Abstract
The preference for nitrate over chloride through regulation of transporters is a fundamental feature of plant ion homeostasis. We show that Medicago truncatula MtNPF6.5, an ortholog of Arabidopsis thaliana AtNPF6.3/NRT1.1, can mediate nitrate and chloride uptake in Xenopus oocytes but is chloride selective and that its close homologue, MtNPF6.7, can transport nitrate and chloride but is nitrate selective. The MtNPF6.5 mutant showed greatly reduced chloride content relative to wild type, and MtNPF6.5 expression was repressed by high chloride, indicating a primary role for MtNPF6.5 in root chloride uptake. MtNPF6.5 and MtNPF6.7 were repressed and induced by nitrate, respectively, and these responses required the transcription factor MtNLP1. Moreover, loss of MtNLP1 prevented the rapid switch from chloride to nitrate as the main anion in nitrate‐starved plants after nitrate provision, providing insight into the underlying mechanism for nitrate preference. Sequence analysis revealed three sub‐types of AtNPF6.3 orthologs based on their predicted substrate‐binding residues: A (chloride selective), B (nitrate selective), and C (legume specific). The absence of B‐type AtNPF6.3 homologues in early diverged plant lineages suggests that they evolved from a chloride‐selective MtNPF6.5‐like protein.
Keywords: chloride uptake, MtNLP1, nitrate preference, NPF, NRT1.1
Subject Categories: Membranes & Trafficking, Plant Biology
The identification of the chloride selective ion transporter MtNPF6.5 in Medicago truncatula roots illustrates how ion homeostasis between chloride and nitrate is maintained in the root.

Introduction
Ion homeostasis is intrinsic to cellular function and dictates fundamental processes including nutrient uptake and cell turgor. Plants and other eukaryotes have a large array of anion and cation pumps and channels to modulate cytoplasmic ion concentrations, maintain cellular pH, and sequester ions in vacuoles to maintain cell turgor (Feng et al, 2020). In plants, one such protein family is the NRT1/PTRs (NPFs). The NPFs are part of a large family of proton‐coupled transporters having 92 members in medicago (Medicago truncatula), 70 in rice (Oryza sativa), and 52 in arabidopsis (Arabidopsis thaliana; Léran et al, 2014; Longo et al, 2018). Numerous NPF substrates have been identified, including NO3 −, basic amino acids, di‐, and tripeptides, Cl−, glucosinolates, dicarboxylic acids, and several plant hormones (Rentsch et al, 1995; Almagro et al, 2008; Krouk et al, 2010; Nour‐Eldin et al, 2012; Andersen et al, 2013; Chiba et al, 2015; Wen et al, 2017, reviewed in Corratgé‐Faillie & Lacombe, 2017). The most well‐studied NPF, arabidopsis AtNPF6.3/NRT1.1, was the first NO3 − transporter identified (Tsay et al, 1993). AtNPF6.3 transcript levels are induced by NO3 −, and this response is directly dependent on the transcription factor NIN‐LIKE PROTEIN7 (NLP7), a central regulator of N homeostasis that controls expression of many NO3 − responsive genes (Tsay et al, 1993; Marchive et al, 2013; Zhao et al, 2014).
AtNPF6.3 has mostly been studied for its role in NO3 − uptake. NO3 − uptake by AtNPF6.3 in plants is proton‐coupled, with a symport ratio of 2H+/NO3 − (Meharg & Blatt, 1995; Miller & Smith, 1996), and its transport function requires an ExxER/K motif and a proton‐coupling residue, which is conserved across much of the NPF family (Jørgensen et al, 2015). Considerable progress has been made in understanding the co‐transport mechanism of AtNPF6.3. Crystal structure analysis of AtNPF6.3 revealed that H356, as well as a salt bridge predicted between K164 and E476 are required for NO3 − uptake (Parker & Newstead, 2014; Sun et al, 2014). It was found that proton movement could be separated from substrate recognition, which was suggested to be a key feature of NPFs, providing them with increased freedom to evolve new substrate specificities (Parker et al, 2017). One example of this flexibility in substrates is AtNPF6.3 and its maize homologue ZmNPF6.6, which can transport Cl− in the absence of NO3 −, while another close homologue, ZmNPF6.4, can transport NO3 − but shows strong preference for Cl− (Wen et al, 2017). Outside of their transport activities in Xenopus oocytes the importance of the ZmNPFs in plant Cl− transport remains unexplored. Notably, ZmNFP6.6 contains a His at the position corresponding to AtNPF6.3:H356, while ZmNPF6.4 contains a Thr at this position, and the corresponding Thr to His substitution in ZmNFP6.4 resulted in a switch from Cl− to NO3 − selectivity.
AtNPF6.3 is characterized by its ability to transport NO3 − at both high (low affinity) and low (high affinity) external NO3 − concentrations (Liu, Huang & Tsay, 1999). A conserved Thr (T101) in AtNPF6.3 was shown to be important for NO3 − transport in the high‐affinity range, and phosphorylation of this residue has been suggested to act as a mechanism to switch between high‐ and low‐affinity transport modes (Liu & Tsay, 2003; Ho et al, 2009). The model suggests that when NO3 − availability is low, phosphorylation of T101 by CIPK23 switches AtNPF6.3 from a low‐ to high‐affinity NO3 − transporter, which is proposed to occur through decoupling of AtNPF6.3 dimers (Sun & Zheng, 2015). A role for AtNPF6.3 in NO3 − signaling has been proposed as mutation of T101 results in altered responses to NO3 −, including changes in the expression of other NO3 − transporters and a long noncoding RNA involved in the NO3 − response (Muños et al, 2004; Walch‐Liu & Forde, 2008; Ho et al, 2009; Liu et al, 2019). Furthermore, AtNPF6.3 has been implicated in NO3 −‐dependent changes in root system architecture, root hair development, and seed germination (Liu et al, 1999; Liu & Tsay, 2003; Alboresi et al, 2005; Remans et al, 2006; Ho et al, 2009; Krouk et al, 2010; Vatter et al, 2015; Bouguyon et al, 2016; Canales et al; 2017; Chai et al, 2020; Maghiaoui et al, 2020).
The contribution of AtNPF6.3 to plant NO3 − uptake varies across NO3 − concentrations and conditions. When external NO3 − is low, its contribution appears to be relatively minor, as discussed by Glass and Kotur (2013). Under low NO3 − availability, NO3 −uptake is mainly mediated by members of the NRT2 family; 15NO3 − ‐uptake in triple or quadruple mutants lacking specific NRT2s was reduced to 5% and 3% of wild‐type levels, and plant growth was severely compromised (Kiba et al, 2012; Lezhneva et al, 2014). However, when plants are grown with NH4 + uptake of 15NO3 − is greatly reduced in the atnpf6.3 mutant (Liu, et al, 1999; Ho et al, 2009; Wang et al, 2009; Zhao et al, 2014). Conversely, if plants are grown with NO3 −‐only or NO3 − as the main N source, AtNPF6.3’s contribution to high‐affinity uptake is abolished (Touraine & Glass, 1997; Muños et al, 2004; Remans et al, 2006), and in some cases 15NO3 − uptake in the mutant was actually increased (Muños et al, 2004; Ye et al, 2019). A recent study directly addressed this issue using triple mutants lacking AtNPF6.3 and the two principal high‐affinity NRT2‐type NO3 − transporters NRT2.1 and NRT2.2. With a mixed N pre‐treatment, the authors estimated that the loss of AtNPF6.3 reduced high‐affinity 15NO3 −‐uptake by about 12% (Ye et al, 2019). Similarly, low‐affinity 15NO3 − uptake in the atnpf6.3 single mutant was not severely compromised, but using a triple mutant, the contribution of AtNPF6.3 was estimated to be 25%. This suggests that NRT2 transporters can compensate for loss of AtNPF6.3 for NO3 − uptake under both high and low NO3 − supply with mixed N. Further distinguishing it from other NO3 − transporters, AtNPF6.3 was also shown to have NO3 − efflux activity, although the biological importance of this is not known (Léran et al, 2013).
In addition to its role in NO3 − transport, AtNPF6.3 appears to be involved in other cellular systems. In particular, loss of AtNPF6.3 greatly affects root proton secretion in the presence of NO3 −, resulting in a reduced ability to elevate rhizosphere pH (Fang et al, 2016). Arabidopsis npf6.3 also displays decreased Cd2+ tolerance, and enhanced resistance to Pb2+ toxicity (Hachiya et al, 2011; Zhu et al, 2019). Notably, Pb2+ resistance in arabidopsis npf6.3 was dependent on the presence of both NO3 − and NH4 + in the medium but was not observed for other high and low‐affinity NO3 − transport mutants (Zhu et al, 2019). AtNPF6.3 was also shown to be involved in NH4 + toxicity in a NO3 − independent manner (Hachiya, & Noguchi, 2011; Jian et al, 2019). Related to this finding, an unexpected role for AtNPF6.3 in Cl− uptake has recently become apparent. The initial breakthrough came from Wen et al (2017), revealing the capacity for AtNPF6.3 to mediate Cl− uptake Xenopus oocytes. Then, studies on the mechanism of ammonia toxicity by Liu et al (2020) revealed a central role for AtNPF6.3 in the overaccumulation of Cl− in ammonia fed plants. This overaccumulation was not observed in NO3 − fed plants. Together these studies suggest that in the absence of NO3 −, AtNPF6.3 plays a central role in Cl− uptake from the environment.
NO3 − availability is often growth limiting, and its levels in soil rise and fall constantly with changing conditions. In contrast, Cl− is usually abundant in soils, and deficiencies are uncommon. Both, however, play important roles as osmotica, and in this role, they are interchangeable, but only NO3 − is assimilated and is therefore usually more readily taken up by roots, with plants exhibiting a strong preference for uptake of NO3 − over Cl− (Glass & Siddiqi, 1985), but the mechanisms underlying this are poorly understood. Here we study the evolution, biochemical function, and biological roles of three legume orthologs of AtNPF6.3. We show that both MtNPF6.5 and MtNPF6.7 can mediate uptake of NO3 − and Cl− but with different selectivity and that MtNPF6.5 largely determines plant Cl− levels in Medicago truncatula. We show that MtNPF6.5 is suppressed by NO3 −, while MtNPF6.7 is induced and that this regulation requires the transcription factor MtNLP1, providing insight into how plants facilitate their preference for NO3 − over Cl−.
Results
Legume AtNPF6.3 paralogues differ in their substrate binding residues
The closest homologues of AtNPF6.3 were identified in medicago using a BLASTP search and were designated MtNPF6.5/NRT1.1A (Medtr4g101380), MtNPF6.7/NRT1.1B (Medtr5g012290), and MtNPF6.6/NRT1.1C (Medtr5g012270) which were highlighted by Murray et al (2017). MtNPF6.7 and MtNPF6.6 are tandemly arranged on chromosome 5, presumably due to a gene duplication. To establish their relationship with NPF6 family members studied in other plant species we constructed a phylogenetic tree using protein sequence alignments of all NPFs from rice, arabidopsis and medicago. Two previously characterized Zea mays homologues, ZmNPF6.4/6.6, were included for reference. For practical reasons, just NPF6 clade members are shown in Fig 1A. The sequences used for the analysis were previously identified by Longo et al (2018) and their identifiers are provided in Dataset EV1 (tab A). Three NPF6 subclades are evident. Two of these subclades, represented by AtNPF6.2 and AtNPF6.4, have just one member in each the species examined. However, in the third sub‐clade, represented by AtNPF6.3, medicago and rice are each represented by three members and A. thaliana has just one, suggesting either gene loss in arabidopsis or gene gain in medicago and rice. We will hereafter refer to this group, which contains the three M. truncatula proteins of interest, as the AtNPF6.3 sub‐clade. To more closely examine the evolution of the AtNPF6.3 sub‐clade, a second phylogenetic tree was constructed using closest homologues of AtNPF6.3 from several rosid species. The following legumes were included: medicago, pea (Pisum sativum), red clover (Trifolium pratense), common bean (Phaseolus vulgaris) and soybean (Glycine max). This tree reveals that members of the legume Hologalegena clade (medicago, pea, L. japonicus, and red clover) have three distinct orthologs of AtNPF6.3, while other legumes have just two (Fig 1B; gene IDs provided in Dataset EV1 (tab B). This analysis indicates that the MtNPF6.7‐MtNFP6.6 duplication is legume‐specific, and is restricted to hologalegoids which are typified by temperate herbaceous species. The other rosid species examined, representing the orders Fagales, Fabales, Rosales, Malpighiales and Cucurbitales, have from one to three AtNPF6.3 sub‐clade members, while the monocot rice (Oryza sativa) has three.
Figure 1. Phylogenetic analysis of M. truncatula NPF6 proteins.

