Abstract
Brain tumors and brain metastases induce changes in brain tissue remodeling that lead to immunosuppression and trigger an inflammatory response within the tumor microenvironment. These immune and inflammatory changes can influence invasion and metastasis. Other neuroinflammatory and necrotic lesions may occur in patients with brain cancer or brain metastases as sequelae from treatment with radiotherapy. Glioblastoma (GBM) is the most aggressive primary malignant brain cancer in adults. Imaging methods such as positron emission tomography (PET) and different magnetic resonance imaging (MRI) techniques are highly valuable for the diagnosis and therapeutic evaluation of GBM and other malignant brain tumors. However, differentiating between tumor tissue and inflamed brain tissue with imaging protocols remains a challenge. Here, we review recent advances in imaging methods that have helped to improve the specificity of primary tumor diagnosis versus evaluation of inflamed and necrotic brain lesions. We also comment on advances in differentiating metastasis from neuroinflammation processes. Recent advances include the radiosynthesis of 18F‐FIMP, an L‐type amino acid transporter 1 (LAT1)‐specific PET probe that allows clearer differentiation between tumor tissue and inflammation compared to previous probes, and the combination of different advanced imaging protocols with the inclusion of radiomics and machine learning algorithms.
Keywords: brain metastases, brain tumor, glioblastoma, imaging, magnetic resonance imaging, positron emission tomography
Brain tumors induce immune and inflammatory changes in the brain tissue that influence invasion and metastasis. Neuroinflammatory and necrotic lesions also occur in patients with brain cancer, as a result of radiotherapy. We review advances in imaging techniques help to differentiate primary brain tumors and metastases from neuroinflammation.
INTRODUCTION
Neuroimaging techniques are crucial tools for diagnosing, staging and monitoring the treatment effects in patients with brain cancers [1]. Structural magnetic resonance imaging (MRI) helps to identify, classify and grade brain tumors, as well as to guide surgery [2]. Complementary information is obtained with positron emission tomography (PET) imaging where insights on tissue metabolism can be evaluated, which is particularly valuable for the measurement of fast cell proliferation in tumors and investigation of early‐stage tumors [3, 4, 5, 6].
Despite the current efficiency of these imaging techniques in brain tumor detection, some limitations remain to be overcome. For example, differentiating among relapsed brain tumors, solitary brain metastases and inflammatory and necrotic lesions resulting from chemo‐ and radiotherapy remains a challenge. For example, the most commonly used PET probe for tumor imaging is 2‐deoxy‐2‐18F‐fluoro‐D‐glucose, which is actively transported into cells via glucose transporters (GLUTs), accumulating not only in tumor tissue, which contains inflammatory processes itself, but also in other inflamed areas, where glucose metabolism is crucial for neuroinflammatory and neuroimmune responses. This can lead to false‐positive results for tumor diagnosis [7, 8, 9]. Also, both tumors and metastatic sites can display MRI patterns featuring peritumoral hyperintensities with similar intratumoral texture, thus representing a potential confounder for neuroradiologists [10]. In this review, we will discuss selected recent advances in imaging approaches aimed at improving the evaluation of brain tumors and brain metastases in relation to immunomodulation and neuroinflammatory processes. We will focus specifically upon one type of brain tumor, glioblastoma (GBM), given that it represents the most aggressive primary malignant brain cancer in adults.
GLIOBLASTOMA
The current World Health Organization (WHO) classification divides GBM tumors into three groups, depending on the status of the isocitrate dehydrogenase (IDH) gene: IDH wild‐type (IDHwt) GBM, mutated IDH or not specified GBM (NOS, unevaluated status). IDHwt GBMs represent approximately 90% of cases and typically appear in older patients, whereas approximately 10% of patients present tumors with an IDH mutation, which correspond to secondary GBMs which originate from lower‐grade gliomas, occur in younger patients and have a better prognosis. Furthermore, GBMs are defined by other genetic biomarkers, including O6‐methylguanine DNA methyltransferase (MGMT) promoter methylation, chromosome 1p/19q deletion, mutations of telomerase reverse transcriptase (TERT) promoter, tumor protein P53 (TP53) and phosphatase and tensin homolog (PTEN) and amplification of epidermal growth factor receptor (EGFR) and platelet‐derived growth factor receptor A (PDGFRA). The prognosis for patients with GBM is dismal; current treatment, based on combining surgical resection, radiotherapy and adjuvant chemotherapy, still results in a median overall survival of fewer than 2 years [11, 12, 13, 14, 15].
IMMUNOREGULATION AND INFLAMMATION IN GLIOBLASTOMA: BRAIN TUMORS AS NEUROINFLAMMATORY DISEASES
Inflammation is an integral component of cancer pathology, contributing to carcinogenesis and tumor progression. One common feature of GBM is tissue necrosis accompanied by microenvironment inflammation. Immunosuppressive inflammation with associated necrosis is typical of GBMs that display higher resistance to therapies and a worst prognosis [16, 17, 18, 19, 20]. GBM cells express and secrete immune suppressive chemokines and cytokines, including interleukin (IL)‐6, IL‐10, transforming growth factor (TGF)‐β, galectin‐1 and prostaglandin‐E, which act on infiltrating immune cells to hijack them by inducing a protumor cellular phenotype. Thus, a population of non‐neoplastic cells consisting mainly of tumor‐associated macrophages is established in the tumor site. These immunosuppressive changes, enabled by inflammatory mediators, stimulate GBM cell proliferation, migration, angiogenesis and resistance to treatment. For example, signaling mediated by C‐X‐C motif chemokine receptor 3 (CXCR3) and C‐C chemokine receptor type 2 (CCR2) receptors recruit tumor‐promoting immune cells such as T cells and myeloid‐derived suppressor cells [16, 21, 22, 23, 24, 25, 26, 27, 28]. In GBM cancer stem cells (CSCs), hypoxic tissue triggers expression of genes of the inflammatory reparative response [25, 29]. Cytokines such as IL‐1β and TGF‐β co‐operate to induce inflammatory gene expression and a proinflammatory phenotype in GBM CSCs which, in turn, facilitates cellular proliferation and migration [30].
NEUROINFLAMMATION AS A CONSEQUENCE OF GBM TREATMENT
Inflammation and necrosis of healthy brain tissue is one of the main unwanted effects of radiotherapy used to treat GBM. Studies have found incidences of radiation‐induced necrosis ranging from 2.5 to approximately 30% of GBM patient cohorts [31, 32, 33]. Necrosis can include underlying inflammation together with occlusive vasculopathy and perivascular parenchymal changes. Alterations in the integrity of the blood–brain barrier in inflamed sites leads to immune cell infiltration, fluid transudation into the interstitial space and brain edema. Infiltrated immune cells and reactive glial cells increase expression of mediators, amplifying the response [31, 34, 35, 36].
