Key Points
Question
Is there an increased risk of developing Guillain-Barré Syndrome (GBS) following vaccination with the recombinant zoster vaccine (RZV)?
Findings
In an observational study of Medicare beneficiaries, a medical record–based, self-controlled analysis of GBS cases after RZV vaccination identified a rate ratio of 2.84 between the risk and control windows, resulting in an attributable risk of 3 cases per million RZV (Shingrix) doses.
Meaning
These findings suggest that there is an increased risk of developing GBS following vaccination with RZV.
This case series cohort study examines the association of onset of Guillain-Barré Syndrome following vaccination with the recombinant zoster vaccine in Medicare beneficiaries.
Abstract
Importance
Guillain-Barré syndrome can be reported after vaccination. This study assesses the risk of Guillain-Barré syndrome after administration of recombinant zoster vaccine (RZV or Shingrix), which is administered in 2 doses 2 to 6 months apart.
Objective
Use Medicare claims data to evaluate risk of developing Guillain-Barré syndrome following vaccination with zoster vaccine.
Design, Setting, and Participants
This case series cohort study included 849 397 RZV-vaccinated and 1 817 099 zoster vaccine live (ZVL or Zostavax)-vaccinated beneficiaries aged 65 years or older. Self-controlled analyses included events identified from 2 113 758 eligible RZV-vaccinated beneficiaries 65 years or older. We compared the relative risk of Guillain-Barré syndrome after RZV vs ZVL, followed by claims-based and medical record-based self-controlled case series analyses to assess risk of Guillain-Barré syndrome during a postvaccination risk window (days 1-42) compared with a control window (days 43-183). In self-controlled analyses, RZV vaccinees were observed from October 1, 2017, to February 29, 2020. Patients were identified in the inpatient, outpatient procedural (including emergency department), and office settings using Medicare administrative data.
Exposures
Vaccination with RZV or ZVL vaccines.
Main Outcomes and Measures
Guillain-Barré syndrome was identified in Medicare administrative claims data, and cases were assessed through medical record review using the Brighton Collaboration case definition.
Results
Amongst those who received RZV vaccinees, the mean age was 74.8 years at first dose, and 58% were women, whereas among those who received the ZVL vaccine, the mean age was 74.3 years, and 60% were women. In the cohort analysis we detected an increase in risk of Guillain-Barré syndrome among RZV vaccinees compared with ZVL vaccinees (rate ratio [RR], 2.34; 95% CI, 1.01-5.41; P = .047). In the self-controlled analyses, we observed 24 and 20 cases during the risk and control period, respectively. Our claims-based analysis identified an increased risk in the risk window compared with the control window (RR, 2.84; 95% CI, 1.53-5.27; P = .001), with an attributable risk of 3 per million RZV doses (95% CI, 0.62-5.64). Our medical record–based analysis confirmed this increased risk (RR, 4.96; 95% CI, 1.43-17.27; P = .01).
Conclusions and Relevance
Findings of this case series cohort study indicate a slightly increased risk of Guillain-Barré syndrome during the 42 days following RZV vaccination in the Medicare population, with approximately 3 excess Guillain-Barré syndrome cases per million vaccinations. Clinicians and patients should be aware of this risk, while considering the benefit of decreasing the risk of herpes zoster and its complications through an efficacious vaccine, as risk-benefit balance remains in favor of vaccination.
Introduction
The US Food and Drug Administration (FDA) approved the recombinant zoster vaccine (RZV), Shingrix, as a 2-dose series administered 2 to 6 months apart to prevent herpes zoster (HZ) in adults aged 50 years or older on October 20, 2017. Herpes zoster, or shingles, is a painful rash caused by reactivation of the varicella zoster virus from latency and especially affects elderly and immunocompromised patients. Herpes zoster and associated complications can cause significant morbidity, including long-term nerve pain and vision loss.1 Results from 2 randomized, placebo-controlled, clinical studies conducted in parallel in 18 countries demonstrated RZV reduced the risk of developing HZ by 97.2% in participants aged 50 years or older and 89.8% in participants aged 70 years or older when compared with placebo.2 After evaluation of available safety and efficacy data, the Advisory Committee on Immunization Practices (ACIP) gave RZV a preferential recommendation over zoster vaccine live (ZVL), Zostavax, for the prevention of HZ in immunocompetent individuals aged 50 years and older.3
Guillain-Barré syndrome (GBS) is a rare, immune-mediated polyneuropathy leading to muscle weakness and paralysis. It affects 3000 to 6000 people each year in the United States (1 to 2 cases per 100 000 person-years).4,5 Guillain-Barré syndrome is thought to result when an individual’s immune response to an antecedent event cross-reacts with peripheral nerve proteins.6,7 A preceding respiratory or gastrointestinal infection has been associated with approximately two-thirds of cases, but GBS has been reported following certain vaccinations.7
The Centers for Disease Control and Prevention (CDC) conducted postlicensure safety surveillance of RZV in the Vaccine Safety Datalink (VSD) by monitoring prespecified adverse events, including GBS. The VSD used International Statistical Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes to identify GBS in the emergency department, inpatient, and outpatient settings, and found GBS incidence following RZV vaccination was higher than expected compared with a historical ZVL cohort, with a statistically significant rate ratio (RR) of 5.06.8
To evaluate the VSD GBS statistical signal, the FDA, the Centers for Medicare & Medicaid Services (CMS), and CDC investigated GBS risk following RZV administration in the Medicare claims database.
