Abstract
Background:
Among animal phyla, some of the least studied nervous systems are those of the phylum Echinodermata. Part of the problem lies in that most of their nervous components are embedded in the body wall that has calcareous skeletal components.
New method:
We have developed a novel technique for the successful isolation of the radial nerve cords (RNCs) and an in vitro system where the isolated RNCs can be cultured and are amenable to experimental manipulation. Here we use this system to isolate the RNC of the sea cucumber Holothuria glaberrima as a way to extend our studies on its regeneration capabilities.
Results:
The RNCs can be isolated from the surrounding tissues by collagenase treatment. The explants obtained following enzymatic dissociation can be kept in culture for up to 2 weeks.Histological and immunohistochemical studies show that the explants maintain a stable number of cells with little proliferation or apoptosis throughout the culture incubation period. The main change observed in RNCs in vitro is a progressive dedifferentiation of radial glia-like cells. This dedifferentiation corresponds to the first step in the regeneration response to injury that has been described in vivo.
Comparison with existing methods:
There are no existing methods to isolate and culture echinoderm radial nerve cord.
Conclusions:
The described protocol provides a unique tool to obtain easily accessible RNC from holothurians to perform cellular, biochemical, and genomic experiments in the echinoderm nervous system without interference of adjacent tissues. The technique provides a unique opportunity to study the dedifferentiation response associated with the regeneration of the nervous system in echinoderms.
Keywords: nerve cord, CNS, regeneration, radial glia-like, echinoderm, dedifferentiation
1. Introduction
Echinoderms possess a remarkable evolutionary advantage of undergoing extensive tissue and organ regeneration upon injury (Hyman, 1955; García-Arrarás and Greenberg, 2001). Their incredible regenerative capacities place them as ideal model systems to understand and elucidate the cellular and molecular mechanisms involved in regeneration processes. Holothurians, popularly known as sea cucumbers, regenerate their central nervous system, as well as their gastrointestinal tract and longitudinal muscle pairs (García-Arrarás and Dolmatov, 2010; Quispe-Parra et al., 2021; San Miguel-Ruiz and García-Arrarás, 2007; San Miguel-Ruiz et al., 2009). Previous studies from our group have shown that holothurians are able to fully regenerate their radial nerve cords (RNCs) without scarring (Mashanov et al., 2008; San Miguel-Ruiz et al., 2009). Our group has continued to utilize the sea cucumber Holothuria glaberrima as a model system to study the cellular and molecular events associated with this regeneration process. In addition to their impressive regenerative capacity, it is important to highlight an additional advantage of the sea cucumber model: the close phylogenetic relationship between echinoderms and chordates. Echinoderms, although primitive looking with their radial symmetry, comprise one of the few invertebrate deuterostomes in the evolutionary branch leading to the Chordata. As such, experimental findings on their capacity for nervous system regeneration, when compared to the limited regeneration of vertebrates, are of great interest to regeneration biology and to the study of nervous system evolution.
Our understanding of the underlying cellular and molecular events that take place during central nervous system regeneration in H. glaberrima is greatly hindered by the complexity of the model system and the lack of in vitro whole-tissue explant culture techniques. The adult echinoderm nervous system is comprised by five radial RNCs that extend along the body axis (anterior to posterior) and come together anteriorly forming a nerve ring (Hyman, 1955). The RNCs are ganglionated nerve cords similar to the vertebrate spinal cord. They are mainly composed of two cell populations: glia and neurons in approximately 2:1 ratio (Mashanov et al., 2010). The RNCs do not have any vessels within them, but are separated into two subdivisions, ectoneural and hyponeural, by a thin layer of connective tissue. The neuronal population has been shown to express various nervous system markers including markers of catecholaminergic and gabaergic neurons (Díaz-Balzac et al., 2016). The glia population is mainly comprised by radial glia-like cells (Mashanov et al., 2010). Among the markers that have been used to define the cellular populations, the two most important are: (1) the RN1 antibody that labels all known neuronal cells, particularly their neurites (Díaz-Balzac et al., 2007). The antigen recognized by this antibody has been determined to be a holothurian STARD protein (Rosado-Oliveri et al. 2017) and (2) the ERG1antibody, shown to label the radial glia-like cells (Mashanov et al., 2010). The antigen recognized by this antibody remains undetermined.
Various studies from multiple labs have developed and utilized echinoderm tissue cultures (Bello et al., 2015; Candia et al., 1998; Di Benedetto et al., 2014; Mercurio et al., 2014; Sharlaimova et al., 2010, 2012; Wang et al., 2020). However, most if not all, are tailored for a particular species, organ, or cell-type and mainly non-neuronal tissues. This lack of available echinoderm nervous system culture techniques is likely due to the echinoderm nervous component being an experimentally inaccessible tissue. In all echinoderm classes, with the exception of the Echinoidea (sea urchins), the RNCs are embedded within the body wall (Díaz-Miranda et al., 1995; Hyman, 1955; San Miguel-Ruiz et al., 2009), which hinders direct access to the nervous tissue for tissue-specific studies.
Here, we report a novel dissection technique that successfully isolates the RNCs from neighboring tissues and an in vitro system where isolated RNCs can be kept in culture for approximately 2-weeks. This in vitro system is thus, amenable to experimental manipulations aimed at studying the central nervous system of H. glaberrima and extend our studies on its regenerative capabilities. Thus, we describe here the procedure to isolate and culture the RNCs. Moreover, as an experimental validation, we analyze the explants for several cellular processes that take place within the first 2-weeks in culture and show an increase in the dedifferentiation of the radial glia-like cells, but no change in apoptosis or cell proliferation. This finding suggests that dedifferentiation, which has been previously shown to be the initial in vivo response to injury, can be separated from other cellular responses and studied in vitro.
2. Materials and Methods
2.1. Animal Collection
Adult H. glaberrima specimens were collected from the rocky shores of San Juan, Puerto Rico and kept in salt-water aquaria at room temperature as previously described (Mashanov et al., 2017).