CONSEQUENCES OF INFLAMMATION FOR IMAGING DIAGNOSIS: DIFFERENTIATING BETWEEN TUMORS AND INFLAMMATORY LESIONS
Conventional neuroradiological techniques do not always allow a differentiation between radiation necrosis and GBM recurrence, given that both recurrent tumors and necrosis (pseudoprogressions) may appear as similar lesions in MRI protocols such as T2‐ and gadolinium‐enhanced T1‐weighted imaging, with an increase in contrast enhancement and the image of a mass [31, 37, 38, 39, 40, 41, 42]. PET imaging using 18F‐fluorodeoxyglucose PET, in turn, can be useful in differentiating radiation‐induced lesions from recurrent GBM [31, 43]. However, with this method false‐positive and ‐negative PET scan results can lead to low sensitivity and pose a severe limitation [44].
Imaging advances for the evaluation of brain tumors and neuroinflammation
The measurement of metabolic and cell physiology parameters using improved MRI techniques provides detailed information on tissue biochemical composition and blood perfusion, thus facilitating the distinction between tumor tissues and necrosis. Proton magnetic resonance (MR) spectroscopic imaging is used to evaluate cell metabolism through the detection of proton metabolites and their distribution. Chemicals detected include compounds containing choline, creatine, lactate, lipid and N‐acetylaspartate. Choline, a component of cell membrane phospholipids, is particularly useful for differentiating tumors from non‐tumor tissues because its content is increased in highly proliferative cell populations. Overall, higher choline levels are associated with disease progression or recurrence, whereas low levels of choline are found in necrotic lesions. The analysis of increased ratios of choline content in relation to other chemicals can result in an accuracy of up to 97% in separating tumors from non‐tumoral necrotic tissues [31, 42].
Conventional MRI, with the T1/T2 mismatch criterion, had a specificity of 75% and a sensitivity of only 44% in distinguishing between tumors and inflamed lesions. A PET scan combined the best sensitivity and specificity, respectively, of 92 and 69%. PET remained superior compared to NMR spectroscopy for choline/N‐acetylaspartate and choline/creatine ratios across different thresholds [45]. A retrospective study of 57 GBM patients examined with T2*‐weighted dynamic susceptibility‐weighted contrast material‐enhanced MRI found that mean, maximum and minimum relative peak height and relative cerebral blood volume were significantly higher in GBM cases compared to radiation necrosis cases. In contrast, mean, maximum and minimum relative percentages of signal intensity recovery values were significantly lower in recurrent GBM compared to radiation necrosis [46]. Proton MR spectroscopy showed a temporary elevation of choline in four of nine cases of necrosis, creating a confounding factor that could result in false‐positive findings for tumor recurrence [47]. Another study, using proton MR spectroscopy in 11 patients who received high‐dose radiotherapy, revealed that cases of radiation necrosis had either increased lactate/creatine and phosphocreatine ratio and decreased choline‐containing compounds/phosphocreatine ratio compared to recurrent GBM, or reductions in all major metabolites [48]. A meta‐analysis of 397 patients in 13 studies examined roles of several metabolites. MR spectroscopy and MR perfusion using choline/N‐acetylaspartate and choline/phosphocreatine ratios and relative cerebral blood volume (rCBV) may increase the accuracy of differentiating necrosis from recurrent tumor in patients with primary brain tumors or brain metastases [49].
A meta‐analysis of six studies with 118 patients and 134 scans indicated 11C‐choline PET as an accurate diagnostic method for the differentiation of tumor relapse from radiation‐induced necrosis in gliomas [50]. A study with 55 patients followed‐up for at least 11 months with suspected brain tumor recurrence or necrosis after radiotherapy, examined MRI, F18‐fluorodeoxyglucose and 11C‐choline PET/computerized tomography (CT), deciding on the superiority of 11C‐choline PET/CT [51]. In a F98 orthotopic rat model of GBM, PET using F18‐fluorodeoxyglucose and [18F]fluoro‐ethyl‐tyrosine PET were effective in discriminating GBM from radiation necrosis, with F18‐fluorodeoxyglucose delayed PET being particularly useful [34]. A study with 50 patients showed that (11)C‐methionine‐PET was superior to both (11)C‐choline and F18‐fluorodeoxyglucose‐PET for distinguishing GBM recurrence from radiation necrosis [52]. L‐type amino acid transporter 1 (LAT1) tumor‐specific PET tracer 2‐[18F]‐2‐fluoroethyl‐L‐phenylalanine PET is able to differentiate GBM from radiation necrosis and 2–2‐[18F]‐2‐fluoroethyl‐L‐phenylalanine uptake is less likely to be contaminated by the presence of inflammation than the F18‐fluorodeoxyglucose signal [35] (Table 1).
TABLE 1.
Summary of selected studies on imaging approaches for distinguishing brain tumors from neuroinflammation
| Technique | Main findings | References |
|---|---|---|
| Proton MR spectroscopic imaging | Higher levels of choline are associated with GBM tumor progression or recurrence, whereas low levels of choline indicate necrotic tissue | [31, 42] |
| The analysis of increased ratios of choline content in relation to other chemicals can separate tumors from necrosis with an accuracy of up to 97% | ||
| Conventional MRI with the T1/T2 mismatch criterion | Differentiates tumors from neuroinflammation with a specificity of 75% and a sensitivity of 44% | [45] |
| PET scan | Differentiates tumors from neuroinflammation with a specificity of 69% and a sensitivity of 92% and is superior to NMR spectroscopy for choline/N‐acetylaspartate and choline/creatine ratios across different thresholds | [45] |
| T2*‐weighted dynamic susceptibility‐weighted contrast material‐enhanced MRI | Mean, maximum and minimum relative peak height and relative cerebral blood volume were significantly higher in GBM compared to radiation‐induced necrosis in a retrospective study (n = 57 patients) | [46] |
| Mean, maximum and minimum relative percentage of signal intensity recovery values were significantly lower in recurrent GBM compared to radiation necrosis | ||
| Proton MR spectroscopy | Increase in choline levels in patients with necrosis (4 of 9 cases) | [47] |
| Proton MR spectroscopy | Increased lactate/creatine and phosphocreatine ratio and decreased choline /phosphocreatine ratio compared, or reductions in all major metabolites, to recurrent GBM patients (n = 11) with radiation necrosis | [48] |
| MR spectroscopy and MR perfusion using choline/N‐acetylaspartate and choline/phosphocreatine ratios and rCBV | Enhanced ability to differentiate necrosis from recurrent GBM in meta‐analysis of 13 studies involving 397 patients | [49] |
| 11C‐choline PET | Good ability to differentiate GBM relapse from radiation necrosis in meta‐analysis of 6 studies involving 118 patients | [50] |
| MRI, F18‐fluorodeoxyglucose and 11C‐choline PET/CT | 11C‐choline PET/CT is superior in differentiating GBM recurrence from necrosis (n = 55) | [51] |
| (11)C‐methionine‐PET was superior to both (11)C‐choline and F18‐fluorodeoxyglucose‐PET | (11)C‐methionine‐PET is superior to (11)C‐choline or F18‐fluorodeoxyglucose‐PET for distinguishing GBM recurrence from necrosis (n = 50) | [52] |
| F18‐fluorodeoxyglucose and [18F]fluoro‐ethyl‐tyrosine PET | PET were effective in discriminating GBM from radiation necrosis, with F18‐fluorodeoxyglucose delayed PET is particularly useful in discriminating GBM tumors from radiation necrosis in an orthotopic rat model of GBM | [34] |
| [18F]‐2‐fluoroethyl‐L‐phenylalanine PET | LAT1 tumor‐specific PET tracer 2‐[18F]‐2‐fluoroethyl‐L‐phenylalanine PET is able to differentiate GBM from radiation necrosis and shows less contamination by inflammation compared to F18‐fluorodeoxyglucose signals | [35] |
Abbreviations: MR = magnetic resonance; GBM = glioblastoma; MRI = magnetic resonance imaging; PET = positron emission tomography; NMR = nuclear magnetic resonance; rCBV = relative cerebral blood volume; CT = computerized tomography; LAT1 = L‐type amino acid transporter 1.