Methods
Overview
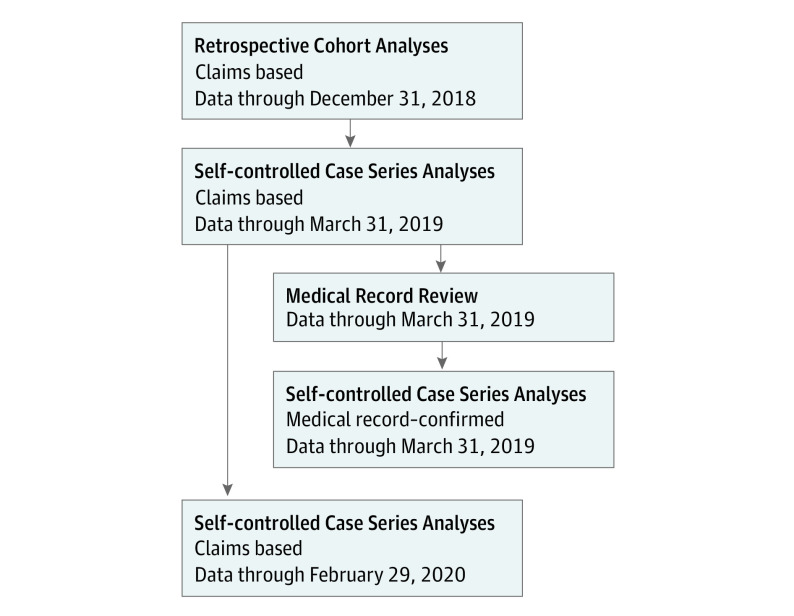
First, we replicated the VSD analysis by conducting a retrospective cohort analysis comparing GBS incidence following RZV and ZVL vaccinations. Second, we completed a self-controlled case series (SCCS) analysis based on cases confirmed by medical record review from October 1, 2017, to March 31, 2019.9 The SCCS design uses cases as their own controls, so it is not susceptible to the same sources of potential bias between comparison groups as with the cohort study. Finally, we did another SCCS analysis with an extended study period using claims-identified cases through February 29, 2020, to assess whether the observed GBS risk changed as more people received RZV (Figure 1). Full study protocols are available in Supplement 1. This study was approved by the FDA’s Research Involving Human Subjects Committee, and the use of Medicare administrative data was approved by the CMS privacy board under a data use agreement.
Figure 1. Shingrix Safety Analysis Workflow.
Data Source
This study used Medicare enrollment and claims databases, the Enrollment Database (EDB) and the Common Working File (CWF), to identify enrolled Medicare beneficiaries who received RZV or ZVL vaccinations and then had an incident case of GBS. Demographic information on age, sex, and race were also obtained from the EDB.
Study Population
Participant RZV and ZVL vaccinations were identified using National Drug Codes (NDCs) from Medicare Part D claims (eTables 1 and 2 in Supplement 2). To ensure complete capture of RZV vaccinations, we required continuous Medicare Fee-for-Service (FFS; Parts A, B, and not C) and Part D enrollment from October 1, 2017, through 6 months after the last RZV vaccination, the end of the study period, or death, whichever occurred earlier. Beneficiaries must have aged into Medicare, be aged 65 years or older on October 1, 2017. To reduce potential confounding from specific frail Medicare populations that could have a different association between vaccination and GBS, beneficiaries could not be undergoing long-term dialysis therapy, or admitted to a nursing home, skilled nursing facility, or in hospice at any point during the study period. In addition, we excluded any beneficiaries with a historical GBS diagnosis in any diagnosis position from any provider within 6 months prior to the first RZV vaccination, and any beneficiaries receiving RZV administration inconsistent with recommended dosing in the package insert2 (ie, received more than 2 administrations of RZV; or received the second administration within 42 days of the first) (eTables 3 and 4 in Supplement 2).
The population varied slightly between different analyses, and complete specifications for the cohort analysis can be found in the eAppendix in Supplement 2.
Outcome
We collected GBS diagnoses from inpatient (IP), outpatient (OP), and carrier (PB) (ie, office) claims using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 357.0 (until September 30, 2015) and the ICD-10-CM code G61.0. Generally, OP claims arise from outpatient procedural and emergency department settings, whereas the PB, or carrier, claims are submitted by professional clinicians in office settings. An incident GBS case was defined as either (1) the first occurrence of a primary discharge diagnosis of GBS in the IP setting postvaccination, or (2) the first occurrence of a GBS diagnosis in any setting and any position followed by a hospitalization within the subsequent 7 days where GBS was the primary discharge diagnosis. In the latter case, the case’s onset date was the date of the earlier GBS claim in the 7 days prior.10
We used a risk window of 42 days after vaccination because this is generally recommended by the Brighton Collaboration GBS case definition. This risk window is frequently used in postvaccination studies, and the number of GBS cases observed would not permit the use of scanning statistics to find an optimal risk window after zoster vaccination.11
Medical Record Review
We requested medical records for all inpatient GBS cases identified during the original study period (October 1, 2017, to March 31, 2019) and conducted medical record review (MRR) for all returned records (eAppendix in Supplement 2). Trained abstractors extracted specific information from the medical records, and 2 independent neurologists independently adjudicated the abstracted data using the Brighton Collaboration’s case definition for GBS (eTable 5 in Supplement 2). Abstractors and neurologists were blinded to vaccination histories. Cases identified by this process as Brighton Level 1, 2, or 3 are medical record–confirmed GBS cases and were included in a medical record–confirmed SCCS analysis. We also collected information on preceding illnesses potentially associated with GBS, such as respiratory and gastrointestinal illness, to investigate potential confounding. The positive predictive value (PPV) of the claims algorithm for medical record–confirmed GBS (ie, medical record confirmation rate) was computed and applied to subsequent analyses.