- NPF6 clade from M. truncatula, O. sativa, and A. thaliana, including two Z. mays NPFs. All NPFs from M. truncatula, O. sativa, and A. thaliana were used for tree construction, and only NPF6s are presented. Sequences were aligned using ClustalOmega, and the phylogenetic tree was constructed using PhyML.
- The AtNPF6.3 subclade in rosids can be grouped into three haplotypes with residues corresponding to AtNPF6.3 T101, H356, T360, and F511, designated as Types A, B, and C. All legumes have Types A and B, while a subset also have Type C, which is found only in legumes of the Hologalegena clade. All NPF6s from M. truncatula, and A. thaliana were used for tree construction and only the AtNPF6.3 subclade is presented. Rosid orders represented: Cucurbitales: Cucumis sativa; Rosales: Prunus persica, Trema orientalis, Parasponia andersonii; Fagales: Morella rubra; Malpighiales: Populus alba; Fabales: Trifolium pratense, Lotus japonicus, Phaseolus vulgaris, and Glycine max. Sequences were aligned using MUSCLE, the phylogenetic tree was constructed using PhyML. Branches with maximum likelihood support > 0.80 are indicated by filled circles.
To gain insight into the potential functions of the three medicago members of the AtNPF6.3 sub‐clade, we used their protein alignments and information from the crystal structure analysis of AtNPF6.3 to predict functionally important residues including those involved in substrate binding (Dataset EV1, tab A). We refer to these non‐contiguous amino acid groupings as “protein haplotypes”, as previously defined (Spooner et al, 2018). The haplotypes of AtNPF6.3 homologues were highly conserved across their ExER motifs and interface residues, but could be distinguished from each other or from other NPFs, by three predicted substrate binding residues (corresponding to residues H356, T360 and F511 of AtNPF6.3) and the phosphoregulatory residue corresponding to AtNPF6.3:T101. Based on these differences AtNPF6.3 orthologs can be grouped into three haplotypes, designated as types A (TYTF), B (THTF), and C (NQMF). By this classification MtNPF6.5, ZmNPF6.4 and OsNPF6.3/6.4 (OsNRT1.1A/C) are type A, while MtNPF6.7, AtNPF6.3 and ZmNPF6.6 are type B haplotypes, and type C variants (e.g., MtNPF6.6) are specific to the legume Hologalegena clade, indicated by their presence in medicago, pea, and red clover, and their absence in soybean and common bean (Fig 1B; Dataset EV1, tab B). A survey of NPF sequences of basal plant lineages revealed that a type‐A NPF is present in the basal angiosperm Amborella trichopoda (AtriNPF6.3) and the liverwort Marchantia polymorpha (MpNPF6.2), but no type‐B NPFs were present in either species (Dataset EV1, tab C). Furthermore, while the more anciently diverged lycophyte Selaginella moellendorffii and the moss Physcomitrella patens lacked type‐A and type‐B proteins, they both had a type A‐like (TYLF) protein, suggesting the founding member of the AtNPF6.3 sub‐clade was type‐A (Dataset EV1, tab C). Consistent with this idea, type A proteins are present in all the angiosperm lineages examined while type B proteins are found in monocots, but are absent in most dicot lineages, the exceptions being legumes and brassicas (Fig 1B; Dataset EV1, tab D). Interestingly, like arabidopsis, Capsella rubella and Brassica rapa also lack a Type‐A protein, which makes the Brassicaceae unique (Dataset EV1, tab D). In conclusion, legumes have either two or three types of AtNPF6.3 homologues, one “ancestral” A‐type, a B‐type resembling AtNPF6.3, and a third C‐type that is present only in a subset of legumes. Moreover, the three legume isoforms differ across substrate binding and regulatory residues, indicating further diversification of this sub‐clade of NPFs and presenting a special opportunity to study their function.
MtNPF6.5 and MtNPF6.7 can transport nitrate
The preceding analyses of predicted substrate binding residues suggest that MtNPF6.7 may be functionally orthologous to AtNPF6.3, while MtNPF6.5 may possess similar qualities as ZmNPF6.4. To assess their ability to uptake NO3 −, we expressed these Medicago proteins in Xenopus oocytes. Protein sequence alignment indicates that residues corresponding to AtNPF6.3: T101 are also found at position 101 in MtNPF6.5/6.7. To determine the importance of T101 in MtNPF6.5/6.7 we also tested alanine and aspartate (phosphomimetic) substituted variants. AtNPF6.3 was included as a positive control. We found that AtNPF6.3, MtNPF6.5, and the phosphomimic MtNPF6.7‐T101D were able to transport NO3 − in all conditions tested using a 14 h incubation period and that NO3 − uptake was always higher at pH 5.5 than at pH 7.5 (Fig 2A and B). For MtNPF6.7, the T101A mutation reduced NO3 − transport in the 10 mM range, suggesting the importance of T101 phosphorylation. In contrast, a small intake of NO3 − by MtNPF6.5 in the high‐affinity range was detected at pH 7.5 but not at pH 5.5, which was absent in the two mutant versions. When supplied with 10 mM NO3 −, MtNPF6.5 transported NO3 − only at pH 5.5, which again was eliminated by the T101A and T101D substitutions. We also compared the ratio of each treatment to the water injected oocytes since there were differences in 15N accumulation in the water injected controls at the two pHs, especially for the high‐affinity range. The relative results show exactly the same pattern (Appendix Fig S1A).
Figure 2. Nitrate transport activities of M. truncatula orthologs of AtNPF6.3 and mutant variants in Xenopus laevis oocytes.

-
A, BcRNA or water as a control was injected into oocytes and uptake was assessed two days after injection for 14 h in N free MBS.
Data information: Biological replicates: n = 5 with 2 oocytes in each sample. Asterisks denote significant difference: **P < 0.01, *P < 0.05 (Student’s t‐test).
We also compared 10 mM NO3 − uptake by AtNPF6.3 and MtNPF6.7 as well as the T101 phosphomimetic variants but with 8 h exposure. Substitution of alanine and aspartate at T101 both reduced the NO3 − accumulation in oocytes compared with the wild types for both genes. However, there was no significant difference between the variants for AtNPF6.3 and MtNPF6.7 (Appendix Fig S1B).
We then tested NO3 −‐uptake by AtNPF6.3 and MtNPF6.7 and its variants using a range of NO3 − concentrations in both the high‐ and low‐affinity ranges at pH 5.5 using a 1.5 h incubation period. In this experiment all three versions of MtNPF6.7 were able to transport nitrate at concentrations ≥ 150 μM (Fig EV1). Figure 3 shows the net NO3 − accumulation in oocytes (with subtraction of the water injected controls). At the low‐affinity range (Fig 3B), the data matched well with linear regression curves with the R 2 values all above 0.95 except for MtNPF6.7‐T101D (R 2 = 0.86) (Table EV1). For the high‐affinity range, we compared both Michaelis–Menten and linear regression fits to the curves (Fig 3A). In general, the R 2 values for linear regression fits were all higher than with Michaelis–Menten curves (Table EV1). In the low‐affinity range the T101A/D variants showed equally reduced NO3 − uptake for both AtNPF6.3 and MtNPF6.7. But these mutations had little effect in the high‐affinity range, contrasting with the results observed using longer term exposures.
Figure EV1. 15NO3 − uptake in Xenopus oocytes at high (left) and low (right) affinity range.

Values are mean ± SE (biological replicates: n = 3) with 3–4 oocytes in each sample. Asterisks denote significant difference: **P < 0.01, *P < 0.05 (Student’s t‐test).
Figure 3. Kinetic study of NO3 − uptake in AtNPF6.3 and MtNPF6.7 expressing oocytes.

-
A, BThe oocytes were exposed to different concentrations of NO3 − in N free MBS for 1.5 h at pH5.5. Net NO3 − accumulation was fitted with both Michaelis–Menten and Linear Regression in A for high‐affinity range and Linear Regression in B for low affinity. Values are the mean ± SE (biological replicates: n = 3) with 3–4 oocytes in each sample.
In summary, MtNPF6.5, MtNPF6.7, but not MtNPF6.6 (for 14 h exposure), showed NO3 − uptake activity. A T101A substitution reduced the high‐ and low‐affinity NO3 −‐uptake activities in MtNPF6.5 or MtNPF6.7 when measured after 14 h exposure to NO3 −, but no reduction of high‐affinity NO3 −‐uptake was seen for MtNPF6.7 (T101A) using a 1.5 h exposure. For MtNPF6.5, T101D substitutions abolished high and low‐affinity NO3 − uptake activity, but the same substitution had no effect on the NO3 −‐uptake activity of MtNPF6.7 using a 14h exposure but reduced its activity in the 1.5 h assay in the low‐affinity range.
MtNP6.5/6.6/6.7 are expressed in roots
To evaluate the expression of these NPFs in M. truncatula roots, their promoters were cloned upstream of the β‐glucoronidase (GUS) gene and these constructs were transformed into seedlings by Agrobacterium rhizogenes‐mediated hairy‐root transformation. For all three genes the reporters were expressed in the root cortex and root hairs and had strongest expression in the apices of the primary and lateral roots and emerging lateral root primordia (Fig 4A). This pattern is similar to what was described for AtNPF6.3 expression in A. thaliana roots (Huang et al, 1996; Remans et al, 2006). Since AtNPF6.3 is known to be NO3 −‐induced, we then tested the short‐term (30 min) transcriptional response of the MtNPFs to NO3 −. Expression of MtNPF6.7 increased by more than > 8‐fold in the 10 mM NO3 − sample, MtNPF6.5 responded to 2mM KNO3 with a 4‐fold increase, while MtNPF6.6 did not respond (Fig 4B).
Figure 4. Expression of MtNPFs and localization of their encoded proteins in Medicago truncatula roots.

- Histochemical stained roots from composite plants transformed with proMtNPF6.5:GUS (left column), proMtNPF6.7:GUS (middle column), proMtNPF6.6:GUS (right column).
- Expression of MtNPFs in roots after transfer to media containing different levels of NO3 − for 30 min. C = KCl control, N = KNO3 (Biological replicates: n = 3 (3 pooled root organs in each replicate), asterisk denotes significance P < 0.05, Student’s t‐test). The means of all samples were compared with that of their concentration‐matched KCl controls. Expression is shown relative to Ubiquitin.
- Scanning confocal image of MtNPF6.5‐GFP (top view) and MtNPF6.7‐GFP (side view) expressed in root epidermal cells near the tips of M. truncatula transgenic hairy roots with FM‐464 used as a PM marker. The gene fusions were expressed from the Lotus japonicus Ubiquitin promoter. Bars represents 10 μm.
MtNPF6.5 and MtNPF6.7 are plasma membrane proteins
To investigate the subcellular localization of MtNPF6.5 and MtNPF6.7 we generated constructs to constitutively express C‐terminal GFP fusions. MtNPF6.6 was not investigated due to its apparent toxicity in bacteria. The constructs were then transformed into M. truncatula using hairy root transformation. Both MtNPF6.5‐GFP and MtNPF6.7‐GFP showed typical plasma membrane localization in root epidermal cells (Fig 4C).
Chloride‐dependent currents in MtNPF6.5 injected Xenopus oocytes
Two maize NPFs belonging to the AtNPF6.3 sub‐clade, ZmNPF6.4 and ZmNPF6.6, were shown to transport both NO3 − and Cl− (Wen et al, 2017). To test the Cl− transport ability of the MtNPFs we studied their electrophysiological characteristics in Xenopus oocytes exposed to Cl−. The steady‐state currents of the transporter activity were measured as a function of membrane voltage and Cl− concentration in oocytes that had been injected with water or cRNAs of the three MtNPFs. AtNPF6.3 was also studied for comparison. No Cl−‐elicited currents for water, AtNPF6.3, MtNPF6.7 and MtNPF6.6 injected oocytes could be measured when exposed to 40 mM of Cl− at pH 5.8 (see Fig 5A, Appendix Fig S2) for results without subtraction of the water injected controls). In contrast, voltage dependent currents were recorded for MtNPF6.5 expressing oocytes (Fig 5A, Appendix Fig S2A). However, there was no difference in the I‐V response between the water injected controls and any of the cRNA injected oocytes exposed to 40 mM Cl− at pH 7.4 (Fig 5B, Appendix Fig S2B). This suggested that MtNPF6.5 can transport Cl− and the mechanism is facilitated by a proton gradient across the plasma membrane. We further exposed the water injected and the MtNPF6.5 expressing oocytes to Cl− ranging from 1 to 120 mM at pH 5.8. The water injected oocytes were exposed to different concentrations of Cl− and, based on the I‐V response, no obvious Cl−‐elicited currents were recorded (Fig 5C). The currents elicited in the MtNPF6.5 injected oocytes (corrected for the currents in water injected controls at the same Cl− concentrations) are shown in Fig 5D (see Appendix Fig S2C for the results without subtracting the water‐injected controls). Consistent with Fig 5A, Cl− (> 20 mM) related I‐V response were recorded and the current elicited increased with increasing Cl− concentrations. These I‐V difference curves could be fitted to a Michaelis–Menten function and the fit for −140 mV is shown in Appendix Fig S2D. The voltage dependence of the kinetic parameters for −20 mV to −140 mV are shown in Appendix Fig S2E and F. Both Km and imax were voltage independent. The Km values for Cl− between −60 to −140 mV ranged from about 90 mM to above 150 mM.
Figure 5. Chloride‐elicited currents in MtNPF6.5 injected Xenopus oocytes.

