Abstract
OBJECTIVE:
Many ICU survivors suffer disabling long-term cognitive impairment (LTCI) after critical illness. We compared EEG characteristics during critical illness with patients’ one-year neuropsychological outcomes.
METHODS:
We performed a post-hoc analysis of patients in the BRAIN-ICU study (Pandharipande, NEJM 2013) who had undergone EEG for clinical purposes during admission (N=10). All survivors underwent formal cognitive assessments at 12-month follow-up. We evaluated EEGs by conventional visual inspection and computed 10 quantitative features. We explored associations between EEG and patterns of LTCI using Wilcoxon Rank-Sum tests and Spearman’s rank correlations.
RESULTS:
Of 521 Vanderbilt patients enrolled in the parent study, 24 had EEG recordings during admission. Ten survivors had EEG tracings available and completed follow-up cognitive testing. All but one inpatient EEG showed generalized background slowing. All patients demonstrated cognitive impairment in at least one domain at follow-up. The most common deficits occurred in delayed memory (DM – median index 62) and visuospatial/constructional (VC – median index 69) domains. Relative alpha power correlated with VC score (rho=0.78, p=0.008). Peak interhemispheric coherence correlated negatively with DM (rho=−0.81, p=0.018).
CONCLUSIONS:
Quantitative EEG features during critical illness correlated with domain-specific cognitive performance in our small cohort of ICU survivors. Further study in larger prospective cohorts is required to determine whether these relationships hold.
SIGNIFICANCE:
EEG may serve as a prognostic biomarker predicting patterns of long-term cognitive impairment.
Keywords: critical care, electroencephalography, cognition, dementia, quantitative EEG, delirium
1. INTRODUCTION
Many survivors of critical illness suffer lasting cognitive impairment after hospital discharge.1–3 These newly acquired cognitive deficits impact employment, interpersonal relationships, mental health and overall quality of life.1,4,5 There is also an increased risk of depression and rehospitalization among those who suffer the most severe cognitive decline.6,7
Risk factors for new long-term cognitive impairment (LTCI) after critical illness include older age at admission, presence and duration of delirium and multi-organ system failure.8,9 The underlying pathophysiology of critical illness-related LTCI is poorly understood. An understanding of the pathophysiologic mechanisms leading to critical illness-related brain injury will direct the development of therapeutic and preventative interventions and help identify the patients most likely to benefit from them.10
Electroencephalography (EEG) provides a noninvasive evaluation of neurophysiologic activity. EEG features during critical illness vary from person to person, depending on presence and degree of brain dysfunction. EEG features such as background slowing, generalized periodic discharges with triphasic morphology (also referred to as “triphasic waves”) and disorganization (absence of a normal posterior dominant rhythm) are typically seen in delirium.11–13 Additionally, quantitative features derived from computational analysis of the EEG signals (quantitative EEG, or qEEG) may give insight to the underlying physiologic processes. Quantitative EEG has been evaluated extensively to help prognosticate neurologic function after cardiac arrest,14 traumatic brain injury15,16 and stroke.17,18 To our knowledge no study has examined the relationship between EEG features during critical illness and the detailed patterns of cognitive impairment.
We aimed to characterize conventional and quantitative EEG findings in patients with and without LTCI after critical illness. We hypothesized that EEG features associated with delirium would be more prevalent among patients with subsequent long-term cognitive impairment than in those with normal cognition at 12 month follow-up. We sought to test this hypothesis in a convenience sample of critically ill patients for whom EEGs were performed during their inpatient hospitalization and who completed formal neuropsychological testing 12 months after their hospitalization. We further sought to characterize the relationships between quantitative EEG features and performance in the most strongly affected domains of cognition in the same patients. Our results will inform future studies using EEG to investigate the pathophysiologic link between critical illness and long-term cognitive impairment.
2. METHODS
2.1. Participants and Cognitive Evaluation
This is a nested cohort study of patients at Vanderbilt University Hospital who were previously enrolled in the Bringing to Light the Risk Factors And Incidence of Neuropsychological dysfunction in ICU survivors (BRAIN-ICU) study, in which long term neuropsychological outcomes of critical illness were prospectively examined in patients with respiratory failure, cardiogenic shock or septic shock. Study procedures were approved by the Institutional Review Board of Vanderbilt University Medical Center. Written informed consent was provided by participants or their surrogates.