Differentiation of brain tumors from brain infections and abscesses
Brain abscesses caused by aerobic bacteria can mimic GBM tumors in MRI image appearance and single voxel proton MR spectroscopy, leading to misdiagnosis. Based on the hypothesis that metabolite levels of choline would be decreased in the ring‐enhancing portion of abscesses compared to GBM, one study using MR spectroscopic imaging found that metabolite ratios and maximum choline/choline‐n, choline/creatine and choline/N‐acetylaspartate ratios of the contrast‐enhancing rim could be useful differentiating aerobic abscesses from GBM [53]. In MRI procedures, the apparent diffusion coefficient (ADC) may be useful in differentiating GBM from brain fungal abscess. Combining DTI and dynamic susceptibility contrast perfusion‐weighted imaging provides better results in differentiating lesions from brain infections than each technique used alone [54, 55] (Table 2).
TABLE 2.
Summary of selected studies on imaging approaches for distinguishing brain tumors from brain infections and abscesses
| Technique | Main findings | References |
|---|---|---|
| MR spectroscopic imaging | Metabolite ratios and maximum choline/choline‐n, choline/creatine, and choline/N‐acetylaspartate ratios of the contrast‐enhancing rim could differentiate abscesses from GBM tumors (n = 15) | [53] |
| MRI | The apparent diffusion coefficient (ADC) MRI may differentiate GBM from brain fungal abscess in retrospective study (n = 90) as well as in MRI an investigation of 10 other patients | [54, 55] |
| MRI | The combination of DTI and dynamic susceptibility contrast perfusion‐weighted imaging improves the differentiation between tumors and brain infections |
Abbreviations: DTI = diffusion tensor imaging; GBM = glioblastoma; MRI = magnetic resonance imaging.
BRAIN METASTASES, INFLAMMATION AND IMAGING
It is worth noting that CSCs constitute the main cell population mediating metastasis [56]. Brain metastases are more common than tumors originating primarily in the brain and confer a grave prognosis for patients with various types of primary cancers originating in other sites, such as lung, breast, colorectal and melanoma, given that available treatments show very limited efficacy. The tumor microenvironment is crucial to determine the establishment of brain metastases and, within this context, neuroinflammation plays a central role [57].
Brain metastases can produce a tissue lesion that induces a response comprising persistent astrocyte and microglia activation with cytokine and chemokine release, increased blood vessel permeability and recruitment of immune cells, resulting in neuroinflammation [57, 58, 59, 60]. Also, non‐neoplastic inflamed sites in the brain may facilitate the adhesion of circulating tumor cells from peripheral tumors to the activated endothelium in brain vessels, which is one of the possible mechanisms promoting metastasis initiation [61]. Inflammation at distant sites promotes adhesion of circulating tumor cells to the activated endothelium and then initiates the formation of metastases. Many different types of mediators and immune cells are involved in brain metastasis depending, among other factors, on the type of primary tumor of origin, metastatic site in the brain and differential astrocyte and microglial activation, resulting in high biological heterogeneity [57, 62, 63, 64, 65, 66].
In terms of imaging, differentiating brain tumors from brain metastases pose a challenge in itself, as both present imaging patterns with similar peritumoral hyperintensities and intratumoral texture on MRI. Improvements have been obtained with the use of relative cerebral blood flow and volume analyses, diffusion tensor imaging, neurite orientation dispersion, density imaging to examine intracellular volume fraction, isotropic volume fraction and extracellular volume fraction and metabolite analysis with MR spectroscopy. Also, intratumoral creatine suggests GBM, whereas its absence indicates metastasis when single‐voxel proton MR spectroscopy is used for imaging [10, 67]. A study examining the use of the mean apparent diffusion coefficient and absolute standard deviation derived from apparent diffusion coefficient measurements based on cellularity levels could not find marked differences between GBM tumors from brain metastasis [68]. However, other studies found that the apparent diffusion coefficient could differentiate GBM from metastasis, and that homogeneity and the inverse difference moment of GBM were significantly higher than those of metastases in the regions of interest placements examined [10, 69].
Assessing the heterogeneity of both the tumor masses and peritumoral edema with MRI texture analysis revealed that the heterogeneity of the GBMs peritumoral edema was significantly higher than the edema surrounding metastasis, differentiating them with a sensitivity of 80% and specificity of 90% [70]. Combining arterial spin labeling perfusion (ASL)‐ and DTI‐derived metrics showed to be promising in differentiating GBM and solitary brain metastases [71]. The use of 2D texture features extracted from images obtained with MRI may be a useful alternative for discriminating between GBM and brain metastases [72]. Computational‐aided quantitative analysis of MRI images may improve the accuracy in differentiating GBM from metastases, and texture features are more significant than fractal‐based features for that purpose [73]. Increasingly, machine learning algorithms have been applied to imaging data to improve the differentiation between GBM and brain metastases [74, 75, 76, 77, 78]. Novel diagnostic support systems based on radiomic features extracted from post‐contrast 3 diffusion tensor imaging (DT1) MR images may help improving the distinction between solitary brain metastases and GBM with high diagnosis performance and generalizability [74]. Machine learning and deep learning‐based models applied to conventional MR images may support pre‐operative discrimination between GBM and solitary brain metastasis [75, 76, 77], and deep learning network models that allow automated, on‐site analysis of resected tumor specimens based on confocal laser endoscopic techniques image data sets have been developed [78]. Other parameters, such as the cerebral blood volume gradient in the peritumoral brain zone, may enable the differentiation of GBMs from metastases [79]. Another approach is to evaluate peritumoral areas with color map of phase difference enhanced imaging (Color PADRE) [80].
