Key Points
Question
Is physical activity associated with all-cause mortality in individuals with Parkinson disease (PD)?
Findings
In this nationwide population-based cohort study of 10 699 individuals with PD, all physical activity intensities were associated with reduced all-cause mortality, with an inverse dose-response association between the total amount of physical activity and mortality. Participants who performed physical activity before and after the PD diagnosis had the lowest mortality risk.
Meaning
In this analysis, physical activity and all-cause mortality had a dose-response association in individuals with PD; although reverse causality might exist, modifications to increase and maintain physical activity may be beneficial for reducing mortality in PD.
This cohort study uses a national sample of Korean individuals with Parkinson disease to evaluate associations between physical activity, including amount and maintenance, and mortality.
Abstract
Importance
The protective effects of physical activity (PA) against Parkinson disease (PD) development have been suggested; however, the association of PA with mortality in PD has rarely been investigated.
Objective
To evaluate the association between PA and mortality in individuals with PD and determine how the amount and maintenance of PA are associated with mortality.
Design, Setting, and Participants
This nationwide population-based cohort study used Korean National Health Insurance System data. Participants were included from January 1, 2010, and December 31, 2013, and were followed up until December 31, 2017. Data were analyzed from September 2020 to March 2021. Individuals who were newly diagnosed with PD were selected using the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision code G20 and registration code V124 in the program for rare intractable diseases in 2010 through 2013. Individuals who underwent health checkups within 2 years before and after the PD diagnosis were enrolled. Those aged younger than 40 years or with missing data were excluded.
Exposures
Physical activity levels were collected using self-reported questionnaires.
Main Outcomes and Measures
All-cause mortality.
Results
A total of 45 923 individuals were identified; 10 987 were enrolled, and 34 individuals younger than 40 years and 254 with missing data were excluded. A total of 10 699 individuals with PD were included; 4925 (46%) were male and 5774 (54%) were female, and the mean (SD) age was 69.2 (8.8) years. During the 8-year follow-up period, there were 1823 deaths (17%). The mortality rate was lower among individuals who were physically active vs inactive at all PA intensities (vigorous: hazard ratio [HR], 0.80 [95% CI, 0.69-0.93]; moderate: HR, 0.66 [95% CI, 0.55-0.78]; light: HR, 0.81 [95% CI, 0.73-0.90]). There was a significant inverse dose-response association between the total amount of PA and mortality (HRs: vigorous, 0.80 [95% CI, 0.69-0.93]; moderate, 0.66 [95% CI, 0.55-0.78]; light, 0.81 [95% CI, 0.73-0.90]; P < .001). Moreover, maintenance of PA was associated with the mortality rate. Individuals with PD who were physically active both before and after the PD diagnosis had the greatest reduction in mortality rate across all PA intensities (HRs: vigorous, 0.66 [95% CI, 0.50-0.88]; moderate, 0.49 [95% CI, 0.32-0.75]; light, 0.76 [95% CI, 0.66-0.89]). Individuals who started PA after receiving the PD diagnosis had a lower mortality rate than those who remained physically inactive (HRs: vigorous, 0.82 [95% CI, 0.70-0.97]; moderate, 0.69 [95% CI, 0.57-0.83]; light, 0.86 [95% CI, 0.78-0.98]).
Conclusions and Relevance
This analysis found a dose-response association between PA and all-cause mortality in PD. Reverse causality may exist, and future prospective randomized clinical trials are warranted to determine the effect of PA on mortality in PD.
Introduction
Parkinson disease (PD), the second most common neurodegenerative disease, is characterized by cardinal motor signs, including bradykinesia, resting tremor, rigidity, and postural instability. Although dopaminergic medication is the mainstay of relieving the symptoms of PD, effective disease-modifying treatments are lacking. The causative mechanism of PD remains poorly understood, and multifactorial origins, including both genetic and environmental factors, have been suggested.1,2,3 Thus, adopting a positive lifestyle could be a possible disease-modifying therapeutic option.
Lifestyle factors, including physical activity (PA), alcohol consumption, and smoking habits, have been suggested to be associated with PD development.4,5,6,7,8 Although some lifestyle factors, such as alcohol consumption, have shown controversial results,4,5 PA has demonstrated relatively consistent protective outcomes in PD development.9,10,11,12 A recent meta-analysis6 in 2008 including 544 336 participants from 8 previous studies showed an approximately 20% reduced risk of PD development in individuals who were physically active.
Some studies have examined the association of PA with PD progression.13,14 Regular PA as well as competitive sports demonstrated protective outcomes against cognitive decline and balance impairment in PD.14 Various exercises, including treadmill training, dance, and tai chi, have shown beneficial outcomes against PD progression15,16,17; however, which exercise type or intensity is more beneficial for preventing PD progression requires further investigation. Moreover, there have been few reports on the associations of PA with mortality in PD.14 Therefore, this study aimed to evaluate the association of PA with all-cause mortality in individuals with PD. We also investigated how PA maintenance and total amount are correlated with mortality in participants with PD using a nationwide, population-based cohort in Korea.
Methods
Data Source
The Korean National Health Insurance Service (NHIS) is a single-payer system that provides mandatory universal comprehensive medical coverage for more than 97% of citizens of Korea and medical aid to approximately 3% of the population at the lowest income level. The NHIS database includes a unique anonymous number for each patient and summarizes demographic variables, such as age, sex, insurance type, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) diagnoses, and health care utilization. The NHIS provides a free biannual national health screening program for all beneficiaries older than 40 years. The national health screening program includes a self-reported health behavior questionnaire, medical history, anthropometric measurements, and laboratory tests. This study was approved by the institutional review board of the Bundang Jesaeng General Hospital, which waived the need for informed consent because of the retrospective design and use of encrypted participant data.