2.2. Radial Nerve Cord Dissection
Specimens were anesthetized in 0.2% 1,1,1-tricholoro-2-methyl-2-propanol (Sigma-Aldrich) for 1hr at room temperature. Animals were then disinfected by submersion in individual beakers containing 95% ethanol, 10% commercial bleach, and sterile ultrapure water for 1 minute each. Discoloration of the animal is expected. Animals were placed dorsal side up on an autoclaved dissection pad underneath a sterile dissecting microscope. Using a 20mm scalpel blade, animals were incised below the ring canal and above the cloaca. An incision was made following an anatomical line between the ambulacral feet and the dorsal side from the anterior to posterior end. The initial incisions spared the longitudinal muscles, thus avoiding incising the RNCs (located between the longitudinal muscle pairs) during the dissection. The body wall was then cleaned of the viscera and placed with pushpins on a dissection pad (Figure 1A-B). Tissues were always maintained hydrated with filtered sea water. Four out of five longitudinal muscle pairs were identified for dissection. About three-fourths of each longitudinal muscle pairs were then removed, using a Vannas spring scissor, from the anterior to posterior edges to avoid damaging the RNCs (Figure 1C). The remaining longitudinal muscle and nerve complex were dissected by an additional incision in the body wall connective tissue. Thus, the dissected muscle-nerve-body wall complex contained RNCs embedded between remaining longitudinal muscle (ventral) and body wall connective tissues (dorsal). These tissues were then stretched using fine serrated forceps and transferred to a 15mL sterile tube with 5mL antibiotic/antifungal solution for 1hr on ice prior to enzymatic digestion. The antibiotic/antifungal solution contains penicillin/streptomycin (30 U/mL and 300 μg/mL, respectively), gentamicin (150 μg/mL), and amphotericin B (7.5μg/mL). Filtered natural sea water was used as the solvent and added up to 50mL. The pH of solution was adjusted to 7.7 – 7.8. The solution was sterilized by filtration using 0.22 μm filters and stored at 4 °C.
Figure 1. Dissection protocol overview.
A) Diagram showing the initial dissection to expose the internal side of the body wall. B) Photo depicting the exposed interior of the animal with the radial nerve cord (RNC)-longitudinal muscle areas identified with large black rectangles. C) Diagram of a transverse section of the body wall including RNC, longitudinal muscles and coelomic cavity. Dashed lines represent the three incisions made to excise the RNC from the animal. D) Photograph of RNC explants isolated from surrounding tissue after 24 hours of enzymatic dissection. BW, body wall; RNC, radial nerve cord; LM, longitudinal muscle; RC, radial canal; CC, coelomic cavity.
2.3. Dissected Tissue Enzymatic Digestion
After removing the antibiotic/antifungal solution, the dissected tissues were rinsed in extra antibiotic/antifungal solution. Two to four of the dissected muscle-nerve-body wall complexes were transferred to a 15mL tube containing 5mL solution of 0.15% collagenase Type IA from Clostridium histolyticum (Sigma) (the pH of the solution had been previously adjusted to 7.7 – 7.8, sterilized by filtration using 0.22 μm filters and stored at 4 °C). Tubes were covered with aluminum foil as solution is light sensitive. A compact rocker platform at room temperature was used to shake the 15 mL tubes for different time periods ranging from 8 to 48 hours.
2.4. Radial Nerve Cord Explant Culture
Following tissue digestion with the collagenase solution, the RNCs were cleaned from debris using Dumont super fine forceps and transferred to an autoclaved glass petri dish with 1mL of fresh L-15 supplemented media using a Moria mini perforated spoon. After RNCs explants were washed twice with media (Figure 1D), they were placed in 1mL of media per well in 4-well plates. RNC explants were then cultured in a sterile modular incubator chamber at room temperature. Changes of the culture medium were made every 3 days. RNC explants were checked daily under an inverted microscope for morphological changes or possible contamination.
2.5. L-15 Medium and L-15 Supplemented Medium
The L-15 medium (Leibovitz, Sigma) was conditioned for marine species by adding salts, glucose and amino acids to the original composition (Bello et al., 2015). The medium contains 6.9g of L-15 powder, 6.25g NaCl, 3.12g D-glucose, 1.58g MgSO4, 172mg KCl, 96mg NaHCO3, 1.33g MgCl2), 150mg L-glutamine, 745 mg CaCl2•2H2O. Five hundred (500) mL of ultrapure water were added, and the contents diluted by magnetic stirring. Medium was sterilized by filtration using 0.22μm filters. The L-15 medium was then supplemented with antibiotics (100 U/mL penicillin, 100 μg/mL streptomycin, 50 μg/mL gentamicin), antifungal (2.5 μg/mL amphotericin B), 1x MEM non-essential amino acids, 1mM sodium pyruvate and 1.75mg/mL α-tocopherol acetate. The pH was adjusted to 7.7–7.8. Supplemented L-15 medium was sterilized by filtration using 0.22 μm filters.
2.6. Tissue sections
For the in vivo cross sections, samples were obtained from the ventro-lateral ambulacrum region and dorso-lateral body wall (Díaz-Balzac et al., 2016). Body wall portions and RNC explants were fixed in 4% paraformaldehyde at 4°C for approximately 12hrs. Tissues were rinsed 3 times for 15min with 0.1 M phosphate-buffered saline (PBS), and left in a 30% sucrose solution at 4°C for at least 24hrs before proceeding to embed them in Tissue-Tek (Sakura Finetek, Torrance, CA). Cryostat tissue sections (20 μm) were cut and mounted on Poly-L-lysine-coated slides.
2.7. Immunohistochemistry
The indirect immunofluorescence method previously described (García-Arrarás, 1993) was followed. Sections were incubated in goat serum 1:50 (Invitrogen, Carlsbad, CA) for 1 hr and rinsed once for 15 min in 1% Triton X, followed by three rinses of 15 minutes each in 0.1M PBS. Subsequently, sections were treated with primary antibodies and incubated overnight at room temperature. The primary antibodies used include the RN1 monoclonal antibody diluted 1:50,000 (Díaz-Balzac et al., 2007 and 2016; Rosado-Olivieri et al., 2017); the monoclonal anti-collagen antibody IBA2 diluted 1:1000 (Nieves-Ríos et al., 2019) and the monoclonal antibody ERG1 used directly from stock solution (Mashanov et al., 2010, Developmental Studies Hybridoma Bank). The following day, sections were treated with the secondary antibody, a Cy3-conjugated goat anti-mouse antibody used at a 1:1,000 dilution. Cell nuclei were stained by adding 2 μM 4′,6-diamidino-2-phenylindole (DAPI) to the buffered glycerol solution used to mount the slides. Tissues were examined and photomicrographs taken on a Nikon Eclipse E600 fluorescent microscope.
