Abstract
Overexpression of the ErbB2 receptor, a major component of the ErbB receptor signaling network, contributes to the development of a number of human cancers. ErbB2 presents itself, therefore, as a target for antibody-mediated therapies. In this respect, anti-ErbB2 monoclonal antibody 4D5 specifically inhibits the growth of tumor cells overexpressing ErbB2. We have analyzed the effect of 4D5-mediated ErbB2 inhibition on the cell cycle of the breast tumor cell line BT474. 4D5 treatment of BT474 cells resulted in a G1 arrest, preceded by rapid dephosphorylation of ErbB2, inhibition of cytoplasmic signal transduction pathways, accumulation of the cyclin-dependent kinase inhibitor p27Kip1, and inactivation of cyclin-Cdk2 complexes. Time courses demonstrated that 4D5 treatment redirects p27Kip1 onto Cdk2 complexes, an event preceding increased p27Kip1 expression; this correlates with the downregulation of c-Myc and D-type cyclins (proteins involved in p27Kip1 sequestration) and the loss of p27Kip1 from Cdk4 complexes. Similar events were observed in ErbB2-overexpressing SKBR3 cells, which exhibited reduced proliferation in response to 4D5 treatment. Here, p27Kip1 redistribution resulted in partial Cdk2 inactivation, consistent with a G1 accumulation. Moreover, p27Kip1 protein levels remained constant. Antisense-mediated inhibition of p27Kip1 expression in 4D5-treated BT474 cells further demonstrated that in the absence of p27Kip1 accumulation, p27Kip1 redirection onto Cdk2 complexes is sufficient to inactivate Cdk2 and establish the G1 block. These data suggest that ErbB2 overexpression leads to potentiation of cyclin E-Cdk2 activity through regulation of p27Kip1 sequestration proteins, thus deregulating the G1/S transition. Moreover, through comparison with an ErbB2-overexpressing cell line insensitive to 4D5 treatment, we demonstrate the specificity of these cell cycle events and show that ErbB2 overexpression alone is insufficient to determine the cellular response to receptor inhibition.
The ErbB family of type I receptor tyrosine kinases has four members, ErbB1/epidermal growth factor receptor, ErbB2/Neu, ErbB3, and ErbB4. Although these receptors share common structural elements, including an extracellular ligand-binding domain and an intracellular tyrosine kinase domain, ligands have been identified only for ErbB1, ErbB3, and ErbB4 (for a review, see reference 16). ErbB2 remains an orphan receptor, with no diffusible ErbB2-specific ligand identified. However, ErbB2 can be transactivated through heterodimerization with other ErbB family members (11, 62) and appears to be their preferred heterodimerization partner (23, 30). ErbB2-containing heterodimers couple potently to major mitogenic signaling cascades, such as the mitogen-activated protein (MAP) kinase and phosphatidylinositol 3-kinase (PI3-kinase) pathways (16). Moreover, ErbB2 plays a role in the potentiation and prolongation of ErbB receptor signaling (4, 22, 30, 49).
The role of growth factors and their cognate receptors in cell growth and differentiation is now well established. Additionally, deregulation of growth factor receptors and/or elements of their signaling pathways occurs during the stepwise progression of a normal cell to a malignant phenotype. In this respect, two ErbB family members, ErbB1 and ErbB2, are involved in the development of many human cancers, including ovary and breast cancers. Indeed, amplification of the gene encoding ErbB2, leading to overexpression of the receptor, was one of the first consistent genetic alterations found in primary human breast tumors (6, 70, 71). Furthermore, overexpression of ErbB2 correlates with a poor patient prognosis not only in breast cancer (24, 59, 70, 71) but also in other malignancies, such as ovarian (71) and gastric (84) cancers. These observations suggest that ErbB2 overexpression provides tumor cells with a growth advantage leading to a more aggressive phenotype. It seems likely, therefore, that an ErbB2-dependent sustained mitogenic stimulus may contribute to the uncontrolled cell growth associated with tumor progression. This phenomenon is presumably due to the formation of active receptor dimers which signal even in the absence of ligand. In agreement with this hypothesis, treatment with ErbB2-specific antibodies has been shown to selectively inhibit the growth of tumor cells which overexpress ErbB2 (26, 27, 29, 37, 38). However, despite the obvious involvement of ErbB2 in tumor progression, the underlying mechanisms by which overexpression of this receptor potentiates tumor cell growth remain poorly understood.
In addition to perturbations in signal transduction networks, aberrant expression of key cell cycle regulators also contributes to deregulated cell proliferation during tumor development (reviewed in references 18 and 28). In nonimmortalized, somatic cells genetic integrity during cell division is maintained through the proper execution of an intrinsic cell cycle machinery. The replication, repair, and segregation of DNA must be accurately performed in order to prevent the genetic changes associated with malignant transformation. The major regulators of cell cycle progression are the cyclin-dependent kinases (Cdks), the periodic activation and inactivation of which regulate not only progression through each cell cycle stage but also transitions from one cell cycle stage into another (for a review, see reference 47). In G1, for example, passage of cells from growth factor dependency to growth factor independence (through the restriction point) is mediated by the sequential activation of cyclin D-dependent kinases Cdk4 and -6 and cyclin E-dependent kinase Cdk2 (for a review, see reference 68). These kinases phosphorylate and inactivate growth suppressor proteins of the retinoblastoma protein (pRb) family, allowing the expression of genes whose activities are required for S-phase entry (75). The importance of this pathway in growth control is highlighted by the fact that many of its components are commonly mutated, deleted, or aberrantly expressed in human cancer (for reviews, see references 18 and 28).
The activity of G1 Cdks is stringently regulated, not only by association with specific regulatory cyclin subunits and phosphorylation/dephosphorylation events but also through association with specific Cdk inhibitors (CKIs) (47, 67, 68). There are two classes of CKIs, the INK4 proteins (INK4a to -d), which act specifically on cyclin D-dependent kinases, and the CIP/KIP family (p21Cip1/Waf1, p27Kip1, and p57Kip2), which bind all G1 cyclin-Cdk complexes (67, 68). In fibroblasts, regulation of p27Kip1 function is an essential step in the pathway linking mitogenic signals to passage through the restriction point (14). This is thought to be due to p27Kip1-mediated regulation of cyclin E-Cdk2 activity. Indeed, in vitro, p27Kip1 is a more effective inhibitor of cyclin E-Cdk2 than of cyclin D-Cdk4 (56, 76). Additionally, in vivo, p27Kip1 mediates inhibition of cyclin E-Cdk2 in cells that are exposed to growth-inhibitory agents (57, 72).
Although it was first assumed that CKIs act solely as inhibitors of Cdk complexes, members of the CIP/KIP family also promote the assembly of Cdk4-cyclin D complexes (34). Indeed, both p21Cip1/Waf1 and p27Kip1 are essential for Cdk4-cyclin D activity (13), being found in active kinase complexes in proliferating cells (8, 34, 73, 85). Furthermore, the sequestration of p27Kip1 (and p21Cip1/Waf1) into higher-order complexes with cyclin D-dependent kinases appears to play a role in the activation of cyclin E-Cdk2 as cells progress through late G1. In this respect, the proto-oncogene c-myc, which is clearly involved in the regulation of cyclin E-Cdk2 activity (7, 36), has been shown to play a major role in p27Kip1 sequestration through modulation of cyclin D protein levels (10, 54), as well as possibly other unknown p27Kip1 sequestration proteins (2, 77). This suggests that a number of p27Kip1-sequestering proteins may exist. The relative contribution of each to cell cycle control may depend on cellular context.
In this study, we have addressed the question of why ErbB2 overexpression in tumors is associated with more aggressive growth characteristics. In this regard, an anti-ErbB2 monoclonal antibody (MAb 4D5), directed to the extracellular domain of the receptor, has been previously shown to specifically inhibit the growth of tumor cells overexpressing the ErbB2 receptor (27, 37, 38). These observations suggest that the growth of tumors overexpressing ErbB2 may be potentiated by increased ErbB2 receptor signaling. To gain insight into the consequence of ErbB2 overexpression for tumor development, we have examined how receptor overexpression impinges on cytoplasmic signaling pathways and elements of cell cycle control by analyzing the molecular mechanism of action of this growth-inhibitory antibody. We show that 4D5 treatment of BT474 cells, a human breast carcinoma cell line overexpressing ErbB2, results in a stable G1 accumulation. This correlates with rapid downregulation of ErbB2 receptor signaling, increased p27Kip1 levels, and inactivation of the cyclin E-Cdk2 complex. We further demonstrate that ErbB2 receptor inhibition leads to a redistribution of p27Kip1 protein onto Cdk2 complexes. This event precedes increases in p27Kip1 expression, paralleling the loss of proteins involved in p27Kip1 sequestration, and is sufficient to totally inhibit Cdk2 activity and establish the G1 block. These data suggest that in breast tumor cells ErbB2 overexpression provides an essential signaling element, leading to the potentiation of cyclin E-Cdk2 activity through sequestration of the CKI p27Kip1. Analysis of a second overexpressing cell line (SKBR3), which exhibits reduced proliferation in response to 4D5 treatment, supports this hypothesis. Furthermore, through comparison with an ErbB2-overexpressing, gastric carcinoma cell line (MKN7) insensitive to 4D5 treatment, we demonstrate that the growth response to 4D5-mediated inhibition of ErbB2 receptor function is tumor specific and may correlate with ErbB receptor expression profiles and/or the absence of compensatory mitogenic signaling pathways.
MATERIALS AND METHODS
Cell culture, growth assays, lysate preparation, and flow cytometry.
Breast carcinoma (BT474, T47D, and SKBR3) cells were obtained from the American Type Culture Collection (Manassas, Va.) and grown in Dulbecco's modified Eagle's medium (GIBCO BRL, Gaithersburg, Md.), supplemented with 10% fetal calf serum, at 37°C and 5% CO2. Gastric carcinoma (MKN7) cells were kindly provided by C. Benz (University of California, San Francisco) and cultured as described above except that the Dulbecco's modified Eagle's medium was mixed 1:1 with Ham's F-12 (GIBCO BRL). BT474, SKBR3, and MKN7 cells were considered ErbB2 overexpressors by the criterion that they express approximately 0.5 × 106 to 1.0 × 106 receptors/cell (I. Harwerth and N. E. Hynes, unpublished results; see also reference 37). For growth assays, cells were plated at a density of 2,000 cells/cm2 or as stated in the text. After 24 h of incubation, the medium was changed and either the purified mouse MAb 4D5 (kindly supplied by Genentech, Inc., South San Francisco, Calif.) or FRP5 (25) was added to a final concentration of 10 μg/ml. Both of these antibodies are of the isotype immunoglobulin G1. Cells were trypsinized at the times stated and counted in a hemocytometer. Cells grown for more than 4 days were refed with fresh medium, with or without antibody, on day 4.
For direct measurements of DNA synthesis, cells were seeded onto acid-washed glass coverslips and cultured in the presence of antibody as described above. After the times stated, bromodeoxyuridine (BrdU) was added for 4 h, the cells were fixed, and BrdU incorporation into nuclei was revealed by immunofluorescence as previously described (35). Cells were counted, and the percentage with BrdU-labeled nuclei was calculated.
For preparation of protein lysates, cells were plated at a density of 3 × 104 cells/cm2. After 24 h of incubation, the medium was changed and 4D5 or FRP5 was added to a final concentration of 10 μg/ml. At the times indicated, cells were first washed with ice-cold phosphate-buffered saline (PBS) containing 1 mM phenylmethylsulfonyl fluoride and then with buffer containing 50 mM HEPES (pH 7.5), 150 mM NaCl, 25 mM β-glycerophosphate, 25 mM NaF, 5 mM EGTA, 1 mM EDTA, 15 mM pyrophosphate, 2 mM sodium orthovanadate, 10 mM sodium molybdate, leupeptin (10 μg/ml), aprotinin (10 μg/ml), and 1 mM phenylmethylsulfonyl fluoride (protease inhibitors from Sigma Chemical, St. Louis, Mo.). Cells were extracted in the same buffer containing 1% NP-40. After homogenization, lysates were clarified by centrifugation and frozen at −80°C. Protein concentrations were determined with the Bio-Rad (Munich, Germany) protein assay reagent.
To analyze the cell cycle profile of cells, cultures were seeded and treated with antibody as for the preparation of protein lysates. At the times indicated, cells were trypsinized, washed twice with ice-cold PBS, and resuspended in propidium iodide buffer (1 mM sodium citrate [pH 4.0], 1.5 mM NaCl, 5 mM EDTA, 5 mM EGTA, 0.1% NP-40, 4 μg of propidium iodide/ml, and 80 μg of RNase A/ml in PBS). After 30 min of incubation on ice, cell cycle distribution was monitored with a Becton Dickinson FACScan flow cytometer.