As with tumors originating in the brain metastases are treated with radiotherapy and stereotactic radiosurgery, making incidence of radiation necrosis an important issue, and the distinction between metastasis and inflamed and necrotic tissue by MRI can be challenging. One study examined the hypothesis that methionine levels could be increased in metastatic tissue, whereas the inflammation marker translocator protein peripheral benzodiazepine receptor‐translocator protein (PBR‐TSPO), which can be quantified with specific PET tracers, would be increased in necrosis. Thus, the use of the [11C]methionine and [11C]PBR28 tracers in PET was evaluated in five patients’ previously treated brain metastases showing regrowth. The use of [11C]methionine could accurately identify pathologically confirmed tumor regrowth in all seven lesions examined, whereas [11C]PBR28 could only identify three of seven lesions, indicating that the former, but not the latter, tracer can be used as a reliable marker [81] (Table 3).
TABLE 3.
Summary of selected studies on imaging approaches for identifying brain metastases
| Technique | Main findings | References |
|---|---|---|
| Single‐voxel proton MR spectroscopy | The presence of intratumoral creatine indicates GBM, whereas its absence indicates metastasis, in samples consisting of 11 anaplastic gliomas, 20 GBMs and 25 metastases | [67] |
| MRI | No differentiation between GBM tumors and brain metastases when using the mean apparent diffusion coefficient and absolute standard deviation derived from apparent diffusion coefficient measurements based on cellularity levels (n = 34 patients) | [68] |
| MRI | Use of the apparent diffusion coefficient could differentiate GBM from metastasis | [69] |
| Higher homogeneity and inverse difference moment in GBM compared to metastases (n = 36 GBMs and 26 metastases) | ||
| MRI | Heterogeneity of the GBMs peritumoral edema was significantly higher than the edema surrounding MET by texture analysis, allowing a differentiation with a sensitivity of 80% and specificity of 90% (n = 22 GBM tumors and 21 metastases) | [70] |
| MRI | Combining arterial spin labeling perfusion (ASL)‐ and diffusion tensor imaging (DTI)‐derived metrics could differentiate GBM from brain metastases (n = 36 patients with provisional diagnosis of GBM or metastasis) | [71] |
| MRI | The use of 2D texture features extracted from images obtained with MRI enable the discrimination between GBM and brain metastases (n = 50 patients with GBM and 50 with metastasis) | [72] |
| MRI | Computational‐aided quantitative analysis of MRI images showed high accuracy in differentiating GBM from metastases, with texture features being more relevant than fractal‐based features (n = 30 patients with GBM and 25 with metastasis) | [73] |
| MRI | High sensitivity and specificity obtained in the distinction between GBM and solitary brain metastases with the use of post‐contrast 3 DT1 MR images selection and optimized by a machine learning classifier with a nested cross‐validation (n = 71 patients with GBM and 72 with metastasis) | [74] |
| MRI | Development of an efficient deep learning‐based model validated using images from 498 patients, from a total sample of records from 598 patients with confirmed GBM or metastasis analyzed retrospectively | [75] |
| MRI | A trained multi‐class multi‐layer perceptron model using parameters from preoperative MR images could differentiate GBM, brain metastasis and central nervous system lymphoma provides approximately 19% increase in diagnostic yield | [76] |
| MRI | Multi‐class multi‐player models trained with tumor volumes could discriminate among GBM, brain metastasis and central nervous system lymphoma with a maximum accuracy of 69.2%. Using the MLP model as a computer‐aided diagnosis for cases in which human reviewers disagreed on the diagnosis resulted in correct diagnoses 19.2% additional cases (n = GBM, 9 metastasis; and 8 central nervous system lymphoma | [77] |
| High‐resolution confocal laser endoscopic images | A trained residual network model that allows automated, on‐site analysis of tumor specimens based on confocal laser endoscopic image data sets achieved a test accuracy of 90.9% after applying a two‐class prediction for GBMs versus brain metastases, whereas for three class predictions the model achieved a ratio of correct predicted label of 85.8% in the test set | [78] |
| A prediction accuracy to 98.6% was reached by applying a confidence rate of 0.999 when images with substantial artifacts were excluded (n = 25 patients with GBM, brain metastasis or meningioma) | ||
| Contrast perfusion MRI | The cerebral blood volume gradient in the peritumoral brain zone was the most efficient parameter to differentiate GBM tumors from metastases | [79] |
| Phase difference enhanced imaging (PADRE) MRI | Evaluation of peritumoral areas on Color PADRE helped to differentiate GBM tumors from metastases (n = 17 patients with GBM, 11 patients with brain metastases and 9 with diffuse astrocytoma) | [80] |
| PET with use of sequential tracers | The use of [11C]methionine as a PET tracer could identify GBM regrowth in all lesions examined, whereas [11C]PBR28 could only identify 3 of 7 lesions (n = 5 patients previously treated brain metastases showing regrowth) | [81] |
Abbreviations: MR = magnetic resonance; DT1 = diffusion tensor imaging (DT1); GBM = glioblastoma; MRI = magnetic resonance imaging; PADRE = map of phase difference enhanced imaging.
CONCLUDING REMARKS
As discussed above, the differentiation between GBM recurrence and radiation necrosis remains a crucial challenge (Figure 1), given that the need for additional surgery in a previously operated brain region increases surgical risk in addition to raising treatment costs. Multi‐parametric MRI is an important current tool, using a combination of perfusion and diffusion parameters, although previous studies [82, 83, 84, 85, 86, 87, 88] found that apparent diffusion coefficient, volume transfer constant and relative cerebral blood volume are extremely useful, and the latter is the strongest parameter for differentiating radiation necrosis from GBM tumors [89]. Nael et al. [90] achieved a diagnostic accuracy of 92.8% by combining the use of volume transfer constant and relative cerebral blood volume.
FIGURE 1.