-
A, BI–V responses in oocytes (subtracted of the water injected controls) exposed to 40 mM Cl at pH5.8 (A) and pH7.4 (B). The currents were measured while the oocyte plasma membrane was clamped at −60 mv to between 20 and −140 mv for 120 ms with −20 mv increments for each Cl− concentration. The Cl− ‐elicited current values in D were obtained by subtracting the currents in water‐injected oocytes from the MtNPF6.5 injected oocytes at each Cl− concentration.
-
C, DI–V responses in the (C) water and (D) MtNPF6.5 (subtracted of the water injected controls) injected oocytes exposed to different concentrations of Cl− at pH5.8. The currents were measured while the oocyte plasma membrane was clamped at −60 mv to between 20 and −140 mv for 120 ms with −20 mv increments for each Cl− concentration. The Cl− ‐elicited current values in D were obtained by subtracting the currents in water‐injected oocytes from the MtNPF6.5 injected oocytes at each Cl− concentration.
Data information: Value = mean ± SEM, biological replicates: n = 4 for A and B, n = 6 for C and D.
MtNPFs can transport chloride
We found that upon addition of Cl−, NO3 −‐uptake in MtNPF6.5‐expressing oocytes was strongly reduced, indicating that Cl− competes for NO3 −‐uptake (Fig 6A). Cl−‐addition significantly reduced NO3 −‐uptake in AtNPF6.3 and MtNPF6.5‐injected oocytes but not in MtNPF6.7‐injected oocytes. Interestingly, in this experiment MtNPF6.6 showed a weak NO3 − uptake activity when 2 h exposure was used, which was abolished in the presence of Cl−. However, the difference for MtNPF6.6 between the two conditions was likely due to the difference between the water‐injected controls at the two conditions. This difference illustrates the variation in the endogenous transport properties of different batches of oocytes. There was no difference between the two conditions for MtNPF6.6 comparing the ratio of cRNA injected oocytes with the water injected controls (Appendix Fig S3A).
Figure 6. MtNPF6.5/6.6/6.7 can transport Cl− .

- 15NO3 − ‐uptake (δ15N‰) in X. laevis oocytes in the presence of equal concentrations of Cl− or gluconate at ~pH 5.5. The oocytes were exposed to 10 mM Na15NO3 for 2 h. Biological replicates: n = 4 (2 oocytes in each sample).
- Oocytes were exposed to 1.17 mM 36Cl− for 2 h in Cl free condition. Biological replicates: n = 6 (4 oocytes in each sample).
- Oocytes were exposed to 1.17 mM 36Cl− for 2 h with 1 and 10 mM NO3 −. Biological replicates: n = 6 (4 oocytes in each sample).
- The same data as in C are shown without AtNPF6.3 to allow for better comparison between MtNPFs. Biological replicates: n = 6 (4 oocytes in each sample).
Data information: Asterisks denote significant difference: **P < 0.01, *P < 0.05 (Student’s t‐test). In the boxplots, horizontal black bars represent the median, horizontal red bars in A and B and black cross in C and D represent the mean. Boxes represent the middle 50% of the distribution, and whiskers represent the entire spread of the data. Means were compared using Student’s t‐test, α = 0.05.
Since MtNPF6.6 shows a very weak NO3 − uptake without Cl− in the uptake solution, we then set up a more detailed experiment for MtNPF6.6 for uptake in 200 µM and 10 mM NO3 − in solutions with and without Cl− at pH5.5 with a 6 h exposure. With the presence of Cl−, MtNPF6.6 showed NO3 − import at both high and low affinity, though the oocytes accumulated much less NO3 − compared with AtNPF6.3 (Appendix Fig S3C). However, we did not see NO3 − uptake by MtNPF6.6 in the Cl−‐free environment. This indicated that although MtNPF6.6 showed NO3 − influx under certain conditions, the transport ability is weak and highly dependent on the experimental conditions and quality of the oocytes. We also found that expression of MtNPF6.6 was lethal in E. coli, so it is possible that MtNPF6.6 is also toxic for oocytes. The variation we observed in its activity could therefore result from variation in its expression and protein turnover, particularly since MtNPF6.6 shows only very weak transport ability.
36Cl− (1.17 mM) was used to test if the MtNPFs can transport Cl−. Figure 6B showed that at pH5.5, all three MtNPF transporters were able to accumulate more 36Cl− in the oocytes compared with water injected oocytes, but they had much lower 36Cl− influx when compared with the AtNPF6.3 expressing oocytes. At pH7.5, none of the three medicago transporters were able to influx 36Cl− which was distinct from AtNPF6.3 (Fig 6B). We also tested the 36Cl− uptake in MBS solution. High Cl in MBS strongly suppressed the 36Cl− accumulation in oocytes. Only AtNPF6.3 injected oocytes showed significant increase compared with the water injected controls in these conditions (Appendix Fig S3B).
We then did a 36Cl− influx experiment in competition with NO3 − at pH5.5. In this experiment 1 mM NaCl was included into the uptake solution to minimize the impact of endogenous X. laevis oocyte Cl− channels on the measured Cl− uptake (Wen et al, 2017). As shown in Fig 6C and D (with and without AtNPF6.3 plotted). AtNPF6.3, MtNPF6.5 and MtNPF6.7 expressing oocytes all showed significantly increased 36Cl− accumulation when compared to the water injected oocytes with and without NO3 − added. Adding 1 mM of NO3 − decreased the 36Cl− accumulation in AtNPF6.3 expressing oocytes by 62.8% when compared with the NO3 − free condition and adding of 10 mM NO3 − further decreased the 36Cl− accumulation by 92.8%. MtNPF6.7 expressing oocytes showed no reduction of 36Cl− accumulation at 1 mM NO3 − but 10 mM NO3 − significantly decreased the 36Cl− accumulation by 63.1% compared with NO3 − free solution. For MtNPF6.5, 1 mM and 10 mM NO3 − decreased the 36Cl− accumulation by 34 and 29.3% respectively. In this assay, there was no difference between MtNPF6.6 expressing oocytes and the controls, showing no evidence of Cl− transport. These results demonstrate that MtNPFs can mediate cellular uptake of Cl−, and indicate that MtNPF6.5 is Cl‐selective.
We then used the Medicago Gene Expression Atlas (MtGEAv3) to investigate the expression of the NPFs under short‐term salt‐stress reported by Li et al (2009), which showed that upon addition of 180 mM NaCl, the expression levels of MtNPF6.5 and MtNPF6.7 progressively decreased over time (no data was available for MtNPF6.6). We then carried out a similar experiment, which showed strong suppression of MtNPF6.5 expression in M. truncatula roots within 8 h after transfer to liquid media containing 50 or 100 mM NaCl (Fig 7A). In comparison, the expression of MtNPF6.7 and MtNPF6.6, which is typically much lower than that of MtNPF6.5 in the absence of NO3 −, remained low in response to the NaCl treatments (Fig 7B). We then investigated whether KCl affects MtNPF expression, and found that MtNPF6.5 was progressively suppressed by increasing levels of KCl (Fig 7C), while expression of MtNPF6.7 and MtNPF6.6 was not changed (Fig 7D).
Figure 7. The effects of Cl− on the NO3 − transport activities of NPFs and NPF gene expression.

-
A, BRelative expression of NPFs in M. truncatula roots grown in FP and exposed to different NaCl treatments.
-
CRelative expression of NPFs in M. truncatula roots grown in FP and exposed to different concentration of KCl for 48 h.
-
DRelative expression of NPFs in M. truncatula roots grown in FP and exposed to 100 mM KCl treatments for different times.
Data information: *P < 0.05, Student’s t‐test; biological replicates: n = 3 (3 pooled root organs in each replicate) ± SEM.
Overall, our analyses of transport activities suggest that MtNPF6.7 and AtNPF6.3, whose substrate‐binding haplotypes match those of OsNPF6.5 (OsNRT1.1B), and ZmNPF6.6, are able to selectively transport NO3 −, a characteristic associated with conserved residues corresponding to AtNPF6.3:H356. Our results indicate that all three MtNPFs can also mediate Cl‐uptake into Xenopus oocytes, but that only MtNPF6.5 can produce Cl‐elicited currents and shows Cl‐selectivity. We found that MtNPF6.5 shares its haplotype with ZmNPF6.4 and OsNPF6.3/6.4. Like MtNPF6.5, ZmNPF6.4 can transport both Cl− and NO3 −, with selectivity for Cl−, the latter feature which in ZmNPF6.4 is determined by a Tyr at the position equivalent to AtNPF6.3:H356 (Wen et al, 2017).
Isolation of NPF6.3 mutants from medicago
To further investigate the role of the MtNPFs we isolated homozygous mutants from a M. truncatula Tnt1 transposon insertion mutant population (Cheng et al, 2011, 2017). Three alleles each were isolated for MtNPF6.5, and MtNPF6.7, and two for MtNPF6.6. All of these mutants had insertions in exons that result in premature stop codons (Fig EV2). The gene structure analysis required for the mutant isolation showed medicago MtNPF6.5/6.6 genes have five exons like AtNPF6.3, while the MtNPF6.7 gene has just four, which suggests this exon fusion (as well as the noted changes in the substrate binding residues of MtNPF6.6) occurred after the tandem gene duplication event in the common ancestor of the Hologalegoids.
Figure EV2. Medicago truncatula Tnt1 insertion mutants for MtNPF6.5, MtNPF6.7, and MtNPF6.6 .

Gene structure of M. truncatula AtNPF6.5/6.6/6.7. Arabidopsis NPF6.3 is shown for reference. The Tnt1‐transposon insertion positions are indicated by red arrows.
MtNPF6.5 is required for Cl− uptake
Our data in oocytes suggest that all three medicago NPFs can transport Cl−, but only MtNPF6.5 can produce a current in response to Cl. To determine whether MtNPFs are important contributors to net Cl−‐uptake we tested Cl− levels in MtNPF mutant seedlings in FP (~1 mM Cl−) and high NaCl media. We first determined the tolerance levels of M. truncatula for NaCl in our system; NaCl concentrations of 50 mM and 100 mM allowed root growth, while 150 mM strongly stunted it (Fig EV3A). When grown in FP medium (which does not contain NO3 −), the Cl− concentration of the seedlings, as determined by ion chromatography, was reduced by 70% in mtnpf6.5‐3 but was unchanged in mtnpf6.7‐1 and mtnpf6.6‐2 (Fig 8A). This phenotype was also present in a second MtNPF6.5 allele (mtnpf6.5‐1; Fig EV3B). Under saline (50 mM NaCl) and highly saline (100 mM NaCl) conditions, the Cl− content of the mtnpf6.5‐3 roots and shoots was reduced by 48‐55% and 22–26%, respectively, while mtnpf6.7‐1 and mtnpf6.6‐2 resembled wild type (Figs 8A and EV3C). Cl− accumulated to similar levels in wild type seedlings across the range of NaCl concentrations tested. We then grew the mutants on media containing either KNO3 or NH4NO3. In these conditions, root Cl− levels in wild type roots decreased to 22–25% of those grown in NO3 − ‐free media, indicating a strong preference for NO3 − over Cl− in M. truncatula, as has been reported for other plants (Fig 8B). When grown with KNO3, no difference in Cl− content was observed for any of the mutants, and growth on NH4NO3 revealed a relatively small decrease in root Cl− content in mtnpf6.5‐3 (Fig 8B). The attenuation of the phenotype when NO3 − is present may reflect that the Cl− content under these conditions may be near base‐line levels. The mtnpf6.7‐1 and mtnpf6.6‐2 mutants showed an increase in Cl− content with NH4NO3. We then investigated Cl− in mtnpf6.5‐3 roots using X‐ray fluorescence. This confirmed that the mutant has lower Cl− levels in NO3 − deficient conditions and revealed that the largest differences occurred near the root tip, where MtNPF6.5 is most highly expressed (Fig 8C).
Figure EV3. NaCl responses of wild‐type (R108‐C3) Medicago truncatula seedlings.

- Relative growth of wild‐type M. truncatula seedlings on FP with increasing levels of NaCl.
- Cl− content of roots and shoots of seedlings grown on FP. biological replicates: Biological replicates: n = 4 (3 pooled organs in each replicate), *P < 0.05, Student’s t‐test.
- Cl− content of roots and shoots of seedlings grown on FP supplemented with 50 or 100 mM NaCl. biological replicates: n = 4 (3 pooled organs in each replicate), *P < 0.05, Student’s t‐test.
- Medicago truncatula seedlings grown on FP supplemented with 50 or 100 mM NaCl. These 50 mM NaCl treatments and controls also are shown in Fig 9B and are provided here as a reference.
Data information: Roots were rearranged for measurements and display, i.e., no agravitropic root curvature was seen.
Figure 8. Cl− and NO3 − content of Medicago truncatula npf6.5/6.6/6.7 mutants. Seedlings were grown on FP or FP supplemented with the indicated salts.