Methods for the BRAIN-ICU study are described in detail elsewhere.9 Briefly, patients or their surrogates were recruited on admission to one of the critical care units at Vanderbilt University Hospital or Saint Thomas Hospital in Nashville, TN. Participants were assessed for delirium daily during hospitalization by trained research staff using the Confusion Assessment Method for the ICU (CAM-ICU)19 and underwent neurocognitive assessments, including the Repeatable Battery for Assessment of Neurological Status (RBANS)20 by trained psychology professionals at 3 and 12 months after discharge. Participants were screened for prior cognitive impairment at enrollment through structured interview questions and the AD8.21 Those who were over 50 years old or who reported problems with memory or thinking prior to their illness were screened for dementia using the Short Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE)22 and the Clinical Dementia Rating (CDR) Scale.23 Participants with a score of 3.3 or more on the IQCODE22 and score greater than 2.0 on the Clinical Dementia Rating Scale were excluded.
For the current study, we included Vanderbilt participants on whom EEG was recorded for clinical purposes during the inpatient stay and for whom 12-month follow-up RBANS data were available. The RBANS is a validated neuropsychometric evaluation of global cognition. It includes subtests spanning 5 domains: immediate memory, delayed memory, visuospatial/construction, language and attention. Global cognitive impairment was defined as a total RBANS score less than 1.5 standard deviation (SD) below the age-adjusted population mean. Domain-specific cognitive impairment was defined as an indexed score less than 1.5 SD below the age adjusted population mean for that specific domain. These cutoffs were based on prior literature suggesting 1.5 SD as an optimal screening indicator for Alzheimer’s disease.24
2.2. EEG Analysis
EEGs were recorded as clinically indicated using a commercial Nicolet data acquisition system. All EEG recordings used a sampling rate of 250Hz and a 19-channel montage with ground and reference according to the International 10–20 system of electrode placement.
EEG traces were reviewed by a neurologist blinded to the original report (SWR), using NicoletOne EEG Reader™ (Natus Medical Inc., Pleasanton, CA). The following conventional EEG abnormalities were noted: absence of posterior dominant rhythm (PDR), slow PDR (defined as less than 8Hz), focal irregular slowing (delta or theta activity), generalized irregular slowing, focal rhythmic slowing, generalized or bilaterally synchronous rhythmic slowing, generalized periodic discharges with triphasic morphology, burst suppression, epileptiform discharges, electrographic seizures and absence of background reactivity. Slow PDR was differentiated from generalized irregular slowing when theta activity was present in the posterior regions with preserved anterior-to-posterior gradient of frequencies. In case of discrepancy between the original EEG report and visual assessment, a second clinical neurophysiologist (RST) adjudicated the findings.
Additionally, EEG data underwent quantitative analysis as follows: recordings were exported from the clinical archives in European Data Format (EDF) and imported to EEGLAB25 for preprocessing. Recordings were detrended and band-pass filtered to 0.1Hz and 50Hz using a finite impulse response filter. The signals thus obtained were visually inspected and channels as well as periods with excessive artifact excluded. The remaining EEG was then referenced to a common average reference to remove global effects, e.g. due to reference contamination. Recordings were then divided into three-second consecutive, nonoverlapping epochs for subsequent feature calculation.
We used proprietary MATLAB functions based on the Chronux toolkit (http://chronux.org)26 to compute a series of quantitative features, averaging them across epochs and across channels. We computed relative spectral power and spectral variability27 in the delta (1–4Hz), theta (4–8Hz), alpha (8–13Hz) and beta (13–25Hz) ranges. Relative power was computed as the ratio of band power to the total absolute power in the 1–40Hz range. Spectral variability was computed as the ratio of standard deviation (across epochs) in relative power to the mean (across epochs) relative power in each frequency band. as well as peak interhemispheric coherence between anterior temporal channels (F7, F8) in the 1–8Hz range. We also computed approximate entropy (ApEn), a measure of the complexity of the EEG amplitude over time. ApEn can take on any value greater than 0, with higher values indicating increased complexity. Lower values are seen in sleep, dementia, anesthetic states and delirium. Consistent with prior literature28 we estimated ApEn using window length of 1 and tolerance of 0.25 times the signal standard deviation, averaged across all electrode contacts for each patient. Other authors have concluded that using these parameters, physiological data produce good statistical validity of ApEn.29
2.3. Statistical Analysis
We used the Wilcoxon rank sum test to compare cognitive test scores among patients with and without each conventional EEG abnormality. We used Spearman’s rank correlations to look for associations between qEEG features and cognitive test scores. To minimize effects of multiple comparisons, we selected the global score and the two most strongly affected subdomains for statistical analysis. All statistical analyses were performed using standard functions in MATLAB R2018b (MathWorks, Natick, MA).