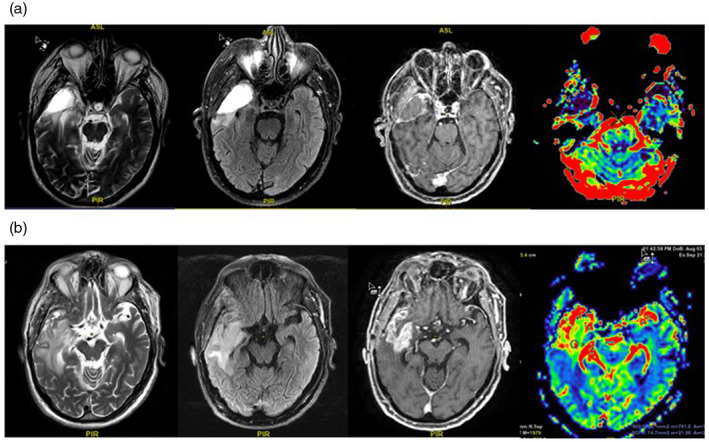
Illustrations of (a). A pseudoprogression resulting from radiation‐induced inflamed necrosis. Magnetic resonance (MR) images taken post‐operatively show axial T2, fluid attenuated inversion recovery (FLAIR), T1 contrast‐enhanced and perfusion, with enhancement in the right temporal lobe and areas of T2/FLAIR hyperintensity in the surrounding parenchyma, indicating a lesion induced by radiation therapy. There is no increase in relative cerebral blood volume in the enhancing portion of the lesion, which further suggests pseudoprogression. (b) A tumor relapse shown by images taken from the same patient 3 months later. MR images taken post‐operatively show axial T2, FLAIR, T1 contrast‐enhanced and perfusion, with heterogeneous enhancement in the right temporal lobe and great extension of the T2/FLAIR hyperintensity area, indicating a combination of tumor spread and vasogenic edema involving the right peritrigonal and insular regions. There is increased relative cerebral blood volume in the enhancing portion of the lesion, representing tumoral progression. Anonymised image.
In daily neuro‐oncological practice, multi‐parametric MRI is the most used tool to achieve an accurate differential diagnosis between GBM, inflamed sites and other brain diseases. Despite its advantages, the use of PET scans remains limited in many regions of the world due to economic and logistic shortcomings. Overall, the integrated use of different MR and PET approaches, including multi‐parametric MRI, in addition to perfusion and diffusion parameters [91] may enable increasingly accurate diagnosis based on brain imaging.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors contributed to the conception, writing and revision of this article.
ACKNOWLEDGEMENTS
The writing of this article was supported by the National Council for Scientific and Technological Development (CNPq, MCTI, Brazil) grant 305647/2019‐9 (R.R.) and the Center for Advanced Neurology and Neurosurgery (CEANNE)‐Brazil (S.A.D., G.R.I.).
Roesler R, Dini SA, Isolan GR. Neuroinflammation and immunoregulation in glioblastoma and brain metastases: Recent developments in imaging approaches. Clin Exp Immunol. 2021;206:314–324. 10.1111/cei.13668
OTHER ARTICLES PUBLISHED IN THIS REVIEW SERIES
Imaging immune responses in neuroinflammatory diseases. Clinical and Experimental Immunology 2021, 206: 248–250.
Magnetic resonance imaging in neuromyelitis optica spectrum disorder. Clinical and Experimental Immunology 2021, 206: 251–265.
Clinical and neuroimaging findings in MOGAD‐MRI and OCT. Clinical and Experimental Immunology 2021, 206: 266–281.
Towards PET imaging of the dynamic phenotypes of microglia. Clinical and Experimental Immunology 2021, 206: 282–300.
Imaging immunological processes from blood to brain in amyotrophic lateral sclerosis. Clinical and Experimental Immunology 2021, 206: 301–313.
‘A picture is worth a thousand words’: The use of microscopy for imaging neuroinflammation. Clinical and Experimental Immunology 2021, 206: 325–345.
Contributor Information
Rafael Roesler, Email: rafaelroesler@hcpa.edu.br.
Gustavo R. Isolan, Email: gustavo.isolan@fempar.edu.br.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Drake LR, Hillmer AT, Cai Z. Approaches to PET imaging of glioblastoma. Molecules. 2020;25:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reza SMS, Samad MD, Shboul ZA, Jones KA, Iftekharuddin KM. Glioma grading using structural magnetic resonance imaging and molecular data. J Med Imaging. 2019;6:1. 10.1117/1.JMI.6.2.024501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chiang GC, Kovanlikaya I, Choi C, Ramakrishna R, Magge R, Shungu DC. Magnetic resonance spectroscopy, positron emission tomography and radiogenomics – relevance to glioma. Front Neurol. 2018;9. 10.3389/fneur.2018.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frosina G. Positron emission tomography of high‐grade gliomas. J Neurooncol. 2016;127:415–25. [DOI] [PubMed] [Google Scholar]
- 5. Holzgreve A, Albert NL, Galldiks N, Suchorska B. Use of PET imaging in neuro‐oncological surgery. Cancers. 2021;13:2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moreau A, Febvey O, Mognetti T, Frappaz D, Kryza D. Contribution of different positron emission tomography tracers in glioma management: focus on glioblastoma. Front Oncol. 2019;9:1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cook GJ, Maisey MN, Fogelman I. Normal variants, artefacts and interpretative pitfalls in PET imaging with 18‐fluoro‐2‐deoxyglucose and carbon‐11 methionine. Eur J Nucl Med. 1999;26:1363–78. [DOI] [PubMed] [Google Scholar]
- 8. Culverwell AD, Scarsbrook AF, Chowdhury FU. False‐positive uptake on 2‐[18F]‐fluoro‐2‐deoxy‐D‐glucose (FDG) positron‐emission tomography/computed tomography (PET/CT) in oncological imaging. Clin Radiol. 2011;66:366–82. [DOI] [PubMed] [Google Scholar]
- 9. Nozaki S, Nakatani Y, Mawatari A, Shibata N, Hume WE, Hayashinaka E, et al. 18F‐FIMP: a LAT1‐specific PET probe for discrimination between tumor tissue and inflammation. Sci Rep. 2019;9:15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fordham A‐J, Hacherl C‐C, Patel N, Jones K, Myers B, Abraham M, et al. Differentiating glioblastomas from solitary brain metastases: an update on the current literature of advanced imaging modalities. Cancers. 2021;13:2960. 10.3390/cancers13122960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brennan C, Verhaak R, McKenna A, Campos B, Noushmehr H, Salama S, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella‐Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803–20. [DOI] [PubMed] [Google Scholar]
- 13. Roesler R, Brunetto AT, Abujamra AL, de Farias CB, Brunetto AL, Schwartsmann G. Current and emerging molecular targets in glioma. Exp Rev Anticancer Ther. 2010;10:1735–51. [DOI] [PubMed] [Google Scholar]
- 14. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. [DOI] [PubMed] [Google Scholar]
- 15. Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. [DOI] [PubMed] [Google Scholar]