Immunological techniques.
For immunoblot analysis of cell cycle regulators and signal transducers, clarified protein lysates (30 to 50 μg) were electrophoretically resolved on denaturing sodium dodecyl sulfate (SDS)-polyacrylamide gels (7.5 to 14%), transferred to polyvinylidene difluoride (Boehringer Mannheim GmbH, Mannheim, Germany), and probed with the following primary antibodies: anti-cyclin A and -p45SKP2 (kindly supplied by W. Krek, Friedrich Miescher Institute, Basel, Switzerland); anti-c-Myc (9E10), -cyclin E (C-19), -cyclin D2 (C-17), -cyclin D3 (C-16), -Cdk2 (M2), and -Cdk4 (C-22; from Santa Cruz Biotechnology, Santa Cruz, Calif.); anti-cyclin D1 (DCS-6; Novocastra Laboratories Ltd., Newcastle upon Tyne, United Kingdom); anti-pRb (G3-245; Pharmingen, San Diego, Calif.); anti-p27Kip1 (Transduction Laboratories, Lexington, Ky.); and anti-protein kinase B (PKB), -phospho-PKB (serine 473), -Erk1/2, and -phospho-Erk1/2 (threonine 202/tyrosine 204; New England Biolabs, Inc., Beverley, Mass.). Decorated proteins were revealed using horseradish peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulins followed by enhanced chemiluminescence (ECL kit; Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom).
For Cdk2-p27Kip1 and Cdk4-p27Kip1 coimmunoprecipitation experiments, clarified protein lysates (70 to 150 μg for Cdk2 and 350 μg for Cdk4) were precleared with 10 μl of protein A Sepharose (Sigma) and then precipitated with 2 μg of anti-Cdk2 (M2) or anti-Cdk4 (H-22; Santa Cruz Biotechnology) antibody previously coupled to protein A-Sepharose. Beads were washed thoroughly in extraction buffer, and Cdk-p27Kip1 levels were analyzed by immunoblotting as described above.
For analysis of ErbB receptor protein and phosphotyrosine levels, protein extracts were either precipitated with an antibody specific for ErbB1 (500 μg with antibodies 528 and R-1 mixed 1:1), ErbB3 (400 μg with antibody C-17), ErbB4 (500 μg with antibody C-18; from Santa Cruz Biotechnology), or ErbB2 (60 to 200 μg with antibody 21N; specific for the intracellular domain of the receptor [49]). ErbB protein and tyrosine phosphorylation levels were analyzed by immunoblotting as described above, using anti-ErbB1 (1005; Santa Cruz Biotechnology), ErbB3 (C-17), ErbB4 (C-18), or ErbB2 (21N) antibody and a phosphotyrosine-specific MAb as previously described (49). Stripping of membranes for reprobing was performed as previously described (49).
Histone H1 kinase assays.
Histone H1 kinase assays were performed using Cdk2 (M-2) immunoprecipitates (from 50 μg of lysate protein) or cyclin E (C-19) immunoprecipitates (from 75 μg of lysate protein) as previously described (81) except that the amount of histone H1 (Boehringer Mannheim) per assay was increased to 5 μg and the final reaction volume was reduced to 20 μl. Phosphorylated proteins were resolved by SDS-polyacrylamide gel (10%) electrophoresis (PAGE) and analyzed by autoradiography and scintillation counting.
p27Kip1 antisense assays.
Antisense and mismatch p27Kip1 phosphorothioate oligonucleotides, modified by the addition of a propynyl group to the pyrimidine bases, were prepared and purified by reversed-phase chromatography by Microsynth (Balgach, Switzerland). Due to sequence conservation between the murine and human p27Kip1 genes, the antisense and mismatch sequences utilized were the same as those previously used for the inhibition of p27Kip1 expression in murine fibroblasts (14). BT474 cells were plated as stated above for the preparation of cell lysates. After 24 h of incubation, cells were treated with 50 nM oligonucleotides mixed with LipofectAMINE (or LipofectAMINE alone as a control) for 5.5 h as instructed by the manufacturer (GIBCO BRL). After this time, cells were washed and refed with normal culture medium. The cells were then allowed to recover for 3 to 5 h before the addition of antibody 4D5 (10 μg/ml). At the times stated (i.e., subsequent to antibody addition), cells were either extracted for lysate production or trypsinized for flow cytometry as described above.
In vivo [32P]orthophosphate-labeled tryptic phosphopeptide mapping of the ErbB2 receptor.
Cells were cultured as stated above for the preparation of cell lysates. After 24 h of incubation, cells were deprived of phosphate for 12 h (using phosphate-free medium supplemented with 10% normal phosphate-containing medium) and labeled with [32P]orthophosphate (Amersham) for 4 h. In the continued presence of [32P]orthophosphate, cells were subsequently treated with antibody 4D5 or FRP5 (10 μg/ml) for 1 h; equal cell numbers were extracted for each treatment (twice as many MKN7 as BT474 cells were extracted due to the lower stoichiometry of ErbB2 phosphorylation in this cell line), and the ErbB2 receptor was immunoprecipitated as described above. Phosphorylated ErbB2 was excised from the gel, and tryptic phosphopeptide mapping was performed as previously described (49).
RESULTS
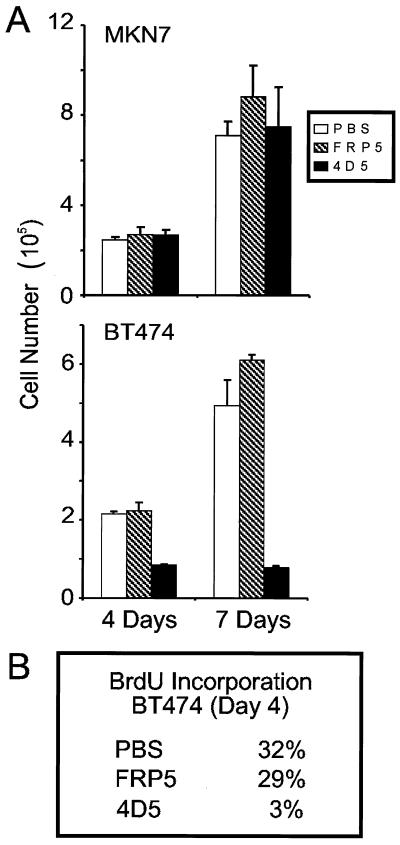
Treatment of BT474 cells, but not MKN7 cells, with MAb 4D5 induces a stable growth arrest.
Primary tumors overexpressing ErbB2 show a more aggressive phenotype which is associated with poor patient prognosis. The precise role of ErbB2 overexpression in tumor development, however, is not determined. To address this question, we have screened a number of ErbB2-overexpressing tumor cell lines for MAb 4D5 sensitivity. An isotype-matched MAb (FRP5), which recognizes the extracellular domain of ErbB2 but is not growth inhibitory (25, 26), was used as a negative control. From this analysis, and in agreement with previous work (37, 38), the growth of the breast carcinoma cell line BT474 was found to be drastically inhibited by 4D5 treatment over a 7-day period (Fig. 1A, bottom). This correlated with a 10-fold decrease in S-phase fraction, as determined by pulse-labeling of antibody-treated cells with BrdU followed by immunofluorescence (Fig. 1B). In contrast, the growth of MKN7 cells, an ErbB2-overexpressing, gastric carcinoma cell line, was unaffected by 4D5 (Fig. 1A, top; see also reference 37). In both cell lines, FRP5 had little effect; after 7 days of incubation, FRP5-treated cells displayed slightly increased cell numbers compared to untreated controls (Fig. 1A), possibly due to the partially agonistic effects of this antibody on the ErbB2 receptor (25, 41). These data indicate, therefore, that although both of these cell lines overexpress ErbB2 (Fig. 2A; see Materials and Methods), they exhibit quite different responses to 4D5 treatment. This variability between cellular responses to 4D5 treatment has been previously reported (37, 38) and may reflect different dependencies on ErbB2 overexpression among tumor cell lines.
FIG. 1.
Proliferation assays of BT474 and MKN7 cells treated with anti-ErbB2 antibodies. MKN7 and BT474 cells were seeded at a density of 2,000 cells/cm2; after 24 h of incubation, the medium was changed and either MAb FRP5 or MAb 4D5 was added to a final concentration of 10 μg/ml, or an equal volume of PBS was added. After 4 and 7 days of incubation, cells were trypsinized and total cell number was calculated (A), or after 4 days, cells were pulse-labeled with BrdU for 4 h and BrdU incorporation into nuclei was revealed by immunofluorescence (B). Shown are the percentages of nuclei labeled with BrdU during the pulse period.
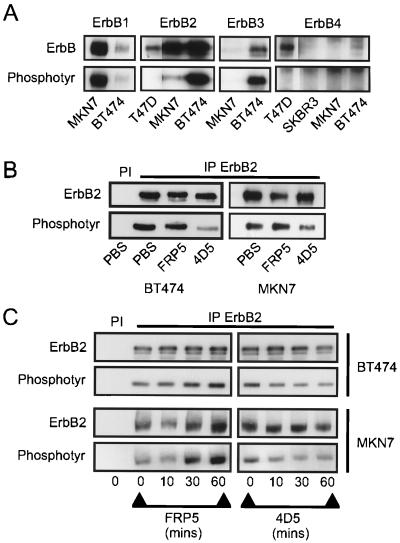
FIG. 2.
Screen of ErbB receptor protein and tyrosine phosphorylation levels and effects of anti-ErbB2 antibody treatment on tyrosine phosphorylation of the ErbB2 receptor in BT474 and MKN7 cells. Cells were seeded at a density of 3 × 104 cells/cm2. After 24 h of incubation, extracts were made and ErbB receptor protein (ErbB) and phosphotyrosine (Phosphotyr) levels were analyzed following immunoprecipitation of the appropriate receptor (A). Cells were seeded as described above, and either MAb FRP5 or MAb 4D5 was added to a final concentration of 10 μg/ml or an equal volume of PBS was added. Extracts were prepared, and ErbB2 protein and phosphotyrosine levels were assessed after 48 h of incubation (B) or at the times indicated (C) following immunoprecipitation (IP) with the ErbB2-specific polyclonal antibody 21N. Preimmune control precipitations are indicated (PI). In panels B and C, longer exposures were required for MKN7 cells due to the lower ErbB2 tyrosine phosphorylation levels in these cells than in BT474 cells. The exposures shown, therefore, do not represent a quantitative comparison of the two cell lines but were chosen to most clearly represent phosphorylation changes induced as a result of 4D5 treatment.
MAb 4D5-mediated growth inhibition is independent of effects on receptor phosphorylation.
Both MAb FRP5 and 4D5 efficiently bind the extracellular domain of ErbB2 (25, 37). The growth defect seen, therefore, is presumably caused by a sustained antibody-specific effect on the ErbB2 receptor, which in BT474 cells is manifested by growth inhibition. Immunoblot analysis of ErbB receptor immunoprecipitates revealed that BT474 and MKN7 cells express quite different ErbB receptor complements (Fig. 2A). While both clearly overexpressed ErbB2, in contrast to a low-expressing breast tumor cell line (T47D), ErbB2 derived from BT474 cell extracts was highly phosphorylated in comparison with that derived from MKN7 (Fig. 2A, compare top and bottom panels). This elevated ErbB2 tyrosine phosphorylation correlated with coexpression of ErbB3 (Fig. 2A, top), the preferred and most potent heterodimerization partner of ErbB2 (55, 79), and suggests that ErbB2 is more active as a tyrosine kinase in BT474 cells than in MKN7 cells. Interestingly, although ErbB3 protein could not be detected by immunoblotting in MKN7 cells, ErbB1 was highly expressed and highly phosphorylated in this cell line (Fig. 2A, top and bottom panels). In BT474 cells, however, ErbB1 expression was equivalent to that of a moderately expressing cell line (data not shown). ErbB4 protein, in contrast, was just detectable in BT474 cells compared to a known nonexpressing (SKBR3) and moderately expressing (T47D) cell line. No ErbB4 was detected in MKN7 cells, and in all cell lines, tyrosine phosphorylation was undetectable (Fig. 2A, compare top and bottom panels), indicating that ErbB4 is not a major signaling element in these cells in the culture conditions used. These observations suggest that the relative activity of overexpressed ErbB2 may depend on the coexpression of other ErbB receptors, such as ErbB3. Furthermore, in MKN7 cells, overexpression of ErbB1 alone is insufficient to fully activate ErbB2.