Study Population
In 2001, the Korean government started a registration program for rare intractable diseases, including PD, to assist patients with medical expenses. To be registered in the program with PD, the physician must confirm the patient meets strict criteria (eAppendix in the Supplement), which are almost the same as the those of the UK Parkinson’s Disease Society Brain Bank. In addition, the NHIS program conducts regular cross-checking by reviewing medical records to prevent miscoding or inaccurate medical claims; therefore, the rare intractable diseases registry data are considered valid and reliable.
For this study, we selected individuals who had been newly diagnosed with PD between January 1, 2010, and December 31, 2013, with a primary diagnosis of PD (ICD-10 code G20) and a registration code for PD (V124) in the registration program. Of these individuals, we included only those who attended health checkups within 2 years before and after receiving the PD diagnosis, to evaluate the association between PA maintenance and mortality. Individuals younger than 40 years and those with missing data were also excluded.
Physical Activity
Data on PA were collected using self-reported structured questionnaires using a 7-day recall method at the time of health checkup, similar to the International Physical Activity Questionnaire-Short form,18 in which participants were asked how many days per week they spent performing each activity by intensity level. High-intensity PA, such as running, aerobics, fast biking, and climbing, for more than 20 minutes was defined as vigorous activity. Moderate-intensity PA, such as fast walking, riding a bicycle at a normal speed, and doubles tennis, for more than 30 minutes was defined as moderate activity. Light-intensity PA, such as walking to and from work or for leisure, for more than 30 minutes was regarded as light activity. Various other activities were included and categorized into the corresponding intensity groups. Individuals who reported performing vigorous PA 3 or more times or moderate or light PA for 5 or more times a week were considered physically active according to American College of Sports Medicine guidelines.19 Otherwise, the participants were considered physically inactive.
We calculated the total metabolic equivalent of task (MET) minutes per week to quantify the total PA amount for each individuals. The MET ratings of 3, 5, and 8 were assigned for light-intensity, moderate-intensity, and vigorous-intensity activities based on the 2011 Updates on Compendium of Physical Activity.20 The values of MET-minutes per week were calculated by summing the frequency, intensity, and duration of PA, and the total PA amount was categorized into quartiles.
Other Variables
All-cause mortality up to December 31, 2017, was evaluated based on nationwide death certificate data from the Korea National Statistical Office. Residential areas were categorized into urban and rural. The NHI premium was used as a proxy measure of income because it is proportional to monthly income, including earnings and capital gains. Individuals who drank heavily were defined as those who consumed 30 g of alcohol per day. Anthropometric data, including height, weight, and blood pressure (systolic and diastolic), were assessed. Body mass index (BMI) was calculated as weight divided by height squared. Venous blood samples were drawn after an overnight fast to determine total cholesterol, high-density lipoprotein, low-density lipoprotein, and triglyceride levels. Comorbidities were measured using the Charlson Comorbidity Index.
Statistical Analyses
For statistical analyses, age, sex, income level, residential area, and comorbidities were obtained from the nationwide claims database, and lifestyle-factor, anthropometric, and laboratory data were obtained from the health checkup database. Baseline characteristics after the most recent health checkup within 2 years of PD diagnosis were used to compare individuals with PD who died with those who did not die. Baseline demographic and medical data are presented as mean (SD) or median (IQR) values for continuous variables and numbers (percentages) for categorical variables. The variables in the 2 groups were compared using t tests or Mann-Whitney U tests for continuous variables or χ2 tests for categorical variables. The primary outcome was all-cause mortality, according to the intensity, total amount, and maintenance of PA in individuals with PD. Cox proportional hazard models were used to assess mortality risk by PA level using 3 models with adjustment for confounding variables. Kaplan-Meier curves for all-cause mortality were constructed and compared using log-rank tests. Subgroup analyses were performed to evaluate whether the associations of PA with mortality were consistent. Differences were considered statistically significant at 2-sided P < .05. We used SAS for Windows version 9.4 (SAS Institute Inc) to perform the statistical analyses. Data were analyzed from September 2020 to March 2021.
Results
Demographic and Medical Characteristics
At total of 10 699 individuals with PD were included in the analysis (eFigure in the Supplement). The baseline demographic and medical characteristics of the participants are shown in Table 1. The participants with PD included 4925 men (46%) and 5774 women (54%), and the mean (SD) age was 69.2 (8.8) years. Among the 10 699 individuals with PD who were followed up for up to 8 years, there were 1823 deaths (mortality rate, 17%). The mean (SD) follow-up duration was 4.5 (1.4) years. There were significant differences in age and sex between individuals who did and did not die (mean [SD] ages: those who did not die, 68.3 [8.8] years vs those who died, 73.2 [7.5] years; P < .001; percentage of women: those who did not die, 5053 of 8876 [56.9%] vs those who died, 721 of 1823 [39.6%]; P < .001). The median (IQR) total PA was 270 (0-630) and 450 (0-900) MET-minutes per week for those who did and did not die, respectively. Detailed information on PA status is presented in eTable 1 in the Supplement.