- Seedling Cl− content determined by ion chromatography 2 h after treatment with 50 mM NaCl. *P < 0.05, Student’s t‐test; biological replicates: n = 4.
- Cl− content determined by ion chromatography of seedlings supplemented with 5mM KNO3 or NH4NO3. *P < 0.05, Student’s t‐test; biological replicates: n = 4.
- On the left, relative Cl− content determined by XRF of NO3 − starved seedlings after KNO3 supplementation. *P < 0.05, Student’s t‐test; biological replicates: n = 3–5. On the right, a representative XRF image of Cl− signal across WT and mutant roots at the 0 h time point. Five roots per genotype are shown. The outlines indicate the measurement areas. The color legend indicates relative Cl− concentration.
- Cl− and NO3 − content determined by ion chromatography of transgenic roots of composite plants overexpressing MtNPF6.5 compared with control roots transformed with an empty vector (EV). The plants were grown on FP (NO3 − starved) and then supplemented with 5 mM KNO3 and sampled a 0 and 48 h. *P < 0.05, Student’s t‐test; biological replicates: n = 3.
To further investigate its potential role in Cl uptake, we overexpressed MtNPF6.5 in roots of NO3 −‐starved M. truncatula composite plants after NO3 − treatment. The overexpression roots showed only a slight decrease in Cl− content 48h after NO3 − treatment, while Cl− content of control roots was strongly reduced (Fig 8D). The NO3 − content showed the opposite trend, with the overexpression roots accumulating significantly less NO3 − than the controls. Our results suggest that MtNPF6.5 has a positive role in Cl− uptake, and that this is diminished by high concentrations of NaCl and in the presence of NO3 −.
MtNPF mutants have altered root system architecture
When grown on FP media (NO3 − free) the fresh weight of the mutant roots was mostly normal, except for a slight decrease in the shoot mass of mtnpf6.7‐1 (Fig 9A). However, differences in root architecture were observed in the mutants. When grown on FP or high NaCl medium mtnpf6.5 and mtnpf6.7 mutants developed noticeably longer primary roots (Figs 9B and C, and EV3D) and all three mutants developed more lateral roots than wild type at 100 mM NaCl (Figs 9D and EV3D) suggesting functional importance of these proteins under highly saline conditions. The increase was verified using additional mutant alleles of mtnpf6.5 and mtnpf6.7, and no overall increase in lateral root density was observed (Appendix Figs S4 and S5). These experiments were repeated using KCl in place of NaCl. We observed that shoot fresh weights were increased for all three mutants, in contrast to NaCl treatments which either reduced or did not affect shoot weights (Appendix Fig S6A). All three mutants showed an increase in the number of lateral roots, and mtnpf6.5 and mtnpf6.7 had longer primary roots (Appendix Fig S6B and C). Interestingly, the longer primary root length and increased lateral root number phenotypes induced by 50mM NaCl were lost in the presence of NO3 − (Fig EV4). These results indicate that loss of any one of these NPF transporters results in changes in root system architecture under high salt (NaCl or KCl).
Figure 9. Root phenotypes of Medicago truncatula mtnpf6.5/6.6/6.7 mutants.
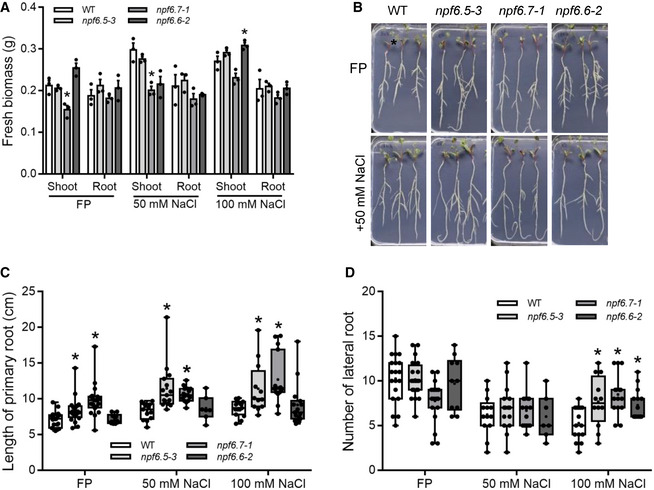
- Biomass (fresh weight) of seedlings grown on FP or FP supplemented with 50 or 100 mM NaCl. *P < 0.05, Student’s t‐test; biological replicates: n = 3 (2–5 pooled organs in each replicate).
- Image of seedlings grown on FP or FP + 50 mM NaCl.
- Primary root length of seedlings grown on FP or FP supplemented with 50 or 100 mM NaCl. *P < 0.05, Student’s t‐test.
- Lateral root number of seedlings grown on FP or FP supplemented with 50 or 100 mM NaCl. *P < 0.05, Student’s t‐test.
Data information: Note that in B roots were rearranged to better display their length, they did not exhibit curling/agravitropic growth. For C, D biological replicates: n = 7–20.
Figure EV4. Root phenotypes with KNO3 or a combination of KNO3 and NaCl.

- Biomass (fresh weight) with FP supplemented with either 5 mM KNO3, or 5 mM KNO3 and 50 mM NaCl. Biological replicates: n = 3 (5 pooled organs in each replicate), *P < 0.05, Student’s t‐test.
- Primary root length with FP supplemented with either 5 mM KNO3, or 5 mM KNO3 and 50 mM NaCl. Biological replicates: n = 15, *P < 0.05, Student’s t‐test.
- Lateral root number FP supplemented with either 5 mM KNO3, or 5 mM KNO3 and 50 mM NaCl. Biological replicates: n = 15, *P < 0.05, Student’s t‐test.
MtNPF6.5 and MtNPF6.7 expression correlate with changes in the NO3 − to Cl− ratio.
Considering the importance of MtNPF6.5 in Cl− ‐uptake we sought to test whether regulation of MtNPF6.5 plays a role in the well‐recognized plant preference for NO3 − over Cl− (Glass & Siddiqi, 1985). We grew plants in the absence of NO3 − and treated them with 5mM KNO3 and determined relative gene expression and Cl− and NO3 − content over a two‐day period. The expression of MtNPF6.7 was induced by NO3 − by 12 h, and stayed high throughout the experiment, while MtNPF6.5 was rapidly and persistently repressed (Fig 10A). MtNPF6.6 initially did not respond to the NO3 −‐treatment but showed increased expression at 48h (Fig 10A). These effects on gene expression also occurred using NaNO3, suggesting they were NO3 −‐mediated (Appendix Fig S7). The NO3 −‐starved plants initially had a higher Cl− to NO3 − ratio, but KNO3 addition resulted in a rapid switchover to NO3 − as the main anion that was maintained throughout the experiment, with NO3 − content exceeding Cl− content in all genotypes by 12 h, which further increased at 24h (Figs 10B and EV5A and B). Unexpectedly, this increase in NO3 − correlated with a substantial decline of Cl− content in all genotypes, indicating that the net uptake of Cl− was diminished (Figs 10B and EV5A and B). This “Cl− dumping” response was still present, but was less severe, in the mtnpf6.5 single and mtnpf6.5 mtnpf6.7 double mutants, but the mutants still had substantially lower Cl− content than wild type throughout the experiment. Surprisingly, while mtnpf6.7 accumulated slightly less NO3 − than wild type at 12 h, at later time points it was substantially increased (Fig 10B). By 48 h, the NO3 − content of mtnpf6.5 was also elevated, reaching a similar level to that of mtnpf6.7. The behavior of the mtnpf6.5 mtnpf6.7 double mutant was not additive, with similar NO3 − levels to that of wild type at 48 h, suggesting an interaction between these transporters with regards to plant NO3 − ‐content (Fig 10B). The anion levels of the mtnpf6.6 mutant did not differ from wild type at any of the time points sampled (Fig EV5A). The levels of Cl− in the mtnpf6.5 and the mtnpf6.5 mtnpf6.7 double mutant were always much lower than wild type (Fig 10B), and their NO3 −/Cl− ratios were always higher than wild type (Fig 10C). In contrast, mtnpf6.7 and mtnpf6.6 seedlings maintained anion ratios similar to wild type (Fig 10C). Notably, by 48h the total anion content [NO3 − + Cl−] of the double mutant was substantially lower than that of either single mutant. Given that both MtNPF6.5 and MtNPF6.7 can transport NO3 −, this result suggests that the elevated NO3 − levels in the single mutants could be due to compensation by the other transporter. However, no increased expression of either MtNPF6.5 in mtnpf6.7 or MtNPF6.7 in mtnpf6.5 was observed under these conditions, suggesting that if compensation occurs, it is likely to act at the post‐translational level, or through other transporters (Appendix Fig S8). In summary, the persistent transcriptional suppression of MtNPF6.5 under high NO3 − is consistent with a role for it in NO3 − preference over Cl−, while loss of either or both MtNPF6.5 and MtNPF6.7 did not reduce NO3 − uptake under these conditions.
Figure 10. Changes in anion content and expression of MtNPFs in NO3 − ‐starved plants after KNO3 or NaCl treatments.

- Relative MtNPF expression following treatment with 5 mM KNO3.
- Changes in NO3 − and Cl− content (mol/g dry weight) of NO3 − starved plants after NO3 − provision.
- Data from “B” expressed as NO3 − to Cl− ratios.
- Relative MtNPF expression following treatment with 100 mM NaCl.
- Relative MtNPF expression following treatment with 5 mM KNO3 +100 mM NaCl.
Data information: In A, C, D, and E expression shown is relative to Ubiquitin; *P < 0.05, Student’s t‐test; biological replicates: n = 3 (3 pooled root organs in each replicate) ± SEM. In B, *P < 0.05, Student’s t‐test; biological replicates: n = 4 ± SEM.
Figure EV5. NO3 − and Cl− content of medicago roots of NO3 −‐starved plants after KNO3 treatments.

- NO3 − and Cl− content of medicago roots.
- The same data expressed as NO3 − to Cl− ratios.
Data information: Filled symbols indicate that the mean of the mutant is significantly different (P < 0.05, Student’s t‐test) than wild type (R108‐C3). Biological replicates: n = 4 ± SEM.
High NaCl suppresses MtNPF6.5 and MtNPF6.7 in the presence of nitrate
To investigate potential interactions between NO3 − and Cl− in regulating the expression of MtNPFs we tested the effects of high NaCl and combination of high NaCl and KNO3. In NO3 − ‐starved plants, addition of 100 mM NaCl suppressed expression of MtNPF6.5 by ~50% (Fig 10D), while addition of both KNO3 and NaCl resulted in a more severe repression (~20 fold; Fig 10E), down to similar levels as MtNPF6.7 in the absence of NO3 −. Both treatments largely suppressed the induction of MtNPF6.7 and MtNPF6.6 by NO3 − (Fig 10D and E). This suggests an interaction between NO3 − and Cl− in the regulation of MtNPF expression, and further suggests that suppression of MtNPF6.5 is important to limit Cl− uptake in high NaCl conditions.
The regulation of MtNPF6.5 and MtNPF6.7 by nitrate requires MtNLP1
Many NO3 −‐dependent changes in gene expression are mediated by NLP transcription factors so we tested these responses in the M. truncatula nlp1 mutant. In NO3 −‐free media, the expression of MtNPF6.5 was similar to that of wild type in mtnlp1. However, after NO3 − addition, while MtNPF6.7 was induced and MtNPF6.5 was repressed in wild type, these effects were absent in mtnlp1 (Fig 11A). Moreover, MtNPF6.5 transcript levels increased by ~4‐fold in the NO3 −‐induced samples in mtnlp1, suggesting either its direct repression by MtNLP1, or the existence of a negative feedback mechanism downstream of MtNLP1. Based on these results we conclude that MtNLP1 controls the NO3 −‐induced changes in MtNPF6.5 and MtNPF6.7 expression in M. truncatula.
Figure 11. MtNLP1 mediates the preference for NO3 − over Cl− .