To address the question of whether differences in cellular response to 4D5 treatment are reflected by differential effects on ErbB2 receptor signaling capacity, immunoblot analyses of ErbB2 receptor protein and phosphotyrosine levels after antibody treatment were performed. After 48 h of 4D5 treatment, a dramatic decrease in ErbB2 tyrosine phosphorylation was observed in BT474 cells (Fig. 2B, left). This was accompanied by a resultant increase in the electrophoretic mobility of the receptor. However, no significant decrease in ErbB2 receptor levels was observed, even after these long treatment times. Furthermore, a similar analysis of 4D5-treated MKN7 cells also revealed receptor dephosphorylation with no effect on protein levels (Fig. 2B, right). To analyze the kinetics of receptor dephosphorylation, time courses of 4D5 treatment of BT474 and MKN7 cells, with FRP5 as a control, were performed. Strikingly, decreased ErbB2 tyrosine phosphorylation was observed within 10 min of 4D5 treatment in both cell lines, and this lower level was maintained for 1 h (Fig. 2C). Decreases in total receptor phosphorylation were also observed if cells were cultured in medium containing 32Pi and subsequently treated for 1 h with 4D5, followed by immunoprecipitation of the ErbB2 receptor (Fig. 3A). Additionally, in agreement with previous reports (25, 41), FRP5 treatment of both cell lines rapidly induced ErbB2 phosphorylation (Fig. 2C and 3A). Taken together, these data indicate that treatment of BT474 or MKN7 cells with MAb 4D5 or FRP5 results in comparable effects on ErbB2 phosphorylation.
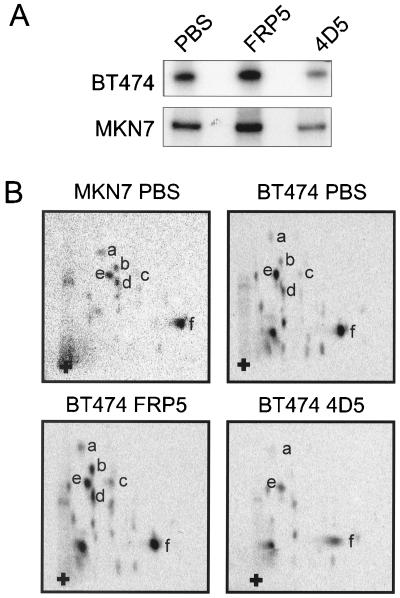
FIG. 3.
In vivo [32P]orthophosphate labeling of the ErbB2 receptor in BT474 and MKN7 cells after anti-ErbB2 antibody treatment; tryptic phosphopeptide mapping. Cells were seeded as in Fig. 2. Twice as many MKN7 cells as BT474 cells were seeded (see Materials and Methods). After 24 h of incubation, cells were deprived of phosphate for 12 h and then prelabeled with [32P]orthophosphate followed by addition of MAb FRP5, MAb 4D5, or an equal volume of PBS for 1 h. Equal amounts of BT474 cell extracts (doubled in the case of MKN7 extracts) were immunoprecipitated with the anti-ErbB2 antibody 21N, and labeled proteins were identified by separation using SDS-PAGE (7.5% gel) (A). The labeled ErbB2 receptor protein was excised and analyzed by tryptic phosphopeptide mapping (B). Specific tryptic phosphopeptides are indicated by letters, and the origin is shown by a plus sign. Gels and tryptic phosphopeptide maps were analyzed with a phosphorimager. Although MKN7 samples were exposed for longer than BT474 samples, due to lower stoichiometry of phosphorylation, the same exposure is shown for each treatment protocol. The amount of label incorporated into the immunoprecipitated ErbB2 protein in panel A was quantified by scintillation counting. BT474 cells treated with MAb FRP5 and MAb 4D5 had 158 and 60% 32P incorporation, respectively, compared to the control (PBS)-treated cells. MKN7 cells treated with MAb FRP5 and MAb 4D5 had 139 and 75% 32P incorporation, respectively, compared to the control (PBS)-treated cells.
To determine whether the 4D5-induced changes in ErbB2 receptor phosphorylation was due to dephosphorylation of specific sites or represented a general effect on receptor phosphorylation, tryptic phosphopeptide mapping of 32Pi-labeled ErbB2 immunoprecipitates from MAb-treated cell lines, as in Fig. 3A, was performed. In vivo-labeled ErbB2 from untreated, asynchronously growing BT474 cells exhibited phosphopeptide maps similar to those observed in MKN7 cells (Fig. 3B, compare top panels). As expected from the above results, FRP5 treatment of BT474 cells induced increases in the phosphorylation of a number of phosphopeptides (i.e., a through d) while not affecting the relative phosphorylation state of others (i.e., e and f; Fig. 3B, bottom left). In contrast, 4D5 treatment of BT474 cells led to the disappearance of b, c, and d and to a decrease in the intensity of a, e, and f, suggesting that it caused a general dephosphorylation of the ErbB2 receptor (Fig. 3B, bottom right). Quantitatively similar results were observed in MKN7 cells (data not shown). In conclusion, therefore, treatment with 4D5 induces general receptor dephosphorylation in both BT474 and MKN7 cells.
MAb 4D5 treatment inhibits cytoplasmic signaling in BT474 cells but not in MKN7 cells.
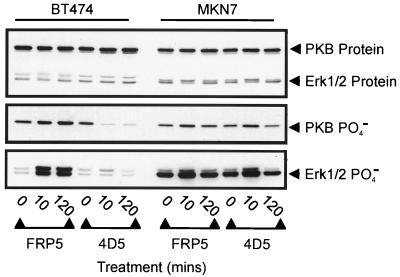
ErbB2 plays a pivotal role in ErbB receptor-mediated activation of the major cytoplasmic, mitogenic signaling pathways, such as the MAP kinase and PI3-kinase pathways (4, 16, 22, 30, 49). We therefore investigated the effects of 4D5 treatment on these pathways in both BT474 and MKN7 cells (Fig. 4). As a readout for the MAP kinase and PI3-kinase pathways, the activation states of the Erk1/2 protein kinases and PKB, respectively, were measured by immunoblotting with antibodies specific for activating phosphorylation sites (see Materials and Methods). Consistent with effects on ErbB2 receptor phosphorylation (Fig. 2C), a dramatic decrease in PKB phosphorylation was observed within 10 min of 4D5 treatment in BT474 cells (Fig. 4, middle). This reduction was maintained for at least 4 h (Fig. 4 and data not shown). Equivalent effects on Erk1/2 phosphorylation were not observed, although a reduction in phosphorylation was seen at later times (Fig. 4, bottom, and data not shown). In this respect, however, in contrast to PKB phosphorylation, Erk1/2 phosphorylation appeared to be comparatively low in BT474 cells and was dramatically induced by FRP5 treatment (Fig. 4, middle and bottom; compare BT474 and MKN7). This indicates that the MAP kinase pathway may not be optimally activated under normal growth conditions in these cells. Surprisingly, a similar analysis of MKN7 cells gave no indication of 4D5-mediated downregulation of either of these pathways (Fig. 4, middle and bottom). A slight increase in Erk1/2 and PKB phosphorylation was observed after 10 min of 4D5 treatment. However, this was not apparent at later times and was shown to be due to the medium change at the beginning of the experiment (Fig. 4 and data not shown). These data demonstrate that despite similar effects of 4D5 treatment on receptor phosphorylation levels in both BT474 and MKN7 cells, only BT474 cells exhibit downstream effects on cytoplasmic signaling molecules. This suggests that receptor dephosphorylation alone is insufficient to determine the cellular response of ErbB2-overexpressing tumor cells to 4D5-induced receptor inhibition.
FIG. 4.
Analysis of PKB and Erk1/2 phosphorylation after treatment of BT474 and MKN7 cells with anti-ErbB2 antibodies. Cells were seeded and treated with MAb FRP5 or MAb 4D5 as in Fig. 2. At the times indicated, cells were extracted and the protein levels (top) and phosphorylation (PO4−) states of PKB (middle) and Erk1/2 (bottom) were evaluated by immunoblotting. Untreated cells (t = 0) were included as controls.
MAb 4D5 treatment induces a G1 arrest in BT474 cells which correlates with accumulation of p27Kip1 and Cdk2 inactivation.
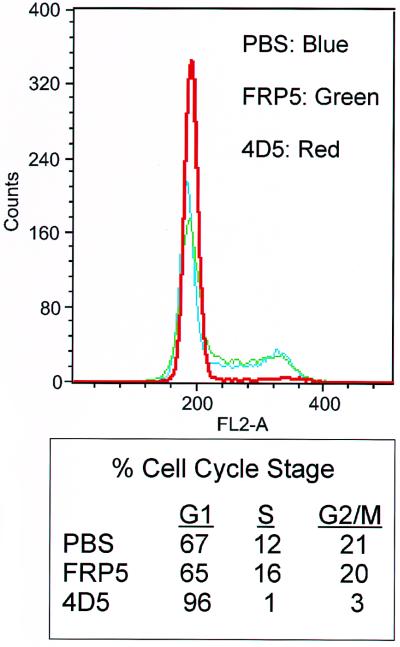
To more accurately define the 4D5-specific growth suppression of BT474 cells, the cell cycle profiles of antibody-treated cells were analyzed after 48 h treatment using flow cytometry. As expected from previous proliferation assays (Fig. 1), FRP5 had no effect on cell cycle distribution compared to untreated controls (Fig. 5). However, 96% of 4D5-treated cells accumulated in G1 (Fig. 5). This G1 arrest was stable for up to a week (data not shown), indicating that these cells were blocked in the ability to progress into S phase of the cell cycle. No evidence of apoptosis was observed. Indeed, if 4D5 was removed from the culture medium, the cells were able to reenter the cell cycle (data not shown), confirming that this is a cytostatic rather than cytotoxic agent.
FIG. 5.
Analysis of cell cycle distribution after treatment of BT474 cells with anti-ErbB2 antibodies. BT474 cells were seeded as in Fig. 2. After 24 h of incubation, the medium was changed, and MAb FRP5, MAb 4D5, or PBS was added as in Fig. 2. After 48 h, cells were harvested by trypsinization and nuclei were stained with propidium iodide. Shown is flow cytometry analysis of antibody-treated cells compared to PBS-treated controls (top). Percentages of cells in each cell cycle stage are indicated (bottom).
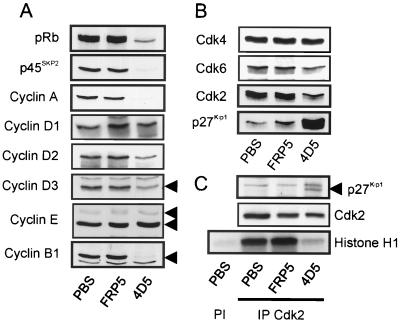
To identify the molecular basis of this G1 arrest, an initial screen of effects on the levels and activity of G1 regulators was performed. For this, BT474 cell extracts were prepared after 48 h treatment with MAb 4D5 or FRP5 and analyzed by immunoblotting (Fig. 6A and B). As expected, markers of S-phase progression (cyclin A and p45SKP2) as well as G2/M (cyclin B1) were almost completely absent in 4D5-treated cells. Additionally, pRb was found exclusively in its hypophosphorylated, active, growth suppressor state. FRP5 treatment, in contrast, had no effect on the expression of these proteins or on pRb phosphorylation (Fig. 6A). Analysis of G1 Cdk and cyclin levels demonstrated no changes in Cdk4, Cdk6, cyclin E, or cyclin D1 expression, whereas cyclin D2 and D3 levels were reduced in 4D5-treated cells (Fig. 6A and B). Most strikingly, however, the CKI p27Kip1 was dramatically increased in 4D5-treated cells, a phenomenon correlating with the disappearance of the faster-migrating, active form of Cdk2 (Fig. 6B). Immunoprecipitation of Cdk2, followed by either immunoblotting for associated p27Kip1 or by histone H1 kinase assay, showed an increase in Cdk2-p27Kip1 association and almost complete Cdk2 inactivation (Fig. 6C). No effects on the protein levels of other CKIs (including p21Cip1/Waf1, p57Kip2, p15INK4b, and p16INK4a) were observed (data not shown). Surprisingly, multiple independent kinase assays revealed that Cdk4 activity (the major cyclin D-dependent kinase expressed in BT474 cells) remained little affected in these experiments despite decreased cyclin D levels (data not shown). As cyclin D proteins are not completely lost, this maintenance of activity could be due to there being sufficient cyclin D to maintain Cdk4 activity. It should also be noted that FRP5-treated cells showed reproducible increases in cyclin D1 protein expression as well as increased Cdk2 activity (Fig. 6A and C). The partial agonistic effect of this antibody could explain this phenomenon (25, 41). Taken together, these data indicate that 4D5 treatment of BT474 cells induces increased p27Kip1 protein levels, leading to the inhibition of Cdk2, events which do not occur with a noninhibitory control antibody.