3. RESULTS
3.1. Demographic and Clinical Profiles
Of 521 patients enrolled at Vanderbilt in the parent study, 24 had EEGs recorded for clinical purposes during their index admission. Eight patients died and 4 were lost to 12-month follow-up. Two patients were excluded as we were unable to obtain their EEG tracings from hospital archives. Table 1 describes the clinical and demographic features for the remaining 10 participants. Of these, 6 had EEGs performed prior to ICU discharge. None of the patients had preexisting neurologic disease and all participants screened negative for evidence of premorbid memory or cognitive dysfunction. Median age was 45 and 50% of the patients were male. Seven screened positive for delirium or coma on the day of the EEG recording. Delirium screening occurred a median of 53 minutes prior to EEG recordings, with two participants having delirium screening during the EEG. The most common indication for EEG was altered mental status, with or without a shaking spell concerning for seizure. Median ICU length of stay (LOS) was 12 days, and median hospital LOS was 29.5 days.
Table 1:
Clinical and Demographic Characteristics
| ID | Age (years)/ Sex |
Reason for Intubation | Charlson Score | Delirium | ICU LOS / Hosp LOS (days) |
Reason for EEG | EEG Location | EEG Duration (min) | Time from CAM-ICU (hr:min) | Sedatives |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 43/F | Sepsis/ARDS | 0 | Positive | 20 / 28 | Agitation | ICU | 20 | 0:36 | Fentanyl, Propofol |
| 2 | 72/F | Sepsis/ARDS | 2 | Positive | 11 / 12 | AMS | ICU | 25 | 0:41 | None |
| 3 | 63/F | Sepsis/ARDS | 1 | Positive | 13 / 44 | AMS | floor | 21 | 1:12 | None |
| 4 | 41/M | Respiratory | 2 | Negative | 7 / 30 | Shaking spell | ICU | 150 | 0:00 | Propofol |
| 5 | 61/M | CHF | 4 | Negative | 111 / 115 | AMS | ICU | 25 | 3:10 | None |
| 6 | 31/M | Sepsis/ARDS | 0 | UTA | 18 / 29 | AMS | ICU | 20 | 0:50 | Midazolam |
| 7 | 47/M | Airway | 1 | Positive | 12 / 51 | Coma | ICU | 20 | 0:55 | None |
| 8 | 65/M | Colonic Surgery | 6 | Positive | 11 / 28 | Shaking spell | floor | 21 | 4:47 | None |
| 9 | 21/F | Respiratory | 1 | Negative | 12 / 54 | Seizure | floor | 20 | 0:00 | Hydromorphone, Oxycodone, Lorazepam |
| 10 | 35/F | Respiratory | 0 | Positive | 6 / 12 | AMS | floor | 27 | 7:13 | None |
Clinical and demographic characteristics of BRAIN-ICU participants for whom EEG tracings were recorded during hospitalization and cognitive testing completed at 12 months. Delirium assessments were performed using the CAM-ICU. Time from CAM-ICU reflects timespan between delirium assessment and EEG recording. Sedatives reflect sedating medications administered within 24h of EEG recording. AMS: Altered Mental Status; ARDS: Acute Respiratory Distress Syndrome; CHF: Congestive Heart Failure or Cardiogenic Shock; F: Female; ICU: Intensive Care Unit; LOS: Length of Stay; M: Male; Other: Respiratory failure not attributable to sepsis or ARDS; UTA: Unable to Assess (due to coma)
3.2. Conventional EEG features
Of the ten conventional EEG abnormalities evaluated, 7 were present in at least one EEG (Table 2). The most common abnormality was generalized irregular slowing, followed by slow posterior dominant rhythm (PDR).
Table 2:
Conventional EEG Abnormalities
| Participant | No PDR | Slow PDR | Focal Irregular Slowing |
Generalized Irregular Slowing | Generalized Rhythmic Slowing | No Reactivity | Low Amplitude |
|---|---|---|---|---|---|---|---|
| 1 | X | X | |||||
| 2 | X | X | |||||
| 3 | X | ||||||
| 4 | X | X | X | ||||
| 5 | X | X | X | ||||
| 6 | X | X | |||||
| 7 | X | X | X | X | |||
| 8 | X | X | |||||
| 9 | X | X | X | ||||
| 10 |
Presence of common EEG abnormalities on visual inspection. Ratings required agreement by at least two clinical neurophysiologists. All but one study had background slowing (generalized irregular slowing or slow PDR). None of this cohort of patients had focal rhythmic slowing, triphasic waves, burst suppression, interictal epileptiform discharges or seizures. One participant (4) did have normal sleep architecture – this EEG was recorded for 2.5 hours. PDR: Posterior Dominant Rhythm
3.3. Cognitive Outcomes
Table 3 details the 12-month cognitive testing results as global and subdomain scores on the RBANS. Roughly half the participants exhibited global cognitive impairment. The most commonly affected domains were Delayed Memory (median 62, range 48–81) and Visuospatial/Constructional (median 69, range 50–84), whereas Immediate Memory and Language remained intact in most participants.