- The induction of MtNPF6.7 and repression of MtNPF6.5 by NO3 − requires MtNLP1. Plants were grown on FP plates and then treated with 5 mM KNO3. expression shown is relative to UBQ; *P < 0.05, Student’s t‐test; biological replicates: n = 3 (3 pooled root organs in each replicate).
- The switch from Cl− to NO3 − as the major anion upon NO3 − addition is lost in mtnlp1. Plants were grown on FP plates and then treated with 5 mM KNO3 treatment. *P < 0.05, Student’s t‐test; biological replicates: n = 4 (3 pooled root organs in each replicate).
- Model for the regulation of NO3 − preference in legumes. NO3 − induces the increased expression of MtNPF6.7 and other NO3 − transporters through MtNLP1 resulting in a net uptake of NO3 −. MtNPF6.5 is repressed by NO3 − in an MtNLP1‐dependent manner, contributing to a net loss of Cl−. The associated Cl− ‐efflux could be mediated by transporters such as orthologs of AtNPF2.5 and occurs independently of MtNLP1. When NO3 − is scarce, MtNPF6.5 mediates the influx of Cl−, while MtNPF6.7 expression remains relatively low.
MtNLP1 mediates the switch from chloride to nitrate as the major anion
Our results suggested that the repression of MtNPF6.5 by NO3 − treatment may play a role in the displacement of Cl− by NO3 − in medicago roots. To test this, we determined the ion content of NO3 −‐starved wild type and mtnlp1 mutant plants 48 after NO3 − provision. After NO3 − treatment, the NO3 − content of mtnlp1 mutant roots remained lower than that of Cl−, in contrast to wild type where the anion ratio shifted to greatly favor NO3 − (Fig 11B). This resulted partly from a higher Cl− content in mtnlp1 roots (31% higher than wild type) which was accompanied by a strongly diminished ability to uptake/retain NO3 − (Fig 11B). Our results show that MtNLP1 mediates the preference for NO3 − over Cl− in M. truncatula, and that this occurs though its complementary regulation of NO3 − and Cl− uptake.
Discussion
Cl− is an essential micronutrient as a cofactor of PSII and is also a macronutrient, acting as an important osmoticum which is required for optimal leaf growth (Franco‐Navarro et al, 2016). It also helps improve NO3 − utilization and contributes to both water and N use efficiency (Iglesias et al, 2004; Raven, 2017, 2020; Wege et al, 2017; Colmenero‐Flores, et al, 2019; Rosales et al, 2020). In addition, Cl− is the major anion in the apoplast and cytoplasm, and its flux is implicated in the stabilization of membrane potential and the regulation of pH gradients. However, despite decades of research on salt stress and Cl− homeostasis, the key transporters required for Cl− uptake from the soil remained elusive (Wege et al; 2017; Isayenkov & Maathuis, 2019). An initial breakthrough came when Wen et al (2017) reported that maize orthologs of AtNPF6.3 can uptake Cl−. This was followed up recently with evidence showing that when using ammonia as a N source, loss of AtNPF6.3 results in decreased Cl− content in arabidopsis. Here, we show that MtNPF6.5, which is closely related to AtNPF6.3 but has distinct biochemical features and regulation, plays a similar role in nitrate‐free conditions in M. truncatula. The exposure of these phenotypes required nitrate free conditions, reflecting the dominant preference for plants to accumulate NO3 − over Cl−; when NO3 − is replete, Cl− uptake is essentially abolished, making Cl− uptake phenotypes difficult to detect. Several other key Cl− transporters have been identified, including AtNPF2.4 and AtNPF2.5, which passively efflux Cl−, and CLC transporters involved in Cl− and NO3 − compartmentalization in the vacuole (Geelen et al, 2000; De Angeli et al, 2006; Jossier et al, 2010; Li et al, 2016, 2017; Hu et al, 2017; Demes et al, 2020). It was not until recently that ZmNPF6.4 and ZmNPF6.6 from maize were shown to have both NO3 − and Cl− uptake activities in Xenopus oocytes, but their role in Cl− transport in plants was not explored (Wen et al, 2017). Our investigation demonstrates that one M. truncatula homologue of these proteins, MtNPF6.7, is involved in NO3 − uptake, while another, MtNPF6.5, mediates Cl− uptake and largely determines plant Cl− content, filling a gap in our knowledge of plant anion homeostasis.
We show that MtNPF6.5 mediates the unidirectional uptake of Cl− in roots and is required for Cl− accumulation in the root and shoot. The suppression of MtNPF6.5 transcripts by NO3 −, which is accompanied by increased expression of MtNPF6.7, suggests a mechanism by which plants can exert a preference for NO3 − over Cl−. On the other hand, loss of either MtNPF6.5, MtNPF6.7, or both did not greatly diminish NO3 − accumulation, instead both single mutants over accumulated NO3 −. This suggests that other NO3 − transporters, such as NRT2s or other unidentified transporters, play a more important role in the Cl−‐to‐NO3 − switchover. We found that the reciprocal regulation of MtNPF6.5 and MtNPF6.7 is coordinated by MtNLP1 (Fig 11C). NLPs are the key regulators of NO3 − responses, and MtNLP1 was recently shown to be required for certain NO3 − induced changes in gene expression (Lin et al, 2018). Our analysis demonstrated that mutation of MtNLP1 leads to a major decrease in NO3 − uptake while reducing the loss of Cl− after NO3 − provision, suggesting that its role in anion homeostasis extends beyond NO3 − regulation. Interestingly, based on publicly available gene expression data, NLP1 appears to be regulated by long term, but not short‐term N availability, with high expression at lower N‐supply (Appendix Fig S9). Such regulation is consistent with a role in exchanging NO3 − for Cl− after relief of nitrate starvation. It remains to be determined whether the strong de‐repression of MtNPF6.5 in mtnlp1 occurs directly through MtNLP1 or through a downstream repressor. The rise in NO3 − content and drop in MtNPF6.5 expression was accompanied by a ~50% decrease in root Cl− content in wild type. To our knowledge, a net decrease in Cl− content in response to NO3 − provision (“Cl− ‐dumping”) has not been previously described, but Cl− efflux in response to increasing [Cl−] has been reported for carrot and maize (Cram, 1968; Weigl, 1969). It is possible that NO3 − starvation resulted in “overloading” of Cl− to maintain cell osmotic pressure, which, when NO3 − became available, was resolved by Cl− ‐efflux. This apparent adaptation to low NO3 − may be widespread in plants, or could be a special feature of legumes, which are capable of growth and reproduction in environments that are virtually NO3 − ‐free. Nonetheless, the net efflux of Cl− in response to NO3 −, although attenuated, still occurred in mtnlp1 (~38% decrease, compared to ~52% decrease in wild type at 24h; Fig 8B). Our results suggest that NLP1 does not directly control Cl− ‐dumping per se, but that it facilitates the process through its suppression of MtNPF6.5. The Cl− dumping response was intact or even slightly enhanced in the mtnpf6.5 and mtnpf6.5 mtnpf6.7 double mutants (~53‐56% decrease compared with ~49% in wild type; Fig 7B), but the absolute decrease in Cl− content was smaller in the mutants, probably reflecting their lower initial Cl− levels.
Using a phylogenetic approach and predicted substrate binding residues, we can group AtNPF6.3‐like proteins into three types: A, Cl− ‐selective; B, NO3 − ‐selective; and C, a legume‐specific form which appears to transport little or no NO3 − or Cl−. A broader survey of NPFs proteins across angiosperm lineages showed that the A/B/C haplotypes are completely specific to the AtNPF6 clade, and mostly specific to the AtNPF6.3 subclade. Our analysis suggests that type‐B proteins, such as AtNPF6.3 and MtNPF6.7, arose from an A‐type ancestor. It appears that the number of amino acid changes required for such an alteration of substrate specificity is minimal, with a single amino acid change being sufficient to convert a Cl− selective transporter into a NO3 −‐selective transporter (Wen et al, 2017). All legumes appear to have at least one type‐A and one type‐B protein, while the other rosids examined have a single type‐A, with the exception of arabidopsis, where AtNPF6.3, the only member of the subclade, is a type‐B. The absence of A‐type transporters from the Brassicas is consistent with the demonstrated role of AtNPF6.3 in Cl− uptake in A. thaliana (Liu et al, 2020). This conclusion is further supported by our findings and the original report by Wen et al (2017) showing it can mediate Cl− uptake in Xenopus oocytes. This suggests that AtNP6.3 may represent a dual‐function protein that combines the NO3 − and Cl− related functions, a feature that appears to be unique to Brassicas. This idea is further supported by the clustering of AtNPF6.3 with A‐type transporters, consistent with its role in net Cl− uptake and suggesting it evolved independently from the legume B‐type transporters (Fig 1B). Our electrophysiological and uptake studies point to several key differences between these two Cl− transporters: AtNPF6.3 showed a higher Cl−‐uptake capacity, and only MtNPF6.5‐injected cells showed a Cl− induced current. These differences, along with their opposing regulation by nitrate, suggest these transporters have distinct functions and mechanisms.
MtNPF6.5 showed Cl− and NO3 − uptake activities with selectivity for Cl−, similar to ZmNPF6.4 (Wen et al, 2017). These proteins have a AtNPF6.3:T101 equivalent but have a Tyr in place of the residue corresponding to AtNPF6.3:H356, which in AtNPF6.3 binds NO3 − and is required for its NO3 − transport activity (Parker & Newstead, 2014; Sun et al, 2014). Indeed, this is the only predicted substrate binding residue that differs between type A and type B members of the AtNPF6.3 subclade (TYTF vs THTF). A total of 15 other arabidopsis NPFs have been reported to have NO3 − influx activity (reviewed by Corratgé‐Faillie & Lacombe, 2017). Our analysis indicates that none of these NPFs have the conserved His corresponding to AtNPF6.3:H356, suggesting its role in NO3 − binding/selectivity is specific to members of the AtNPF6.3 subclade (Dataset EV1, tab E). A Tyr‐His substitution at this position in ZmNPF6.4 was sufficient to confer NO3 − selectivity (Wen et al, 2017). Whether the converse substitution in AtNPF6.3 or MtNPF6.7 would be sufficient to confer Cl− ‐selectiveness is unknown, but the importance of this Tyr and the residue corresponding to AtNPF6.3:T101 is suggested by the fact that they are conserved in all early plant lineages, and in many green algae (Dataset EV1, tab C). The localization of MtNPF6.5 in the plasma membrane and expression of MtNPF6.5 in root hairs suggest that a NPF6.5 homologue may be responsible, at least in part, for the electrogenic 2H+/Cl− symporter activity in the plasma membrane of root hair cells (Sanders, 1980; Felle, 1994). No changes in Cl− content were found for mtnpf6.7, or mtnpf6.6 even in the absence of NO3 −, suggesting that MtNPF6.5 may be the primary transporter for bulk uptake of Cl− from the soil in M. truncatula under the conditions tested. The importance of MtNPF6.5 in uptake of Cl− is further reinforced by the repression of MtNPF6.5 transcripts by high salinity. This finding is consistent with an earlier report in barley showing that Cl− pre‐treatments inhibit Cl− influx (Deane‐Drummond & Glass, 1982). High [Cl−] can cause toxicity in plants, and our results suggest that the suppression of MtNPF6.5 by NaCl may serve a protective role.
MtNPF6.7 features conserved residues corresponding to both AtNPF6.3:T101 and :H356. MtNPF6.7 resembles AtNPF6.3 in its ability to influx NO3 −, and like AtNPF6.3, MtNPF6.7 was able to take up Cl− in Xenopus oocytes. Their ability to transport Cl− resembles ZmNPF6.6, which transports both NO3 − and Cl− with selectivity for NO3 −. Our results support the importance of the T101 residue for NO3 − transport in both low‐ and high‐affinity ranges, for both MtNPF6.5 and MtNPF6.7 (Fig 2A and B). In line with these observations, Ho and Frommer (2014) reported that the T101A substitution in AtNPF6.3 abolished NO3 − transport/NiTrac1 in the high‐affinity range and reduced it in the low‐affinity range. Indeed, we found that all but one of the NPFs known to influx NO3 − have the conserved Thr (Dataset EV1, tab E). Indeed, the only exception, AtNPF4.6, was shown to have a much higher affinity for abscisic acid than for NO3 − (Kanno et al, 2013; Léran et al, 2020). The importance of this residue in NO3 − transport seems clear, and our analysis indicates that it may be a useful predictor of NO3 − transport ability within the NPF family. A phosphomimetic version of MtNPF6.7 (T101D) was unaltered in its NO3 − transport activity in Xenopus oocytes, similar to what was reported for the analogous version of AtNPF6.3 (Martín et al, 2008).