Table 1. Demographic and Medical Characteristics.
| Variables | Mortality, No. (%) | P value | |
|---|---|---|---|
| No (n = 8876) | Yes (n = 1823) | ||
| Age, mean (SD), y | 68.3 (8.8) | 73.2 (7.5) | <.001 |
| Sex | |||
| Male | 3823 (43.1) | 1102 (60.5) | <.001 |
| Female | 5053 (56.9) | 721 (39.6) | |
| Income levels | |||
| Medical aid | 95 (1.1) | 20 (1.1) | .71 |
| Quartile 1 (lowest) | 1724 (19.4) | 335 (18.4) | |
| Quartile 2 | 1600 (18.0) | 315 (17.3) | |
| Quartile 3 | 2209 (24.9) | 463 (25.4) | |
| Quartile 4 (highest) | 3248 (36.6) | 690 (37.9) | |
| Urban residential area | 3671 (41.4) | 623 (34.2) | <.001 |
| Disability registration | 394 (4.4) | 201 (11.0) | <.001 |
| Comorbidities | |||
| Charlson Comorbidity Index score, median (IQR) | 2 (1-4) | 3 (2-5) | <.001 |
| Diabetes | 2797 (31.5) | 774 (42.5) | <.001 |
| Hypertension | 4793 (54.0) | 1065 (58.4) | <.001 |
| Dyslipidemia | 4268 (48.1) | 807 (44.3) | .003 |
| Current smoking | 449 (5.1) | 116 (6.4) | .02 |
| Heavy drinking | 86 (1.0) | 26 (1.4) | .08 |
| Physically active | 3699 (41.7) | 515 (28.3) | <.001 |
| MET-min/wk, median (IQR) | 450 (0-900) | 270 (77-148) | <.001 |
| BMI, mean (SD) | 24.0 (3.1) | 23.1 (3.31) | <.001 |
| Blood pressure, mean (SD), mm Hg | |||
| Systolic | 126.1 (15.2) | 126.5 (16.5) | .32 |
| Diastolic | 76.4 (9.7) | 76.1 (10.2) | .21 |
| Laboratory findings, mean (SD), mg/dL | |||
| Total cholesterol | 185.9 (38.3) | 180.5 (39.7) | <.001 |
| High-density lipoprotein cholesterol | 52.3 (13.1) | 50.4 (13.3) | <.001 |
| Low-density lipoprotein cholesterol | 108.8 (38.9) | 105.4 (35.5) | <.001 |
| Triglycerides | 109 (79-152) | 107 (77-148) | .14 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); MET, metabolic equivalent task.
SI conversion factors: To convert high-density and low-density cholesterol to mmol/L, multiply by 0.0259; triglycerides to mmol/L, multiply by 0.0113.
Association of PA With All-Cause Mortality Risk After PD Diagnosis
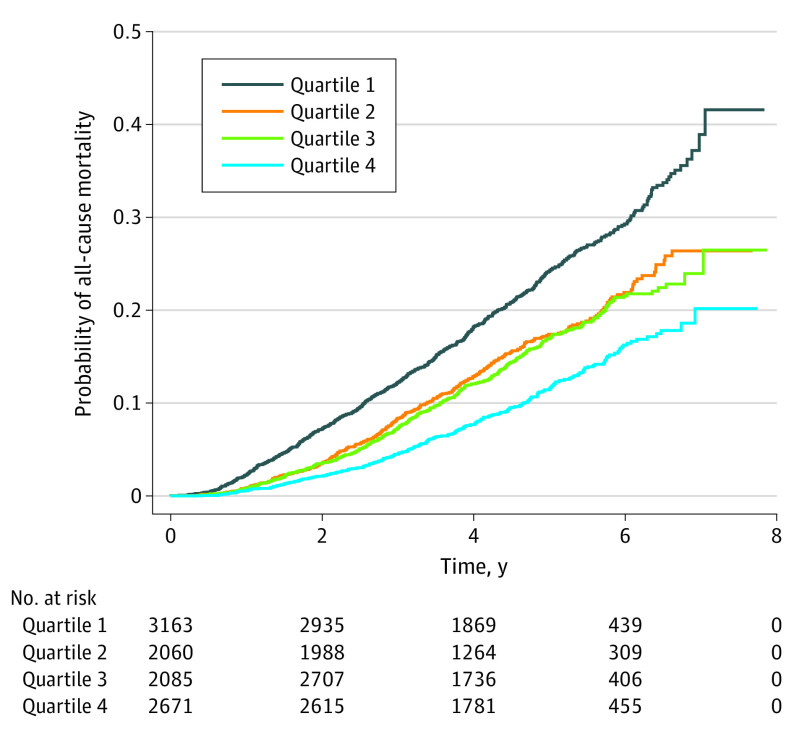
A significantly reduced mortality risk was observed in individuals with PD who were physically active vs physically inactive. After adjustment for confounding variables, the Cox proportional hazard regression models for mortality showed hazard ratios (HRs) of 0.80 (95% CI, 0.69-0.93) for vigorous-intensity PA, 0.66 (95% CI, 0.55-0.78) for moderate-intensity PA, and 0.81 (95% CI, 0.73-0.90) for light-intensity PA (eTable 2 in the Supplement). The log-rank test revealed a significantly lower mortality rate among individuals with PD who were physically active (P < .001) (Figure 1). According to the total amount of PA measured in MET-minutes per week, multivariate Cox proportional hazards regression analysis showed a progressively decreasing risk of mortality from the first quartile (<90 MET-minutes per week) to the fourth quartile (≥820 MET-minutes per week) (HR, 0.61 [95% CI, 0.53-0.70]; P < .001; Table 2). The log-rank test revealed a significant inverse association between mortality rate and amount of PA in individuals with PD (P < .001) (Figure 2).
Figure 1. Kaplan-Meier Curves of All-Cause Mortality in Parkinson Disease by Physical Activity Intensity Level.