- 16. Alghamri MS, McClellan BL, Hartlage MS, Haase S, Faisal SM, Thalla R, et al. Targeting neuroinflammation in brain cancer: uncovering mechanisms, pharmacological targets, and neuropharmaceutical developments. Front Pharmacol. 2021;12. 10.3389/fphar.2021.680021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arimappamagan A, Somasundaram K, Thennarasu K, Peddagangannagari S, Srinivasan H, Shailaja BC, et al. A fourteen gene GBM prognostic signature identifies association of immune response pathway and mesenchymal subtype with high risk group. PLOS ONE. 2013;8:e62042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carro MS, Lim WK, Alvarez MJ, Bollo RJ, Zhao X, Snyder EY, et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 2010;463:318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DeCordova S, Shastri A, Tsolaki AG, Yasmin H, Klein L, Singh SK, et al. Molecular heterogeneity and immunosuppressive microenvironment in glioblastoma. Front Immunol. 2020;11:1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yeo ECF, Brown MP, Gargett T, Ebert LM. The role of cytokines and chemokines in shaping the immune microenvironment of glioblastoma: implications for immunotherapy. Cells. 2021;10:607. 10.3390/cells10030607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crane CA, Ahn BJ, Han SJ, Parsa AT. Soluble factors secreted by glioblastoma cell lines facilitate recruitment, survival, and expansion of regulatory T cells: implications for immunotherapy. Neuro Oncol. 2012;14:584–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Groblewska M, Litman‐Zawadzka A, Mroczko B. The role of selected chemokines and their receptors in the development of gliomas. Int J Mol Sci. 2020;21:3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huettner C, Paulus W, Roggendorf W. Messenger RNA expression of the immunosuppressive cytokine IL‐10 in human gliomas. Am J Pathol. 1995;146:317–22. [PMC free article] [PubMed] [Google Scholar]
- 24. Perng P, Lim M. Immunosuppressive mechanisms of malignant gliomas: parallels at non‐CNS sites. Front Oncol. 2015;5:153. 10.3389/fonc.2015.00153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tafani M, Di Vito M, Frati A, Pellegrini L, De Santis E, Sette G, et al. Pro‐inflammatory gene expression in solid glioblastoma microenvironment and in hypoxic stem cells from human glioblastoma. J Neuroinflamm. 2011;8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Urbantat RM, Vajkoczy P, Brandenburg S. Advances in chemokine signaling pathways as therapeutic targets in glioblastoma. Cancers. 2021;13:2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Van Meir E, Sawamura Y, Diserens AC, Hamou MF, de Tribolet N. Human glioblastoma cells release interleukin 6 in vivo and in vitro . Cancer Res. 1990;50:6683–8. [PubMed] [Google Scholar]
- 28. Waters MR, Gupta AS, Mockenhaupt K, Brown LN, Biswas DD, Kordula T. RelB acts as a molecular switch driving chronic inflammation in glioblastoma multiforme. Oncogenesis. 2019;8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Papale M, Buccarelli M, Mollinari C, Russo MA, Pallini R, Ricci‐Vitiani L, et al. Hypoxia, inflammation and necrosis as determinants of glioblastoma cancer Stem cells progression. Int J Mol Sci. 2020;21:2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang L, Liu Z, Balivada S, Shrestha T, Bossmann S, Pyle M, et al. Interleukin‐1β and transforming growth factor‐β cooperate to induce neurosphere formation and increase tumorigenicity of adherent LN‐229 glioma cells. Stem Cell Res Ther. 2012;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brandes AA, Tosoni A, Spagnolli F, Frezza G, Leonardi M, Calbucci F, et al. Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: pitfalls in neurooncology. Neuro Oncol. 2008;10:361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. DeAngelis LM, Delattre JY, Posner JB. Radiation‐induced dementia in patients cured of brain metastases. Neurology. 1989;39:789–96. [DOI] [PubMed] [Google Scholar]
- 33. Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys. 1980;6:1215–28. [DOI] [PubMed] [Google Scholar]
- 34. Bolcaen J, Descamps B, Deblaere K, Boterberg T, De Vos Pharm F, Kalala J‐P, et al. (18)F‐fluoromethylcholine (FCho), (18)F‐fluoroethyltyrosine (FET), and (18)F‐fluorodeoxyglucose (FDG) for the discrimination between high‐grade glioma and radiation necrosis in rats: a PET study. Nucl Med Biol. 2015;42:38–45. [DOI] [PubMed] [Google Scholar]
- 35. Verhoeven J, Baguet T, Piron S, Pauwelyn G, Bouckaert C, Descamps B, et al. 2‐[18F]FELP, a novel LAT1‐specific PET tracer, for the discrimination between glioblastoma, radiation necrosis and inflammation. Nucl Med Biol. 2020;82–83:9–16. [DOI] [PubMed] [Google Scholar]
- 36. Sonar SA, Lal G. Blood–brain barrier and its function during inflammation and autoimmunity. J Leukoc Biol. 2018;103:839–53. [DOI] [PubMed] [Google Scholar]
- 37. Burger PC, Dubois PJ, Schold SC, Smith KR, Odom GL, Crafts DC, et al. Computerized tomographic and pathologic studies of the untreated, quiescent, and recurrent glioblastoma multiforme. J Neurosurg. 1983;58:159–69. [DOI] [PubMed] [Google Scholar]
- 38. Dooms GC, Hecht S, Brant‐Zawadzki M, Berthiaume Y, Norman D, Newton TH. Brain radiation lesions: MR imaging. Radiology. 1986;158:149–55. [DOI] [PubMed] [Google Scholar]
- 39. Jain R, Narang J, Sundgren PM, Hearshen D, Saksena S, Rock JP, et al. Treatment induced necrosis versus recurrent/progressing brain tumor: going beyond the boundaries of conventional morphologic imaging. J Neurooncol. 2010;100:17–29. [DOI] [PubMed] [Google Scholar]
- 40. Tihan T, Barletta J, Parney I, Lamborn K, Sneed PK, Chang S. Prognostic value of detecting recurrent glioblastoma multiforme in surgical specimens from patients after radiotherapy: should pathology evaluation alter treatment decisions? Hum Pathol. 2006;37:272–82. [DOI] [PubMed] [Google Scholar]
- 41. Martínez‐Bisbal MC, Celda B. Proton magnetic resonance spectroscopy imaging in the study of human brain cancer. Q J Nucl Med Mol Imaging. 2009;53:618–30. [PubMed] [Google Scholar]
- 42. Weybright P, Sundgren PC, Maly P, Hassan DG, Nan B, Rohrer S, et al. Differentiation between brain tumor recurrence and radiation injury using MR spectroscopy. Am J Roentgenol. 2005;185:1471–6. [DOI] [PubMed] [Google Scholar]