FIG. 6.
Analysis of G1 regulators in anti-ErbB2 antibody-treated BT474 cells. BT474 cells were seeded and treated with MAb FRP5, MAb 4D5, or PBS as in Fig. 2. After 48 h, cells were extracted and the protein levels of G1 regulators were evaluated by immunoblotting (A and B). Additionally, p27Kip1 association with Cdk2 complexes, and Cdk2 activity, was assessed through immunoprecipitation (IP) of Cdk2 followed by immunoblotting for associated p27Kip1 protein or in vitro histone H1 kinase assay (C). Preimmune control precipitations are indicated (PI).
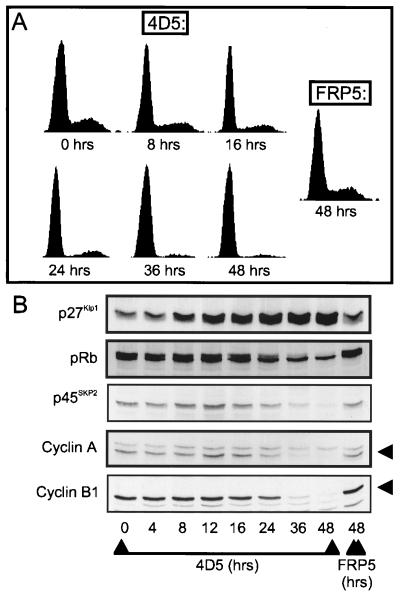
To further determine a possible relationship between increased p27Kip1 expression and the G1 block, BT474 cells were treated for 4 to 48 h with 4D5 and, at the times shown (Fig. 7), either extracted and analyzed for markers of G1 arrest by Western blotting (Fig. 7B) or trypsinized, and the cell cycle profile was determined by flow cytometry (Fig. 7A). Untreated cells (time [t] = 0 h) and cells treated for 48 h with FRP5 were used as controls. The results showed that 4D5-induced effects on pRb phosphorylation, as well as on cyclin A, cyclin B1, and p45SKP2 levels, became apparent only between 24 and 36 h (Fig. 7B), coincident with a large proportion of cells having accumulated in G1 (Fig. 7A; 87 and 96% in G1 at 24 and 36 h, respectively). In contrast, p27Kip1 levels increased after 8 h, well before the G1 block was evident (77.5 and 74% in G1 at 0 and 8 h, respectively). The timing of p27Kip1 accumulation would, therefore, suggest that p27Kip1-mediated inhibition of Cdk2-cyclin E activity is the direct cause of the G1 block.
FIG. 7.
Time course of the effect of MAb 4D5 treatment on the cell cycle distribution and the levels of cell cycle markers in BT474 cells. BT474 cells were seeded and treated with MAb FRP5 or MAb 4D5 as in Fig. 2. At the times shown, cells were trypsinized, and half of the sample was either stained with propidium iodide and analyzed for cell cycle distribution using flow cytometry (A) or extracted for immunoblot analysis of the proteins indicated (B). Untreated cells (t = 0) and cells treated for 48 h with MAb FRP5 were included as controls.
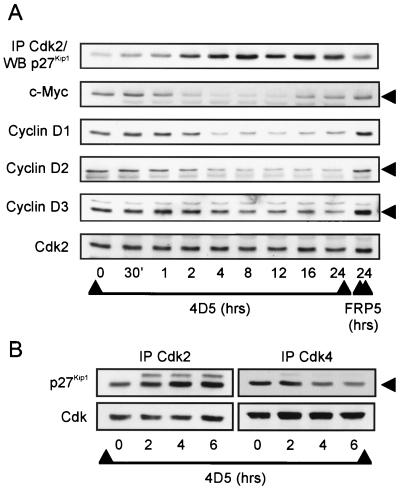
MAb 4D5-induced Cdk2 inactivation parallels increased p27Kip1-Cdk2 association, downregulation of D-type cyclins and the c-Myc transcription factor, and loss of p27Kip1 from Cdk4 complexes.
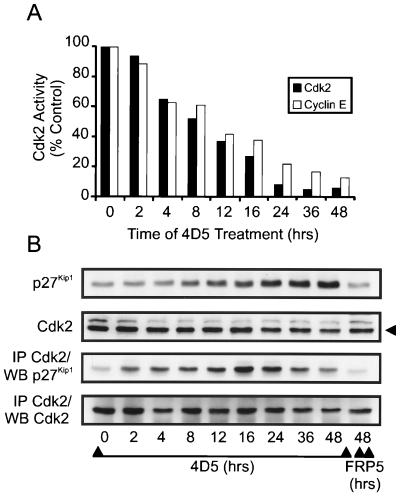
The above data support the hypothesis that in BT474 cells inhibition of ErbB2 receptor function through 4D5 treatment induces p27Kip1 protein accumulation by an unknown mechanism. However, a closer analysis of the kinetics of Cdk2 inactivation demonstrated that after only 2 h of 4D5 treatment, Cdk2 activity had already decreased (Fig. 8A) despite no increase in p27Kip1 levels at this time (Fig. 8B). Indeed, after 8 h, when p27Kip1 levels were just starting to increase, total Cdk2 activity had decreased to approximately 50% of normal levels, reaching minimum levels after 24 h when p27Kip1 protein levels were not yet maximal (Fig. 8). Furthermore, similar kinetics of inactivation were seen if Cdk2 activity was measured after immunoprecipitation of cyclin E (Fig. 8A). Subsequent analysis of Cdk2-p27Kip1 association, in immunoprecipitates from the same samples, revealed that p27Kip1 started to accumulate on Cdk2 within 2 h of 4D5 treatment, reaching a peak after 16 to 24 h (Fig. 8B). At later times (36 to 48 h), less p27Kip1 appeared to be associated with Cdk2. However, this was coincident with decreased Cdk2 levels which, along with reduced pRb expression (Fig. 6A), was always observed after longer treatments with 4D5 and may represent a delayed program of adaptation to the G1 block.
FIG. 8.
Time course of Cdk2 inactivation and Cdk2-p27Kip1 complex formation in MAb 4D5-treated BT474 cells. BT474 cells were seeded and treated with MAb FRP5 or MAb 4D5 as in Fig. 2. At the times indicated, cell extracts were prepared and immunoprecipitated with Cdk2-specific or cyclin E-specific antibodies followed by in vitro histone H1 kinase assay (A). Additionally, the levels of p27Kip1 and Cdk2 protein in the same extracts were either analyzed directly by immunoblotting (WB) or after immunoprecipitation (IP) with Cdk2-specific antibodies (B). Untreated cells (t = 0) and cells treated with MAb FRP5 for 48 h were included as controls. Cdk2 kinase activity is expressed as percentage of control (t = 0) cells.
Increased association of p27Kip1 with Cdk2 correlates with Cdk2 inactivation in 4D5-treated BT474 cells, and both events begin prior to the accumulation of p27Kip1 protein. One must consider, therefore, that 4D5 treatment results in the rapid release of an intracellular pool of sequestered p27Kip1 protein. Cyclin D-dependent kinase complexes, as well as the c-Myc transcription factor, play major roles in the regulation of p27Kip1 sequestration (see the introduction). With this in mind, therefore, we performed a more detailed time course of 4D5 treatment and analyzed the extracts for correlations between changes in p27Kip1-Cdk2 association and c-Myc or cyclin D protein expression (Fig. 9A). Strikingly, c-Myc protein levels decreased within 1 h of 4D5 addition, reaching a minimum level within 2 h. This decrease correlated exactly with the initial accumulation of p27Kip1 on Cdk2 in the same samples (Fig. 9A) but was transient, as c-Myc protein levels began to recover after 16 h, reaching normal levels after 36 to 48 h (Fig. 9A and data not shown). Cyclin D levels also decreased with similar kinetics (Fig. 9A). The previous immunoblot analysis (Fig. 6A) demonstrated that after 48 h of 4D5 treatment, cyclin D1 was present at normal levels. Subsequent analysis of longer time courses indicated that, as with c-Myc, cyclin D1 levels did indeed recover at later times, reaching normal levels by 48 h (not shown). Taken together, the rapid downregulation of c-Myc and cyclin D proteins provides an explanation for the redistribution of p27Kip1 onto Cdk2 complexes after 4D5 treatment. Indeed, in this context, loss of p27Kip1 from cyclin D-Cdk4 complexes was observed after 4D5 treatment and correlated with increased p27Kip1-Cdk2 complex formation (Fig. 9B).
FIG. 9.
Time course of MAb 4D5-induced effects on Cdk/p27Kip1 association and levels of proteins involved in p27Kip1 sequestration in BT474 cells. BT474 cells were seeded and treated with MAb FRP5 or MAb 4D5 as in Fig. 2. At the times indicated, cell extracts were prepared and analyzed by immunoblotting (WB) either directly or after immunoprecipitation (IP) with Cdk2-specific antibodies (A). Additionally, p27Kip1 association with Cdk2 and Cdk4 was analyzed by immunoblotting after immunoprecipitation (IP) with Cdk2- or Cdk4-specific antibodies (B). Untreated cells (t = 0) and cells treated with MAb FRP5 for 24 h were included as controls.
Redirection of p27Kip1 protein onto Cdk2 complexes occurs in the absence of increased p27Kip1 expression in SKBR3 cells: correlation with partial Cdk2 inactivation and reduced proliferation levels.
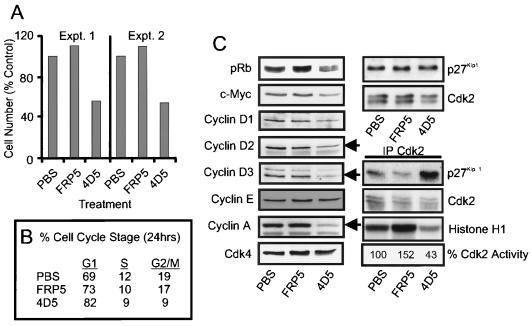
To extend the observations made in BT474 cells, a similar cell cycle analysis was performed on a second ErbB2-overexpressing cell line (SKBR3) sensitive to 4D5 treatment. Intriguingly, growth assays demonstrated that the proliferation rate of these cells was reproducibly reduced by approximately 50% as a result of 4D5 treatment (Fig. 10A), indicating that these cells were less sensitive to antibody-mediated ErbB2 receptor inhibition than BT474 cells. This decrease in proliferation rate was not dependent on cell density (Fig. 10A, compare Expt. 1 with Expt. 2) and correlated with a 10 to 15% increase in the proportion of cells in G1 as judged by flow cytometry (Fig. 10B and data not shown), suggesting a delay in G1-to-S progression. This possibility was supported by the observation that the phosphorylation state of pRb was reduced after 24 h of 4D5 treatment, with a higher proportion being found in the hypophosphorylated, growth suppressor state (Fig. 10C). Additionally, cyclin A protein expression was reduced, but not completely absent, consistent with the fact that these cells were still proliferating at a lower rate in the presence of 4D5 (Fig. 10C). As expected, treatment with the control antibody FRP5 had no growth-inhibitory effects and again appeared to be partially agonistic (Fig. 10).
FIG. 10.
Effects of anti-ErbB2 MAb treatment on SKBR3 cell proliferation and the expression and activity of G1 regulators. SKBR3 cells were seeded as in Fig. 2. (A, Expt. 1; B and C) or at half the density (A, Expt. 2). After 24 h of incubation, PBS, MAb FRP5, or MAb 4D5 was added as in Fig. 2, and cells were treated as follows: (A) incubated for 4 days and trypsinized, after which total cell number was calculated; (B) incubated for 24 h, trypsinized, and treated with propidium iodide, after which cell cycle distribution was analyzed by flow cytometry; (C) incubated for 24 h, after which cell extracts were prepared and the protein levels of G1 regulators were evaluated by immunoblotting, or p27Kip1 association with Cdk2 complexes, and Cdk2 activity, was assessed through immunoprecipitation (IP Cdk2) of Cdk2 followed by immunoblotting for associated p27Kip1 protein or in vitro histone H1 kinase assay. Cdk2 activity is indicated as a percentage of that in control (PBS)-treated cells.