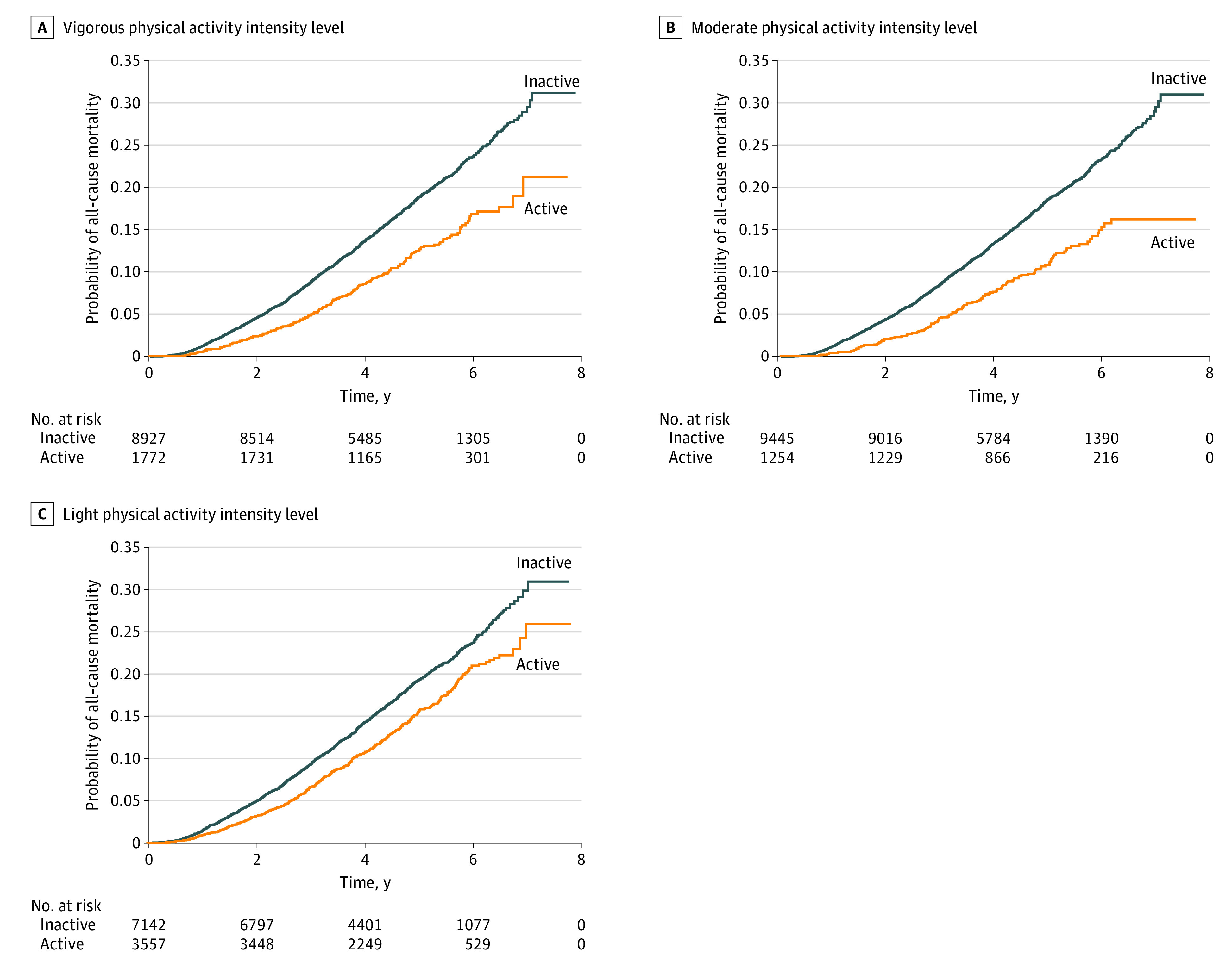
Table 2. Cox Proportional Hazard Regression Analysis of the Risk of All-Cause Mortality Among Individuals With Parkinson Disease by Total Amount of Physical Activity.
| Quartile (MET-min/wk) | Parkinson disease, No. | Mortality, No. | Person-years | Mortality rate per 1000 person-y | Model 1, hazard ratio (95% CI) | P value | Model 2, hazard ratio (95% CI) | P value | Model 3, hazard ratio (95% CI) | P value |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 (<90) | 3163 | 744 | 13 817.0 | 53.847 | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| 2 (<450) | 2060 | 344 | 9250.5 | 37.187 | 0.69 (0.61-0.78) | <.001 | 0.77 (0.67-0.87) | <.001 | 0.81 (0.71-0.92) | .001 |
| 3 (<820) | 2805 | 436 | 12 543.4 | 34.759 | 0.64 (0.57-0.73) | <.001 | 0.71 (0.63-0.80) | <.001 | 0.76 (0.67-0.85) | <.001 |
| 4 (≥820) | 2671 | 299 | 12 350.9 | 24.209 | 0.44 (0.39-0.51) | <.001 | 0.55 (0.48-0.63) | <.001 | 0.61 (0.53-0.70) | <.001 |
Abbreviations: MET, metabolic equivalent task; NA, not applicable.
Mortality rate is the incidence of mortality per 1000 person-years. Model 1 was unadjusted. Model 2 was adjusted for age, sex, income level, and residential area. Model 3 was adjusted for age, sex, income level, residential area, comorbidities, smoking status, alcohol consumption, body mass index (calculated as weight in kilograms divided by height in meters squared), and disability registration.
Figure 2. Kaplan-Meier Curves of All-Cause Mortality in Parkinson Disease by Total Amount of Physical Activity.
Quartile 1 was the group with the lowest amount of activity (<90 metabolic equivalent of task–minutes/week), and quartile 4 was the group with the highest amount of activity (≥820 metabolic equivalent of task–minutes/week).
