubMed] [Google Scholar]
- 24. Pizzuti, A., Frittitta, L., Argiolas, A., Baratta, R., Goldfine, I.D., Bozzali, M., Ercolino, T., Scarlato, G., Iacoviello, L., Vigneri, R., Tassi, V. and Trischitta, V. (1999) A polymorphism (K121Q) of the human glycoprotein PC-1 gene coding region is strongly associated with insulin resistance. Diabetes, 48, 1881–1884. [DOI] [PubMed] [Google Scholar]
- 25. Tanyolaç, S., Bremer, A.A., Hodoglugil, U., Movsesyan, I., Pullinger, C.R., Heiner, S.W., Malloy, M.J., Kane, J.P. and Goldfine, I.D. (2009) Genetic variants of the ENPP1/PC-1 gene are associated with hypertriglyceridemia in male subjects. Metab. Syndr. Relat. Disord., 7, 543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen, G., Shriner, D., Zhou, J., Doumatey, A., Huang, H., Gerry, N.P., Herbert, A., Christman, M.F., Chen, Y., Dunston, G.M. et al. (2010) Development of admixture mapping panels for African Americans from commercial high-density SNP arrays. BMC Genomics, 11, 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuchenbaecker, K., Telkar, N., Reiker, T., Walters, R.G., Lin, K., Eriksson, A., Gurdasani, D., Gilly, A., Southam, L., Tsafantakis, E. et al. (2019) The transferability of lipid loci across African, Asian and European cohorts. Nat. Commun., 10, 4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Viitasalo, A., Schnurr, T.M., Pitkänen, N., Hollensted, M., Nielsen, T.R.H., Pahkala, K., Atalay, M., Lind, M.V., Heikkinen, S., Frithioff-Bøjsøe, C. et al. (2019) Abdominal adiposity and cardiometabolic risk factors in children and adolescents: a Mendelian randomization analysis. Am. J. Clin. Nutr., 110, 1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang, N., Cheng, J., Ning, Z., Chen, Y., Han, B., Li, Q., Chen, C., Zhao, L., Xia, F., Lin, D. et al. (2018) Type 2 diabetes and adiposity induce different lipid profile disorders: a Mendelian randomization analysis. J. Clin. Endocrinol. Metab., 103, 2016–2025. [DOI] [PubMed] [Google Scholar]
- 30. Adeyemo, A.A., Zaghloul, N.A., Chen, G., Doumatey, A.P., Leitch, C.C., Hostelley, T.L., Nesmith, J.E., Zhou, J., Bentley, A.R. and Shriner, D. (2019) ZRANB3 is an African-specific type 2 diabetes locus associated with beta-cell mass and insulin response. Nat. Commun., 10, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wojcik, G.L., Graff, M., Nishimura, K.K., Tao, R., Haessler, J., Gignoux, C.R., Highland, H.M., Patel, Y.M., Sorokin, E.P., Avery, C.L. et al. (2019) Genetic analyses of diverse populations improves discovery for complex traits. Nature, 570, 514–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kettunen, J., Tukiainen, T., Sarin, A.P., Ortega-Alonso, A., Tikkanen, E., Lyytikäinen, L.P., Kangas, A.J., Soininen, P., Würtz, P., Silander, K. et al. (2012) Genome-wide association study identifies multiple loci influencing human serum metabolite levels. Nat. Genet., 44, 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Surakka, I., Horikoshi, M., Mägi, R., Sarin, A.P., Mahajan, A., Lagou, V., Marullo, L., Ferreira, T., Miraglio, B., Timonen, S. et al. (2015) The impact of low-frequency and rare variants on lipid levels. Nat. Genet., 47, 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maddux, B.A. and Goldfine, I.D. (2000) Membrane glycoprotein PC-1 inhibition of insulin receptor function occurs via direct interaction with the receptor alpha-subunit. Diabetes, 49, 13–19. [DOI] [PubMed] [Google Scholar]