2.8. Cell Proliferation and Apoptosis Assay
To determine the percentage of dividing cells in the explants, we added 5-bromo-2-deoxyuridine (BrdU, Sigma) to a final concentration of 50 μg/mL the day prior to fixation (days 2, 6 or 10). Tissues were prepared for immunohistochemistry and tested with mouse anti-BrdU antibody (Sigma Aldrich).
Deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) was performed to identify cells undergoing programmed cell death. For this we used the FragEL DNA Fragmentation Detection Kit, Fluorescent - TdT Enzyme (Calbiochem, QIA39) following the protocol previously adapted in our lab (Bello et al., 2019).
Cell counts for BrdU and TUNEL assays were done by counting BrdU positive cells or TUNEL positive cells per tissue section or, alternatively, by dividing the total number of cells stained by the average number of DAPI-labeled cell nuclei in all tissue sections for a given time point (e.g. BrdU+/average nuclei count of all explant sections for 3 days in culture). Cell counting was performed utilizing FIJI Cell Counter plugin. Differences between groups were analyzed with one-way ANOVA.
2.9. Dedifferentiation Score Algorithm
To determine the degree of radial glia-like cell dedifferentiation in explants we developed a semi-quantitative algorithm based on the presence of cell extensions and their length (Figure 2). As radial glia-like cell dedifferentiation in H. glaberrima has been previously described (Mashanov et al., 2013), we validated our dedifferentiation assay by utilizing the ERG1 monoclonal antibody in tissue sections and scoring dedifferentiation to one of the described five stages. Scores ranged from 0 (no dedifferentiation as found in in vivo non injured animals) to 4 (fully dedifferentiated). Differences between groups were analyzed with one-way ANOVA.
Figure 2. Semi-quantitative method for dedifferentiation of radial glia-like cells.
Diagram displaying the semi-quantitative method used to characterize the degree of glial dedifferentiation in RNCs. Circles represent the cell bodies in the periphery of the RNC while lines represent the glia-like cell radial extensions. Scores are given according to the length of these extensions as they are found in the RNC neuropile.
3. Results
3.1. Collagenase Dissociation
Similar to other holothurians, H. glaberrima, has a pentamerous nervous system organization with five longitudinal RNCs extending from an anterior circumoral nerve ring to the posterior cloaca. RNCs are embedded within the sea cucumber internal cavity body wall, which is comprised of extracellular matrix and muscle. Hence, isolating the RNCs without surrounding tissue debris, significant contamination from neighboring tissues, or severely damaging the tissue of interest is extremely difficult. The surgically excised radial tissue complexes (RNCs and surrounding tissues) were treated with collagenase for different time periods (8, 18, 24, 42 and 48 hours) to assess optimal digestion period for isolated RNCs (Figure 3A-E). Tissues digested for 8-hours showed an excess of neighboring tissues from body wall and longitudinal muscles (Figure 3A) which were tightly adhered to the RNCs. At 18-hours post-collagenase digestion, the RNC is slightly distinguishable from the surrounding tissues (Figure 3B). However, it could not be manually removed without significant contamination or damage. Meanwhile, tissues digested in collagenase for 24-hours resulted in isolated RNCs with little to no presence of surrounding tissue debris (Figure 3C). Notably, RNC explants have a tendency to ‘curl-up’ due to a loss of rigidity following removal of neighboring tissues. After a 42- or 48-hour collagenase digestion, RNC explant structural integrity remained intact with little to no presence of tissue debris (Figure 3D-E). Our results showed that a 24-hour collagenase digestion treatment was sufficient for most of the muscle and body wall tissues surrounding the RNC to dissociate, thus isolating intact RNC explants. We utilized the 24-hour digestion period for further experiments where the RNC explants were cultured in vitro following collagenase treatment.
Figure 3. Enzymatic dissection of radial nerve cords (RNCs).
Strips of body wall containing the RNCs were treated with collagenase for A) 8-, B) 18-, C) 24-, D) 42- and E) 48-hours. A) After digestion for 8 hours in collagenase, the RNC (delineated by stippled lines) is still surrounded by connective tissue and other body wall components. B) The RNC is visible after an 18-hour digestion in collagenase but still shows significant debris associated with it. C) RNCs with peripheral nerves (arrowhead) after 24-hr digestion in collagenase show very little signs of surrounding debris. RNCs have a tendency to curl-up when free of surrounding tissue. D) RNC after a 42-hour or E) 48-hour digestion shows that the expected structure and morphology remains intact. Peripheral nerves are observed (arrowhead). (F-I) RNC cross-section immunostaining using anti-collagen immunohistochemistry. F) Immunohistochemical studies on tissue sections of the body wall and RNC show the collagen (arrows) that separates the RNC from the surrounding body wall for in vivo cross sections. The thin band of connective tissue that separates the ectoneural (Ec) component from the hyponeural (Hy) is also observed (arrowhead). Following collagenase dissection and culture in vitro for G) 3-, H) 7- and I) 11-days little collagen immunoreactivity is associated with the RNC explants. Scale bar = 10 μm in A-E and 20 μm in F-I.