Changes in PA Before and After PD Diagnosis and All-Cause Mortality
Table 3 shows the risk of mortality according to changes in PA before and after the PD diagnosis. Individuals with PD who were physically active both before and after the PD diagnosis showed the greatest reduction in mortality rate for all PA intensities (vigorous: HR, 0.66 [95% CI, 0.50-0.88]; moderate: HR, 0.49 [95% CI, 0.32-0.75]; light: HR, 0.76 [95% CI, 0.66-0.89]). Individuals who started PA after receiving the PD diagnosis had a lower mortality rate than those who remained physically inactive (vigorous: HR, 0.82 [95% CI, 0.70-0.97]; moderate: HR, 0.69 [95% CI, 0.57-0.83]; light: HR, 0.86 [95% CI, 0.78-0.98]). However, those who became inactive after receiving their PD diagnosis did not have a significantly better survival rate than individuals with PD who were continuously inactive, despite being physically active before receiving the PD diagnosis.
Table 3. All-Cause Mortality by Physical Activity Continuity Status Among Individuals With Parkinson Disease.
| Prediagnosis and Postdiagnosis | Parkinson disease, No. | Mortality, No. | Person-years | Mortality rate per 1000 person-y | Model 1, hazard ratio (95% CI) | P value | Model 2, hazard ratio (95% CI) | P value | Model 3, hazard ratio (95% CI) | P value |
|---|---|---|---|---|---|---|---|---|---|---|
| Vigorous | ||||||||||
| Inactive and inactive | 7855 | 1454 | 34 897.4 | 41.665 | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Inactive and active | 1245 | 157 | 5731.5 | 27.392 | 0.65 (0.55-0.77) | <.001 | 0.78 (0.66-0.92) | .003 | 0.82 (0.70-0.97) | .02 |
| Active and inactive | 1072 | 162 | 4912.8 | 32.975 | 0.78 (0.67-0.92) | .003 | 0.80 (0.68-0.94) | .007 | 0.80 (0.69-0.94) | .007 |
| Active and active | 527 | 50 | 2420.2 | 20.660 | 0.49 (0.37-0.65) | <.001 | 0.58 (0.44-0.77) | <.001 | 0.66 (0.50-0.88) | .005 |
| Moderate | ||||||||||
| Inactive and inactive | 8662 | 1560 | 38 564.6 | 40.452 | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Inactive and active | 1007 | 116 | 4725.6 | 24.547 | 0.60 (0.49-0.72) | <.001 | 0.66 (0.55-0.80) | <.001 | 0.69 (0.57-0.83) | <.001 |
| Active and inactive | 783 | 126 | 3526.4 | 35.731 | 0.88 (0.74-1.06) | .18 | 0.87 (0.72-1.04) | .12 | 0.87 (0.73-1.05) | .14 |
| Active and active | 247 | 21 | 1145.4 | 18.335 | 0.45 (0.29-0.69) | <.001 | 0.45 (0.29-0.70) | <.001 | 0.49 (0.32-0.75) | .001 |
| Light | ||||||||||
| Inactive and inactive | 5427 | 983 | 24 239.8 | 40.553 | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Inactive and active | 2130 | 306 | 9661.9 | 31.671 | 0.78 (0.69-0.89) | <.001 | 0.81 (0.71-0.92) | .002 | 0.86 (0.75-0.98) | .02 |
| Active and inactive | 1715 | 328 | 7652.7 | 42.861 | 1.06 (0.93-1.20) | .38 | 1.00 (0.88-1.13) | .97 | 1.02 (0.90-1.15) | .19 |
| Active and active | 1427 | 206 | 6407.5 | 32.150 | 0.80 (0.68-0.92) | .003 | 0.76 (0.66-0.89) | <.001 | 0.76 (0.66-0.89) | <.001 |
Abbreviation: NA, not applicable.
Mortality rate is the incidence of mortality per 1000 person-years. Model 1 was unadjusted. Model 2 was adjusted for age, sex, Income level, and residential area. Model 3 was adjusted for age, sex, income level, residential area, comorbidities, smoking, alcohol consumption, body mass index (calculated as weight in kilograms divided by height in meters squared), and disability registration.
Subgroup Analyses
The results of subgroup analyses of age, sex, comorbidities, alcohol consumption, smoking, and BMI for the association between PA and mortality are presented in eTable 3 in the Supplement. The interaction term for all variables was nonsignificant, and the inverse association between PA and mortality remained consistent. Overall, age (HR, 1.07 [95% CI, 1.06-1.07]; P < .001), disability registration (HR, 1.92 [95% CI, 1.66-2.23]; P < .001), and Charlson Comorbidity Index (HR, 1.13 [95% CI, 1.11-1.16]; P < .001) were associated with increased mortality, whereas female sex (HR, 0.53 [95% CI, 0.48-0.58]; P < .001) and BMI (HR, 0.94 [95% CI, 0.92-0.95]; P < .001) were associated with reduced mortality (eTable 4 in the Supplement).
Discussion
In this nationwide population-based longitudinal large-scale cohort study, we analyzed 10 699 individuals with new-onset PD to evaluate the association between PA and all-cause mortality. Individuals with PD who are physically active had a lower mortality rate than those who were physically inactive for all PA intensities. Moreover, the total amount of PA showed an inverse dose-response association with all-cause mortality in individuals with PD. In addition, PA maintenance was associated with the mortality rate; individuals who were physically active before and after the PD diagnosis had a greater reduction in mortality rate than those who were consistently physically inactive. Performing PA after receiving the PD diagnosis was associated with a decreased mortality rate, even in individuals with PD who were physically inactive before receiving their PD diagnosis.