Further analysis of 4D5-treated cells demonstrated that p27Kip1 protein was indeed redirected onto Cdk2 complexes (Fig. 10C). This correlated with a reduction in c-Myc and cyclin D protein levels, a reduction in the intensity of the faster migrating, active form of Cdk2 and a reduction in Cdk2 activity to 43% of that seen in untreated cells (Fig. 10C). Interestingly, no increase in p27Kip1 levels was observed after either 24 h (Fig. 10C) or 48 h (not shown) of 4D5 treatment. SKBR3 cells, therefore, exhibited 4D5-induced effects on p27Kip1 sequestration protein levels and p27Kip1-Cdk2 complex formation similar to those observed in BT474 cells. In contrast, redirection of p27Kip1 onto Cdk2 complexes in SKBR3 cells was insufficient to totally inactivate Cdk2, a situation reflected in the growth characteristics of these cells. These data suggest that the extent of growth inhibition elicited by 4D5 treatment is cell type specific and correlates with the degree of Cdk2 inactivation. Moreover, an increase in p27Kip1 expression is not a universal response to ErbB2 inhibition in 4D5-sensitive cells.
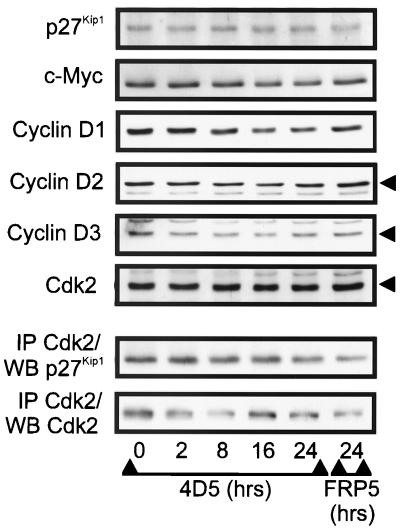
An equivalent response to MAb 4D5 treatment is not observed in MKN7 cells.
If the molecular events observed in BT474 and SKBR3 cells were indeed related to growth inhibition, it would be expected that they would not occur in MKN7 cells, as these cells were not growth inhibited by treatment with 4D5 (Fig. 1A). To address this question, a time course of 4D5 treatment was performed with MKN7 cells. Here, no increase in p27Kip1 protein levels or p27Kip1-Cdk2 association was observed, even after 24 h (Fig. 11) or 48 h (not shown) of 4D5 treatment. Furthermore, c-Myc and cyclin D protein levels were little affected (Fig. 11). These data suggest that the cell cycle effects observed in BT474 and SKBR3 cells are related to growth inhibition, rather than being nonspecific events.
FIG. 11.
Time course of the effects of MAb 4D5 treatment on G1 regulators in MKN7 cells. MKN7 cells were seeded at a density of 3 × 104 cells/cm2. After 24 h of incubation, the medium was changed and MAb FRP5 or MAb 4D5 was added to a concentration of 10 μg/ml for the times indicated. Cell extracts were prepared and analyzed by immunoblotting (WB) either directly or after immunoprecipitation (IP) with Cdk2-specific antibodies. Untreated cells (t = 0) and cells treated with MAb FRP5 for 24 h were included as controls.
Increased p27Kip1 levels are not required for MAb 4D5-induced p27Kip1-Cdk2 association, Cdk2 inactivation, and G1 arrest in BT474 cells.
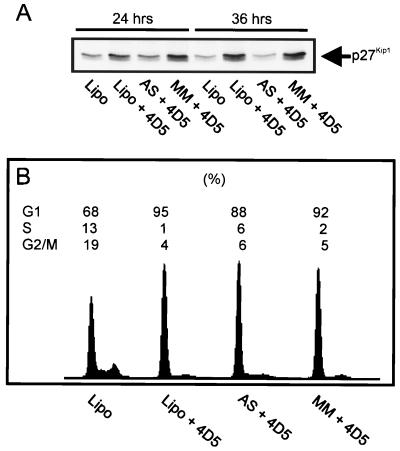
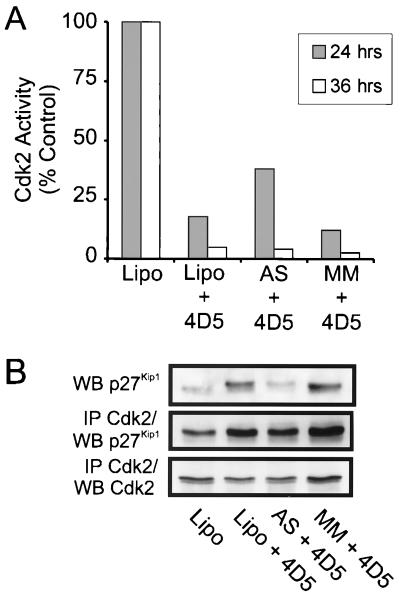
In BT474 cells, increased p27Kip1-Cdk2 complex formation correlated with Cdk2 inactivation and preceded increased p27Kip1 expression. This observation prompted the question of whether increased p27Kip1 expression is an essential component in mediating the G1 block induced by ErbB2 receptor inhibition in BT474 cells, or whether it is simply a consequence of 4D5-induced Cdk2 inactivation. This question was particularly relevant considering the absence of p27Kip1 induction in SKBR3 cells, which displayed only partial Cdk2 inhibition in response to 4D5 treatment (Fig. 10C). To address this issue, therefore, we used an antisense approach to assess the cell cycle effects of preventing 4D5-specific increases in p27Kip1 protein levels in BT474 cells. For this, a specific 15-base p27Kip1 antisense oligonucleotide and a mismatch control oligonucleotide were constructed as previously described (14) (see Materials and Methods) and introduced into BT474 cells by lipofection. Immunoblots of lipofection-treated BT474 cells, subsequently treated for 24 or 36 h with 4D5, revealed that although p27Kip1 levels increased as a result of 4D5 treatment in both untreated and mismatch controls, p27Kip1 protein levels were unaffected by 4D5 in cells treated with antisense oligonucleotide (Fig. 12A). These data demonstrate the efficacy of antisense-mediated inhibition of p27Kip1 protein expression in this system. Strikingly, when the cell cycle profile of the same cells was analyzed by flow cytometry, antisense-treated cells were still found to be blocked in G1, as a result of 4D5 treatment, to an extent similar to that for untreated or mismatch control-treated cells (Fig. 12B). Importantly, cells treated with oligonucleotide displayed a normal cell cycle profile when cultured for the same time in the absence of 4D5 (data not shown).
FIG. 12.
Effect of antisense-mediated inhibition of MAb 4D5-induced p27Kip1 accumulation on the cell cycle of BT474 cells. BT474 cells were seeded at a density of 3 × 104 cells/cm2. After 24 h of incubation, cells were treated with LipofectAMINE alone (Lipo), p27Kip1 antisense oligonucleotide (AS), or a mismatch control oligonucleotide (MM) as outlined in Materials and Methods. Cells were subsequently refed with normal growth medium; after 3 to 5 h, MAb 4D5 (+ 4D5) was added (10 μg/ml) for 24 or 36 h. After these times, cell extracts were prepared and p27Kip1 protein levels were examined by immunoblotting (A). After 36 h, cells were trypsinized and treated with propidium iodide, and cell cycle distribution was analyzed by flow cytometry (B). Cells treated with LipofectAMINE alone followed by no addition of MAb 4D5 were included as controls. The LipofectAMINE procedure itself had no effect on cell cycle distribution compared to untreated controls, as assessed after 36 h of incubation (not shown).
Consistent with the presence of a G1 block, Cdk2 activity was also decreased as a result of 4D5 treatment in all cases (Fig. 13A). Indeed, although Cdk2 inactivation appeared to be slightly delayed in antisense-treated cells (38%, compared to 18 and 12% in untreated and mismatch-treated cells, respectively, after 24 h incubation with 4D5), by 36 h almost total Cdk2 inactivation had occurred (4%, compared to 5 and 2.5% in untreated and mismatch-treated cells, respectively). Further analysis of Cdk2 immunoprecipitations for p27Kip1 association indicated that after 24 h of treatment with 4D5 (a time when Cdk2 levels were unaffected), similar levels of p27Kip1 protein became associated with Cdk2 in antisense-treated cells compared to controls (Fig. 13B). These data confirm that 4D5 treatment of BT474 cells induces the relocation of p27Kip1 protein onto Cdk2 complexes. Furthermore, this movement is independent of increased p27Kip1 protein levels and is sufficient to potentiate Cdk2 inactivation and, hence, establish a G1 block. Additionally, no induction of the expression of the CKI p21Cip1/Waf1 was observed after lipofection of either the antisense or mismatch control oligonucleotide (data not shown). This attests to the specificity of this effect, ruling out nonspecific effects of single-stranded DNA on Cdk2 activity.
FIG. 13.
Effect of antisense-mediated inhibition of MAb 4D5-induced p27Kip1 accumulation on Cdk2 activity and p27Kip1-Cdk2 complex formation in BT474 cells. BT474 cells were seeded, treated with either LipofectAMINE alone (Lipo), antisense p27Kip1 oligonucleotide (AS), or mismatch (MM) control oligonucleotide, and treated with MAb 4D5 (+ 4D5) as outlined in Fig. 12. After 24 and 36 h of incubation, cells were extracted and immunoprecipitated with Cdk2-specific antibodies followed by in vitro histone H1 kinase assay (A). After 24 h of incubation, the same extracts were analyzed for p27Kip1 protein levels by immunoblotting (WB) directly or after immunoprecipitation (IP) of Cdk2 complexes. Cells treated with LipofectAMINE alone followed by no addition of MAb 4D5 were used as controls, and Cdk2 activity is expressed as a percentage of this control.
DISCUSSION
ErbB2 overexpression potentiates cyclin E-Cdk2 activity in breast tumor cells.
Examination of primary tumors overexpressing the ErbB2 receptor tyrosine kinase has revealed more aggressive tumor phenotypes, associated with poor patient prognosis (24, 59, 70, 71, 84). The extracellular accessibility and involvement in tumor malignancy suggest ErbB2, therefore, as an appropriate target for tumor-directed therapies. For this reason, elucidating the molecular mechanisms by which ErbB2 overexpression potentiates tumor cell growth is a priority. In this work, we have shown that MAb 4D5 treatment of the ErbB2-overexpressing breast tumor cell line BT474 results in a rapid reduction in ErbB2 receptor phosphorylation. Consistent with the relationship between tyrosine phosphorylation and receptor activity, a concomitant decrease in the activity of downstream cytoplasmic signaling pathways was also demonstrated. These observations imply antibody-mediated interference of receptor function in 4D5-treated cells. Consequently, BT474 cells respond to antibody treatment by growth arrest, suggesting that they are dependent on elevated ErbB2 receptor activity for proliferation. More specifically, 4D5 treatment results in a block of the G1/S transition, characterized by a rapid increase in p27Kip1 levels and inactivation of the cyclin E-Cdk2 complex.
Increased p27Kip1 protein levels, with an associated G1 accumulation, have been previously observed in ErbB1-overexpressing human carcinoma cell lines after treatment with the ErbB1 growth-inhibitory MAb 225 (53, 81, 83), as well as in an ErbB2-overexpressing ovarian carcinoma cell line treated with 4D5 (83). This relationship could point to a general role for ErbB receptor overexpression in maintaining cyclin E-Cdk2 activity by directly controlling p27Kip1 protein levels, thus deregulating control mechanisms regulating the G1/S transition. The consequences of such a role for tumor development are obvious and would explain the more aggressive growth characteristics of tumors overexpressing ErbB2 receptors. However, through the work presented here, we provide evidence disputing this simple interpretation. First, a second ErbB2-overexpressing cell line (SKBR3), which is also growth inhibited as a result of 4D5 treatment, did not exhibit increased p27Kip1 expression. Additionally, a more in-depth analysis of the effects of 4D5 treatment on p27Kip1 function in BT474 cells demonstrated that the most immediate effect (within 2 h) of 4D5 treatment was to increase the availability of p27Kip1, allowing it to interact with cyclin-Cdk2 complexes. This occurred prior to increases in p27Kip1 protein levels and paralleled Cdk2 inactivation kinetics. A similar shift of p27Kip1 protein onto Cdk2 complexes was also observed in SKBR3 cells, correlating with reduced Cdk2 activity. We postulate, therefore, that elevated ErbB2 receptor signaling in overexpressing tumor cells potentiates G1/S progression by impeding p27Kip1 association with cyclin E-Cdk2 complexes. This hypothesis is supported by further experiments, using an antisense p27Kip1 oligonucleotide to prevent 4D5-induced increases in p27Kip1 protein levels in BT474 cells. Here, increased p27Kip1-Cdk2 complex formation was still observed after 4D5 treatment, correlating with Cdk2 inactivation. Additionally, although increased p27Kip1 levels may have contributed to the stability of the G1 arrest at later times, this event was found not to be required to establish the G1 block induced by 4D5 treatment in these cells.