Cohort Analysis
The cohort analysis compared the postvaccination GBS rate for RZV (October 1, 2017, to December 31, 2018) to the rate for ZVL when it was predominantly administered (October 1, 2012, to September 30, 2017) within the 42-day postvaccination window. We used a Poisson regression model with an offset to account for variable amounts of person-time observed. A detailed description of the cohort analysis is in the eAppendix in Supplement 2.
Self-controlled Case Series Analysis
The SCCS analysis estimates RR by comparing incidence during different time periods for the same individuals rather than across cohorts, thus using individual beneficiaries as their own control, which accounts for time-fixed confounders12 (eAppendix in Supplement 2). Only eligible RZV vaccinees with an incident GBS outcome in the risk or control windows were included in the analysis.
The risk window was days 1 to 42 postvaccination after the first or second dose of RZV, whereas the control window was days 43 to 183 after a first dose (or until second dose receipt) and days 43 to 183 after a second dose (or until end of study period, disenrollment, or death). The control window was bound at 6 months because additional control time would increase enrollment requirements, thus decreasing the size of our eligible population and reducing the power of analyses. Owing to influenza vaccinations’ known association with GBS,13 we produced descriptive statistics for influenza vaccinations occurring 42 days before or after RZV vaccination and that were associated with an identified GBS case (influenza vaccine codes are described in eTable 6 in Supplement 2).
Both claims-identified and medical record–confirmed analyses of hospitalized GBS cases were conducted after all doses. We used a conditional Poisson regression model to calculate the RR, using 95% CIs and 2-tailed P values (P ≤ .05), offset by person-time. The unadjusted attributable risk (AR) was directly derived from the model with a standard error estimated from bootstrap resampling 10 000 times.14 To obtain an AR that better represents the true underlying rate of GBS, we used the PPV obtained from the MRR to impute GBS status for all claims-identified cases and reported the number of excessive cases as the PPV-adjusted AR (eAppendix in Supplement 2). For the secondary analyses, GBS risk was assessed following the first and second RZV doses independently.
Three claims-based sensitivity analyses were conducted: (1) adjusting for seasonality of GBS incidence using background influenza rates (eAppendix in Supplement 2),13 (2) using the adjusted SCCS approach proposed by Farrington that accounts for rare exposures after an outcome (ie, second doses of RZV after GBS),15 and (3) assuming full observability of both the risk and control windows for any individuals who died or disenrolled before the end of the control window. We conducted additional sensitivity analyses using the medical record–confirmed GBS onset dates for medical record–based analyses. To account for unreturned records, PPV-based quantitative bias sensitivity analyses, which included both medical record–confirmed and imputed GBS cases in a conditional Poisson regression model, were conducted (eAppendix in Supplement 2). We also investigated changes in postvaccination GBS risk over time and patterns of GBS case severity (eAppendix, eFigures 1 and 2, and eTables 7-9 in Supplement 2).
All analyses were conducted using R (version 3.6.0; R Foundation) and SAS statistical software (version 9.4; SAS Institute, Inc).
Results
Cohort Analysis
We identified a total of 1 318 004 eligible RZV vaccinations (849 397 beneficiaries) and 1 817 099 eligible ZVL vaccinations (1 817 099 beneficiaries; Table 1; eAppendix in Supplement 2). Amongst the RZV vaccinees, the mean age was 74.8 years at first dose, and 58% were female, whereas among those who received the ZVL vaccine, the mean age was 74.3 years, and 60% were female. After adjusting for age and sex, GBS risk after RZV compared with ZVL was elevated with an RR of 2.34, (95% CI, 1.01-5.41; P = .047) (Figure 2; Table 2).
Table 1. Demographic Characteristics for the Cohorts of RZV and ZVL Vaccinees Included in the Analysisa.
| Characteristic | No. (%) | |
|---|---|---|
| RZV (Oct 1, 2017-Dec 31, 2018) | ZVL (Oct 1, 2012-Sep 30, 2017) | |
| Total | 849 397 (100) | 1 817 099 (100) |
| Age (categories)b | ||
| 65-69 | 199 113 (23.44) | 532 652 (29.31) |
| 70-74 | 261 960 (30.84) | 525 539 (28.92) |
| 75-79 | 197 831 (23.29) | 360 392 (19.83) |
| 80-84 | 113 180 (13.32) | 227 336 (12.51) |
| 85-89 | 55 685 (6.56) | 125 642 (6.91) |
| ≥90 | 21 628 (2.55) | 45 538 (2.51) |
| Sex | ||
| Male | 353 126 (41.57) | 730 515 (40.20) |
| Female | 496 271 (58.43) | 1 086 584 (59.80) |
| Race | ||
| Asian | 23 497 (2.77) | 62 222 (3.42) |
| Black | 18 631 (2.19) | 69 489 (3.82) |
| Hispanic | 6135 (0.72) | 26 557 (1.46) |
| White | 757 480 (89.18) | 1 599 453 (88.02) |
| Unknown/other | 43 654 (5.14) | 59 378 (3.27) |
Abbreviations: RZV, recombinant zoster vaccine; ZVL, zoster vaccine live.
Column totals may add up to 99.9% due to rounding.
Age at the first eligible vaccination.
Figure 2. Distribution of GBS Cases Following RZV and ZVL Vaccinations.