- 35. Pan, W., Ciociola, E., Saraf, M., Tumurbaatar, B., Tuvdendorj, D., Prasad, S., Chandalia, M. and Abate, N. (2011) Metabolic consequences of ENPP1 overexpression in adipose tissue. Am. J. Physiol. Endocrinol. Metab., 301, E901–E911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chikka, M.R., McCabe, D.D., Tyra, H.M. and Rutkowski, D.T. (2013) C/EBP homologous protein (CHOP) contributes to suppression of metabolic genes during endoplasmic reticulum stress in the liver. J. Biol. Chem., 288, 4405–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kheradpour, P. and Kellis, M. (2013) Systematic discovery and characterization of regulatory motifs in ENCODE TF binding experiments. Nucleic Acids Res., 42, 2976–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Denechaud, P.-D., Lopez-Mejia, I.C., Giralt, A., Lai, Q., Blanchet, E., Delacuisine, B., Nicolay, B.N., Dyson, N.J., Bonner, C., Pattou, F., Annicotte, J.S. and Fajas, L. (2016) E2F1 mediates sustained lipogenesis and contributes to hepatic steatosis. J. Clin. Invest., 126, 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lai, Q., Giralt, A., Le May, C., Zhang, L., Cariou, B., Denechaud, P.-D. and Fajas, L. (2017) E2F1 inhibits circulating cholesterol clearance by regulating Pcsk9 expression in the liver. JCI Insight, 2, e89729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eberlé, D., Hegarty, B., Bossard, P., Ferré, P. and Foufelle, F. (2004) SREBP transcription factors: master regulators of lipid homeostasis. Biochimie, 86, 839–848. [DOI] [PubMed] [Google Scholar]
- 41. Moya, M., Benet, M., Guzmán, C., Tolosa, L., García-Monzón, C., Pareja, E., Castell, J.V. and Jover, R. (2012) Foxa1 reduces lipid accumulation in human hepatocytes and is down-regulated in nonalcoholic fatty liver. PLoS One, 7, e30014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rotimi, C.N., Chen, G., Adeyemo, A.A., Furbert-Harris, P., Parish-Gause, D., Zhou, J., Berg, K., Adegoke, O., Amoah, A., Owusu, S. et al. (2004) A genome-wide search for type 2 diabetes susceptibility genes in West Africans: the Africa America Diabetes Mellitus (AADM) Study. Diabetes, 53, 838–841. [DOI] [PubMed] [Google Scholar]
- 43. Rotimi, C.N., Dunston, G.M., Berg, K., Akinsete, O., Amoah, A., Owusu, S., Acheampong, J., Boateng, K., Oli, J., Okafor, G. et al. (2001) In search of susceptibility genes for type 2 diabetes in West Africa: the design and results of the first phase of the AADM study. Ann. Epidemiol., 11, 51–58. [DOI] [PubMed] [Google Scholar]
- 44. Friedewald, W.T., Levy, R.I. and Fredrickson, D.S. (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem., 18, 499–502. [PubMed] [Google Scholar]
- 45. Adeyemo, A.A., Tekola-Ayele, F., Doumatey, A.P., Bentley, A.R., Chen, G., Huang, H., Zhou, J., Shriner, D., Fasanmade, O., Okafor, G. et al. (2015) Evaluation of genome wide association study associated type 2 diabetes susceptibility loci in Sub Saharan Africans. Front. Genet., 6, 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu, Z., Shriner, D., Hansen, N.F., Rotimi, C.N., Mullikin, J.C. and Program, N.C.S. (2020) Admixture mapping identifies genetic regions associated with blood pressure phenotypes in African Americans. PLoS One, 15, e0232048–e0232048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Willer, C.J., Li, Y. and Abecasis, G.R. (2010) METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics, 26, 2190–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Adeyemo, A., Gerry, N., Chen, G., Herbert, A., Doumatey, A., Huang, H., Zhou, J., Lashley, K., Chen, Y., Christman, M. and Rotimi, C. (2009) A genome-wide association study of hypertension and blood pressure in African Americans. PLoS Genet., 5, e1000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Han, B. and Eskin, E. (2011) Random-effects model aimed at discovering associations in meta-analysis of genome-wide association studies. Am. J. Hum. Genet., 88, 586–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ramos, E., Chen, G., Shriner, D., Doumatey, A., Gerry, N.P., Herbert, A., Huang, H., Zhou, J., Christman, M.F., Adeyemo, A. and Rotimi, C. (2011) Replication of genome-wide association studies (GWAS) loci for fasting plasma glucose in African-Americans. Diabetologia, 54, 783–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