- 43. Chen W. Clinical applications of PET in brain tumors. J Nucl Med. 2007;48:1468–81. [DOI] [PubMed] [Google Scholar]
- 44. Ricci PE, Karis JP, Heiserman JE, Fram EK, Bice AN, Drayer BP. Differentiating recurrent tumor from radiation necrosis: time for re‐evaluation of positron emission tomography? Am J Neuroradiol. 1998;19:407–13. [PMC free article] [PubMed] [Google Scholar]
- 45. Menoux I, Noël G, Namer I, Antoni D. PET scan and NMR spectroscopy for the differential diagnosis between brain radiation necrosis and tumour recurrence after stereotactic irradiation of brain metastases: Place in the decision tree. Cancer Radiother. 2017;21:389–97. [DOI] [PubMed] [Google Scholar]
- 46. Barajas RF, Chang JS, Segal MR, Parsa AT, McDermott MW, Berger MS, et al. Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility‐weighted contrast‐enhanced perfusion MR imaging. Radiology. 2009;253:486–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nakajima T, Kumabe T, Kanamori M, Saito R, Tashiro M, Watanabe M, et al. Differential diagnosis between radiation necrosis and glioma progression using sequential proton magnetic resonance spectroscopy and methionine positron emission tomography. Neurol Med Chir. 2009;49:394–401. [DOI] [PubMed] [Google Scholar]
- 48. Kamada K, Houkin K, Abe H, Sawamura Y, Kashiwaba T. Differentiation of cerebral radiation necrosis from tumor recurrence by proton magnetic resonance spectroscopy. Neurol Med Chir. 1997;37:250–6. [DOI] [PubMed] [Google Scholar]
- 49. Chuang MT, Liu YS, Tsai YS, Chen YC, Wang CK. Differentiating radiation‐induced necrosis from recurrent brain tumor using MR perfusion and spectroscopy: a meta‐analysis. PLOS ONE. 2016;11:e0141438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gao L, Xu W, Li T, Zheng J, Chen G. Accuracy of 11C‐choline positron emission tomography in differentiating glioma recurrence from radiation necrosis: a systematic review and meta‐analysis. Medicine. 2018;97:e11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tan H, Chen L, Guan Y, Lin X. Comparison of MRI, F‐18 FDG, and 11C‐choline PET/CT for their potentials in differentiating brain tumor recurrence from brain tumor necrosis following radiotherapy. Clin Nucl Med. 2011;36:978–81. [DOI] [PubMed] [Google Scholar]
- 52. Takenaka S, Asano Y, Shinoda J, Nomura Y, Yonezawa S, Miwa K, et al. Comparison of (11)C‐methionine, (11)C‐choline, and (18)F‐fluorodeoxyglucose‐PET for distinguishing glioma recurrence from radiation necrosis. Neurol Med Chir. 2014;54:280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lai PH, Weng HH, Chen CY, Hsu SS, Ding S, Ko CW, et al. In vivo differentiation of aerobic brain abscesses and necrotic glioblastomas multiforme using proton MR spectroscopic imaging. Am J Neuroradiol. 2008;29:1511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Aziz K, Nawaz A. 1411. Differentiation of fungal abscess of brain from brain glioblastoma by MRI scan ADC value. Open Forum Infect Dis. 2019;6:S514. [Google Scholar]
- 55. Bink A, Gaa J, Franz K, Weidauer S, Yan B, Lanfermann H, et al. Importance of diffusion‐weighted imaging in the diagnosis of cystic brain tumors and intracerebral abscesses. Zentralbl Neurochir. 2005;66:119–25. [DOI] [PubMed] [Google Scholar]
- 56. Nandy SB, Lakshmanaswamy R. Cancer stem cells and metastasis. Prog Mol Biol Transl Sci. 2017;151:137–76. [DOI] [PubMed] [Google Scholar]
- 57. Doron H, Pukrop T, Erez N. A Blazing landscape: neuroinflammation shapes brain metastasis. Cancer Res. 2019;79:423–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gyoneva S, Ransohoff RM. Inflammatory reaction after traumatic brain injury: therapeutic potential of targeting cell–cell communication by chemokines. Trends Pharmacol Sci. 2015;36:471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. O’Callaghan JP, Sriram K, Miller DB. Defining ‘neuroinflammation’. Ann N Y Acad Sci. 2008;1139:318–30. [DOI] [PubMed] [Google Scholar]
- 60. Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sikpa D, Whittingstall L, Fouquet JP, Radulska A, Tremblay L, Lebel R, et al. Cerebrovascular inflammation promotes the formation of brain metastases. Int J Cancer. 2020;147:244–55. [DOI] [PubMed] [Google Scholar]
- 62. Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81:229–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Doron H, Amer M, Ershaid N, Blazquez R, Shani O, Lahav TG, et al. Inflammatory activation of astrocytes facilitates melanoma brain tropism via the CXCL10–CXCR3 signaling axis. Cell Rep. 2019;28:1785–98.e6. [DOI] [PubMed] [Google Scholar]
- 64. Klein A, Schwartz H, Sagi‐Assif O, Meshel T, Izraely S, Ben Menachem S, et al. Astrocytes facilitate melanoma brain metastasis via secretion of IL‐23. J Pathol. 2015;236:116–27. [DOI] [PubMed] [Google Scholar]
- 65. Seike T, Fujita K, Yamakawa Y, Kido MA, Takiguchi S, Teramoto N, et al. Interaction between lung cancer cells and astrocytes via specific inflammatory cytokines in the microenvironment of brain metastasis. Clin Exp Metastasis. 2011;28:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xing F, Kobayashi A, Okuda H, Watabe M, Pai SK, Pandey PR, et al. Reactive astrocytes promote the metastatic growth of breast cancer stem‐like cells by activating Notch signalling in brain. EMBO Mol Med. 2013;5:384–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ishimaru H, Morikawa M, Iwanaga S, Kaminogo M, Ochi M, Hayashi K. Differentiation between high‐grade glioma and metastatic brain tumor using single‐voxel proton MR spectroscopy. Eur Radiol. 2001;11:1784–91. [DOI] [PubMed] [Google Scholar]
- 68. Beig Zali S, Alinezhad F, Ranjkesh M, Daghighi MH, Poureisa M. Accuracy of apparent diffusion coefficient in differentiation of glioblastoma from metastasis. Neuroradiol J. 2021;34:205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang G, Chen X, Zhang S, Ruan X, Gao C, Liu Z, et al. Discrimination between solitary brain metastasis and glioblastoma multiforme by using ADC‐based texture analysis: a comparison of two different ROI placements. Acad Radiol. 2019;26:1466–72. [DOI] [PubMed] [Google Scholar]