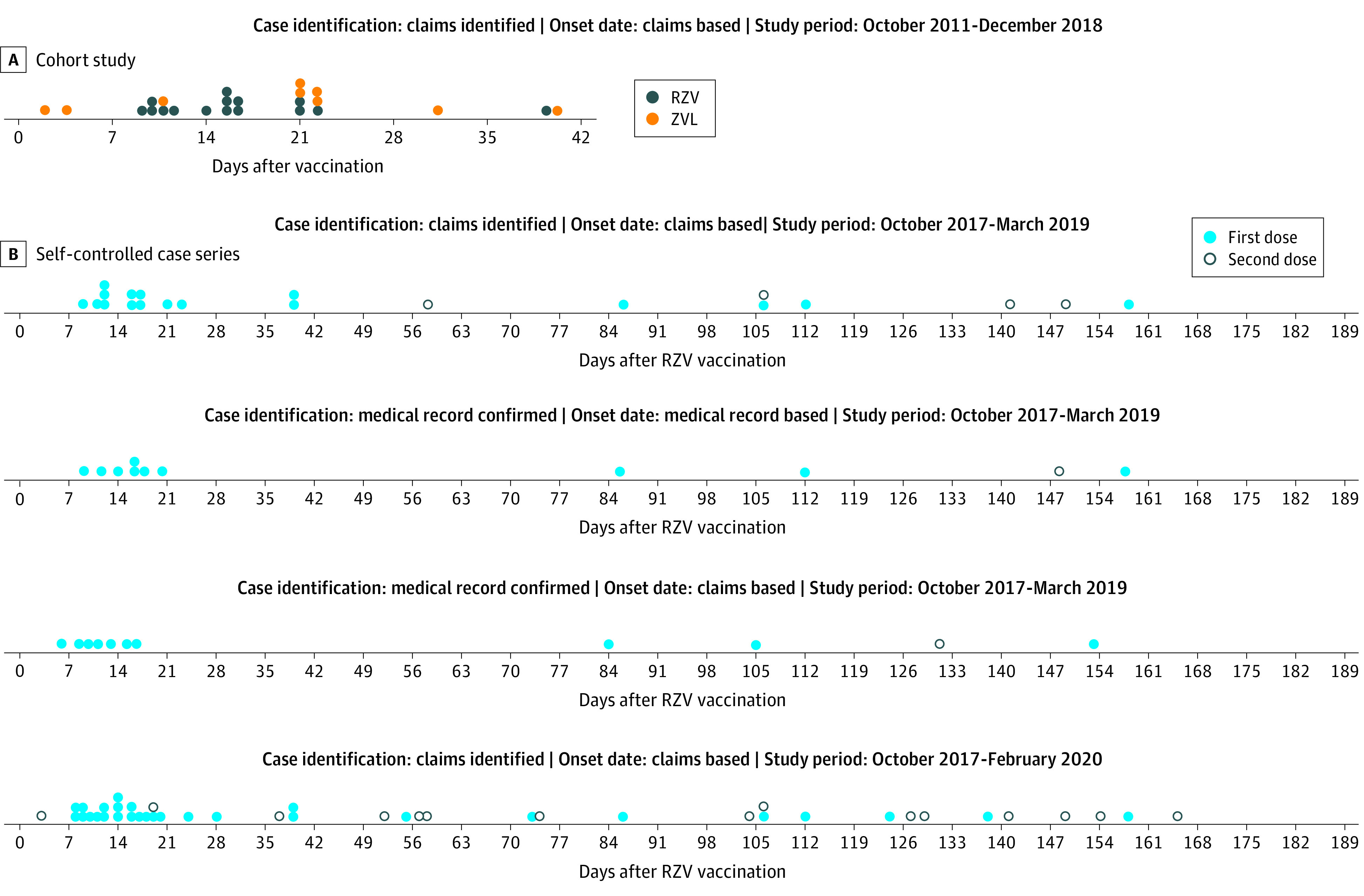
GBS indicates Guillain-Barré Syndrome; RZV, recombinant zoster vaccine; ZVL, zoster vaccine live. A, the cohort analyses, blue dots represent individual cases observed following RZV vaccination, and the green dots represent cases observed following ZVL vaccination. B, the self-controlled analyses, the red dots represent individual cases observed following a first dose of RZV, and the grey dots represent cases observed following a second dose of RZV.
Table 2. Cohort Analysis Results—Comparison of GBS Rates Among RZV vs ZVL Vaccinees in Claims Data Within the 42 Day Risk Window.
| Model | Eligible vaccinations | Cases | Total person time, person days, millions | Outcome rate per million doses | Rate ratio (95% CI) | Attributable risk per million doses (95% CI) |
|---|---|---|---|---|---|---|
| Unadjusted | ||||||
| RZV | 1 318 004 | 15 | 51.49 | 0.29 | 2.44 (1.07 to 5.57) | 6.74 (0.20 to 13.27) |
| ZVL | 1 817 099 | 9 | 75.34 | 0.12 | 1 [Reference]a | 1 [Reference]a |
| Adjusted for Age and Sex | ||||||
| RZV | 1 318 004 | 15 | 51.49 | 0.29 | 2.34 (1.01 to 5.41) | 6.54 (−0.11 to 13.19) |
| ZVL | 1 817 099 | 9 | 75.34 | 0.12 | 1 [Reference]a | 1 [Reference]a |
Abbreviations: GBS, Guillain-Barré syndrome; RZV, recombinant zoster vaccine; ZVL, zoster vaccine live.
ZVL-vaccinated individuals served as the reference group to calculate the rate ratio and attributable risk within the RZV-vaccinated group.
Self-controlled Case Series Analyses
Out of all eligible RZV vaccinees, we observed 13 claims-based GBS cases during the risk period, and 8 during the control period (Figure 2) (eAppendix in Supplement 2). The primary analysis identified an increased risk of GBS in the risk window compared with the control window (RR, 4.30; 95% CI, 1.76-10.53; P = .001; Table 3). We found that there were 6.47 excess cases of GBS in the risk window compared with the control window (95% CI, 2.50-10.45; P = .001) per million vaccinations. The PPV-adjusted AR calculation showed 5.08 excess cases per million vaccinations (95% CI, 1.04-9.11; P = .01). Only 2 of the 21 observed claims-based GBS cases received an influenza vaccination in the 42 days prior to RZV vaccination; both cases occurred in the control window, so no GBS cases identified in the risk window were preceded by influenza vaccination. In the extended analysis, of the 44 claims-based GBS cases, 6 received an influenza vaccination in the 42 days prior to RZV vaccination; 3 cases each in the risk and control windows.