It is known that p27Kip1 levels are regulated principally by degradation (51). Furthermore, (i) phosphorylation of p27Kip1 on threonine 187 by Cdk2 kinase and (ii) stable trimeric complex formation with cyclin-Cdk2 complexes act as signals for ubiquitination and hence target p27Kip1 to the proteasome degradation machinery (43, 44, 66). From the results presented here, p27Kip1 accumulation in BT474 cells appears to be a secondary effect of 4D5-induced p27Kip1-Cdk2 complex formation, as the latter was sufficient to almost totally inhibit Cdk2 activity. In the absence of Cdk2 activity, p27Kip1 protein would be inefficiently phosphorylated and stabilized. This supposition is supported by two observations. First, no p27Kip1 induction was observed in 4D5-treated SKBR3 cells, which exhibited only partial Cdk2 inactivation as a result of p27Kip1 redirection. Second, using [35S]methionine pulse-chase techniques after 16 h of 4D5 treatment, we have shown a doubling of p27Kip1 protein half-life, with no specific effect on p27Kip1 translation (unpublished data).
With these data in mind, therefore, we propose that the initial effect of inhibiting ErbB2 receptor function in 4D5-sensitive breast carcinoma cells is to redirect p27Kip1 onto Cdk2 complexes resulting in inhibition of G1/S progression. The extent of Cdk2 inactivation following receptor inhibition appears to be cell type specific and in some cases is sufficient to instigate a complete G1 block and induce increased p27Kip1 protein expression. More specifically, we speculate that in some tumors ErbB2 overexpression promotes constitutive intracellular signaling leading to sequestration of p27Kip1 away from Cdk2 complexes.
ErbB2 overexpression regulates the expression of proteins involved in p27Kip1 sequestration.
The D-type cyclins and the transcription factor c-Myc are involved in regulating p27Kip1 sequestration in proliferating cells (8, 34, 73, 77, 85). In this respect, a reduction in the level of these proteins was observed in 4D5-treated BT474 and SKBR3 cells, correlating with p27Kip1-Cdk2 complex accumulation. Loss of p27Kip1 sequestration proteins would provide an explanation for 4D5-induced p27Kip1 relocation onto Cdk2 complexes. However, we acknowledge that alternative mechanisms of regulating p27Kip1 availability in tumor cells may also be affected (50). Previous reports have shown that activation of the MAP (Erk1/2) kinase pathway leads to stabilization of the c-Myc protein (65) and increased cyclin D transcription (42). Furthermore, activation of the PI3-kinase/PKB pathway has been implicated in the translational induction of c-Myc (80) and stabilization of the D-type cyclins (13, 17). Here, we have shown that 4D5-treated BT474 cells exhibit a rapid and dramatic reduction in PKB phosphorylation, with less dramatic effects on Erk1/2, suggesting an effect on the activation state of these kinases. Additionally, effects on both PKB and Erk1/2 phosphorylation have also been demonstrated in SKBR3 cells (data not shown). Based on the literature, therefore, these observations could account for the decreased expression of c-Myc and D-type cyclins in cells sensitive to 4D5 treatment. Recently, the role of c-Myc in the regulation of cyclin D1 and D2 expression has been demonstrated (10, 54). Whether in our experiments downregulation of the D-type cyclins after 4D5 treatment was purely a consequence of reduced c-Myc protein levels is a matter for debate, particularly as in BT474 cells c-Myc protein levels recovered at later times (36 to 48 h) of 4D5 treatment, whereas cyclin D2 (and D3) levels did not. Intriguingly, changes in cyclin D1 protein levels did mirror fluctuations in c-Myc protein, suggesting that cyclin D1 expression is downstream of the c-Myc transcription factor in BT474 cells.
It should also be noted that no effect of 4D5-induced c-Myc downregulation was seen at the level of expression of cyclin E or the G1 Cdk-regulatory phosphatase Cdc25A (Fig. 6A and data not shown). Both of these proteins have been postulated to be downstream transcriptional targets of c-Myc (2, 48). For Cdc25A, this now seems unlikely (48). Moreover, the exact relationship between c-Myc and cyclin E expression is not established. Indeed, the cyclin E promoter has no consensus c-Myc binding sites, and in some systems c-Myc has been shown to increase cyclin E-Cdk2 activity in the absence of changes in cyclin E expression (61, 74). An additional point to consider is that cyclin E is constitutively overexpressed in breast tumor cells (31, 32). It is possible, therefore, that cyclin E levels were maintained after 4D5 treatment as a result of tumor-specific deregulation of cyclin E expression.
Differential responses to MAb 4D5 treatment indicate differences in growth dependency in ErbB2-overexpressing tumor cells.
In vitro screening of ErbB2-overexpressing cell lines for 4D5-mediated growth inhibition has revealed variability in the response of tumor cells to antibody treatment (37, 38). Here, we have also shown that two overexpressing breast tumor cell lines, BT474 and SKBR3, respond to antibody-mediated inhibition of ErbB2 signaling to differing extents. Moreover, effects on cell proliferation appear to correlate with the extent of Cdk2 inhibition induced by antibody treatment. In this context, cyclin E-Cdk2 kinase activity is known to be heavily deregulated in breast tumor cells (31, 32). Indeed, increased cyclin E expression has been associated with a high proliferative capacity, highly aggressive tumors, and poor patient prognosis (45, 46, 58). Furthermore, cyclin E-Cdk2 activation levels in primary breast tumors correlate with the phosphorylation status of pRb and with proliferation rates (40), a finding which corroborates the observations presented here. The reason for the differences between the overall responses of BT474 and SKBR3 cells to 4D5 treatment is not known. However, we note that SKBR3 cells express approximately fourfold less p27Kip1 protein than BT474 cells, as well as significantly higher levels of cyclin D2 (sevenfold), cyclin D3 (twofold), Cdk6 (fourfold), and c-Myc (twofold) proteins (unpublished data). It is possible, therefore, that cell-type-specific differences in the expression of p27Kip1 and p27Kip1 sequestration proteins may determine the potency of the growth response to ErbB2 receptor inhibition. This important question will be addressed in the future.
An additional consideration is that ErbB2-overexpressing tumor cells may exhibit graded responses to ErbB2 inhibition due to different dependencies on elevated ErbB2 receptor expression for the maintenance of mitogenic signaling pathways (discussed below). This possibility is exemplified in MKN7 cells, which also overexpress ErbB2 but are not growth inhibited by 4D5 treatment. Indeed, 4D5 treatment of MKN7 cells had no effect on p27Kip1 sequestration or p27Kip1 protein levels. Accordingly, major cytoplasmic signaling pathways, as well as c-Myc and cyclin D protein levels, remained essentially unchanged. These data indicate that the effects on the cell cycle machinery observed in 4D5-treated BT474 and SKBR3 cells are indeed related to growth inhibition. Furthermore, despite the fact that MKN7 cells dramatically overexpress ErbB2 to levels similar to those observed in BT474 cells (Fig. 2A; see also references 21 and 37) and also exhibit a general reduction in receptor phosphorylation as a result of 4D5 treatment, impaired ErbB2 receptor signaling does not seem to affect the maintenance of p27Kip1 sequestration proteins or stimulate p27Kip1-Cdk2 complex formation in this case. The role of ErbB2 overexpression in the potentiation of Cdk2 activity in tumors, therefore, is not universal.
ErbB2 receptor overexpression alone does not determine growth dependency.
Consistent with downstream effects on cytoplasmic signaling pathways, we have demonstrated that 4D5 treatment of BT474 cells results in a rapid and general reduction in ErbB2 phosphorylation. In contrast to a previous report examining the effect of 4D5 treatment on ovarian cancer cells (83), we observed no gross downregulation of ErbB2 protein levels. It is possible that slight decreases in ErbB2 expression were not detected by the immunoblotting approach that we used. With this in mind, therefore, we cannot rule out the possibility that partial receptor downregulation may have occurred after prolonged 4D5 treatment, as previously shown (27, 33). Until now, receptor analyses showing 4D5-induced reductions in ErbB2 phosphorylation were performed after long treatment periods, and tryptic phosphopeptide mapping was not carried out (27, 33). Moreover, it has also been suggested that 4D5 induces ErbB2 phosphorylation in BT474 cells (63). From detailed time courses of 4D5 treatment of BT474 cells, however, we have detected decreased receptor phosphorylation levels within 10 min of 4D5 addition. Furthermore, through phosphopeptide mapping, receptor dephosphorylation was shown to be general, including sites stimulated by treatment with MAb FRP5, a known ErbB2 partial agonist (25, 41). The lack of correlation between our observations and those of Scott and coworkers (63) could be due to differences in cell culture conditions before 4D5 addition. In our experiments, cells were treated with antibody at low densities (see Materials and Methods) when a normal cell cycle profile was evident (Fig. 7A). In contrast, 80 to 100% confluent cells were used by the above authors, which could have resulted in differences in response to antibody treatment.
Taken together, our data show that 4D5-induced inhibition of ErbB2 receptor signaling in BT474 cells affects downstream signaling events required for the maintenance of p27Kip1 sequestration proteins and, hence, Cdk2 activity. It is tempting to propose, therefore, that the general dephosphorylation of ErbB2 induced by 4D5 treatment is sufficient to inhibit growth in ErbB2-overexpressing cell lines. However, as 4D5 treatment of the insensitive tumor cell line MKN7 also induced receptor dephosphorylation in a similar fashion, this event cannot be considered a marker for cellular response to 4D5 treatment. Consequently, one has to consider that other cell-type-specific factors may determine whether tumor cells become dependent on elevated ErbB2 signaling for proliferation.
It has been suggested that long-term resistance to 4D5 treatment may be due to intracellular expression of the extracellular domain of ErbB2, which interferes with internalized ErbB2-4D5 complexes (64). However, from the rapid kinetics of receptor dephosphorylation observed in both MKN7 and BT474 cells after addition of 4D5, this hypothesis would not explain the resultant differential effects on nuclear proteins. From the data presented in this paper, therefore, we speculate on a number of more plausible explanations for why there are differences between cellular responses to 4D5-induced ErbB2 receptor inhibition. First, although ErbB2 is overexpressed in MKN7 cells to levels similar to those in BT474 cells, the receptor is minimally phosphorylated in the former case (Fig. 2A). This implies that ErbB2 is not as active a signaling moiety in MKN7 cells as in BT474 cells. Of all ErbB receptor interactions, the ErbB2-ErbB3 heterodimer is considered the preferred and most potent signaling module (55, 79), coupling efficiently to the PI3-kinase pathway (20, 60). It is significant, therefore, that ErbB3 is overexpressed and active in BT474 cells but is undetectable in MKN7 cells. Moreover, the PI3-kinase pathway, as measured by PKB phosphorylation, is dramatically downregulated in BT474 as a result of 4D5 treatment but remains unaffected in MKN7 cells. Previously, ErbB3 expression has been shown to enhance ErbB2-mediated transformation and tumorigenic growth of NIH 3T3 cells (1, 78, 88). Furthermore, ErbB2-ErbB3 coexpression has been observed in human breast tumors (9, 69), where it has been postulated to play a critical role in tumor progression (69). ErbB3 may, therefore, collaborate with ErbB2, contributing to tumor development. The above data, together with the finding that 4D5-sensitive SKBR3 cells express similar levels of ErbB3 protein (as well as ErbB1 and ErbB2) as BT474 cells (data not shown) and reports that ErbB2-overexpressing cell lines with no or low ErbB3 expression are minimally growth inhibited by 4D5 treatment (37, 38), make it tempting to postulate that the strong proliferative signal resulting from an ErbB2-ErbB3 collaboration could lead to growth dependency during tumor development.
A second possible explanation for resistance to 4D5 treatment is the presence of alternative signaling pathways with the capacity to override ErbB2 receptor inhibition. In MKN7 cells a likely candidate is the ErbB1 receptor, which, in contrast to the situation in BT474 and SKBR3 cells, is overexpressed (see Fig. 2A and reference 37) and highly activated in these cells (Fig. 2A). The observations that ErbB-mediated signaling pathways overlap (5, 16), that epidermal growth factor rescues growth inhibition caused by 4D5 treatment (reference 83 and our unpublished data), and that the anti-ErbB1 MAb 225 augments 4D5-induced growth inhibition (83) provide compelling evidence for alternative routes by which tumor cells ensure maintenance of their proliferative capacity. With these two possibilities in mind, therefore, the involvement of all ErbB receptor family members in determining cellular responses to 4D5 treatment should be considered.
Implications for tumor development.