Table 3:
Cognitive Testing Scores at 12 Months
| Participant | Immediate Memory | Visuospatial Constructional | Language | Attention | Delayed Memory | GLOBAL |
|---|---|---|---|---|---|---|
| 1 | 106 | 50 | 103 | 106 | 78 | 84 |
| 2 | 106 | 64 | 96 | 94 | 81 | 84 |
| 3 | 109 | 81 | 87 | 112 | 60 | 85 |
| 4 | 103 | 84 | 100 | 106 | 75 | 90 |
| 5 | 97 | 69 | 108 | 106 | 68 | 85 |
| 6 | 81 | 78 | 89 | 100 | 48 | 74 |
| 7 | 109 | 66 | 85 | 72 | 56 | 72 |
| 8 | 100 | 69 | 101 | 72 | 64 | 77 |
| 9 | 103 | 69 | 96 | 68 | 60 | 74 |
| 10 | 81 | 60 | 74 | 53 | 52 | 55 |
Normalized RBANS scores at 12 months after hospital discharge. Scores more than 1.5 standard deviation below norm are indicated in bold red. Five of the 10 participants demonstrated global cognitive impairment. Delayed Memory (80% impaired, median 62) and Visuospatial/Constructional (70% impaired, median score 69) were the most commonly affected domains in this cohort.
3.4. Comparison between EEG Features and RBANS Scores
We found no statistically significant differences in cognitive test scores between patients with and without any of the identified conventional EEG abnormalities (Supplementary Table). The Figure depicts correlation coefficients between qEEG features and 12-month global, visuospatial/constructional and delayed memory scores. Relative alpha power correlated with visuospatial/constructional ability (rho=0.78, p=0.008). Peak interhemispheric coherence correlated inversely with delayed memory (rho = −0.81, p=0.018). Because EEG findings are impacted by severity of neurologic impairment and because neurologic status often fluctuates during the course of admission, we considered that the relationship between EEG findings and long term cognition may be influenced by the setting in which EEG was acquired (during hyperacute/critical phase vs during subacute/early recovery phase). We thus used location of EEG (ICU vs floor) as a proxy for phase of illness at the time of EEG recording and conducted post-hoc sub analyses restricted to participants whose EEGs were recorded in the ICU. In this subset, peak interhemispheric coherence again correlated inversely with delayed memory (rho=−1.0, p=0.017) and theta-range spectral variability correlated negatively with delayed memory (rho=−0.89, p=0.033).