A subset of legumes, the Hologalegina, have a third AtNPF6.3 paralog originating from a tandem gene duplication that gave rise to MtNPF6.7 and MtNPF6.6. MtNPF6.6 is therefore specific to legumes. MtNPF6.6 showed no ability to transport NO3 − with the exception of one assay where it showed a small influx activity that could be inhibited by Cl−. MtNPF6.6 has apparently neofunctionalized through several changes in the residues predicted to be associated with NO3 − binding, in particular, the loss of conserved residues corresponding to AtNPF6.3:H356 and :T101. Also, the expression of MtNPF6.6 is only weakly induced by NO3 −, further indicating a change in its role. MtNPF6.6’s functional importance is suggested by its active expression in roots, the conservation of this haplotype across Hologalegoid legumes, and the root system architecture phenotypes of the mtnpf6.6 mutants. Moreover, the conservation of the ExxER/K motif and proton‐coupling residue (equivalent to AtNPF6.3:E476) indicate that it encodes an active anion transporter, and the overlap of root system architecture phenotypes with mtnpf6.5 and mtnpf6.7 mutants suggest its function is allied with that of MtNPF6.5 and MtNPF6.7. Legumes stand out in their ability to nodulate with rhizobia, and while the roles of MtNPFs remain to be investigated in this context, several other NPFs have already been implicated in nodulation (Yendrek et al, 2010; Bagchi et al, 2012; Valkov et al, 2017; Wang et al, 2020).
The discovery in legumes of three AtNPF6.3 homologues, each with different transport properties, reveals an unexpected diversity of function of these proteins. The variation in number and types of these transporters across plant species may reflect evolutionary adjustments to changing soil environments such as variations in salinity and NO3 − availability. Further study into how plants have evolved to exclude Cl− when sufficient NO3 − is available will enable more effective crop management and breeding.
Materials and Methods
Gene expression analysis
Medicago truncatula wild‐type (R108C3) seedlings were grown on FP plates (pH6.0) for 7 days, then the seedlings were transferred to FP liquid media (pH6.0) with indicated treatments for 0.5 h or 2 days. For the NO3 − induction test, the samples were treated with 0.2, 2, or 10 mM KNO3 for 0.5 h. For the NaCl test, the seedlings were treated with 50 or 100 mM NaCl for 2 days. Total RNA was extracted from whole roots using RNAprep Pure Cell Kit (Tiangen) and quantified using a Spectrophotometer (DeNovix). Reverse transcript cDNA was synthesized using ReaHifair®Ⅱ1st Strand cDNA Synthesis Kit (Yeasen). Real‐time RT–PCR was done with the RealStar Green Fast Mixture (SYBR Green; GenStar) and detected using an ABI StepOne PCR system (Thermo Fisher). Relative gene expression was normalized to the reference gene Ubiquitin. Standard errors and statistical significance based on three biological replicates were calculated using the 2‐ΔΔCt method. Primers used for RT–qPCR of MtNPFs:
MtNPF6.5: 5’‐AGCAGGTGTTGCCACATAGC‐3', 5'‐TCGTTGTGGCCCATATTGGTA‐3';
MtNPF6.7: 5'‐GTTGACAACACTCGGCATCG‐3' 5'‐CGGAACCAAAGCCAGAGACA‐3';
MtNPF6.6: 5'‐GGTAACGCTTCCTCTGCCAA‐3' 5'‐TGAACCAAAGCCAGGGACAC‐3'.
cRNA synthesis and transport assays in Xenopus laevis oocytes
Residues corresponding to T101 are conserved in MtNPF6.5 and MtNPF6.7, but not in MtNPF6.6. To test the importance of these residues, we made versions of MtNPF6.5 and MtNPF6.7 with T101A, T101D (phosphomimetic) substitutions. The coding regions of MtNPF6.5, MtNPF6.5‐T101A, MtNPF6.5‐T101D, MtNPF6.7, MtNPF6.7‐T101A, and MtNPF6.7‐T101D were synthesized by Life Technologies. For cloning of the genes in the oocyte expression vector, the open reading frames (ORFs) were amplified from the synthesized genes with the following primers, which had either a Bgl II or a Spe I restriction enzyme site. MtNPF6.5 genes: forward 5’‐GAA GAT CTA TGA GTA CTC TCC CTC AAA CTC AGG GGC AAA CAA TTC‐3’ and reverse 5’‐CTA TGC ATG GCA AGC TGT ATC TGC T‐3’; MtNPF6.7 genes: forward 5’‐GAA GAT CTA TGA GCA CCC TCC CTA CAA CAC AAG‐3’ and reverse 5’‐CTA TGC ATG GAA TGT AGA GGC‐3’. The PCR products were ligated into the PCR‐Blunt II‐TOPO vector (Thermo Fisher Scientific), and the sequences were confirmed (Eurofin). Then, the MtNPF6.5 and MtNPF6.7 ORFs were sub‐cloned into the in vitro transcription vector pT7TS/flag (Chen et al, 2017) vector between Bgl II and Spe I. We also synthesized the MtNPF6.6 coding region but failed to clone it into any vectors after several attempts in which only clones with premature stop codons were isolated, which suggests MtNPF6.6 is toxic when expressed in E. coli. Therefore, a three step PCR‐based strategy was used to acquire the template for MtNPF6.6 cRNA synthesis. First, we used a forward primer 5’‐TAA TAC GAC TCA CTA TAG GGA CTA GTA TGG ACA GTC TCC CCA CAA CAA CAC‐3’ (containing a T7 promoter followed by the first 24 bp of the MtNPF6.6 ORF) and a reverse primer 5’‐CTA GTC AGT CAC TAG TTT ACT CTT CCA TGC CTT CCT CAG CAA‐3’ (corresponding to the 3’ terminus of the MtNPF6.6 ORF and an Spe I site followed by several other nucleotides) to amplify the MtNPF6.6 ORF using the synthesized MtNPF6.6 as the template (fragment 1). Second, the 3’‐UTR of Xenopus Gamma globulin (fragment 2) was amplified from the pT7TS/flag vector with a forward primer containing a 5’ Spe I site: 5’‐GGA CTA GTG ACT GAC TAG GAT CTG GTT ACC ACT AAA CC‐3’, and a reverse primer: 5’‐GGG CCT CTT CGC TAT TAC GC‐3’. The two fragments were each digested with Spe I and then ligated together. The ligation solution was used directly in the third PCR to generate a product containing both fragment 1 and fragment 2 using the forward primer used to amplify fragment 1 and the reverse primer from fragment 2 (SP6). The resulting product, which contains T7 promoter upstream of MtNPF6.6:globulin 3’‐UTR was confirmed by sequencing and used in the cRNA synthesis.
For selection and handling of Xenopus laevis oocytes, cRNA in‐vitro synthesis and injection was done as previously described (Chen et al, 2017). Two days after cRNA injection, NO3 − uptake experiments were done by exposing the oocytes to Na15NO3. Concentrations of the Na15NO3 used were listed in the text and figure legend. After treatments, oocytes were washed six times and dried out at 60°C for 3 days. Oocyte incubation, NO3 − transport experiments and oocyte washing were all done in the modified saline solution (MBS) without nitrogen (Ca(NO3)2 replaced with CaCl2). The 15N was measured by a Finnigan Delta plus XP isotope ratio mass spectrometer (IRMS; Thermo Fisher Scientific).
36Cl− uptake was done as previously described (Wen et al, 2017) with minor modifications. Two days after cRNA injection, the oocytes were exposed to 0.1 μCi/ml equal to 1.17 mM of basal solution (Wen et al, 2017) for 2 h and the 36Cl−/NO3 − competition was done in basal solution with 1 mM NaCl at pH5.5 for 2 h. The oocytes were washed 5 times with ice cold basal solution. Four pooled oocytes were dissolved in 50 µl H2O2, then mixed with 4 ml of scintillating solution. Incorporated radioactivity was measured using a liquid scintillation analyzer (Tris‐Carb® 2910TR, PerkinElmer). Note that significantly different amounts of 36Cl− uptake relative to water‐injected controls could only be measured in a low Cl− or Cl−‐free solution. High Cl− in MBS significantly reduces the Cl− uptake in oocytes. We have previously noted that the high concentrations of Na+ and Cl− found in oocyte saline can inhibit the activity of plant sugar transporters expressed in oocytes (Zhou & Miller, 2000).
Electrophysiology
Oocytes current were measured using two‐microelectrode voltage‐clamp method (Zhou et al, 1998), and the basal solution used in the perfusion system was described previously (Wen et al, 2017). Cl− was added as HCl in the basal solution, and pH was adjusted by Bis‐Tris propane. Only oocytes with an initial membrane potential below −30 mV in MBS were used in the study. The oocytes were impaled with the two micro‐electrodes in MBS (pH7.4) and perfused with the basal solution (pH7.4) and further with the basal solution (pH5.8). In each step, the membrane potential of the oocyte was reached stable before changing to the next solution. Each I‐V curve was performed at the maximum steady‐state current before, during and after each treatment and only in the cases where identical results from before and after treatment were obtained were data used for further analysis. Clamping and measurements were done by an Axoclamp 900A (Axon) clamp and Digidata 1440A digitizer (Axon) with the pCLAMP program. At any given membrane potential, steady‐state currents elicited by Cl− exposure in the MtNPF6.5 expressing oocytes were fitted to single Michaelis–Menten functions in SigmaPlot software (version 14.0, Systat Software Inc.).
Phylogenetic and haplotype analyses
The NPF protein sequences for rice, medicago, and arabidopsis used for phylogenetic and haplotype analyses were obtained from the Phytozome database (Goodstein et al, 2012) based on the lists compiled by Longo, Miles, and Dickstein (2018). Pea CDS and corresponding genomic sequences are available from the pea RNA‐seq Gene Atlas (Alves‐Carvalho et al, 2015) and https://urgi.versailles.inra.fr/Species/Pisum (Kreplak et al, 2019), respectively. The phylogenetic analysis was carried out using the Phylogeny.fr server (Dereeper et al, 2008) as follows. Protein sequences were aligned using MUSCLE (Edgar, 2004), and the phylogenetic tree was constructed using PhyML (v3.0; Guindon & Gascuel, 2003). For haplotype analysis, the protein sequences were aligned using MAFFT (Katoh, Rozewicki, & Yamada, 2019) or PAGAN (Löytynoja et al, 2012) and visualized using WASABI (Veidenberg, Medlar, & Löytynoja, 2016).
Sequences
The gene models relevant to this paper are MtNPF6.5 (Medtr4g101380), MtNPF6.7 (Medtr5g012290), MtNPF6.6 (Medtr5g012270), PsNPF6.5 (Psat0s3925g0040), PsNPF6.7 (Psat2g178360), PsNPF6.6 (Psat2g178520), ZmNPF6.6 (PWZ54021), ZmNPF6.4 (PWZ44521), OsNPF6.3 (LOC_Os08g05910), OsNPF6.4 (LOC_Os03g01290), OsNPF6.5 (LOC_Os10g40600).
Plant material and growth conditions
For M. truncatula, ecotype R108‐C3 was used WT control. Tnt1 insertional mutants for MtNPF6.5, MtNPF6.7, and MtNPF6.6 were identified in the R108 background from the Noble Institute mutant population using reverse‐ and forward‐genetic screening. Homozygotes for each mutant were identified and used for this study. Seeds were scarified using sandpaper and then washed with sterilized deionized and then treated with bleach for 4 mins, and then washed 6 times with distilled deionized water (ddH20). After imbibition, the seeds were transferred to water agar plates and left in the dark for three days at 4°C, then the plates were inverted to allow roots to extend at 22°C overnight.
Chloride and nitrate content
Seedlings were grown on Fahräeus medium (FP) containing 0.8% agarose for two weeks on petri dishes and then were transferred to liquid FP containing indicated treatments (50 or 100 mM NaCl, 5 mM KNO3, 5 mM Ca(NO3)2, or 5 mM NH4NO3) for 2h. Then, the shoot and root samples were harvested and dried at 60°C for 2 days. The ion content assays followed Colmenero‐Flores et al (2007). In short, samples were ground to a fine powder with a tissue grinder (JingXing JXFSTPRP‐48). Then, samples were extracted with deionized water and then filtered (0.45 µm). Filtrates were analyzed using ion chromatography (Thermo Fisher Dionex ICS‐5000+) by the Instrumental Analysis Center of Shanghai, Jiao Tong University, China. For the X‐ray fluorescence (XRF) analysis, the Medicago seedlings were grown in vitro on FP conditions for one week, then were transferred to hydroponics with FP containing 5 mM KNO3. The roots were harvested at 0, 12, 24 and 48 h. The Micro‐XRF Spectrometer (M4 tornado, Bruker) was used to visualize chloride in the roots.
Agrobacterium rhizogenes‐mediated transformation of Medicago
Agrobacterium rhizogenes ARqua1 strain was electroporated with binary vectors and used to generate composite plants comprising a transgenic hairy root system with non‐transformed shoots and leaves (Boisson‐Dernier et al, 2001).
Promoter‐GUS assays