Alterations targeting and, therefore, deregulating the G1 Cdk/pRb phosphorylation pathway are commonly found in human cancers. In this respect, numerous correlations between abnormal p27Kip1 expression and advanced tumor grade have been made (for examples, see references 12, 19, 39, 58, and 82). Enhanced proteasome-dependent p27Kip1 degradation has been postulated to be a major influencing factor in these tumors (19, 39). Here, we show that ErbB2 overexpression can provide an additional level of p27Kip1 deregulation during tumor development; maintaining p27Kip1 sequestration proteins and, thus, potentiating cyclin E-Cdk2 activity. However, we further demonstrate that receptor overexpression levels alone cannot predict to what extent elevated ErbB2 receptor signaling will contribute to deregulation of the G1/S transition. Bearing this in mind, we note that even though all patients treated with a humanized version of 4D5 (Herceptin) presented with metastatic breast carcinomas overexpressing ErbB2, not all responded to treatment (3, 15, 52). It is tempting to speculate, therefore, that the ability of a given tumor cell to elicit p27Kip1 relocation and, hence, Cdk2 inactivation in response to ErbB2 receptor inhibition may determine the potency of the clinical response to Herceptin. This ability may depend on the relative contribution of other growth factor receptors to ErbB2 activation and to the maintenance of specific intracellular, mitogenic signaling pathways. In some tumor cells, ErbB1 or ErbB3 expression is coamplified with ErbB2, leading to the suggestion that they may collaborate in the induction of human malignancies. The determination of whether such relationships do, indeed, exist has an impact not only on elucidating the mechanisms by which ErbB2 overexpression contributes to malignant transformation but also on therapeutic and screening strategies used in the clinic.
ACKNOWLEDGMENTS
This work was supported in part by a grant from the Swiss Cancer League to H.A.L. and J.M.D. R.M.N. was supported in part by a grant from The Basel Cancer League. A.B.M. acknowledges support from the Stipendium Kommission für Nachwuchskräfte aus Entwicklungsländern, Baselstadt, Switzerland.
We thank P. Dennis, G. Orend, and M. Gstaiger for critically reading the manuscript and all members of the laboratory for valuable discussions. We also thank I. Hoffman (DKFZ, Germany) for continued support, W. Krek (FMI, Basel, Switzerland) for supplying anti-cyclin A and p45SKP2 antibodies, C. Benz (UCSF, San Francisco, Calif.) for supplying MKN7 cells, and M. X. Sliwkowski and Genentech Inc. (South San Francisco, Calif.) for kindly supplying the 4D5 monoclonal, without which this work would not have been possible.
REFERENCES
- 1.Alimandi M, Romano A, Curia M C, Muraro R, Fedi P, Aaronson S A, Di Fiore P P, Kraus M H. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene. 1995;10:1813–1821. [PubMed] [Google Scholar]
- 2.Amati B, Alevizopoulos K, Vlach J. Myc and the cell cycle. Frontiers Biosci. 1998;3:250–268. doi: 10.2741/a239. [DOI] [PubMed] [Google Scholar]
- 3.Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz C C, Dantis L, Sklarin N T, Seidman A D, Hudis C A, Moore J, Rosen P P, Twaddell T, Henderson I C, Norton L. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol. 1996;14:737–744. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]
- 4.Beerli R R, Graus-Porta D, Woods-Cook K, Chen X, Yarden Y, Hynes N E. Neu differentiation factor activation of ErbB-3 and ErbB-4 is cell specific and displays a differential requirement for ErbB-2. Mol Cell Biol. 1995;15:6496–6505. doi: 10.1128/mcb.15.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beerli R R, Hynes N E. Epidermal growth factor-related peptides activate distinct subsets of ErbB receptors and differ in their biological activities. J Biol Chem. 1996;271:6071–6076. doi: 10.1074/jbc.271.11.6071. [DOI] [PubMed] [Google Scholar]
- 6.Berger M S, Locher G W, Saurer S, Gullick W J, Waterfield M D, Groner B, Hynes N E. Correlation of c-erbB-2 gene amplification and protein expression in human breast cancer with nodal status and nuclear grading. Cancer Res. 1988;48:1238–1243. [PubMed] [Google Scholar]
- 7.Berns K, Hijmans E M, Bernards R. Repression of c-Myc responsive genes in cycling cells causes G1 arrest through reduction of cyclin E/CDK2 kinase activity. Oncogene. 1997;15:1347–1356. doi: 10.1038/sj.onc.1201280. [DOI] [PubMed] [Google Scholar]
- 8.Blain S W, Montalvo E, Massague J. Differential interaction of the cyclin-dependent kinase (Cdk) inhibitor p27Kip1 with cyclin A-Cdk2 and cyclin D2-Cdk4. J Biol Chem. 1997;272:25863–25872. doi: 10.1074/jbc.272.41.25863. [DOI] [PubMed] [Google Scholar]
- 9.Bodey B, Bodey B, Jr, Groger A M, Luck J V, Siegel S E, Taylor C R, Kaiser H E. Clinical and prognostic significance of the expression of the c-erbB-2 and c-erbB-3 oncoproteins in primary and metastatic malignant melanomas and breast carcinomas. Anticancer Res. 1997;17:1319–1330. [PubMed] [Google Scholar]
- 10.Bouchard C, Thieke K, Maier A, Saffrich R, Hanley-Hyde J, Ansorge W, Reed S, Sicinski P, Bartek J, Eilers M. Direct induction of cyclin D2 by Myc contributes to cell cycle progression and sequestration of p27. EMBO J. 1999;18:5321–5333. doi: 10.1093/emboj/18.19.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carraway K L, III, Cantley L C. A neu acquaintance for erbB3 and erbB4: a role for receptor heterodimerization in growth signaling. Cell. 1994;78:5–8. doi: 10.1016/0092-8674(94)90564-9. [DOI] [PubMed] [Google Scholar]
- 12.Catzavelos C, Bhattacharya N, Ung Y C, Wilson J A, Roncari L, Sandhu C, Shaw P, Yeger H, Morava-Protzner I, Kapusta L, Franssen E, Pritchard K I, Slingerland J M. Decreased levels of the cell-cycle inhibitor p27Kip1 protein: prognostic implications in primary breast cancer. Nat Med. 1997;3:227–230. doi: 10.1038/nm0297-227. [DOI] [PubMed] [Google Scholar]
- 13.Cheng M, Olivier P, Diehl J A, Fero M, Roussel M F, Roberts J M, Sherr C J. The p21Cip1 and p27Kip1 CDK 'inhibitors' are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 1999;18:1571–1583. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coats S, Flanagan W M, Nourse J, Roberts J M. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science. 1996;272:877–880. doi: 10.1126/science.272.5263.877. [DOI] [PubMed] [Google Scholar]
- 15.Cobleigh M A, Vogel C L, Tripathy D, Robert N J, Scholl S, Fehrenbacher L, Wolter J, Paton V, Shak S, Lieberman G, Slamon D J. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 16.Daly R J. Take your partners, please—signal diversification by the erbB family of receptor tyrosine kinases. Growth Factors. 1999;16:255–263. doi: 10.3109/08977199909069144. [DOI] [PubMed] [Google Scholar]
- 17.Diehl J A, Cheng M, Roussel M F, Sherr C J. Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elledge S J, Winston J, Harper J W. A question of balance: the role of cyclin-kinase inhibitors in development and tumorigenesis. Trends Cell Biol. 1996;6:388–397. doi: 10.1016/0962-8924(96)10030-1. [DOI] [PubMed] [Google Scholar]
- 19.Esposito V, Baldi A, De Luca A, Groger A M, Loda M, Giordano G G, Caputi M, Baldi F, Pagano M, Giordano A. Prognostic role of the cyclin-dependent kinase inhibitor p27 in non-small cell lung cancer. Cancer Res. 1997;57:3381–3385. [PubMed] [Google Scholar]
- 20.Fedi P, Pierce J H, DiFiore P P, Kraus M H. Efficient coupling with phosphatidylinositol 3-kinase, but not phospholipase Cγ or GTPase-activating protein, distinguishes ErbB-3 signaling from that of the Erb/EGFR family members. Mol Cell Biol. 1994;14:492–500. doi: 10.1128/mcb.14.1.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukushige S-I, Matsubara K-I, Yoshida M, Sasaki M, Suzuki T, Semba K, Toyoshima K, Yamamoto T. Localization of a novel v-erbB-related gene, c-erbB-2, on human chromosome 17 and its amplification in a gastric cancer cell line. Mol Cell Biol. 1986;6:955–958. doi: 10.1128/mcb.6.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graus-Porta D, Beerli R R, Hynes N E. Single-chain antibody-mediated intracellular retention of ErbB-2 impairs neu differentiation factor and epidermal growth factor signaling. Mol Cell Biol. 1995;15:1182–1191. doi: 10.1128/mcb.15.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graus-Porta D, Beerli R R, Daly J M, Hynes N E. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16:1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gullick W J, Love S B, Wright C, Barnes D M, Gusterson B, Harris A L, Altman D G. c-erbB-2 protein overexpression in breast cancer is a risk factor in patients with involved and uninvolved lymph nodes. Br J Cancer. 1991;63:434–438. doi: 10.1038/bjc.1991.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harwerth I-M, Wels W, Marte B, Hynes N E. Monoclonal antibodies against the extracellular domain of the erbB-2 receptor function as partial ligand agonists. J Biol Chem. 1992;267:15160–15167. [PubMed] [Google Scholar]
- 26.Harwerth I-M, Wels W, Schlegel J, Müller M, Hynes N E. Monoclonal antibodies directed to the erbB-2 receptor inhibit in vivo tumour cell growth. Br J Cancer. 1993;68:1140–1145. doi: 10.1038/bjc.1993.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hudziak R M, Lewis G D, Winget M, Fendly B M, Shepard H M, Ullrich A. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol. 1989;9:1165–1172. doi: 10.1128/mcb.9.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunter T, Pines J. Cyclins and cancer. II. Cyclin D and CDK inhibitors come of age. Cell. 1994;79:573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 29.Jannot Ch, Beerli R R, Mason S, Gullick W J, Hynes N E. Intracellular expression of a single-chain antibody directed to the epidermal growth factor receptor leads to growth inhibition of tumor cells. Oncogene. 1996;13:275–282. [PubMed] [Google Scholar]
- 30.Karunagaran D, Tzahar E, Beerli R R, Chen X, Graus-Porta D, Ratzkin B J, Seger R, Hynes N E, Yarden Y. ErbB-2 is a common auxiliary subunit of NDF and EGF receptors: implications for breast cancer. EMBO J. 1996;15:254–264. [PMC free article] [PubMed] [Google Scholar]
- 31.Keyomarsi K, Pardee A B. Redundant cyclin overexpression and gene amplification in breast cancer cells. Proc Natl Acad Sci USA. 1993;90:1112–1116. doi: 10.1073/pnas.90.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keyomarsi K, Conte D, Jr, Toyofuku W, Fox M P. Deregulation of cyclin E in breast cancer. Oncogene. 1995;11:941–950. [PubMed] [Google Scholar]
- 33.Kumar R, Shepard H M, Mendelsohn J. Regulation of phosphorylation of the c-erbB-2/HER2 gene product by a monoclonal antibody and serum growth factor(s) in human mammary carcinoma cells. Mol Cell Biol. 1991;11:979–986. doi: 10.1128/mcb.11.2.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LaBaer J, Garrett M D, Stevenson L F, Slingerland J M, Sandhu C, Chou H S, Fattaey A, Harlow E. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- 35.Lane H A, Nigg E A. Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J Cell Biol. 1996;135:1701–1713. doi: 10.1083/jcb.135.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leone G, DeGregori J, Sears R, Jakoi L, Nevins J R. Myc and Ras collaborate in inducing accumulation of active cyclin E/Cdk2 and E2F. Nature. 1997;387:422–426. doi: 10.1038/387422a0. [DOI] [PubMed] [Google Scholar]
