Abstract
Behçet’s syndrome (BS) is a systemic vasculitis with several clinical manifestations. Neutrophil hyperactivation mediates vascular BS pathogenesis, via both a massive reactive oxygen species (ROS) production and neutrophil extracellular traps (NETs) release. Here, we investigated neutrophil‐mediated mechanisms of damage in non‐vascular BS manifestations and explored the in‐vitro effects of colchicine in counteracting these mechanisms. NETs and intracellular ROS production was assessed in blood samples from 80 BS patients (46 with active non‐vascular BS, 34 with inactive disease) and 80 healthy controls. Moreover, isolated neutrophils were incubated for 1 h with an oxidating agent [2,2′‐azobis (2‐amidinopropane) dihydrochloride; 250 nM] and the ability of pure colchicine pretreatment (100 ng/ml) to counteract oxidation‐induced damage was assessed. Patients with active non‐vascular BS showed remarkably increased NET levels [21.2, interquartile range (IQR) = 18.3–25.9 mU/ml] compared to patients with inactive disease (16.8, IQR = 13.3–20.2 mU/ml) and to controls (7.1, IQR = 5.1–8.7 mU/ml, p < 0.001]. Also, intracellular ROS tended to increase in active BS, although not significantly. In active non‐vascular BS, NETs correlated with neutrophil ROS production (p < 0.001) and were particularly increased in patients with active mucosal (p < 0.001), articular (p = 0.004) and gastrointestinal symptoms (p = 0.006). In isolated neutrophils, colchicine significantly reduced oxidation‐induced NET production and cell apoptosis, although not via an anti‐oxidant activity. Neutrophil‐mediated mechanisms might be directly involved in non‐vascular BS, and NETs, more than ROS, might drive the pathogenesis of mucosal, articular and intestinal manifestations. Colchicine might be effective in counteracting neutrophils‐mediated damage in BS, although further studies are needed.
Keywords: autoimmunity, human, neutrophils, reactive oxygen species, vasculitis
Neutrophil‐mediated mechanisms of damage might be directly involved in non‐vascular Behçet's syndrome. Neutrophil extracellular traps (NETs), more than an impaired redox status, might play a central role in the pathogenesis of mucosal, articular and intestinal BS manifestations. Colchicine might be effective to counteract neutrophils‐mediated damage in Behçet's syndrome, via the inhibition of NETs release.
INTRODUCTION
Behçet’s syndrome (BS) is a chronic systemic vasculitis typically involving the oral and genital mucosa and the skin, but often also presenting ocular, articular, vascular, neurological and gastrointestinal manifestations [1, 2]. Although the etiopathogenesis of the disease is not fully understood, BS has been traditionally considered a neutrophilic vasculitis [3, 4, 5, 6].
Neutrophil hyperactivation, probably with a human leukocyte antigen (HLA)‐B51‐related contribution [5], has been shown to directly mediate BS pathogenesis. Indeed, neutrophils are a major source of reactive oxygen species (ROS), mainly produced by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase; in turn, ROS can induce endothelial dysfunction and the migration of inflammatory cells across the endothelial barrier, leading to tissue injury [7, 8, 9].
In particular, neutrophil‐mediated ROS production has been described as a driver of BS vascular manifestations. Indeed, it has been recently shown that neutrophil‐derived ROS can markedly modify fibrinogen secondary structure and hence the overall architecture of the fibrin clot, with a consequent impairment in plasmin‐induced fibrinolysis [6, 10]. On this basis, BS is currently considered as an unusual model of neutrophil‐dependent thrombo‐inflammation, uniquely treated with immunosuppressants rather than anti‐coagulants [8].
Growing data suggest that, in inflammatory and infectious conditions, neutrophils can contribute to thrombo‐inflammation not only via ROS, but also through additional mechanisms, including the release of neutrophil extracellular traps (NETs) [11, 12].