3.2. RNC Morphological and Structural Changes
To show that our enzymatic treatment with collagenase is optimal, we conducted an immunohistochemical analysis of the RNC embedded in the holothurian body wall to demonstrate that the ECM surrounding RNCs contained collagen, and that most of it was lost following enzymatic dissection. In vivo cross sections of the body wall were labeled using anti-collagen antibody, IBA2. Labeled sections clearly showed a large band of collagen surrounding most of the RNC in vivo (Figure 3F) but little if any collagen remained after the explants were obtained and placed in culture (see Figures 3G-I). Collagen traces could also be appreciated in the connective tissue that separates the ectoneural (Ec) from the hyponeural (Hy) nervous components (Figure 3F). This collagen band remains in RNC explants after three day in vitro (Figure 3G), but is gradually lost in 7- and 11-day explants, which could have contributed to changes in morphological and structural integrity of the RNC (Figure 3H-I).
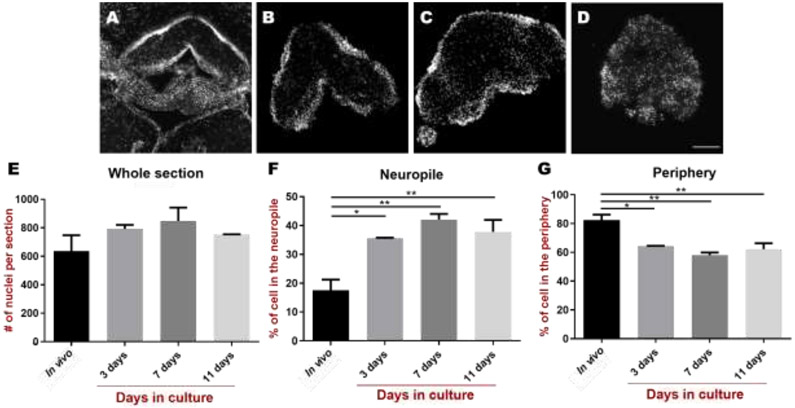
Morphological and structural changes in RNC explants were determined by staining sections with (DAPI) to assess cell numbers and their localization (Figure 4). Previous experiments (Díaz-Miranda et al., 1995, Mashanov et al., 2010) have demonstrated that neuronal somata, as well as glial cell bodies lie in the periphery of the RNC with most of the RNC central region comprised of a neuropile formed by neuronal axons and extensions of radial glia-like cells (Figure 4A-D). The total number of cells in RNC explant sections (as determined by the number of nuclei) at 3-, 7-, and 11-days in culture was counted and averaged 798, 830, and 776 cell nuclei/tissue section, respectively (Figure 4E). While there is no apparent change in the total number of nuclei in the explants, there is a change in their localization. A significant increase in the number of cells in the neuropile was observed at days 3, 7 and 11 in vitro when compared to in vivo cross sections (Figure 4F) while peripheral cell counts showed a significant decrease (Figure 4G).
Figure 4. Cellular dynamics in cultured radial nerve cord (RNC) explants.
Transverse sections of the RNC and the RNC explants using DAPI nuclear staining to determine the number and localization of cells in A) In vivo and in vitro after B) 3-, C) 7-, and D) 11-days in culture. E) Nuclei labeling showed little changes in cell numbers or localization. Cell quantifications determined that the total number of nuclei in sections do not change significantly from controls up to 11 days in vitro. However, there is an apparent change in their localization where more cells are observed within the F) neuropile and less cells are observed in G) the periphery of the RNC. Bars represent the mean ± SD of n = 3, * p-value < 0.05, **p-value < 0.005. Scale bar = 20 μm in A-D.
3.3. Neuronal Cell Populations
The monoclonal antibody RN1 has been shown to identify most neuronal structures in holothurians (Díaz-Balzac et al., 2007, 2016). RNC explants were also labeled by the antibody, particularly the fiber content within the neuropile (Figure 5). Although the antibody is known to overwhelmingly label fibers, some neuronal subpopulations were labeled in our RNC sections; mainly identified by the labeling of cellular bodies surrounding DAPI-stained nuclei (Figure 5B-D’). These cells were prominent in the periphery near the medial, lateral and basal regions of the RNC. RNC explants maintained the number of RN1+ cells in the periphery for the various in vitro time points tested (Figure 5E). RN1 labelling did show an overall decrease at 7- and 11-day timepoints, which suggests that, although the number of RN1+ cells were maintained in the periphery across all timepoints, the labelling of RN1 in the neuropile decreases over time. Whether this is due to a decrease in the STARD protein expression or a reduction in fiber density needs to be determined.
Figure 5. Neuronal fiber and cell labelling in cultured radial nerve cord (RNC) explants.
Immunohistochemistry using the neuronal marker RN1 antibody shows labeling in RNCs: A) In vivo cross section B) 3 days, C) 7 days, and D) 11 days in culture. As observed in vivo the antibody mainly labels the neuropile. Labeled cells are observed (arrows) at higher magnification of the same sections (B’-D’, RN1 green, DAPI-nuclei- blue). E) The percentage of RN1-positive cells per field of view was quantified and shown to remain similar during different days in culture. Bars represent mean (percentage) ± S.D. counts at each stage, n=3. Scale bar = 25 μm in A-D and 5 μm in B’-D’.
3.4. Cell Division and Cell Death
To determine the cellular events that RNC explants undergo in culture, we performed cell division and cell death assays. Using BrdU incorporation assay, we determined that the RNC explant cells undergo minimal cell proliferation under the present culture conditions. After three days in culture, only 1.71% of cells were BrdU-positive, after seven days in culture the number of dividing cells was reduced to 0.83% and, after eleven days down to 0.36% (Figure 6A-C). Cell death ranged from 0 to less than 0.25% of TUNEL-positive cells across sections of 3- and 7-day timepoints (Figure 6E-F). On the 11-day timepoint, there is a significant increase in TUNEL-positive cells when compared to both 3- and 7-day RNC explants (Figure 6G-H). Nonetheless, even at this timepoint, cell apoptosis remained rather low at around 1%.
Figure 6. Cell proliferation and apoptosis in cultured radial nerve cord (RNC) explants.
RNCs explants labeled for BrdU (A-C) at A) 3 days, B) 7 days and C) 11 days in culture. (E-F) RNCs explants labeled using TUNEL assay at E) 3 days, F) 7 days and G) 11 days in culture. Quantification of these results is shown in D) (BrdU) and H) (TUNEL). BrdU and TUNEL labeling (arrows). Both BrdU and TUNEL show a small number of cells labeled within the RNC. Bars represent the mean (percentage) ± SD, n=3, ****P < 0.0001. Scale Bar = 50 μm in A-C and in E-G.