Promoter fragments were amplified from M. truncatula Jemalong A17 genomic DNA (for MtNPF6.5, 3,072 bp, Forward primer: GGG GACAAGTTTGTACAAAAAAGCAGGCTTCGGTTGGAGCTCATGGAGAAG, Reverse primer: GGGGACCACTTTGTACAAGAAAGCTGGGTTAGTACTCATTTGGATATAAG TCG) or from synthesized modules (Life Technologies) for MtNPF6.7 (2,662 bp, Forward primer: GGGGACAAGTTTGTACAAAAAAGCAGGCTTCCCTAAAGAGTAA AGACAC TC, Reverse primer: GGGGACCACTTTGTACAAGAAAGCTGGGTTATTGTAAGATA TGAGAGGTATG) and MtNPF6.6 (2,635 bp, Forward primer: GGGGACAAGTTTGTA CAAAAAAGCAGGCTTCTCTGTGGTCTCAGGAGTAAC; Reverse primer: GGGGACC ACTTTGTACAAGAAAGCTGGGTTTGTTTCAACTTTCAATGAAG) using Phusion High Fidelity Polymerase (NEB) and cloned into pDONR207 by using Gateway BP Clonase (Invitogen). The BP products were then cloned into the destination vector pKGWFS7 using Gateway LR Clonase (Invitrogen) to make pMtNPF6.5:GUS, pMtNPF6.7:GUS, pMtNPF6.6:GUS. The binary vectors were transformed in ARqua1 and used to generate composite plants. Composite plants from each experiment were transferred to sand:terra green 4 weeks after transformation. The roots were harvested 3 weeks later for GUS staining and stained for at least 4 h at 37°C in 1 mg ml−1 X‐Gluc solution with 100 mM potassium phosphate buffer (pH7.0), 1 mM potassium ferricyanide, 1mM potassium ferrocyanide and 10 mM EDTA. Images were obtained using a digital camera mounted on a Nikon Eclipse E800 microscope.
Statistical analysis
All graphs were made using Excel (Microsoft), SigmaPlot (Systat Software Inc.), or Prism 7 (Graphpad Software Inc.). Means were compared using a Student’s t‐test with α = 0.05. The box and whisker plots were created with min to max, and show all data points; the boxes represent the middle 50% of the distribution, and whiskers represent the entire spread of the data, middle black lines represent median, + represents mean.
Subcellular localization
The constructs for MtNPF6.5 and MtNPF6.7 subcellular localization were made by Golden Gate cloning. DNA fragments of MtNPF6.5 and MtNPF6.7 were synthesized by Life Technologies and used as level 0 modules. Level 1 constructs were then assembled to make ProLjUBQ1:MtNPF6.5‐GFP and ProLjUBQ1:MtNPF6.7‐GFP, which were further assembled into EC50507 Level 2 backbone in combination with the pRC:LAP1 gene for use as a visual marker system (Ruan et al, 2021). The constructs were introduced into M. truncatula transgenic roots by A. rhizogenes transformation as described above. For FM4‐64 (US Everbright) staining, the roots were incubated with 2 μM FM4‐64 for 15 min. Then, the signals in roots were detected using a confocal microscope (Olympus FV1000). Fluorescence signals for eGFP (excitation 488 nm, emission 505–550 nm) and FM 4–64 (excitation 561 nm, emission > 575 nm) were detected.
Author contributions
Screening of the medicago mutants: XC, JW, and KM; Other experiment design: JDM, AJM, QX, YC, SR, and C‐WL; Performance of experiment: QX, YC (Xenopus oocyte assays), FR, SR, and C‐WL; Manuscript writing: JDM, AJM, C‐WL, YC, and QX.
Conflict of interest
We declare that there are no competing commercial interests in relation to the submitted work.
Supporting information
Appendix
Expanded View Figures PDF
Table EV1
Dataset EV1
Acknowledgements
Funding for this grant was provided by Ministry of Science and Technology (2019FA0904703), Strategic Priority Research Program of the Chinese Academy of Sciences (XDB27040209) and National Key R&D Program of China (2016YFA0500500), and the Chinese Academy of Science (CAS) (153D31KYSB20160074) and the Biotechnology and Biological Sciences Research Council (BBSRC; grant nos. BB/L010305/1[David Phillips Fellowship]; BB/J004553/1). We thank Ping Xu for her comments on the manuscript, and Fang Xie for providing seeds of Medicago truncatula nlp1‐1, and nlp1‐2. AJM was funded by the UK BBSRC Institute Strategic Program Grants “Molecules from Nature” (BB/P012523/1) and “Plant Health” (BB/P012574/1) and the John Innes Foundation.
The EMBO Journal (2021) 40: e106847.
Contributor Information
Anthony J Miller, Email: tony.miller@jic.ac.uk.
Jeremy D Murray, Email: jeremy.murray@jic.ac.uk.
Data availability
This study does not include data amenable for deposition in external repositories. The authors declare that all data supporting the findings of this study are available within the article and its Supplementary Information Files or are available from the corresponding authors upon request.
References
- Alboresi A, Gestin C, Leydecker MT, Bedu M, Meyer C, Truong HN (2005) Nitrate, a signal relieving seed dormancy in Arabidopsis . Plant Cell Environ 28: 500–512 [DOI] [PubMed] [Google Scholar]
- Almagro A, Lin SH, Tsay YF (2008) Characterization of the Arabidopsis nitrate transporter NRT1.6 reveals a role of nitrate in early embryo development. Plant Cell 20: 3289–3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves‐Carvalho S, Aubert G, Carrère S, Cruaud C, Brochot AL, Jacquin F, Klein A, Martin C, Boucherot K, Kreplak J et al (2015) Full‐length de novo assembly of RNA‐seq data in pea (Pisum sativum L.) provides a gene expression atlas and gives insights into root nodulation in this species. Plant J 84: 1–19 [DOI] [PubMed] [Google Scholar]
- Andersen TG, Nour‐Eldin HH, Fuller VL, Olsen CE, Burow M, Halkier BA (2013) Integration of biosynthesis and long‐distance transport establish organ‐specific glucosinolate profiles in vegetative Arabidopsis . Plant Cell 25: 3133–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi R, Salehin M, Adeyemo OS, Salazar C, Shulaev V, Sherrier DJ, Dickstein R (2012) Functional assessment of the Medicago truncatula NIP/LATD protein demonstrates that it is a high‐affinity nitrate transporter. Plant Physiol 160: 906–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson‐Dernier A, Chabaud M, Garcia F, Bécard G, Rosenberg C, Barker DG (2001) Agrobacterium rhizogenes‐transformed roots of Medicago truncatula for the study of nitrogen‐fixing and endomycorrhizal symbiotic associations. Mol Plant Microbe Interact 14: 695–700 [DOI] [PubMed] [Google Scholar]
- Bouguyon E, Perrine‐Walker F, Pervent M et al (2016) Nitrate controls root development through posttranscriptional regulation of the NRT1.1/NPF6.3 transporter/sensor. Plant Physiol 172: 1237–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canales J, Contreras‐López O, Álvarez JM, Gutiérrez RA (2017) Nitrate induction of root hair density is mediated by TGA1/TGA4 and CPC transcription factors in Arabidopsis thaliana . Plant J 92: 305–316 [DOI] [PubMed] [Google Scholar]
- Chai S, Li E, Zhang Y, Li S (2020) NRT1.1‐mediated nitrate suppression of root coiling relies on PIN2‐ and AUX1‐mediated auxin transport. Front Plant Sci 11: 671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Sun SK, Tang Z, Liu G, Moore KL, Maathuis FJM, Miller AJ, McGrath SP, Zhao FJ (2017) The Nodulin 26‐like intrinsic membrane protein OsNIP3;2 is involved in arsenite uptake by lateral roots in rice. J Exp Bot 68: 3007–3016 [DOI] [PubMed] [Google Scholar]
- Cheng X, Krom N, Zhang S, Mysore KS, Udvardi M, Wen J (2017) Enabling reverse genetics in Medicago truncatula using high‐throughput sequencing for Tnt1 flanking sequence recovery. Methods Mol Biol 1610: 25–37 [DOI] [PubMed] [Google Scholar]
- Cheng X, Wen J, Tadege M, Ratet P, Mysore KS (2011) Reverse genetics in Medicago truncatula using Tnt1 insertion mutants. Methods Mol Biol 678: 179–190 [DOI] [PubMed] [Google Scholar]
- Chiba Y, Shimizu T, Miyakawa S, Kanno Y, Koshiba T, Kamiya Y, Seo M (2015) Identification of Arabidopsis thaliana NRT1/PTR FAMILY (NPF) proteins capable of transporting plant hormones. J Plant Res 128: 679–686 [DOI] [PubMed] [Google Scholar]
- Colmenero‐Flores JM, Franco‐Navarro JD, Cubero‐Font P, Peinado‐Torrubia P, Rosales MA (2019) Chloride as a beneficial macronutrient in higher plants: new roles and regulation. Int J Mol Sci 20: 4686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corratgé‐Faillie C, Lacombe B (2017) Substrate (un)specificity of Arabidopsis NRT1/PTR FAMILY (NPF) proteins. J Exp Bot 68: 3107–3113 [DOI] [PubMed] [Google Scholar]
- Cram WJ (1968) Compartmentation and exchange of chloride in carrot root tissue. Biochim Biophys Acta 163: 339–353 [DOI] [PubMed] [Google Scholar]
- De Angeli A, Monachello D, Ephritikhine G, Frachisse JM, Thomine S, Gambale F, Barbier‐Brygoo H (2006) The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature 442: 939–942 [DOI] [PubMed] [Google Scholar]
- Deane‐Drummond CE, Glass ADM (1982) Studies of nitrate influx into barley roots by the use of 36ClO3 − as a tracer for nitrate. 1. Interactions with chloride and other ions. Can J Bot 60: 2147–2153 [Google Scholar]
- Demes E, Besse L, Cubero‐Font P, Satiat‐Jeunemaitre B, Thomine S, De Angeli A (2020) Dynamic measurement of cytosolic pH and [NO3‐] uncovers the role of vacuolar transporter AtCLCs in cytosolic pH homeostasis. Proc Natl Acad Sci USA 117: 15343–15353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M et al (2008) Phylogeny.fr: robust phylogenetic analysis for the non‐specialist. Nucleic Acids Res 36: W465–W469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32: 1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang XZ, Tian WH, Liu XX, Lin XY, Jin CW, Zheng SJ (2016) Alleviation of proton toxicity by nitrate uptake specifically depends on nitrate transporter 1.1 in Arabidopsis . New Phytol 211: 149–158 [DOI] [PubMed] [Google Scholar]
- Felle HH (1994) The H+/C1− symporter in root‐hair cells of Sinapis alba. An electrophysiological study using ion‐selective electrodes. Plant Physiol 106: 1131–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H, Fan X, Miller AJ, Xu G (2020) Plant nitrogen uptake and assimilation: regulation of cellular pH homeostasis. J Exp Bot 71: 4380–4392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco‐Navarro JD, Brumós J, Rosales MA, Cubero‐Font P, Talón M, Colmenero‐Flores JM (2016) Chloride regulates leaf cell size and water relations in tobacco plants. J Exp Bot 67: 873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geelen D, Lurin C, Bouchez D, Frachisse JM, Lelièvre F, Courtial B, Barbier‐Brygoo H, Maurel C (2000) Disruption of putative anion channel gene AtCLC‐a in Arabidopsis suggests a role in the regulation of nitrate content. Plant J 21: 259–267 [DOI] [PubMed] [Google Scholar]
- Glass AD, Kotur Z (2013) A reevaluation of the role of Arabidopsis NRT1.1 in high‐affinity nitrate transport. Plant Physiol 163: 1103–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass ADM, Siddiqi MY (1985) Nitrate inhibition of chloride influx in barley: implications for a proposed chloride homeostat. J Exp Bot 36: 556–566 [Google Scholar]
- Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Mitros T, Dirks W, Hellsten U, Putnam N et al (2012) Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res 40: D1178–D1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52: 696–704 [DOI] [PubMed] [Google Scholar]
- Hachiya T, Mizokami Y, Miyata K, Tholen D, Watanabe CK, Noguchi K (2011) Evidence for a nitrate‐independent function of the nitrate sensor NRT1.1 in Arabidopsis thaliana . J Plant Res 124: 425–430 [DOI] [PubMed] [Google Scholar]
- Hachiya T, Noguchi K (2011) Mutation of NRT1.1 enhances ammonium/low pH‐tolerance in Arabiopsis thaliana . Plant Signal Behav 6: 706–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C‐H, Frommer WB (2014) Fluorescent sensors for activity and regulation of the nitrate transceptor CHL1/NRT1.1 and oligopeptide transporters. eLife, 3: e01917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CH, Lin SH, Hu HC, Tsay YF (2009) CHL1 functions as a nitrate sensor in plants. Cell 138: 1184–1194 [DOI] [PubMed] [Google Scholar]
- Hu R, Zhu Y, Wei J, Chen J, Shi H, Shen G, Zhang H (2017) Overexpression of PP2A‐C5 that encodes the catalytic subunit 5 of protein phosphatase 2A in Arabidopsis confers better root and shoot development under salt conditions. Plant Cell Environ 40: 150–164 [DOI] [PubMed] [Google Scholar]
- Huang NC, Chiang CS, Crawford NM, Tsay YF (1996) CHL1 encodes a component of the low‐affinity nitrate uptake system in Arabidopsis and shows cell type‐specific expression in roots. Plant Cell 8: 2183–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias DJ, Levy Y, Gómez‐Cadenas A, Tadeo FR, Primo‐Millo E, Talon M (2004) Nitrate improves growth in salt‐stressed citrus seedlings through effects on photosynthetic activity and chloride accumulation. Tree Physiol 24: 1027–1034 [DOI] [PubMed] [Google Scholar]
- Isayenkov SV, Maathuis FJM (2019) Plant salinity stress: many unanswered questions remain. Frontiers in Plant Science 10: 80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian S, Luo J, Liao Q, Liu Q, Guan C, Zhang Z (2019) NRT1.1 regulates nitrate allocation and cadmium tolerance in Arabidopsis . Front. Plant Sci 10: 384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen ME, Olsen CE, Geiger D, Mirza O, Halkier BA, Nour‐Eldin HH (2015) A functional EXXEK motif is essential for proton coupling and active glucosinolate transport by NPF2.11. Plant Cell Physiol 56: 2340–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jossier M, Kroniewicz L, Dalmas F, Le Thiec D, Ephritikhine G, Thomine S, Barbier‐Brygoo H, Vavasseur A, Filleur S, Leonhardt N (2010) The Arabidopsis vacuolar anion transporter, AtCLCc, is involved in the regulation of stomatal movements and contributes to salt tolerance. Plant J 64: 563–576 [DOI] [PubMed] [Google Scholar]