Table 3. Self-controlled Case Series Results From Analysis of GBS Risk Following RZV Administration.
| Analysis | Risk case | Control cases | Risk days | Control days | Rate ratio (95% CI) | Attributable riska | |||
|---|---|---|---|---|---|---|---|---|---|
| Per million doses | Per 100 000 person-years | ||||||||
| Unadjusted (95% CI)b | PPV-Adjusted (95% CI)c | Unadjusted (95% CI)b | PPV-Adjusted (95% CI)c | ||||||
| Original study period (October 1, 2017 to March 31, 2019) | |||||||||
| Primary analysis | |||||||||
| Claims-based (all doses) | 13 | 8 | 1092 | 2928 | 4.30 (1.76-10.53) | 6.47 (2.50-10.45) | 5.08 (1.04-9.11) | 1.98 (0.77-3.20) | 1.56 (0.32-2.79) |
| Secondary analysis | |||||||||
| Claims-based (first dose only) | 13 | 4 | 714 | 2090 | 9.30 (3.00-28.84)d | 12.20 (7.41-17.00)d | 9.50 (4.35-14.66)d | 4.10 (2.49-5.72)d | 3.20 (1.46-4.93)d |
| Medical record–confirmed analysis | |||||||||
| All doses (risk: 1-42; control: 43-183) | 7 | 4 | 546 | 1557 | 4.96 (1.43-17.27) | 5.17 (1.50-8.84) | 1.59 (0.46-2.71) | ||
| Extended study period (October 1, 2017 to February 29, 2020) | |||||||||
| Primary analysis | |||||||||
| Claims-based (all doses) | 24 | 20 | 2489 | 6157 | 2.84 (1.53-5.27) | 4.17 (1.65-6.69) | 3.13 (0.62-5.64) | 1.25 (0.49-2.00) | 0.93 (0.18-1.68) |
| Secondary analysis | |||||||||
| Claims-based | |||||||||
| First dose only | 21 | 8 | 1191 | 3675 | 7.72 (3.39-17.60)d | 8.65 (5.74-11.56)d | 6.48 (3.36-9.60)d | 3.06 (2.03-4.08)d | 2.29 (1.19-3.39)d |
| Second dose only | 3 | 12 | 626 | 1453 | 0.22 (0.04-1.22) | ||||
Abbreviations: GBS, Guillain-Barré syndrome; PPV, positive predictive value; RZV, recombinant zoster vaccine.
When calculating second-dose only attributable risks (ARs), we observed a high variance among the estimates from the bootstrapping simulations; the AR estimate for the individual who dies before the beginning of the control window can be infinity, resulting in drastically inflated CI estimates. We have suppressed second-dose only AR estimates for this reason.
Unadjusted AR estimates are derived using only the claims-based case classifications and do not take into account the outcome PPV or record return rate.
PPV-adjusted AR was calculated by imputing GBS status for all claims-identified cases and cases without medical records using the PPV as the PPV-based quantitative bias sensitivity analysis (eAppendix in Supplement 2).
P < .001.
Our secondary analysis included 950 797 first RZV doses and identified an increased risk of GBS (RR, 9.30; 95% CI, 3.00-28.84; P < .001) (Figure 3). There were 9.50 excess cases for every million vaccinations administered (95% CI, 4.35-14.66; P < .001), adjusted for PPV (Table 3). Seasonality-adjusted analyses produced similar results (eTable 10 in Supplement 2).
Figure 3. Rate Ratio Forest Plot of GBS Incidence During the Risk Window Compared With the Control Window for SCCS Analyses.
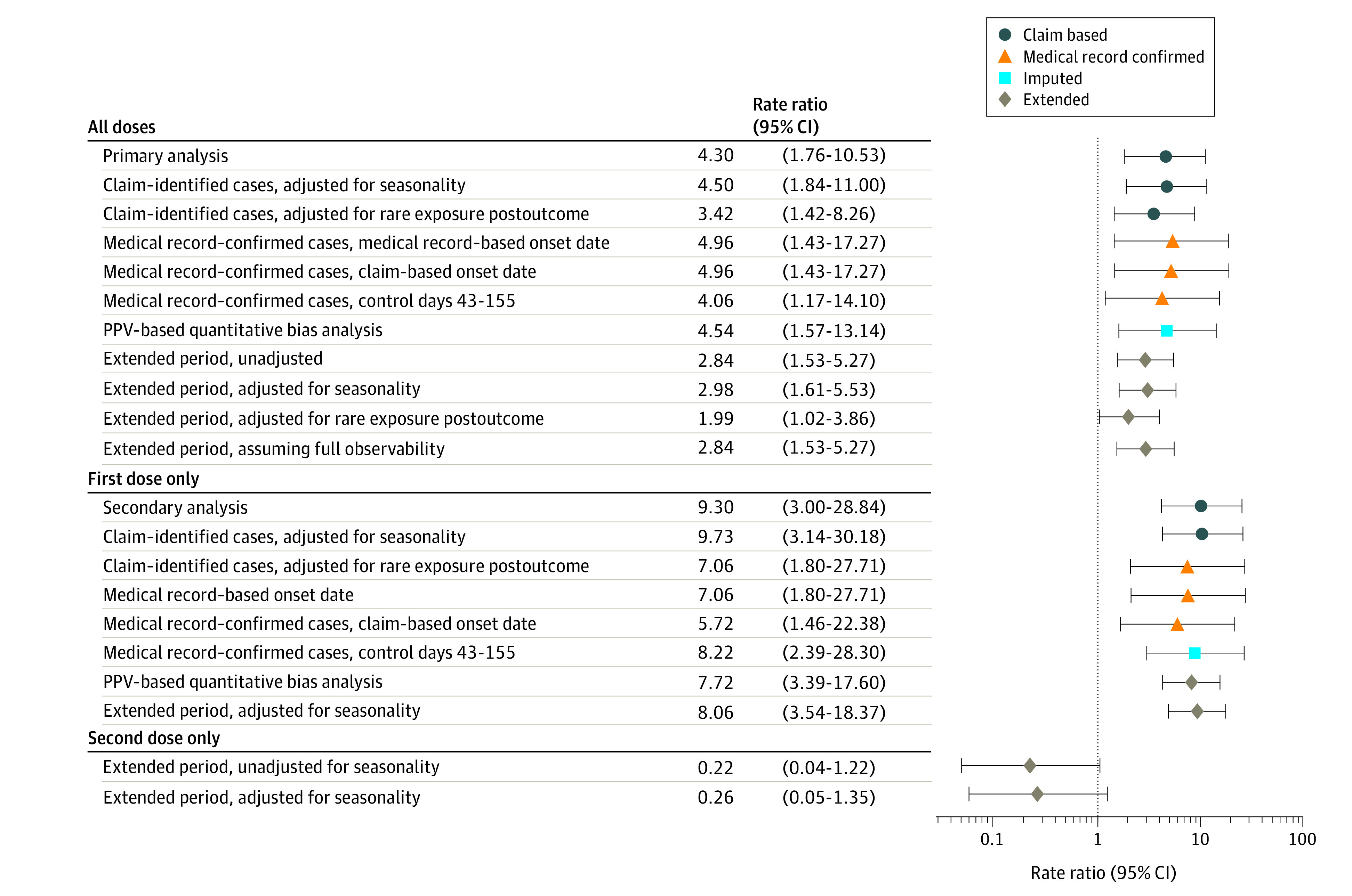
GBS indicates Guillain-Barré Syndrome; PPV, positive predictive value; SCCS, self-controlled case series.