Figure: Summary of Correlations Between Quantitative EEG Features and Cognitive Scores.
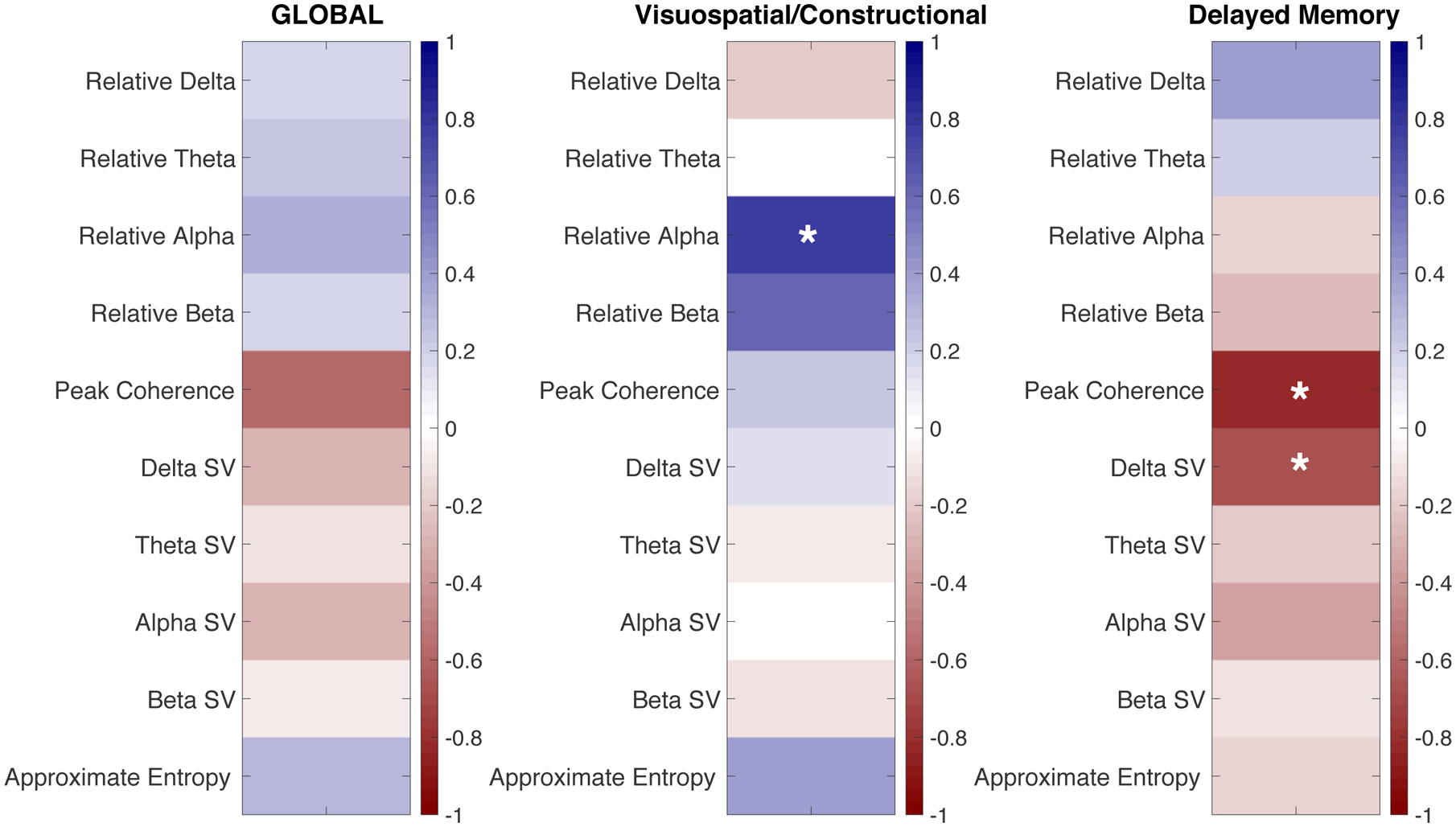
Correlation coefficients using Spearman’s rank correlation between 10 quantitative EEG features and 12-month global RBANS scores (A), visuospatial/constructional scores (B) and delayed memory scores (C). P-Values less than 0.05 are marked with an asterisk (*). Red colors indicate negative correlations, blue indicates positive correlations. Relative alpha power correlated with long-term visuospatial/constructional ability (rho = 0.78, p = 0.008), while peak interhemispheric coherence and spectral variability in the delta range correlated inversely with long-term delayed memory performance (rho = −0.81, p = 0.018 and rho = −0.657, p = 0.039, respectively). For example, persons with higher relative alpha power showed better long-term visuospatial/constructional ability, but those with higher peak coherence showed poor delayed memory at 12 months. RBANS: Repeatable Battery for the Assessment of Neurocognitive Status, SV: Spectral Variability
4. DISCUSSION
This study examined associations between EEG characteristics in the acute setting and cognitive test results at 12 months after discharge in a convenience sample of patients hospitalized for critical illness. Half of our participants scored less than 1.5 standard deviations below the norm for age at 12 months after discharge, indicating at least mild cognitive impairment.20 Specific cognitive tests in this range point to increased risk of developing dementia within the next 4 years among the elderly,30 though it is unknown whether this marker would hold true in the general population. Indeed, an emerging body of literature points toward increased risk of developing dementia after critical illness.4,8,9 There is a pressing need to understand this pathophysiology and remediate its effects.