In recent decades, epidemiologic studies have investigated the association between PA and PD risk and suggested the beneficial associations of PA with PD development.9,10,12 Various neuroprotective mechanisms of exercise and PA, including reducing cellular oxidative stress, mitochondrial dysfunction, and inflammation, have been demonstrated in animal models.21,22,23,24 These neuroprotective mechanisms could also influence progression and even mortality, since PD is a progressive neurodegenerative disease. Progressive resistance training showed beneficial effects on the Unified Parkinson Disease Rating Scale motor score and cognitive function in PD through 2 years of follow-up.25,26 Recently, home-based and remotely supervised aerobic exercise using virtual reality–attenuated off-state motor symptoms and improved cardiovascular fitness in mild PD.27 There have also been reports that physical therapy, including resistance and aerobic exercise, improved nonmotor symptoms, such as mood and sleep, in patients with PD.28,29 However, these studies included small numbers of participants and relatively short follow-up durations. Furthermore, the associations of PA other than physical therapy or specific exercise with PD progression have rarely been investigated, and studies on the association between PA and mortality in patients with PD are limited.14
Physical activity is defined as any body movement caused by the skeletal muscles resulting in energy expenditure.30 In this study, we investigated the association of 3 PA intensities with mortality in individuals with PD and found that all were associated with significantly decreased mortality risks. Although various exercises, including intensive physical therapy and leisure time activities such as tai chi or dance, have shown positive outcomes against PD progression,15,17 the exercise type or intensity that is most beneficial for preventing PD progression remains uncertain. In our results, moderate-intensity PA was more strongly associated with reduced mortality risk than vigorous PA. This could be attributed to the frequency of PA per week. According to the American College of Sports Medicine guidelines, we defined physically active as performing vigorous PA 3 or more times or moderate PA 5 or more times a week; thus, the moderate PA group might perform PA more days a week than the vigorous PA group. This is somewhat in line with a previous study, which suggested that duration of PA is more important than the intensity of PA in European older adults.31 We could not perform a direct comparison between intensities in this study, and future studies to evaluate the most effective PA intensity for individuals with PD are needed.
In this study, we measured the total amount of PA, including exercise, leisure time sports, and daily walking routines, using MET-minutes per week and found a significant inverse dose-response association between the total amount of PA and all-cause mortality. The benefits of vigorous exercise have been well established in the general population,32 and 1 study33 reported that high-intensity treadmill exercise might be feasible and safe in PD. However, individuals with PD often have balance problems and a high risk of falling34; thus, it could be difficult for individuals with PD to perform vigorous exercise. Parkinson disease is a prevalent neurodegenerative disease in elderly individuals older than 60 years,35 and recommendation of frequent light-intensity or moderate-intensity activities that consider each individual’s degree of motor impairment would be more appropriate. There might be reverse causality in that individuals with PD who have relatively severe symptoms did not perform PA and experienced increased mortality. Prospective randomized clinical trials are warranted to confirm a causal association between PA and mortality in PD.
We also evaluated how changes in PA before and after a PD diagnosis were associated with mortality. Individuals with PD who maintained PA before and after receiving the PD diagnosis showed the greatest decrease in mortality rate reduction (up to 50%). Individuals with PD who became physically active at any intensity after receiving the PD diagnosis also showed a decreased mortality risk. However, among individuals with PD who discontinued PA after receiving their PD diagnosis, PA showed a weaker association with mortality risk. This finding suggests that recent PA levels were more important than past PA levels in terms of mortality reduction and PA maintenance could be encouraged in individuals with PD. Although motor function at the time of PD diagnosis could be relatively preserved compared with that of healthy older adults,36 PA levels decrease gradually as PD progresses.37 This PA decrease would make individuals with PD more physically inactive, resulting in an increased all-cause mortality rate. Leisure time exercise or regular walking is less expensive and might be more applicable to individuals with PD than structured and intensive physical therapy programs, and more attention needs to be paid to improve the PA levels of people with PD.
Our results showed an inverse association between PA and mortality in both men and women with PD. Several previous studies have demonstrated the beneficial associations of PA or exercise with reduced PD occurrence only in men.9,38 Moreover, a previous meta-analysis showed an inverse dose-response association between PA and PD risk among men.6 One suggested explanation of the protective effect of PA against PD risk in men was likely because of the lower percentage of women included in most studies. On the other hand, the included studies in the meta-analysis were conducted in Western countries. In this study, we evaluated more than 10 000 individuals with PD with an almost even sex distribution. Future studies to elucidate the protective effects and mechanisms of PA on PD risk and progression according to sex are warranted, and whether a male predominance of the protective outcomes of PA against PD truly exists requires verification.
Limitations
This study has several limitations. First, there may have been selection bias. We only included individuals with PD who underwent health checkups within 2 years before and after receiving the PD diagnosis. Thus, it is possible that only individuals with PD who were relatively healthy or had health-seeking behaviors were enrolled in the analysis. In addition, sampling error could exist in the measurement of PA maintenance based on the PA status at only 2 different points. Second, recall bias was possible. We collected PA information from self-reported questionnaires completed during the health checkup using a 7-day recall method. Third, although this was a nationwide database study, clinical information, such as motor symptoms or PD subtype, associated with PD severity could not be obtained. Therefore, we only included new-onset PD and used disability registration as a proxy for PD severity. Fourth, caffeine consumption and dietary habits, such as dairy food or vitamin E intake, which have been shown to be associated with PD, were not included in this analysis. Therefore, we adjusted for other confounding variables, including comorbidities and BMI. Fifth, we could not include antiparkinsonian medications in this analysis. As for the diagnosis of PD, regarding medication, the response to levodopa was included in the criteria for PD in the rare intractable disease registration program. Nonetheless, the association of medication with PD progression was not considered in the analyses, which might have affected conclusions regarding the mortality rate. Sixth, in this observational study, there could be a reverse causality between PA and mortality. Future prospective randomized clinical trials are warranted to elucidate the causal association between PA and mortality in PD.