3.5. Dedifferentiation of Radial Glia-like Cells
Radial glia-like cells dedifferentiate in vivo following RNC transection by fragmentation of their basal processes that extend into the RNC neuropile (Mashanov et al., 2013). We assessed the degree of radial glia-like cell dedifferentiation in culture using a semi-quantitative analysis. The RNC explants were cultured for up to 11 days and the radial glia-like cells were labeled with ERG1 antibody. Figure 2 shows the dedifferentiation score algorithm used to quantify this data. At day 0 (in vivo cross sections), the radial glia-like extensions elongated from the apical to the basal end of the RNC granting a numerical value of zero in our analytical method. After 3- and 7-days in culture, the radial glia-like extensions within the RNC explants lost their basal processes by degradation or retraction; however, their cell bodies maintained their peripheral organization in the apical region (Figure 7B-C’, 7E). RNC explants for these two time points were granted values ranging from 0-3 depending on the location and density of the apical extensions. After eleven days in culture RNC explant immunohistochemistry showed that the vast majority of ERG1+ cell bodies had few or no radial glia-like extensions (Figure 7D-E) corresponding to values ranging from 2 to 4.
Figure 7. Dedifferentiation of radial glia-like cells in cultured radial nerve cord (RNC) explants.
(A-D) Immunohistochemistry using ERG1 antibody to label radial glia-like cells in RNC sections: A) In vivo cross section, B) 3 days, C) 7 days, and D) 11 days in culture. As observed in vivo the antibody labels the glial radial extensions in the neuropile. The regions within the dashed boxes are shown at higher magnification to highlight the loss of radial extensions (B’-D’, ERG1 green, DAPI-nuclei- blue) E) The dedifferentiation score for every RNC explant was determined. Bars represent the mean ± SD of n=3, ***P < 0.0005, ****P < 0.0001. Scale bar = 35 μm in A-D, 4 μm in B’-D’.
4. Discussion
The method and experimental validations described here are the first reported to successfully isolate the main holothurian central nervous system components from non-neuronal tissues. The technique grants direct access to the holothurian RNCs without the interfering surrounding tissues. Moreover, the isolated RNC explants can be cultured, providing a unique tool to study echinoderm neural and glial cells in vitro, and the changes they undergo. Here we characterize the cellular events that take place early in culture incubation, providing the basis for future experimental possibilities.
4.1. Enzymatic digestion generates a radial nerve cord section, free of surrounding tissues.
The ease of tissue or organ dissections, for use in biochemical, transcriptomic, or other studies, varies greatly depending on the tissue, organ or animal group. In echinoderms, certain organs are easy to collect because their anatomical localization. As examples, gonads or respiratory trees in holothurians can be obtained by a simple dissection from the rest of the viscera. Acquiring samples of other tissues can be a challenge. Such is the case for echinoderm nervous tissue samples, since in most echinoderm classes the main component of the central nervous system (the RNC) is embedded within the body wall and surrounded by other tissues. Here we showed that, in the sea cucumber H. glaberrima, RNCs are embedded within a collagen matrix that can be dissociated with collagenase treatment. This treatment isolates the RNC from neighboring tissues, freeing the RNCs for culture and other experimental studies.
The dissection method, like most in vitro systems, certainly induces some damage on the tissue. First, we were not able to dissect the entirety of the RNCs. Thus, we made several transections to the RNCs to facilitate the dissection. This, in turn, can induce an inflammatory response and additional damage to the tissue as it is removed from the body wall cavity. In addition, the dissection involves transecting the peripheral nerves, thus cutting the axon of some RNC neurons. Furthermore, the tissue is rocked on a moving platform for 24-hours, while digesting in collagenase. The friction prompted by the ‘rocking’ motion and collagenase activity digesting neighboring tissue can induce additional damage on the RNCs. Nonetheless, the isolated RNC explants appear to be quite sturdy and resistant. RNCs maintained their morphological integrity after the 24-hr collagenase treatment, and their cellular components appeared unaltered. Similarly, the collagenase treatment did not appear to have an effect on the RNC’s connective tissue layer that separates the ectoneural (Ec) from the hyponeural (Hy) regions, as the inner connective tissue was labelled with anti-collagen marker as observed in 3-day cultured explants (Figure 2G). In any case, the protocol could be improved in future experiments by decreasing the sources of extracellular stressors, such as by reducing digestion time or utilizing a different enzyme with a higher efficiency to digest surrounding tissues.
4.2. Access to the echinoderm nervous system
Obtaining and maintaining adult echinoderm cells and tissues in culture has been explored by various investigators. The range of echinoderm culture systems extends from limb or organ explants to the culture of single cells. Thus, there are reports of isolated cell cultures such as cultures of coelomocytes, the echinoderm immune cells, that can be easily obtained from the coelomic fluid (Pinsino and Alijagic, 2019; Smith et al., 2018), or of cells dissociated from regenerating holothurian tissues (Bello et al. 2015). Alternatively, explant cultures have been done from organs, such as gonads, that can be easily detached from the rest of the organism (Mercurio et al., 2014) or from severed appendages that can be maintained for long periods in vitro (Di Benedetto et al. 2015). However, the possibility of culturing nervous system cells either as isolated cells or as explants has not been possible until now. Our protocol provides a unique, novel system where the cells of the echinoderm nervous system can be maintained as an explant in vitro. Moreover, the nervous system, free of other tissue or cell types, is easily accessible for different avenues of experimentation.
4.3. Dedifferentiation of radial glia-like cells, but not proliferation or apoptosis, is the main cell event that takes place in the radial nerve cord explants.