Despite the small sample, we found a statistically significant correlation between relative alpha power and visuospatial/constructional ability, and a negative correlation between low frequency interhemispheric coherence and delayed memory. Thus, persons with lower relative alpha power in the hospital showed worse visuospatial/constructional ability at 12 months after discharge, and those with higher interhemispheric coherence showed worse delayed memory performance on follow-up. Recognizing that EEG patterns may change with brain state, and brain state may be impacted by acuity or severity of the current condition, we also performed a post-hoc analysis exploring the subset of EEGs acquired during the hypercritical period (while patients were in the ICU). When the cohort was restricted to those with studies performed in the ICU, spectral variability in the theta range also correlated negatively with delayed memory. These findings are consistent with general principles of clinical electroencephalography wherein lower frequency (delta and theta) activity in the awake state suggests cerebral dysfunction whereas healthier brains typically produce faster frequencies (alpha and beta).31 In a systematic review of studies of Parkinson’s Disease patients, theta and delta activity negatively correlated with cognitive performance while alpha activity showed a positive correlation.32 Low frequency interhemispheric coherence increases with sevoflurane anesthesia in healthy subjects and suggests phase coupling across the cerebral hemispheres. This has been postulated to be a result of unmasked thalamocortical reverberant networks that may interfere with cortical information processing, or it may indicate thalamic deafferentation.33,34
In our cohort, conventional EEG abnormalities ascertained by visual inspection did not demonstrate significant associations with global cognitive function or with cognitive testing scores in individual domains. In larger samples, conventional abnormalities (specifically generalized slowing) were previously shown to correlate with functional outcomes.35 The different results found here may be a function of our smaller sample size, but could also be due to our longer time to follow-up and more specific outcome assessment. Although generalized slowing is a known marker of acute brain dysfunction and in-hospital mortality, it remains to be seen whether this feature can distinguish subtle variations in function among a cohort of long-term ICU survivors. In contrast, using quantitative EEG metrics we were able to identify a relationship with EEG along a continuum of cognitive outcomes even with a small dataset. This may reflect the more nuanced picture of cerebral function that may be derived from continuous, objective descriptors (as opposed to dichotomous features). Taken together, these findings support the notion that quantitative EEG metrics recorded during critical illness hold promise as potential biomarkers of lasting brain injury that commonly occurs in association with critical illness.
EEG patterns at the time of acute illness can be influenced by a number of factors. Baseline cerebral function, sleep-wake cycles, the impact of acute illness on cerebral activity and effect of sedative or neurotropic medications may all play a role. In a series of 214 general ICU patients who received EEGs to evaluate coma of unclear etiology, anoxia was associated with increased in-hospital mortality while other clinical diagnoses (infection, trauma, metabolic compromise) had lower mortality rates. Comatose patients with anoxia had higher odds of EEG suppression, whereas EEGs of patients with metabolic compromise were less likely to show this feature.36 A prospective cohort of nonintubated inpatients referred for EEG to evaluate altered mental status found that generalized EEG slowing was associated with longer hospital stays, worse in-hospital mortality and lower Glasgow Outcome Scores at discharge.35 Both of these studies, like the current investigation, evaluated patients who were referred for EEG based on clinical concerns. In a series of 125 prospectively evaluated mechanically ventilated patients recruited independent of clinical indication for EEG, voltage suppression (burst suppression as calculated by a BIS A1050 EEG monitor) during clinical delirium assessments was associated with an increased risk of death at 6 months (HR 2.04, 95%CI 1.12–3.70), even after adjusting for clinical factors including severity of illness.37 Regardless of whether EEG changes reflect severity of underlying illness, preexisting brain dysfunction or drug effect, the present study suggests that EEG may offer a noninvasive tool for early identification of specific patterns of cognitive deficits. Such a tool may find particular value in guiding early cognitive rehabilitation approaches in a patient population for whom brief neurocognitive screens have failed to predict long-term cognitive outcomes38 and focused participation in more comprehensive neurocognitive testing procedures has proven difficult.39
This study was limited by its small sample size, which precluded statistical controls for potential effects of sedatives and organ failure. Also, given that patients were included based on a clinical indication for EEG, the generalizability of our findings is limited. Necessarily, only those patients who survived with sufficient functional reserve to undergo neurocognitive testing at 12 months were included. This represents a diminished but important subset of ICU survivors. The small number of data points and substantial number of comparisons explored in this analysis raises the risk of both false positive and false negative correlations. These findings underscore the need for larger, prospective studies that can rigorously evaluate potential associations between specific quantitative EEG metrics and patterns of LTCI as well as the implications of specific EEG metrics with regard to physiologic function in the acute setting. An understanding of this physiology will allow us to exploit EEG as a physiologic biomarker of cognitive function and potentially as a prognostic biomarker of long-term cognitive impairment, which could pave the way to targeted cognitive rehabilitation therapies to combat this debilitating condition.