- 70. Skogen K, Schulz A, Helseth E, Ganeshan B, Dormagen JB, Server A. Texture analysis on diffusion tensor imaging: discriminating glioblastoma from single brain metastasis. Acta Radiol. 2019;60:356–66. [DOI] [PubMed] [Google Scholar]
- 71. Abdel Razek AAK, Talaat M, El‐Serougy L, Abdelsalam M, Gaballa G. Differentiating glioblastomas from solitary brain metastases using arterial spin labeling perfusion‐ and diffusion tensor imaging‐derived metrics. World Neurosurg. 2019;127:e593–8. [DOI] [PubMed] [Google Scholar]
- 72. Ortiz‐Ramón R, Ruiz‐España S, Mollá‐Olmos E, Moratal D. Glioblastomas and brain metastases differentiation following an MRI texture analysis‐based radiomics approach. Phys Med. 2020;76:44–54. [DOI] [PubMed] [Google Scholar]
- 73. Petrujkić K, Milošević N, Rajković N, Stanisavljević D, Gavrilović S, Dželebdžić D, et al. Computational quantitative MR image features – a potential useful tool in differentiating glioblastoma from solitary brain metastasis. Eur J Radiol. 2019;119:108634. [DOI] [PubMed] [Google Scholar]
- 74. de Causans A, Carré A, Roux A, Tauziède‐Espariat A, Ammari S, Dezamis E, et al. Development of a machine learning classifier based on radiomic features extracted from post‐contrast 3D T1‐weighted MR images to distinguish glioblastoma from solitary brain metastasis. Front Oncol. 2021;11:638262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Shin I, Kim H, Ahn SS, Sohn B, Bae S, Park JE, et al. Development and validation of a deep learning‐based model to distinguish glioblastoma from solitary brain metastasis using conventional MR images. Am J Neuroradiol. 2021;42:838–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Swinburne NC, Schefflein J, Sakai YU, Oermann EK, Titano JJ, Chen I, et al. Machine learning for semi‐automated classification of glioblastoma, brain metastasis and central nervous system lymphoma using magnetic resonance advanced imaging. Ann Transl Med. 2019;7:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tateishi M, Nakaura T, Kitajima M, Uetani H, Nakagawa M, Inoue T, et al. An initial experience of machine learning based on multi‐sequence texture parameters in magnetic resonance imaging to differentiate glioblastoma from brain metastases. J Neurol Sci. 2020;410:116514. [DOI] [PubMed] [Google Scholar]
- 78. Ziebart A, Stadniczuk D, Roos V, Ratliff M, von Deimling A, Hänggi D, et al. Deep neural network for differentiation of brain tumor tissue displayed by confocal laser endomicroscopy. Front Oncol. 2021;11:668273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. She D, Xing Z, Cao D. Differentiation of glioblastoma and solitary brain metastasis by gradient of relative cerebral blood volume in the peritumoral brain zone derived from dynamic susceptibility contrast perfusion magnetic resonance imaging. J Comput Assist Tomogr. 2019;43:13–7. [DOI] [PubMed] [Google Scholar]
- 80. Doishita S, Sakamoto S, Yoneda T, Uda T, Tsukamoto T, Yamada E, et al. Differentiation of brain metastases and gliomas based on color map of phase difference enhanced imaging. Front Neurol. 2018;9:788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tran TT, Gallezot JD, Jilaveanu LB, Zito C, Turcu G, Lim K, et al. [11C]Methionine and [11C]PBR28 as PET imaging tracers to differentiate metastatic tumor recurrence or radiation necrosis. Mol Imaging. 2020;19. 10.1177/1536012120968669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Asao C, Korogi Y, Kitajima M, Hirai T, Baba Y, Makino K, et al. Diffusion‐weighted imaging of radiation‐induced brain injury for differentiation from tumor recurrence. Am J Neuroradiol. 2005;26:1455–60. [PMC free article] [PubMed] [Google Scholar]
- 83. Hein PA, Eskey CJ, Dunn JF, Hug EB. Diffusion‐weighted imaging in the follow‐up of treated high‐grade gliomas: tumor recurrence versus radiation injury. Am J Neuroradiol. 2004;25:201–9. [PMC free article] [PubMed] [Google Scholar]
- 84. Prager AJ, Martinez N, Beal K, Omuro A, Zhang Z, Young RJ. Diffusion and perfusion MRI to differentiate treatment‐related changes including pseudoprogression from recurrent tumors in high‐grade gliomas with histopathologic evidence. Am J Neuroradiol. 2015;36:877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sugahara T, Korogi Y, Tomiguchi S, Shigematsu Y, Ikushima I, Kira T, et al. Posttherapeutic intraaxial brain tumor: the value of perfusion‐sensitive contrast‐enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast‐enhancing tissue. Am J Neuroradiol. 2000;21:901–9. [PMC free article] [PubMed] [Google Scholar]
- 86. Xu J‐L, Li Y‐L, Lian J‐M, Dou S‐W, Yan F‐S, Wu H, et al. Distinction between postoperative recurrent glioma and radiation injury using MR diffusion tensor imaging. Neuroradiology. 2010;52:1193–9. [DOI] [PubMed] [Google Scholar]
- 87. Young RJ, Gupta A, Shah AD, Graber JJ, Chan TA, Zhang Z, et al. MRI perfusion in determining pseudoprogression in patients with glioblastoma. Clin Imaging. 2013;37:41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yun TJ, Park C‐K, Kim TM, Lee S‐H, Kim J‐H, Sohn C‐H, et al. Glioblastoma treated with concurrent radiation therapy and temozolomide chemotherapy: differentiation of true progression from pseudoprogression with quantitative dynamic contrast‐enhanced MR imaging. Radiology. 2015;274:830–40. [DOI] [PubMed] [Google Scholar]
- 89. Wesseling P, Ruiter DJ, Burger PC. Angiogenesis in brain tumors; pathobiological and clinical aspects. J Neurooncol. 1997;32:253–65. [DOI] [PubMed] [Google Scholar]
- 90. Nael K, Bauer AH, Hormigo A, Lemole M, Germano IM, Puig J, et al. Multiparametric MRI for differentiation of radiation necrosis from recurrent tumor in patients with treated glioblastoma. Am J Roentgenol. 2018;210:18–23. [DOI] [PubMed] [Google Scholar]
- 91. Soni N, Ora M, Mohindra N, Menda Y, Bathla G. Diagnostic performance of PET and perfusion‐weighted imaging in differentiating tumor recurrence or progression from radiation necrosis in posttreatment gliomas: a review of literature. Am J Neuroradiol. 2020;41:1550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