At a macro level, the explants maintained their integrity with small changes in the observed morphology, such as the loss of the wing-shaped RNC to a tubular-like morphology. At the cellular level, some changes induced by the digestion/culture were notable. The radial nerve cords are composed of neurons and glial cell. Most of the cell bodies are organized surrounding the central neuropile, with only a small subset of cell bodies found within the neuropile itself (Mashanov et al., 2006). In the in vitro explants there was no significant effect observed in the total number of cells (nuclei). However, we did observe an apparent redistribution of cells from the periphery to the neuropile suggesting either a migration of cells or an increase in their mobilization. This is further strengthened by an extremely low percentage of proliferation (BrdU-labeling) and apoptosis (TUNEL), the two main processes that could be affecting cell numbers. In fact, the percentage of proliferating or apoptotic cells in the explants resembled those of the uninjured, in vivo, RNCs., where less than 1% of cells undergo either apoptosis or proliferation (Mashanov et al., 2013). It is not clear if cell proliferation or apoptosis took place in a particular cell population (neurons vs glia). Nonetheless, in view that the damage to neurons, due to the loss of their axonal connections, is usually more severe than to glia, we posit that neurons might comprise a larger proportion of the dying cells. However, this remains to be verified by future experiments.
It is indeed surprising that neither cell proliferation nor apoptosis increased significantly in the cultured explants. In vivo, injury to the RNC is followed by an increase in both apoptosis and proliferation to levels of 4-5% of the cells (San Miguel-Ruiz et al., 2009; Mashanov et al., 2013). However, in vitro, no such increase was observed, even up to 11-days in culture. One possible explanation for this lack of response is suggested by experiments using the crinoid Antedon mediterranea arm explants where extensive migration and BrdU incorporation is observed in amoebocytes within the brachial nerve and the coelom of the regenerating arm (Candia Carnevali et al., 1998). Previous studies in this model showed that arm regeneration takes place after coelomocytes, usually surrounding the brachial nerve, migrate through the nerve and proliferate at the tip of the injury site (Candia Carnevali et al., 1993, 1998). This suggests that the regenerative activity of the RNC might be influenced by the presence of migrating cells that are not of nerve origin, such as phagocytes, amoebocytes and granule cells. These non-neural cells have also been documented in holothurian in vivo RNC regeneration (San Miguel-Ruiz et al., 2009) but were not present in the isolated RNCs. Thus, it might be that the presence of non-neural cells is required to activate both apoptotic and proliferation pathways. An alternate explanation might involve the lack of extracellular matrix or local adhesion of the explants. While maintained in vitro, RNC explants do not adhere to the plate in contrast to the nature of this tissue, which is normally embedded within the body wall of the organism. In fact, a study on sea urchin ovaries demonstrated that cell attachment to culture plates coated with poly-L-lysine promoted cell survival (Mercurio et al., 2014).
Our most important finding, nonetheless, is the observed dedifferentiation of the radial glia-like cells in our cultured explants. The dedifferentiation of these cells is one of the main steps that has been observed in the process of echinoderm central nervous system regeneration. In fact, this dedifferentiation characterized by the loss of hallmark basal processes of the radial glia-like cells. is the first observable event that takes place during RNC regeneration (Mashanov et al., 2013). The cultured RNC explants show progressive dedifferentiation up to the 11-day in culture timepoint. In vivo, once the dedifferentiation of radial glia-like cells occurs, these cells migrate and divide extensively to eventually re-differentiate into radial glia-like cells and neurons that form the new RNC segment (Mashanov et al., 2013). A possibility that needs to be studied is if the changes in cell distribution within the RNC explants are aligned to the dedifferentiation and possible migration of the radial glia-like cells. More important, the dedifferentiation process in vitro proceeds in a similar pattern as occurs in vivo and appears to be independent of apoptosis or cell proliferation. This provides the unique opportunity of using our cultured explants to focus on the radial glia-like dedifferentiation without the interference of cell proliferation and apoptotic mechanisms observed in vivo. Thus, following the establishment of this primary RNC explant culture, we aim to use this method as a tool to study neural regenerative processes in vitro and, in particular, the initial dedifferentiation event.
4.4. Comparative studies of radial glial cells
The importance of a radial glia role in developmental and regeneration processes is well known in vertebrates, where certain in vitro systems have been established for their in-depth study. Observations using vertebrate radial glia in vitro systems serve as comparisons to our new holothurian system. Cortical radial glia, or their precursors, have been obtained from fish and from pre- and post-natal mice and cultured. In these in vitro systems, some cells retain or acquire the radial glia morphology and markers and in some cases the cultures serve to study radial glia functions (Culican et al., 1990; Servili et al., 2009, Alvarez et al., 2014). Moreover, these cultures have provided information on the cells differentiation potential by characterizing neurons or other glia that originate from the radial glia in culture. However, it is when compared to Müller cells where strong similarities are observed. Müller cells are considered a type of radial glia found in the vertebrate retina. Similar to the holothurian radial glia-like cells, they have been shown to undergo dedifferentiation to a stem cell/progenitor phenotype (Guidry, 1996, Hamon et al., 2016). In fish, Müller cells acquire an epithelioid morphology, migrate from retinal explants and change their behavior in culture becoming phagocytic (Wagner and Raymond 1991). Similarly, Müller cells from post-natal mammals have been cultured and shown to dedifferentiate in vitro (Hosoki et al., 2015) and even to give rise to retinal neurons (Zhao et al., 2014). Similar changes in Müller cell morphology, proliferation and differentiation potential have been observed in mammalian retinal explants (Ueki and Reh, 2013, Suga et al., 2014, Löffler et al., 2015). Therefore, our RNC explants, showing dedifferentiation of radial glia-like cells provide an interesting system for comparison to vertebrate Müller glia dedifferentiation in the search for those genes and/or proteins that might mediate a common cellular process.
In summary, we developed a novel in vitro system that allows isolated echinoderm RNC explants, without the presence of surrounding tissues, to be maintained in culture for up to 2 weeks. This new in vitro system will lead to innovative studies and important findings of the molecular and cellular processes directing central nervous system regeneration in echinoderms. Furthermore, in view of the close phylogenetic relationship between echinoderms and mammals, it can provide insights into the regulatory mechanisms or key players involved in neurodegeneration and regeneration common to deuterostomes.
Highlights.