5. CONCLUSIONS
We found evidence that quantitative features of the acute, inpatient EEG correlate with domain-specific cognitive performance at 12-month follow-up. These findings suggest that quantitative EEG during critical illness may provide sensitive and viable biomarkers for prediction of specific patterns of long-term cognitive impairment after critical illness. Further study is needed to confirm this hypothesis and to determine the implications of specific qEEG profiles. This line of study is expected to yield a better understanding of the neurophysiology of acute brain injury in critical illness and its link to chronic cognitive dysfunction, informing development of targeted intervention and rehabilitation strategies for millions of survivors of critical illness.
Supplementary Material
6. ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health [grant numbers R01AG027472, R01HL111111]; the Department of Veterans Affairs Tennessee Valley Health Care System Geriatric Research, Education and Clinical Center (GRECC) and VA-MERIT.
Role of the Funder/Sponsor:
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The contents of this paper are solely the responsibility of the authors and do not necessarily represent those of the National Institutes of Health or the Department of Veterans Affairs.
Footnotes
Competing Interests:
Dr. Pandharipande has received a research grant from Hospira. Dr. Ely has received research grants and/or honoraria from Hospira, Orion, Pfizer, Abbott and Masimo. The remaining authors report no financial conflicts of interest.
REFERENCES
- 1.Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED, Larson LV. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. American journal of respiratory and critical care medicine. 1999;160(1):50–56. [DOI] [PubMed] [Google Scholar]
- 2.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA : the journal of the American Medical Association. 2010;304(16):1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilcox ME, Brummel NE, Archer K, Ely EW, Jackson JC, Hopkins RO. Cognitive dysfunction in ICU patients: risk factors, predictors, and rehabilitation interventions. Crit Care Med. 2013;41(9 Suppl 1):S81–98. [DOI] [PubMed] [Google Scholar]
- 4.Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF Jr. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171(4):340–347. [DOI] [PubMed] [Google Scholar]
- 5.Rothenhausler HB, Ehrentraut S, Stoll C, Schelling G, Kapfhammer HP. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: results of an exploratory study. Gen Hosp Psychiat. 2001;23(2):88–94. [DOI] [PubMed] [Google Scholar]
- 6.Hopkins RO, Brett S. Chronic neurocognitive effects of critical illness. Current opinion in critical care. 2005;11(4):369–375. [DOI] [PubMed] [Google Scholar]
- 7.Chodosh J, Seeman TE, Keeler E, et al. Cognitive decline in high-functioning older persons is associated with an increased risk of hospitalization. J Am Geriatr Soc. 2004;52(9):1456–1462. [DOI] [PubMed] [Google Scholar]
- 8.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Critical Care Medicine. 2010;38(7):1513–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. The New England journal of medicine. 2013;369(14):1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fong TG, Inouye SK, Jones RN. Delirium, Dementia, and Decline. JAMA Psychiatry. 2017;74(3):212–213. [DOI] [PubMed] [Google Scholar]
- 11.Romano J, Engel GL. Delirium: Electroencephalographic data. Arch Neurol Psychiatry. 1944;51:356–377. [Google Scholar]
- 12.Kaplan PW. The EEG in metabolic encephalopathy and coma. J Clin Neurophysiol. 2004;21(5):307–318. [PubMed] [Google Scholar]
- 13.Kaplan PW, Rossetti AO. EEG patterns and imaging correlations in encephalopathy: encephalopathy part II. J Clin Neurophysiol. 2011;28(3):233–251. [DOI] [PubMed] [Google Scholar]
- 14.Tiainen M, Poutiainen E, Kovala T, Takkunen O, Happola O, Roine RO. Cognitive and neurophysiological outcome of cardiac arrest survivors treated with therapeutic hypothermia. Stroke. 2007;38(8):2303–2308. [DOI] [PubMed] [Google Scholar]
- 15.Tolonen A, Sarkela MOK, Takala RSK, et al. Quantitative EEG Parameters for Prediction of Outcome in Severe Traumatic Brain Injury: Development Study. Clin EEG Neurosci. 2018;49(4):248–257. [DOI] [PubMed] [Google Scholar]