Conclusions
In this analysis of 10 699 individuals with PD, we demonstrated an inverse association between PA and all-cause mortality. An inverse dose-response association between the total PA amount and mortality was found in both men and women with PD. Those who maintained PA before and after receiving their PD diagnosis showed the greatest mortality reduction, while PA after the PD diagnosis was also associated with reduced mortality. Activity modification to increase and maintain PA would be beneficial for PD management, and future prospective randomized clinical trials to elucidate causal associations between PA and mortality in PD are warranted.
eAppendix. Criteria for Parkinson’s disease in the Registration Program for Rare Intractable Diseases
eFigure. Flowchart of the subject selection process
eTable 1. Physical Activity Status Before and After Diagnosis of Parkinson’s Disease stratified by Mortality
eTable 2. Cox Proportional Hazard Regression Analysis of the Risk of All-Cause Mortality in Patients with Parkinson’s Disease Stratified by Intensity
eTable 3. Subgroup Analyses of Age, Sex, Smoking, Alcohol, Comorbidities, Body Mass Index, and Disability Registration
eTable 4. Multivariate Cox Proportional Hazard Analyses of Covariables for All-cause Mortality in Parkinson’s disease
References
- 1.Nag N, Jelinek GA. A narrative review of lifestyle factors associated with Parkinson’s disease risk and progression. Neurodegener Dis. 2019;19(2):51-59. doi: 10.1159/000502292 [DOI] [PubMed] [Google Scholar]
- 2.Nalls MA, Blauwendraat C, Vallerga CL, et al. ; 23andMe Research Team; System Genomics of Parkinson’s Disease Consortium; International Parkinson’s Disease Genomics Consortium . Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 2019;18(12):1091-1102. doi: 10.1016/S1474-4422(19)30320-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellou V, Belbasis L, Tzoulaki I, Evangelou E, Ioannidis JP. Environmental risk factors and Parkinson’s disease: an umbrella review of meta-analyses. Parkinsonism Relat Disord. 2016;23:1-9. doi: 10.1016/j.parkreldis.2015.12.008 [DOI] [PubMed] [Google Scholar]
- 4.Jiménez-Jiménez FJ, Alonso-Navarro H, García-Martín E, Agúndez JAG. Alcohol consumption and risk for Parkinson’s disease: a systematic review and meta-analysis. J Neurol. 2019;266(8):1821-1834. doi: 10.1007/s00415-018-9032-3 [DOI] [PubMed] [Google Scholar]
- 5.Bettiol SS, Rose TC, Hughes CJ, Smith LA. Alcohol consumption and Parkinson’s disease risk: a review of recent findings. J Parkinsons Dis. 2015;5(3):425-442. doi: 10.3233/JPD-150533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang X, Han D, Cheng Q, et al. Association of levels of physical activity with risk of Parkinson disease: a systematic review and meta-analysis. JAMA Netw Open. 2018;1(5):e182421. doi: 10.1001/jamanetworkopen.2018.2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X, Li W, Liu G, Shen X, Tang Y. Association between cigarette smoking and Parkinson’s disease: a meta-analysis. Arch Gerontol Geriatr. 2015;61(3):510-516. doi: 10.1016/j.archger.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 8.Hernán MA, Takkouche B, Caamaño-Isorna F, Gestal-Otero JJ. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson’s disease. Ann Neurol. 2002;52(3):276-284. doi: 10.1002/ana.10277 [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Zhang SM, Schwarzschild MA, Hernán MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology. 2005;64(4):664-669. doi: 10.1212/01.WNL.0000151960.28687.93 [DOI] [PubMed] [Google Scholar]
- 10.Sääksjärvi K, Knekt P, Männistö S, et al. Reduced risk of Parkinson’s disease associated with lower body mass index and heavy leisure-time physical activity. Eur J Epidemiol. 2014;29(4):285-292. doi: 10.1007/s10654-014-9887-2 [DOI] [PubMed] [Google Scholar]
- 11.Sasco AJ, Paffenbarger RS Jr, Gendre I, Wing AL. The role of physical exercise in the occurrence of Parkinson’s disease. Arch Neurol. 1992;49(4):360-365. doi: 10.1001/archneur.1992.00530280040020 [DOI] [PubMed] [Google Scholar]
- 12.Thacker EL, Chen H, Patel AV, et al. Recreational physical activity and risk of Parkinson’s disease. Mov Disord. 2008;23(1):69-74. doi: 10.1002/mds.21772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaHue SC, Comella CL, Tanner CM. The best medicine? the influence of physical activity and inactivity on Parkinson’s disease. Mov Disord. 2016;31(10):1444-1454. doi: 10.1002/mds.26728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paul KC, Chuang YH, Shih IF, et al. The association between lifestyle factors and Parkinson’s disease progression and mortality. Mov Disord. 2019;34(1):58-66. doi: 10.1002/mds.27577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duncan RP, Earhart GM. Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabil Neural Repair. 2012;26(2):132-143. doi: 10.1177/1545968311421614 [DOI] [PubMed] [Google Scholar]
- 16.Corcos DM, Robichaud JA, David FJ, et al. A two-year randomized controlled trial of progressive resistance exercise for Parkinson’s disease. Mov Disord. 2013;28(9):1230-1240. doi: 10.1002/mds.25380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li F, Harmer P, Fitzgerald K, et al. Tai chi and postural stability in patients with Parkinson’s disease. N Engl J Med. 2012;366(6):511-519. doi: 10.1056/NEJMoa1107911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381-1395. doi: 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 19.Pescatello LS, Riebe D, Thompson PD. ACSM's Guidelines for Exercise Testing and Prescription. Lippincott Williams & Wilkins; 2014. [DOI] [PubMed] [Google Scholar]