Reliable dissection technique for successful isolation of the radial nerve cord
In vitro system where isolated radial nerve cords can be cultured
Cultured RNCs undergo low cell proliferation and cell death in vitro
Progressive dedifferentiation of radial glia-like cells in the cultured explants
Acknowledgements
The authors would like to thank Griselle Valentin for figure preparation and Christian Castro-Ruiz for guidance on developing the dissection protocol. This study was supported by NIH-R21AG057974. EQD was funded by 5T34GM007821-40S1, 5R25GM061151-17, PVFD, RGR and AS were funded by ENDURE NIH-2R25NS080687. We also acknowledge help from the University of Puerto Rico. Part of this work has been presented at the ASBMB Experimental Biology Conference 2019 and the Society for Neuroscience Conference 2018 and 2019.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez Z, Sena E, Mattotti M, Engel E, Alcántara S (2014). An efficient and reproducible method to culture Bergman and cortical radial glia using textured PMMA. J Neuro Meth 232:93–101. [DOI] [PubMed] [Google Scholar]
- Bello SA, Abreu-Irizarry RJ, Garcia-Arraras JE (2015). Primary cell cultures of regenerating holothurian tissues. Methods Mol Biol 1189:283–297. 10.1007/978-1-4939-1164-6_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello SA, Torres-Gutiérrez V, Rodríguez-Flores EJ, Toledo-Román EJ, Rodríguez N, Díaz-Díaz LM, Vázquez-Figueroa LD, Cuesta JM, Grillo-Alvarado V, Amador A, Reyes-Rivera J, García-Arrarás JE (2020). Insights into intestinal regeneration signaling mechanisms. Dev Biol. 458(1):12–31. doi: 10.1016/j.ydbio.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candia Carnevali MD, Lucca E, Bonasoro F (1993). Mechanisms of arm regeneration in the feather star Antedon mediterranea: healing of wound and early stages of development. J Exp Zool 267:299–317 [Google Scholar]
- Candia Carnevali MD, Bonasoro F, Patruno M, Thorndyke MC (1998). Cellular and molecular mechanisms of arm regeneration in crinoid echinoderms: the potential of arm explants. Dev Genes Evol 208: 421–430. [DOI] [PubMed] [Google Scholar]
- Culican SM, Baumrind NL, Yamamoto M, Pearlman AL (1990). Cortical radial glia: identification in tissue culture and evidence for their transformation to astrocytes. J Neurosci 10:684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Benedetto C, Parma L, Barbaglio A, Sugni M, Bonasoro F, Carnevali MD (2014). Echinoderm regeneration: an in vitro approach using the crinoid Antedon mediterranea. Cell Tissue Res. 358(1):189–201. doi: 10.1007/s00441-014-1915-8. [DOI] [PubMed] [Google Scholar]
- Diaz-Balzac CA, Santacana-Laffitte G, San Miguel-Ruiz JE, Tossas K, Valentin-Tirado G, RivesSanchez M, Mesleh A, Torres II, García-Arrarás JE (2007). Identification of nerve plexi in connective tissues of the sea cucumber Holothuria glaberrima by using a novel nerve-specific antibody. Biol Bull 213(1):28–42. 10.2307/25066616 [DOI] [PubMed] [Google Scholar]
- Díaz-Balzac CA, Lázaro-Peña MI, Vázquez-Figueroa LD, Díaz-Balzac RJ, & García-Arrarás JE (2016). Holothurian Nervous System Diversity Revealed by Neuroanatomical Analysis. Plos One, 11(3). doi: 10.1371/journal.pone.0151129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Miranda L, Blanco RE, & García-Arrarás JE (1995). Localization of the heptapeptide GFSKLYFamide in the sea cucumber holothuria glaberrima (echinodermata): A light and electron microscopic study. Journal of Comparative Neurology, 352(4), 626–640. doi: 10.1002/cne.903520410 [DOI] [PubMed] [Google Scholar]
- García-Arrarás JE (1993). Localization of peptides: double labeling immunohistochemistry. Pp. 207–225 in Handbook of Endocrine Research Treatment, De Pablo F, Scanes C, and Weintraub B, eds. Academic Press, San Diego, CA. [Google Scholar]
- García-Arrarás JE, Dolmatov IY (2010). Echinoderms; potential model systems for studies on muscle regeneration. Current Pharmaceutical Design, 16 (8): 942–955. doi: 10.2174/138161210790883426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Arrarás JE, Estrada-Rodgers L, Santiago R, Torres IT, Díaz-Miranda L, & Torres-Avillán I (1998). Cellular mechanisms of intestine regeneration in the sea cucumber, Holothuria glaberrima Selenka (Holothuroidea: Echinodermata). The Journal of Experimental Zoology, 281(4), 288–304. doi: 10.1002/(sici)1097-010x(19980701)281:43.0.co;2-k [DOI] [PubMed] [Google Scholar]
- García-Arrarás JE, Greenberg MJ (2001). Visceral regeneration in holothurians. Microscopy Research and Technique, 55(6), 438–451. doi: 10.1002/jemt.1189 [DOI] [PubMed] [Google Scholar]
- Guidry C (1996). Isolation and characterization of porcine Müller cells. Myofibroblastic dedifferentiation in culture. Inv Ophthalmol Visual Sci 37:740–752. [PubMed] [Google Scholar]
- Hamon A, Roger JE, Yang XJ, Perron M (2016). Müller glial cell-dependent regeneration of the neural retina: An overview across vertebrate model systems. Dev Dyn 245:727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoki A, Oku H, Horie T, Kida T, Sugiyama T, Nakamura K, Ikeda T (2015). Changes in expression of nestin, CD44, vascular epithelial growth factor, and glutamine synthetase by mature Müller cells after dedifferentiation. J Ocul Pharmacol Ther 31:476–481. [DOI] [PubMed] [Google Scholar]
- Hyman LH (1955). The invertebrates: echinodermata. McGraw-Hill, New York [Google Scholar]
- Löffler K, Schäfer P, Völkner M, Holdt T, Karl MO (2015). Age-dependent Múller glia neurogenic competence in the mouse retina. Glia 63:1809–1824. [DOI] [PubMed] [Google Scholar]