- 16.Haveman ME, Van Putten M, Hom HW, Eertman-Meyer CJ, Beishuizen A, Tjepkema-Cloostermans MC. Predicting outcome in patients with moderate to severe traumatic brain injury using electroencephalography. Crit Care. 2019;23(1):401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bentes C, Peralta AR, Viana P, et al. Quantitative EEG and functional outcome following acute ischemic stroke. Clin Neurophysiol. 2018;129(8):1680–1687. [DOI] [PubMed] [Google Scholar]
- 18.Aminov A, Rogers JM, Johnstone SJ, Middleton S, Wilson PH. Acute single channel EEG predictors of cognitive function after stroke. PLoS One. 2017;12(10):e0185841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA : the journal of the American Medical Association. 2001;286(21):2703–2710. [DOI] [PubMed] [Google Scholar]
- 20.Duff K, Hobson VL, Beglinger LJ, O’Bryant SE. Diagnostic accuracy of the RBANS in mild cognitive impairment: limitations on assessing milder impairments. Arch Clin Neuropsychol. 2010;25(5):429–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65(4):559–564. [DOI] [PubMed] [Google Scholar]
- 22.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) - Socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19(4):1015–1022. [DOI] [PubMed] [Google Scholar]
- 23.Berg L Clinical Dementia Rating. Br J Psychiatry. 1984;145:339. [PubMed] [Google Scholar]
- 24.Duff K, Humphreys Clark JD, O’Bryant SE, Mold JW, Schiffer RB, Sutker PB. Utility of the RBANS in detecting cognitive impairment associated with Alzheimer’s disease: sensitivity, specificity, and positive and negative predictive powers. Arch Clin Neuropsychol. 2008;23(5):603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. [DOI] [PubMed] [Google Scholar]
- 26.Mitra P, Bokil H. Observed brain dynamics. Oxford; New York: Oxford University Press; 2008. [Google Scholar]
- 27.van der Kooi AW, Slooter AJ, van Het Klooster MA, Leijten FS. EEG in delirium: Increased spectral variability and decreased complexity. Clin Neurophysiol. 2014;125(10):2137–2139. [DOI] [PubMed] [Google Scholar]
- 28.Abasolo D, Hornero R, Espino P, Poza J, Sanchez CI, de la Rosa R. Analysis of regularity in the EEG background activity of Alzheimer’s disease patients with Approximate Entropy. Clin Neurophysiol. 2005;116(8):1826–1834. [DOI] [PubMed] [Google Scholar]
- 29.Pincus S Approximate entropy (ApEn) as a complexity measure. Chaos. 1995;5(1):110–117. [DOI] [PubMed] [Google Scholar]
- 30.Masur DM, Sliwinski M, Lipton RB, Blau AD, Crystal HA. Neuropsychological prediction of dementia and the absence of dementia in healthy elderly persons. Neurology. 1994;44(8):1427–1432. [DOI] [PubMed] [Google Scholar]
- 31.Semmler A, Widmann CN, Okulla T, et al. Persistent cognitive impairment, hippocampal atrophy and EEG changes in sepsis survivors. J Neurol Neurosurg Psychiatr. 2013;84(1):62–69. [DOI] [PubMed] [Google Scholar]
- 32.Cozac VV, Gschwandtner U, Hatz F, Hardmeier M, Ruegg S, Fuhr P. Quantitative EEG and Cognitive Decline in Parkinson’s Disease. Parkinsons Dis. 2016;2016:9060649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayashi K, Mukai N, Sawa T. Simultaneous bicoherence analysis of occipital and frontal electroencephalograms in awake and anesthetized subjects. Clin Neurophysiol. 2014;125(1):194–201. [DOI] [PubMed] [Google Scholar]
- 34.Akeju O, Westover MB, Pavone KJ, et al. Effects of sevoflurane and propofol on frontal electroencephalogram power and coherence. Anesthesiology. 2014;121(5):990–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimchi EY, Neelagiri A, Whitt W, et al. Clinical EEG slowing correlates with delirium severity and predicts poor clinical outcomes. Neurology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young GB, Kreeft JH, McLachlan RS, Demelo J. EEG and clinical associations with mortality in comatose patients in a general intensive care unit. J Clin Neurophysiol. 1999;16(4):354–360. [DOI] [PubMed] [Google Scholar]
- 37.McHugh D, Cameron CA, Abdenur JE, et al. Clinical validation of cutoff target ranges in newborn screening of metabolic disorders by tandem mass spectrometry: a worldwide collaborative project. Genet Med. 2011;13(3):230–254. [DOI] [PubMed] [Google Scholar]
- 38.Pfoh ER, Chan KS, Dinglas VD, et al. Cognitive screening among acute respiratory failure survivors: a cross-sectional evaluation of the Mini-Mental State Examination. Crit Care. 2015;19:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson JC, Morandi A, Girard TD, et al. Functional brain imaging in survivors of critical illness: A prospective feasibility study and exploration of the association between delirium and brain activation patterns. J Crit Care. 2015;30(3):653.e1–653.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