Of the 36 total cases requested for MRR across both the cohort and SCCS studies, 28 were returned and reviewed, 22 of which were MRR confirmed by neurologists as a GBS case, resulting in a return rate of 77.78% and a medical record–confirmed PPV of 78.57% (95% CI, 63.37%-93.77%). Of the confirmed SCCS cases, 7 were in the risk window and 4 were in the control window (Figure 2). We found an increased risk of GBS (RR, 4.96; 95% CI, 1.43-17.27; P = .01) when comparing incidence in the risk and control windows, resulting in a PPV-adjusted AR of 5.17 per million doses (95% CI, 1.50-8.84; P = .006). Our analysis of infectious illnesses preceding GBS onset did not diminish study findings as preceding infection occurred more frequently among cases in the control window (eTable 11 in Supplement 2).
The extended study period SCCS analysis confirmed earlier results by including additional data, which decreased the observed risk from an RR of 4.30 to 2.84 (95% CI, 1.53-5.27; P = .001); PPV-adjusted AR became 3.13 (95% CI, 0.62-5.64; P = .01). Full results can be found in Table 3; and eTable 10 in Supplement2.
Initially, we did not have sufficient power to conduct second-dose only analyses (0 cases were identified). However, after extending the study period for the claims-based SCCS analysis, we identified 15 GBS events occurring after the second dose; this resulted in an RR of 0.22 (95% CI, 0.04-1.22; P = .08) (Table 3).
Discussion
The CDC VSD signal suggested an association of GBS with RZV. Clinical trial data was not powered to detect a statistical association between the rare event, GBS, and vaccination, and the trials observed an imbalance with more cases occurring after placebo.16 Our current findings confirm an increased risk of GBS following RZV vaccination in the Medicare population aged 65 years and older. The Medicare cohort analysis showed a statistically significant increased risk of GBS among RZV recipients compared with ZVL recipients. The SCCS medical record–confirmed sensitivity analyses, which used the set of GBS cases for which we had the highest degree of certainty, showed an increased risk of GBS following RZV vaccination, with an AR of 5.17 excess GBS cases per million doses during the 42-day risk window following vaccination.
The increased risk of GBS following RZV should be considered in the context of the benefits of the vaccine. Herpes zoster is associated with substantial morbidity, including possible persistent pain, and disseminated disease.17 Prior to HZ vaccine licensure, approximately 1 million cases of HZ occurred annually in the US, resulting in approximately $1.3 billion in medical care costs and $1.7 billion in indirect costs per year.18,19 In clinical trials, RZV reduced the incidence of HZ by 97% in participants aged 50 years and older, and efficacy appeared durable through 4 years after vaccination.2 Comparatively, clinical trials for ZVL reduced incidence of HZ by 51% in participants aged 60 years and older. Further, reports describe the possible association of HZ virus reactivation with subsequent GBS.20,21 If HZ reactivation also triggers cases of GBS, then RZV vaccine could potentially prevent these cases of GBS, and the vaccine could be protective against the outcome. Our study examined the risk of GBS in the 42 days following vaccination, which may not account for potential changes in GBS risk over time owing to wild-type HZ reactivation.