- Mashanov VS, Zueva OR, Heinzeller T, Dolmatov IY (2006). Ultrastructure of the circumoral nerve ring and the radial nerve cords in holothurians (Echinodermata). Zoomorphology 125:27–38 [Google Scholar]
- Mashanov VS, Zueva OR, Heineller T, Aschauer B, Naumann W, Grondona JM, Cifuentes M, García-Arrarás JE (2009). The central nervous system of sea cucumbers (Echinodermata: Holothuroidea) shows positive immunostaining for a chordate glial secretion. Frontiers in Zoology. 6:11. doi: 10.1186/1742-9994-6-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashanov VS, Zueva OR, Heinzeller T (2008). Regeneration of the radial nerve cord in a holothurian: a promising new model system for studying post-traumatic recovery in the adult nervous system. Tissue Cell 40(5):351–372. 10.1016/j.tice.2008.03.004 [DOI] [PubMed] [Google Scholar]
- Mashanov VS, Zueva OR, Garcia-Arraras JE (2010). Organization of glial cells in the adult sea cucumber central nervous system. Glia 58(13): 1581–1593. 10.1002/glia.21031 [DOI] [PubMed] [Google Scholar]
- Mashanov VS, Zueva OR, & García-Arrarás JE (2013). Radial glial cells play a key role in echinoderm neural regeneration. BMC Biology, 11(1). doi: 10.1186/1741-7007-11-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashanov VS, Zueva OR, García-Arrarás JE (2017). Inhibition of cell proliferation does not slow down echinoderm neural regeneration. Front Zool. 14:12. doi: 10.1186/s12983-017-0196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercurio S, Di Benedetto C, Sugni M, Candia Carnevali MD (2014). Primary cell cultures from sea urchin ovaries: a new experimental tool. In Vitro Cell Dev Biol Anim. 50(2):139–45. doi: 10.1007/s11626-013-9686-1. [DOI] [PubMed] [Google Scholar]
- Nieves-Ríos C,. Alvarez-Falcón S, Malavez S, Rodriguez-Otero J, García-Arrarás JE (2020). The nervous system component of the mesentery of the sea cucumber Holothuria glaberrima in normal and regenerating animals. Cell Tissue Res. 380(l):67–77. doi: 10.1007/s00441-019-03142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsino A, Alijagic A (2019). Sea urchin Paracentrotus lividus immune cells in culture: formulation of the appropriate harvesting and culture media and maintenance conditions. Biol Open. bio039289. doi: 10.1242/bio.039289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quispe-Parra D, Valentín G, García-Arrarás JE (2020). A Roadmap for Intestinal Regeneration. Int J Dev Biol, doi: 10.1387/ijdb.200227dq. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosado-Olivieri EA, Ramos-Ortiz GA, Hernandez-Pasos J, Diaz-Balzac CA, Vazquez-Rosa E, Valentin-Tirado G, Vega IE, Garcia-Arraras JE (2017.) A START-domain-containing protein is a novel marker of nervous system components of the sea cucumber Holothuria glaberrima. Comp Biochem Physiol B Biochem Mol Biol 214:57–65. 10.1016/j.cbpb.2017.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Miguel-Ruiz JE, Garcia-Arraras JE (2007). Common cellular events occur during wound healing and organ regeneration in the sea cucumber Holothuria glaberrima. BMC Dev Biol 7:115. 10.1186/1471-213X-7-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Miguel-Ruiz JE, Maldonado-Soto AR, & García-Arrarás JE (2009). Regeneration of the radial nerve cord in the sea cucumber Holothuria glaberrima. BMC Developmental Biology, 9(1), 3. doi: 10.1186/1471-213x-9-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servili A, Bufalino MR, Nishikawa R, Sanchez de Melo L, Muñoz-Cueto JA, Lee LEJ (2009). Establishment of long term cultures of neural stem cells from adult sea bream Dicentrarchus labrax. Comp Biochem Physiol Part A 152:245–254. [DOI] [PubMed] [Google Scholar]
- Sharlaimova NS, Pinaev GP, Petukhova OA (2010). Comparative analysis of behavior and proliferative activity in culture of cells of coelomic fluid and of cells of various tissues of the sea star Asterias rubens L. isolated from normal and injured animals. Cell Tissue Biol. 4:280–288. [Google Scholar]
- Sharlaimova NS, Petukhova OA (2012). Characteristics of populations of the coelomic fluid and coelomic epithelium cells from the starfish Asterias rubens L. able attach to and spread on various substrates. Cell Tissue Biol. 6:176–188. [Google Scholar]
- Smith LC, Hawley TS, Henson JH, Majeske AJ, Oren M, Rosental B (2019). Methods for collection, handling, and analysis of sea urchin coelomocytes. Methods Cell Biol. 150:357–389. doi: 10.1016/bs.mcb.2018.11.009. [DOI] [PubMed] [Google Scholar]
- Suga A, Sadamoto K, Fujii M, Mandai M, Takahashi M (2014). Proliferation potentical of Müller glia after retinal damage varies between mouse strains. PLoS ONE 9(4): e94556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki Y, Reh TA (2013). EGF stimulates Müller glial proliferation via a BMP dependent mechanism. Glia 61:778–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EC, Raymond PA (1991). Müller glial cells of the goldfish retina are phagocytic in vitro but not in vivo. Exp Eye Res 53:583–589. [DOI] [PubMed] [Google Scholar]
- Wang T, Chen X, Xu K, Zhang B, Huang D, Yang J (2020). Apoptosis Induction and Detection in a Primary Culture of Sea Cucumber Intestinal Cells. J Vis Exp. 21;(155). doi: 10.3791/60557. [DOI] [PubMed] [Google Scholar]
- Zhao JJ, Ouyang H, Luo J, Patel S, Xue Y, Quach J, Sfeir N, Zhang M, Fu X, Ding S, Chen S, Zhang K (2014). Induction of retinal progenitors and neurons from mammalian Müller glia under defined conditions. J Biol Chem 289:11945–11951. [DOI] [PMC free article] [PubMed] [Google Scholar]