- Kanno Y, Kamiya Y, Seo M (2013) Nitrate does not compete with abscisic acid as a substrate of AtNPF4.6/NRT1.2/AIT1 in Arabidopsis . Plant Signal Behav 8: e26624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Rozewicki J, Yamada KD (2019) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform 20: 1160–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiba T, Feria‐Bourrellier AB, Lafouge F, Lezhneva L, Boutet‐Mercey S, Orsel M, Bréhaut V, Miller A, Daniel‐Vedele F, Sakakibara H et al (2012) The Arabidopsis nitrate transporter NRT2.4 plays a double role in roots and shoots of nitrogen‐starved plants. Plant Cell 24: 245–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreplak J, Madoui MA, Cápal P, Novák P, Labadie K, Aubert G, Bayer PE, Gali KK, Syme RA, Main D et al (2019) A reference genome for pea provides insight into legume genome evolution. Nat Genet 51: 1411–1422 [DOI] [PubMed] [Google Scholar]
- Krouk G, Lacombe B, Bielach A, Perrine‐Walker F, Malinska K, Mounier E, Hoyerova K, Tillard P, Leon S, Ljung K et al (2010) Nitrate‐regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev Cell 18: 927–937 [DOI] [PubMed] [Google Scholar]
- Léran S, Muños S, Brachet C, Tillard P, Gojon A, Lacombe B (2013) Arabidopsis NRT1.1 is a bidirectional transporter involved in root‐to‐shoot nitrate translocation. Mol Plant 6: 1984–1987 [DOI] [PubMed] [Google Scholar]
- Léran S, Noguero M, Corratgé‐Faillie C, Boursiac Y, Brachet C, Lacombe B (2020) Functional characterization of the Arabidopsis abscisic acid transporters NPF4.5 and NPF4.6 in Xenopus Oocytes. Front Plant Sci 11: 144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léran S, Varala K, Boyer J‐C, Chiurazzi M, Crawford N, Daniel‐Vedele F, David L, Dickstein R, Fernandez E, Forde B et al (2014) A unified nomenclature of nitrate transporter 1/PEPTIDE transporter family members in plants. Trends Plant Sci 19: 5–9 [DOI] [PubMed] [Google Scholar]
- Lezhneva L, Kiba T, Feria‐Bourrellier AB, Lafouge F, Boutet‐Mercey S, Zoufan P, Sakakibara H, Daniel‐Vedele F, Krapp A (2014) The Arabidopsis nitrate transporter NRT2.5 plays a role in nitrate acquisition and remobilization in nitrogen‐starved plants. Plant J 80: 230–241 [DOI] [PubMed] [Google Scholar]
- Li BO, Byrt C, Qiu J, Baumann U, Hrmova M, Evrard A, Johnson AAT, Birnbaum KD, Mayo GM, Jha D et al (2016) Identification of a stelar‐localized transport protein that facilitates root‐to‐shoot transfer of chloride in Arabidopsis . Plant Physiol 170: 1014–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Qiu J, Jayakannan M, Xu B, Li Y, Mayo GM, Tester M, Gilliham M, Roy SJ (2017) AtNPF2.5 Modulates chloride (Cl‐) efflux from roots of Arabidopsis thaliana . Front Plant Sci 7: 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Su Z, Dong J, Wang T (2009) An expression database for roots of the model legume Medicago truncatula under salt stress. BMC Genom 10: 517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JS, Li X, Luo Z, Mysore KS, Wen J, Xie F (2018) NIN interacts with NLPs to mediate nitrate inhibition of nodulation in Medicago truncatula . Nat Plants 4: 942–952 [DOI] [PubMed] [Google Scholar]
- Liu F, Xu Y, Chang K, Li S, Liu Z, Qi S, Jia J, Zhang M, Crawford NM, Wang Y (2019) The long noncoding RNA T5120 regulates nitrate response and assimilation in Arabidopsis . New Phytol 224, 117–131. [DOI] [PubMed] [Google Scholar]
- Liu KH, Huang CY, Tsay YF (1999) CHL1 is a dual‐affinity nitrate transporter of Arabidopsis involved in multiple phases of nitrate uptake. Plant Cell 11: 865–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KH, Tsay YF (2003) Switching between the two action modes of the dual‐affinity nitrate transporter CHL1 by phosphorylation. EMBO J 22: 1005–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XX, Zhu YX, Fang XZ, Ye JY, Du WX, Zhu QY, Lin XY, Jin CW (2020) Ammonium aggravates salt stress in plants by entrapping them in a chloride over‐accumulation state in an NRT1.1‐dependent manner. Sci Total Environ 746: 141244 [DOI] [PubMed] [Google Scholar]
- Longo A, Miles NW, Dickstein R (2018) Genome mining of plant NPFs reveals varying conservation of signature motifs associated with the mechanism of transport. Front Plant Sci 9: 1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löytynoja A, Vilella AJ, Goldman N (2012) Accurate extension of multiple sequence alignments using a phylogeny‐aware graph algorithm. Bioinformatics 28: 1684–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghiaoui A, Bouguyon E, Cuesta C, Perrine‐Walker F, Alcon C, Krouk G, Benková E, Nacry P, Gojon A, Bach L (2020) The Arabidopsis NRT1.1 transceptor coordinately controls auxin biosynthesis and transport to regulate root branching in response to nitrate. J Exp Bot 71: 4480–4494 [DOI] [PubMed] [Google Scholar]
- Marchive C, Roudier F, Castaings L, Bréhaut V, Blondet E, Colot V, Meyer C, Krapp A (2013) Nuclear retention of the transcription factor NLP7 orchestrates the early response to nitrate in plants. Nat Comm 4: 1713 [DOI] [PubMed] [Google Scholar]
- Martín Y, Navarro FJ, Siverio JM (2008) Functional characterization of the Arabidopsis thaliana nitrate transporter CHL1 in the yeast Hansenula polymorpha . Plant Mol Biol 68: 215 [DOI] [PubMed] [Google Scholar]
- Meharg AA, Blatt MR (1995) NO3 − transport across the plasma‐membrane of Arabidopsis‐thaliana root hairs ‐ kinetic control by pH and membrane voltage. J Membr Biol 145: 49–66 [DOI] [PubMed] [Google Scholar]
- Miller AJ, Smith SJ (1996) Nitrate transport and compartmentation in cereal root cells. J Exp Bot 47: 843–854 [Google Scholar]
- Muños S, Cazettes C, Fizames C, Gaymard F, Tillard P, Lepetit M, Lejay L, Gojon A (2004) Transcript profiling in the chl1‐5 mutant of Arabidopsis reveals a role of the nitrate transporter NRT1.1 in the regulation of another nitrate transporter, NRT2.1. Plant Cell 16: 2433–2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JD, Liu C, Chen Y, Miller AJ (2017) Nitrogen sensing in legumes. J Exp Bot 68: 1919–1926 [DOI] [PubMed] [Google Scholar]
- Nour‐Eldin HH, Andersen TG, Burow M, Madsen SR, Jørgensen ME, Olsen CE, Dreyer I, Hedrich R, Geiger D, Halkier BA (2012) NRT/PTR transporters are essential for translocation of glucosinolate defence compounds to seeds. Nature 488: 531–534 [DOI] [PubMed] [Google Scholar]
- Parker JL, Li C, Brinth A, Wang Z, Vogeley L, Solcan N, Ledderboge‐Vucinic G, Swanson JMJ, Caffrey M, Voth GA et al (2017) Proton movement and coupling in the POT family of peptide transporters. Proc Natl Acad Sci USA 114: 13182–13187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JL, Newstead S (2014) Molecular basis of nitrate uptake by the plant nitrate transporter NRT1.1. Nature 507: 68–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JA (2017) Chloride: essential micronutrient and multifunctional beneficial ion. J Exp Bot 68: 359 [DOI] [PubMed] [Google Scholar]
- Raven JA (2020) Chloride involvement in the synthesis, functioning and repair of the photosynthetic apparatus in vivo. New Phytol 227: 334–342 [DOI] [PubMed] [Google Scholar]
- Remans T, Nacry P, Pervent M, Filleur S, Diatloff E, Mounier E, Tillard P, Forde BG, Gojon A (2006) The Arabidopsis NRT1.1 transporter participates in the signaling pathway triggering root colonization of nitrate‐rich patches. Proc Natl Acad Sci USA 103: 19206–19211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentsch D, Laloi M, Rouhara I, Schmelzer E, Delrot S, Frommer WB (1995) NTR1 encodes a high affinity oligopeptide transporter in Arabidopsis . FEBS Lett 370: 264–268 [DOI] [PubMed] [Google Scholar]
- Rosales MA, Franco‐Navarro JD, Peinado‐Torrubia P, Díaz‐Rueda P, Álvarez R, Colmenero‐Flores JM (2020) Chloride improves nitrate utilization and NUE in plants. Front Plant Sci 11: 442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Y, Chen K, Su Y, Jiang S, Xu P, Murray JD (2021) A root tip‐specific expressing anthocyanin marker for direct identification of transgenic tissues by the naked eye in symbiotic studies. Plants 10: 605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D (1980) The mechanism of Cl− transport at the plasma‐membrane of Chara corallina 1. Cotransport with H+ . J Membr Biol 53: 129–141 [Google Scholar]
- Spooner W, McLaren W, Slidel T, Finch DK, Butler R, Campbell J, Eghobamien L, Rider D, Kiefer CM, Robinson MJ et al (2018) Haplosaurus computes protein haplotypes for use in precision drug design. Nat Commun 9: 4128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Bankston JR, Payandeh J, Hinds TR, Zagotta WN, Zheng N (2014) Crystal structure of the plant dual‐affinity nitrate transporter NRT1.1. Nature 507: 73–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Zheng N (2015) Molecular mechanism underlying the plant NRT1.1 dual‐affinity nitrate transporter. Front Physiol 6: 386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touraine B, Glass ADM (1997) NO3 − and ClO3 − fluxes in the chll‐5 mutant of Arabidopsis thaliana: does the CHLI‐5 gene encode a Low‐Affinity NO3 − transporter. Plant Physiol 114: 137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay YF, Schroeder JI, Feldmann KA, Crawford NM (1993) The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate‐inducible nitrate transporter. Cell 72: 705–713 [DOI] [PubMed] [Google Scholar]
- Valkov VT, Rogato A, Alves LM, Sol S, Noguero M, Léran S, Lacombe B, Chiurazzi M (2017) The nitrate transporter family protein LjNPF8.6 controls the N‐fixing nodule activity. Plant Physiol 175: 1269–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatter T, Neuhäuser B, Stetter M, Ludewig U (2015) Regulation of length and density of Arabidopsis root hairs by ammonium and nitrate. J Plant Res 128: 839–848 [DOI] [PubMed] [Google Scholar]
- Veidenberg A, Medlar A, Löytynoja A (2016) Wasabi: an integrated platform for evolutionary sequence analysis and data visualization. Mol Biol Evol 33: 1126–1130 [DOI] [PubMed] [Google Scholar]
- Walch‐Liu P, Forde BG (2008) Nitrate signalling mediated by the NRT1.1 nitrate transporter antagonises L‐glutamate‐induced changes in root architecture. Plant J 54: 820–828 [DOI] [PubMed] [Google Scholar]
- Wang QI, Huang Y, Ren Z, Zhang X, Ren J, Su J, Zhang C, Tian J, Yu Y, Gao GF et al (2020) Transfer cells mediate nitrate uptake to control root nodule symbiosis. Nat Plants 6: 800–808 [DOI] [PubMed] [Google Scholar]
- Wang R, Xing X, Wang Y, Tran A, Crawford NM (2009) A genetic screen for nitrate regulatory mutants captures the nitrate transporter gene NRT1. 1. Plant Physiol 151: 472–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wege S, Gilliham M, Henderson SW (2017) Chloride: not simply a ‘cheap osmoticum’, but a beneficial plant macronutrient. J Exp Bot 68: 3057 [DOI] [PubMed] [Google Scholar]
- Weigl J (1969) [Efflux and transport of Cl(‐) and Rb (+) in corn roots. Action of external KCl, Ca(++), EDTA and IAA] [Article in German]. Planta 84, 311–323. [DOI] [PubMed] [Google Scholar]
- Wen Z, Tyerman SD, Dechorgnat J, Ovchinnikova E, Dhugga KS, Kaiser BN (2017) Maize NPF6 proteins are homologs of Arabidopsis CHL1 that are selective for both nitrate and chloride. Plant Cell 29: 2581–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JY, Tian WH, Jin CW (2019) A reevaluation of the contribution of NRT1.1 to nitrate uptake in Arabidopsis under low‐nitrate supply. FEBS Lett 593: 2051–2059 [DOI] [PubMed] [Google Scholar]
- Yendrek CR, Lee YC, Morris V, Liang Y, Pislariu CI, Burkart G, Meckfessel MH, Salehin M, Kessler H, Wessler H et al (2010) A putative transporter is essential for integrating nutrient and hormone signaling with lateral root growth and nodule development in Medicago truncatula . Plant J 62: 100–112 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Mao G, Liu M, Zhang L, Wang X, Zhang XC (2014) Crystal structure of the E. coli peptide transporter YbgH. Structure 22: 1152–1160 [DOI] [PubMed] [Google Scholar]
- Zhou J‐J, Miller AJ (2000) Comparison of the transport properties of three plant sucrose carriers expressed in Xenopus oocytes. Aust J Plant Physiol 27: 721–728 [Google Scholar]
- Zhou J‐J, Theodoulou FL, Muldin I, Ingemarsson B, Miller AJ (1998) Cloning and functional characterization of a Brassica napus transporter which is able to transport nitrate and histidine. J Biol Chem 273: 12017–12033 [DOI] [PubMed] [Google Scholar]
- Zhu J, Fang XZ, Dai YJ, Zhu YX, Chen HS, Lin XY, Jin CW (2019) Nitrate transporter 1.1 alleviates lead toxicity in Arabidopsis by preventing rhizosphere acidification. J Exp Bot 70: 6363–6374 [DOI] [PMC free article] [PubMed] [Google Scholar]