Recent presentations to ACIP estimated the risk of GBS after HZ reactivation as an RR of 4.0 (95% CI, 1.9-8.7) and an AR of 12.8 cases per million HZ episodes (95% CI, 3.7-31.9). This is statistically similar to the most precise estimates of GBS after RZV identified herein: RR, 2.84 (95% CI, 1.53-5.27) and an AR of 3.13 (95% CI, 0.62-5.64).22,23,24 Using this AR, and the vaccine efficacy of 97%, one would expect 6.26 cases of GBS among a million fully vaccinated individuals (2 doses per individual), whereas one would expect to prevent approximately 7070 HZ reactivations in the same population according to the trial data for those aged 60 to 69 years in the Shingrix Package Insert.2 During an ACIP meeting, a more complex probabilistic model was presented that investigated the risk benefit of RZV vaccination in a population over 20 years. Taking into account risk of GBS after HZ reactivation and RZV, it estimated 6.3 excess GBS cases after RZV vaccination in those aged 60 to 69 years compared with 61 600 HZ cases averted (including 62 potential deaths, 5500 cases of HZ ocular complications, and 9350 cases of postherpetic neuralgia).23 These data convey that the risk-benefit balance for RZV remains favorable, and clinicians should continue to offer and discuss RZV vaccination with their patients.
Strengths and Limitations
This study had several major strengths. First, this is the largest post-market study evaluating GBS risk following RZV vaccination. Further, we use a study population well suited to the analyses being conducted because the Medicare FFS population is the largest cohort of US beneficiaries aged 65 years or older with individually linked data (eg, demographic, diagnostic, and vaccination information). We also completed confirmatory SCCS analyses after the initial cohort analyses to reduce the risk of confounding by time-fixed covariates (eg, sex), which potentially provided more precise estimates of increased GBS risk after RZV vaccination. Finally, we completed an MRR and conducted an analysis of medical record–confirmed cases to increase the reliability of our results; this also helped increase precision of onset dates and case classification.
There are several limitations of this study. First, the cohort and SCCS analyses did not explicitly adjust for preceding illness or other time-varying confounders. However, the potential influence of preceding illnesses was not found to diminish results. Second, using Medicare claims creates the possibility for under-ascertainment of vaccine administration because nontraditional clinical settings may not submit vaccination claims to Medicare, which limits sample size. Third, the risk window for GBS following RZV vaccination is not as well defined as the risk window following influenza vaccination, and we lacked sufficient data to empirically determine the ideal risk window using scanning statistics. Finally, although RZV is approved for individuals aged 50 years or older, our study population only included individuals aged 65 years or older.
This study relied on a rather specific GBS case identification algorithm, which provides additional confidence in identification, but may have limited the number of cases included; we only included cases with hospital discharge diagnoses of GBS in the primary position, which may have excluded some GBS cases. However, GBS is a well-defined acute disease that usually requires hospitalization, and defining cases as claims with a primary GBS hospitalization results in a higher PPV.25
Conclusions
This collaborative effort between the FDA, CMS, and CDC, to examine an early VSD statistical signal illustrates the value of multiagency efforts to conduct GBS surveillance in a timely manner. Despite the elevated risk of GBS observed herein, patients and clinicians should be aware of the risk of GBS, and consider the benefits of avoiding zoster with an efficacious vaccine.
Trial Protocols
eAppendix
eTable 1. National Drug Codes Used to Identify Shingrix Vaccinations in Medicare Claims
eTable 2. National Drug Codes Used to Identify ZVL Vaccinations in Medicare Claims
eTable 3. Cohort Creation Table for RZV and ZVL Vaccinated Populations
eTable 4. Cohort Creation Table for GBS Population
eTable 5. Brighton Collaboration Case Classifications and Criteria for Guillain Barré Syndrome
eTable 6. List of influenza vaccine codes included in surveillance for the 2017-2018, 2018–2019 season
eFigure 1. Self-Controlled Case Series GBS Risk Ratio Over Time (All Doses)
eFigure 2. Length of Stay for Shingrix-Vaccinated GBS Cases (Oct 2017 – Feb 2020)
eTable 7. Respiratory Failure and Intubation Codes
eTable 8. Shingrix-Vaccinated GBS Cases with Respiratory Failure After GBS
eTable 9. Length of Stay for Shingrix-Vaccinated GBS Cases (Oct 2017 – Feb 2020)
eTable 10. Full Set of Self-Controlled GBS Case Series Results
eTable 11. Preceding Illnesses According to Abstraction Results for Chart-confirmed GBS Cases
eReferences
References
- 1.Prevention CfDCa . Shingles | Signs and Symptoms | Herpes Zoster | CDC. Accessed March 9, 2020. https://www.cdc.gov/shingles/about/symptoms.html
- 2.(FDA) FaDA . Shingrix package insert. 2017. Accessed May 16, 2018.https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM581605.pdf
- 3.Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines. MMWR Morb Mortal Wkly Rep. 2018;67(3):103-108. doi: 10.15585/mmwr.mm6703a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(CDC) CfDCaP . Guillain-Barré Syndrome. 2019.Accessed March 9, 2020. https://www.cdc.gov/vaccinesafety/concerns/guillain-barre-syndrome.html