- 37.Lewis G D, Figari I, Fendly B, Wong W L, Carter P, Gorman C, Shepard H M. Differential responses of human tumor cell lines to anti-p185HER2 monoclonal antibodies. Cancer Immunol Immunother. 1993;37:255–263. doi: 10.1007/BF01518520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis G D, Lofgren J A, McMurtrey A E, Huijens A, Fendly B M, Bauer K D, Sliwkowski M X. Growth regulation of human breast and ovarian tumor cells by heregulin: evidence for the requirement of ErbB2 as a critical component in mediating heregulin responsiveness. Cancer Res. 1996;56:1457–1465. [PubMed] [Google Scholar]
- 39.Loda M, Cukor B, Tam S W, Lavin P, Fiorentino M, Draetta G F, Milburn Jessup J, Pagano M. Increased proteosome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med. 1997;3:231–234. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- 40.Lodén M, Nielsen N H, Roos G, Emdin S O, Landberg G. Cyclin E dependent kinase activity in human breast cancer in relation to cyclin E, p27 and p21 expression and retinoblastoma protein phosphorylation. Oncogene. 1999;18:2557–2566. doi: 10.1038/sj.onc.1202488. [DOI] [PubMed] [Google Scholar]
- 41.Marte B M, Graus-Porta D, Jeschke M, Fabbro D, Hynes N E, Taverna D. NDF/heregulin activates MAP kinase and p70/p85 S6 kinase during proliferation or differentiation of mammary epithelial cells. Oncogene. 1995;10:167–175. [PubMed] [Google Scholar]
- 42.Meyerson M, Harlow E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell Biol. 1994;14:2077–2086. doi: 10.1128/mcb.14.3.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montagnoli A, Fiore F, Eytan E, Carrano A C, Draetta G F, Hershko A, Pagano M. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev. 1999;13:1181–1189. doi: 10.1101/gad.13.9.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Müller D, Bouchard C, Rudolph B, Steiner P, Stuckmann I, Saffrich R, Ansorge R, Huttner W, Eilers M. Cdk2-dependent phosphorylation of p27 facilitates its Myc-induced release from cyclin E/Cdk2 complexes. Oncogene. 1997;15:2561–2576. doi: 10.1038/sj.onc.1201440. [DOI] [PubMed] [Google Scholar]
- 45.Nielsen N H, Arnerlov C, Emdin S O, Landberg G. Cyclin E overexpression, a negative prognostic factor in breast cancer with strong correlation to oestrogen receptor status. Br J Cancer. 1996;74:874–880. doi: 10.1038/bjc.1996.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nielsen N H, Lodén M, Cajander J, Emdin S O, Landberg G. G1-S transition defects occur in most breast cancers and predict outcome. Breast Cancer Res Treat. 1999;56:105–112. doi: 10.1023/a:1006208419350. [DOI] [PubMed] [Google Scholar]
- 47.Nigg E A. Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. Bioessays. 1995;17:471–480. doi: 10.1002/bies.950170603. [DOI] [PubMed] [Google Scholar]
- 48.Obaya A J, Mateyak M K, Sedivy J M. Mysterious liaisons: the relationship between c-Myc and the cell cycle. Oncogene. 1999;18:2934–2941. doi: 10.1038/sj.onc.1202749. [DOI] [PubMed] [Google Scholar]
- 49.Olayioye M A, Graus-Porta D, Beerli R R, Rohrer J, Gay B, Hynes N E. ErbB-1 and ErbB-2 acquire distinct signaling properties depending upon their dimerization partner. Mol Cell Biol. 1998;18:5042–5051. doi: 10.1128/mcb.18.9.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Orend G, Hunter T, Ruoslahti E. Cytoplasmic displacement of cyclin E-cdk2 inhibitors p21Cip1 and p27Kip1 in anchorage-independent cells. Oncogene. 1998;16:2575–2583. doi: 10.1038/sj.onc.1201791. [DOI] [PubMed] [Google Scholar]
- 51.Pagano M, Tam S W, Theodoras A M, Beer-Romero P, Del Sal G, Chau V, Yew P R, Draetta G F, Rolfe M. Role of the ubiquitin-proteosome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 52.Pegram M D, Lipton A, Hayes D F, Weber B L, Baselga J M, Tripathy D, Baly D, Baughman S A, Twaddell T, Glaspy J A, Slamon D J. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol. 1998;16:2659–2671. doi: 10.1200/JCO.1998.16.8.2659. [DOI] [PubMed] [Google Scholar]
- 53.Peng D, Fan Z, Lu Y, Deblasio T, Scher H, Mendelsohn J. Anti-epidermal growth factor receptor monoclonal antibody 225 up-regulates p27Kip1 and induces G1 arrest in prostatic cancer cell line DU145. Cancer Res. 1996;56:3666–3669. [PubMed] [Google Scholar]
- 54.Perez-Roger I, Kim S-H, Griffiths B, Sewing A, Land H. Cyclins D1 and D2 mediate Myc-induced proliferation via sequestration of p27Kip1 and p21Cip1. EMBO J. 1999;18:5310–5320. doi: 10.1093/emboj/18.19.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pinkas-Kramarski R, Soussan L, Waterman H, Levkowitz G, Alroy I, Klapper L, Lavi S, Seger R, Ratzkin B J, Sela M, Yarden Y. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J. 1996;15:2452–2467. [PMC free article] [PubMed] [Google Scholar]
- 56.Polyak K, Lee M-H, Erdjument-Bromage H, Koff A, Roberts J M, Tempst P, Massagué J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 57.Poon R Y, Toyoshima H, Hunter T. Redistribution of the CDK inhibitor p27 between different cyclin/CDK complexes in the mouse fibroblast cell cycle and in cells arrested with lovastatin or ultraviolet light. Mol Biol Cell. 1995;6:1197–1213. doi: 10.1091/mbc.6.9.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Porter P L, Malone K E, Heagerty P J, Alaxander G M, Gatti L A, Firpo E J, Daling J R, Roberts J M. Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat Med. 1997;3:222–225. doi: 10.1038/nm0297-222. [DOI] [PubMed] [Google Scholar]
- 59.Press M F, Pike M C, Chazin V R, Hung G, Udove J A, Markowicz M, Danyluk J, Godolphin W, Sliwkowski M, Akita R, Paterson M C, Slamon D J. Her-2/neu expression in node-negative breast cancer: direct tissue quantitation by computerized image analysis and association of overexpression with increased risk of recurrent disease. Cancer Res. 1993;53:4960–4970. [PubMed] [Google Scholar]
- 60.Prigent S A, Gullick W J. Identification of c-erbB-3 binding sites for phosphatidyl 3′kinase and SHC using an EGF receptor/c-erbB-3 chimera. EMBO J. 1994;13:2831–2841. doi: 10.1002/j.1460-2075.1994.tb06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pusch O, Barnaschek G, Eilers M, Hengstschlager M. Activation of c-Myc uncouples DNA replication from activation of G1-cyclin-dependent kinases. Oncogene. 1997;15:649–656. doi: 10.1038/sj.onc.1201236. [DOI] [PubMed] [Google Scholar]
- 62.Riese D J, II, Stern D F. Specificity within the EGF family/ErbB receptor family signaling network. Bioessays. 1998;20:41–48. doi: 10.1002/(SICI)1521-1878(199801)20:1<41::AID-BIES7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 63.Scott G K, Dodson J M, Montgomery P A, Johnson R M, Sarup J C, Wong W L, Ullrich A, Shepard H M, Benz C C. p185HER2 signal transduction in breast tumour cells. J Biol Chem. 1991;266:14300–14305. [PubMed] [Google Scholar]
- 64.Scott G K, Robles R, Park J W, Montgomery P A, Daniel J, Holmes W E, Lee J, Keller G A, Li W-L, Fendly B M, Wood W I, Shepard H M, Benz C C. A truncated intracellular HER2/neu receptor produced by alternative RNA processing affects growth of human carcinoma cells. Mol Cell Biol. 1993;13:2247–2257. doi: 10.1128/mcb.13.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sears R, Lone G, DeGregori J, Nevins J R. Ras enhances Myc protein stability. Mol Cell. 1999;3:169–179. doi: 10.1016/s1097-2765(00)80308-1. [DOI] [PubMed] [Google Scholar]
- 66.Sheaff R J, Groudine M, Gordon M, Roberts J M, Clurman B E. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 1997;11:1464–1478. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- 67.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 68.Sherr C J, Roberts J M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 69.Siegel P M, Ryan E D, Cardiff R D, Muller W J. Elevated expression of activated forms of Neu/ErbB-2 and ErbB-3 are involved in the induction of mammary tumors in transgenic mice: implications for human breast cancer. EMBO J. 1999;18:2149–2164. doi: 10.1093/emboj/18.8.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Slamon D J, Clark G M, Wong S G, Levin W J, Ullrich A, McGuire W L. Human breast cancer: correlation of relapse and survival with the amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 71.Slamon D J, Godolphin W, Jones L A, Holt J A, Wong S G, Keith D E, Levin W J, Stuart S G, Udove J, Ullrich A, Press M F. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 72.Slingerland J M, Hengst L, Pan C H, Alexander D, Stampfer M R, Reed S I. A novel inhibitor of cyclin-Cdk activity detected in transforming growth factor β-arrested epithelial cells. Mol Cell Biol. 1994;14:3683–3694. doi: 10.1128/mcb.14.6.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Soos T J, Kiyokawa H, Yan J S, Rubin M S, Giordano A, DeBlasio A, Bottega S, Wong B, Mendelsohn J, Koff A. Formation of p27-CDK complexes during the human mitotic cell cycle. Cell Growth Differ. 1996;7:135–146. [PubMed] [Google Scholar]
- 74.Steiner P, Philipp A, Lukas J, Godden-Kent D, Pagano M, Mittnacht S, Bartek J, Eilers M. Identification of a Myc-dependent step during the formation of active G1 cyclin-cdk complexes. EMBO J. 1995;14:4814–4826. doi: 10.1002/j.1460-2075.1995.tb00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taya Y. RB kinases and RB-binding proteins: new points of view. Trends Biochem Sci. 1997;22:14–17. doi: 10.1016/s0968-0004(96)10070-0. [DOI] [PubMed] [Google Scholar]
- 76.Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin/cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 77.Vlach J, Hennecke S, Alevizopoulos K, Conti D, Amati B. Growth arrest by the cyclin-dependent kinase inhibitor p27Kip1 is abrogated by c-Myc. EMBO J. 1996;15:6595–6604. [PMC free article] [PubMed] [Google Scholar]
- 78.Wallasch C, Weisse F U, Niederfellner G, Jallal B, Issing W, Ullrich A. Heregulin-dependent regulation of HER2/neu oncogenic signaling by heterodimerization with HER3. EMBO J. 1995;14:4267–4275. doi: 10.1002/j.1460-2075.1995.tb00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weiss F U, Wallasch C, Campiglio M, Issing W, Ullrich A. Distinct characteristics of heregulin signals mediated by HER3 or HER4. J Cell Physiol. 1997;173:187–195. doi: 10.1002/(SICI)1097-4652(199711)173:2<187::AID-JCP19>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 80.West M J, Stoneley M, Willis A E. Translational induction of the c-myc oncogene via activation of the FRAP/TOR signalling pathway. Oncogene. 1998;17:769–780. doi: 10.1038/sj.onc.1201990. [DOI] [PubMed] [Google Scholar]
- 81.Wu X, Rubin M, Fan Z, DeBlasio T, Soos T, Koff A, Mendelsohn J. Involvement of p27Kip1 in G1 arrest mediated by an anti-epidermal growth factor receptor monoclonal antibody. Oncogene. 1996;12:1397–1403. [PubMed] [Google Scholar]
- 82.Yasui W, Kudo Y, Semba S, Yokozaki H, Tahara E. Reduced expression of cyclin-dependent kinase inhibitor p27Kip1 is associated with advanced stage and invasiveness of gastric carcinomas. Jpn J Cancer Res. 1997;88:625–629. doi: 10.1111/j.1349-7006.1997.tb00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ye D, Mendelsohn J, Fan Z. Augmentation of a humanized Anti-HER2 mAb 4D5 induced growth inhibition by a human-mouse chimeric anti-EGF receptor mAb C225. Oncogene. 1999;18:731–738. doi: 10.1038/sj.onc.1202319. [DOI] [PubMed] [Google Scholar]
- 84.Yonemura Y, Ninomiya I, Yamaguchi A, Fushida S, Kimura H, Ohoyama S, Miyazaki I, Endou Y, Tanaka M, Sasaki T. Evaluation of immunoreactivity for erbB-2 protein as a marker of poor short-term prognosis in gastric cancer. Cancer Res. 1991;51:1034–1038. [PubMed] [Google Scholar]
- 85.Zhang H, Hannon G J, Beach D. p21-containing cyclin-kinases exist in both active and inactive states. Genes Dev. 1994;8:1750–1758. doi: 10.1101/gad.8.15.1750. [DOI] [PubMed] [Google Scholar]
- 86.Zhang K, Sun J, Liu N, Wen D, Chang D, Thomason A, Yasinaga S K. Transformation of NIH 3T3 cells by HER3 or HER4 receptors requires the presence of HER1 or HER2. J Biol Chem. 1996;271:3884–3890. [PubMed] [Google Scholar]