- 20.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575-1581. doi: 10.1249/MSS.0b013e31821ece12 [DOI] [PubMed] [Google Scholar]
- 21.Patki G, Lau YS. Impact of exercise on mitochondrial transcription factor expression and damage in the striatum of a chronic mouse model of Parkinson’s disease. Neurosci Lett. 2011;505(3):268-272. doi: 10.1016/j.neulet.2011.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galassetti PR, Nemet D, Pescatello A, Rose-Gottron C, Larson J, Cooper DM. Exercise, caloric restriction, and systemic oxidative stress. J Investig Med. 2006;54(2):67-75. doi: 10.2310/6650.2005.05024 [DOI] [PubMed] [Google Scholar]
- 23.Tsou YH, Shih CT, Ching CH, et al. Treadmill exercise activates Nrf2 antioxidant system to protect the nigrostriatal dopaminergic neurons from MPP+ toxicity. Exp Neurol. 2015;263:50-62. doi: 10.1016/j.expneurol.2014.09.021 [DOI] [PubMed] [Google Scholar]
- 24.Hirsch MA, Iyer SS, Sanjak M. Exercise-induced neuroplasticity in human Parkinson’s disease: what is the evidence telling us? Parkinsonism Relat Disord. 2016;22(suppl 1):S78-S81. doi: 10.1016/j.parkreldis.2015.09.030 [DOI] [PubMed] [Google Scholar]
- 25.Prodoehl J, Rafferty MR, David FJ, et al. Two-year exercise program improves physical function in Parkinson’s disease: the PRET-PD randomized clinical trial. Neurorehabil Neural Repair. 2015;29(2):112-122. doi: 10.1177/1545968314539732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.David FJ, Robichaud JA, Leurgans SE, et al. Exercise improves cognition in Parkinson’s disease: the PRET-PD randomized, clinical trial. Mov Disord. 2015;30(12):1657-1663. doi: 10.1002/mds.26291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Kolk NM, de Vries NM, Kessels RPC, et al. Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: a double-blind, randomised controlled trial. Lancet Neurol. 2019;18(11):998-1008. doi: 10.1016/S1474-4422(19)30285-6 [DOI] [PubMed] [Google Scholar]
- 28.Park A, Zid D, Russell J, et al. Effects of a formal exercise program on Parkinson’s disease: a pilot study using a delayed start design. Parkinsonism Relat Disord. 2014;20(1):106-111. doi: 10.1016/j.parkreldis.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nascimento CM, Ayan C, Cancela JM, Gobbi LT, Gobbi S, Stella F. Effect of a multimodal exercise program on sleep disturbances and instrumental activities of daily living performance on Parkinson’s and Alzheimer’s disease patients. Geriatr Gerontol Int. 2014;14(2):259-266. doi: 10.1111/ggi.12082 [DOI] [PubMed] [Google Scholar]
- 30.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126-131. [PMC free article] [PubMed] [Google Scholar]
- 31.Van Den Brink CL, Picavet H, Van Den Bos GA, Giampaoli S, Nissinen A, Kromhout D. Duration and intensity of physical activity and disability among European elderly men. Disabil Rehabil. 2005;27(6):341-347. doi: 10.1080/09638280400018452 [DOI] [PubMed] [Google Scholar]
- 32.Chave SP, Morris JN, Moss S, Semmence AM. Vigorous exercise in leisure time and the death rate: a study of male civil servants. J Epidemiol Community Health. 1978;32(4):239-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schenkman M, Moore CG, Kohrt WM, et al. Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease: a phase 2 randomized clinical trial. JAMA Neurol. 2018;75(2):219-226. doi: 10.1001/jamaneurol.2017.3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fasano A, Canning CG, Hausdorff JM, Lord S, Rochester L. Falls in Parkinson’s disease: a complex and evolving picture. Mov Disord. 2017;32(11):1524-1536. doi: 10.1002/mds.27195 [DOI] [PubMed] [Google Scholar]
- 35.Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2014;29(13):1583-1590. doi: 10.1002/mds.25945 [DOI] [PubMed] [Google Scholar]
- 36.Mantri S, Fullard ME, Duda JE, Morley JF. Physical activity in early Parkinson disease. J Parkinsons Dis. 2018;8(1):107-111. doi: 10.3233/JPD-171218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amara AW, Chahine L, Seedorff N, Caspell-Garcia CJ, Coffey C, Simuni T; Parkinson’s Progression Markers Initiative . Self-reported physical activity levels and clinical progression in early Parkinson’s disease. Parkinsonism Relat Disord. 2019;61:118-125. doi: 10.1016/j.parkreldis.2018.11.006 [DOI] [PubMed] [Google Scholar]
- 38.Yang F, Trolle Lagerros Y, Bellocco R, et al. Physical activity and risk of Parkinson’s disease in the Swedish National March Cohort. Brain. 2015;138(Pt 2):269-275. doi: 10.1093/brain/awu323 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Criteria for Parkinson’s disease in the Registration Program for Rare Intractable Diseases
eFigure. Flowchart of the subject selection process
eTable 1. Physical Activity Status Before and After Diagnosis of Parkinson’s Disease stratified by Mortality
eTable 2. Cox Proportional Hazard Regression Analysis of the Risk of All-Cause Mortality in Patients with Parkinson’s Disease Stratified by Intensity
eTable 3. Subgroup Analyses of Age, Sex, Smoking, Alcohol, Comorbidities, Body Mass Index, and Disability Registration
eTable 4. Multivariate Cox Proportional Hazard Analyses of Covariables for All-cause Mortality in Parkinson’s disease