- 5.Sejvar JJ, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain-Barré syndrome: a systematic review and meta-analysis. Neuroepidemiology. 2011;36(2):123-133. doi: 10.1159/000324710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuki N, Hartung HP. Guillain-Barré syndrome. N Engl J Med. 2012;366(24):2294-2304. doi: 10.1056/NEJMra1114525 [DOI] [PubMed] [Google Scholar]
- 7.Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976—1977. Am J Epidemiol. 1979;110(2):105-123. doi: 10.1093/oxfordjournals.aje.a112795 [DOI] [PubMed] [Google Scholar]
- 8.Shimabukuro TT. Update on post-licensure safety monitoring of recombinant zoster vaccine. 2019; Atlanta, GA. [Google Scholar]
- 9.Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med. 2006;25(10):1768-1797. doi: 10.1002/sim.2302 [DOI] [PubMed] [Google Scholar]
- 10.Perez-Vilar S, Wernecke M, Arya D, et al. Surveillance for Guillain-Barré syndrome after influenza vaccination among U.S. Medicare beneficiaries during the 2017-2018 season. Vaccine. 2019;37(29):3856-3865. doi: 10.1016/j.vaccine.2019.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sejvar JJ, Kohl KS, Gidudu J, et al. ; Brighton Collaboration GBS Working Group . Guillain-Barré syndrome and Fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2011;29(3):599-612. doi: 10.1016/j.vaccine.2010.06.003 [DOI] [PubMed] [Google Scholar]
- 12.Pérez-Vilar S, Díez-Domingo J, Puig-Barberà J, Gil-Prieto R, Romio S. Intussusception following rotavirus vaccination in the Valencia Region, Spain. Hum Vaccin Immunother. 2015;11(7):1848-1852. doi: 10.1080/21645515.2015.1049787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandhu SK, Hua W, MaCurdy TE, et al. Near real-time surveillance for Guillain-Barré syndrome after influenza vaccination among the Medicare population, 2010/11 to 2013/14. Vaccine. 2017;35(22):2986-2992. doi: 10.1016/j.vaccine.2017.03.087 [DOI] [PubMed] [Google Scholar]
- 14.Cox C, Li X. Model-based estimation of the attributable risk: a loglinear approach. Comput Stat Data Anal. 2012;56(12):4180-4189. doi: 10.1016/j.csda.2012.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farrington CP, Whitaker HJ, Hocine MN. Case series analysis for censored, perturbed, or curtailed post-event exposures. Biostatistics. 2009;10(1):3-16. doi: 10.1093/biostatistics/kxn013 [DOI] [PubMed] [Google Scholar]
- 16.Agger P, Reindel R. BLA Clinical Review Memorandum. U.S. Food and Drug Administration; October 20, 2017. Accessed October 15, 2020. www.fda.gov/media/108786/download
- 17.Tseng HF, Bruxvoort K, Ackerson B, et al. The epidemiology of herpes zoster in immunocompetent, unvaccinated adults ≥50 years old: incidence, complications, hospitalization, mortality, and recurrence. J Infect Dis. 2020;222(5):798-806. doi: 10.1093/infdis/jiz652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82(11):1341-1349. doi: 10.4065/82.11.1341 [DOI] [PubMed] [Google Scholar]
- 19.Curran D, Patterson B, Varghese L, et al. Cost-effectiveness of an adjuvanted recombinant zoster vaccine in older adults in the United States. Vaccine. 2018;36(33):5037-5045. doi: 10.1016/j.vaccine.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 20.Kang JH, Sheu JJ, Lin HC. Increased risk of Guillain-Barré Syndrome following recent herpes zoster: a population-based study across Taiwan. Clin Infect Dis. 2010;51(5):525-530. doi: 10.1086/655136 [DOI] [PubMed] [Google Scholar]
- 21.Roccatagliata L, Uccelli A, Murialdo A. Guillain-Barré syndrome after reactivation of varicella-zoster virus. N Engl J Med. 2001;344(1):65-66. doi: 10.1056/NEJM200101043440117 [DOI] [PubMed] [Google Scholar]
- 22.Anderson T. Zoster Vaccines Session: Summary of the Herpes Zoster Work Group’s Interpretation of Recombinant Zoster Vaccine Safety Data. Paper presented at: Advisory Committee on Immunization Practices; February 25, 2021. [Google Scholar]
- 23.Prosser L. Projected Risks and Health Benefits of Vaccination against Herpes Zoster and Related Complications Interim Results. Paper presented at: Advisory Committee on Immunization Practices; February 25, 2021. [Google Scholar]
- 24.Farrington P, Pugh S, Colville A, et al. A new method for active surveillance of adverse events from diphtheria/tetanus/pertussis and measles/mumps/rubella vaccines. Lancet. 1995;345(8949):567-569. doi: 10.1016/S0140-6736(95)90471-9 [DOI] [PubMed] [Google Scholar]
- 25.Polakowski LL, Sandhu SK, Martin DB, et al. Chart-confirmed guillain-barre syndrome after 2009 H1N1 influenza vaccination among the Medicare population, 2009-2010. Am J Epidemiol. 2013;178(6):962-973. doi: 10.1093/aje/kwt051 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocols
eAppendix
eTable 1. National Drug Codes Used to Identify Shingrix Vaccinations in Medicare Claims
eTable 2. National Drug Codes Used to Identify ZVL Vaccinations in Medicare Claims
eTable 3. Cohort Creation Table for RZV and ZVL Vaccinated Populations
eTable 4. Cohort Creation Table for GBS Population
eTable 5. Brighton Collaboration Case Classifications and Criteria for Guillain Barré Syndrome
eTable 6. List of influenza vaccine codes included in surveillance for the 2017-2018, 2018–2019 season
eFigure 1. Self-Controlled Case Series GBS Risk Ratio Over Time (All Doses)
eFigure 2. Length of Stay for Shingrix-Vaccinated GBS Cases (Oct 2017 – Feb 2020)
eTable 7. Respiratory Failure and Intubation Codes
eTable 8. Shingrix-Vaccinated GBS Cases with Respiratory Failure After GBS
eTable 9. Length of Stay for Shingrix-Vaccinated GBS Cases (Oct 2017 – Feb 2020)
eTable 10. Full Set of Self-Controlled GBS Case Series Results
eTable 11. Preceding Illnesses According to Abstraction Results for Chart-confirmed GBS Cases
eReferences