NETs are structures produced by neutrophil death in response to infectious or inflammatory stimuli; they are mainly composed of extruded cell‐free DNA and histones, microbicidal proteins and proteases, and are used to trap and kill micro‐organisms [13, 14]. In particular, NETs are mainly produced by a specific subset of proinflammatory neutrophils, the low‐density granulocytes (LDGs), which are involved in the pathogenesis of cardiovascular manifestations associated with various autoimmune and autoinflammatory disorders [15, 16, 17]. ROS are directly involved in the mechanisms of NET formation, including the induction of morphological changes, increase in membrane permeability, release of neutrophil elastase from granules and induction of histones degradation and chromatin decondensation [18].
NETs are primarily internalized and degraded by macrophages, leading to macrophage activation with subsequent sustained release of proinflammatory cytokines [19, 20]. Moreover, similarly to ROS, also NETs have been shown to promote a procoagulant state in animal models and in humans, and to contribute to some arterial diseases such as stroke and myocardial infarctions [21].
On this basis, the role of NETs in BS has been mainly investigated as being potentially involved in the pathogenesis of vascular manifestations [19, 21, 22, 23]. BS patients have been shown to be more prone to NETosis compared to healthy volunteers [12, 21]. Le Joncour and colleagues further showed that NET levels were higher in BS patients with active disease or with vascular involvement compared to patients with inactive disease and without vascular symptoms, respectively [21]. Accordingly, Chen and colleagues recently demonstrated that the levels of circulating LDGs (the major NETs sources) were significantly higher in BS patients with active disease, and significantly correlated with the presence of vascular manifestations [17].
Considering that in BS a dense neutrophilic infiltration can be found not only around the vasa vasorum of the large vessel wall, but also at cutaneous [24], articular [25], ocular [26], intestinal [27] and neurological levels [28], we supposed that neutrophils and neutrophil‐derived products might be also involved in the pathogenesis of non‐vascular BS involvement.
The aim of this study was to investigate the role of neutrophil‐mediated mechanisms of damage (NETs and ROS) in non‐vascular BS. Moreover, the study also aimed to investigate, using an in‐vitro approach, the role of colchicine (a standard treatment for BS with a well‐established anti‐neutrophilic activity) in counteracting oxidation‐induced NETs formation and cell apoptosis.
MATERIALS AND METHODS
Study design and population
A case–control study was performed on consecutive adult BS patients referring to the Behçet Center of the University Hospital of Careggi (Florence, Italy) and meeting the International Criteria for Behçet Disease (ICBD) [29]. As this study specifically focused upon non‐vascular BS manifestations, patients with active vascular manifestations were excluded from the study. Conversely, the past history of vascular BS manifestations did not represent an exclusion criterion for this study.
BS patients were matched 1:1 for age and sex with healthy control (HC) subjects, randomly recruited from the general population. Control subjects were excluded if they had immune‐mediated disorders, active infections, history of cerebro‐ and/or cardiovascular diseases, atherothrombotic events or cancer.
All subjects provided written informed consent for the inclusion in the study. The study was approved by the University of Florence ethics committee and was conducted in accordance with the ethical principles of the Declaration of Helsinki.
Clinical data collection
Clinical data for BS patients were retrospectively collected from medical charts. Patients were characterized in terms of demographic features, disease duration, clinical history (including disease manifestations in their whole medical history and past pharmacological therapies), active disease manifestations and ongoing corticosteroid and immunomodulating/immunosuppressive treatments.
Disease activity at the time of inclusion in the study was assessed by means of the Behçet’s Disease Current Activity Form (BDCAF) score [30]. Patients with a BDCAF > 0 were defined as having active disease, whereas patients with a BDCAF = 0 were classified as having inactive disease.
Sample collection
Blood samples from BS patients and HC were collected by venipunture of antecubital vein into vacutainers (BD Vacutainer Systems, Plymouth, UK) containing ethylenediamine tetraacetate 0.17 mol/l. Blood was used immediately for neutrophil isolation and intracellular ROS assessment.
Assessment of neutrophil‐mediated mechanisms of damage
Neutrophil‐mediated mechanisms of damage were assessed in purified neutrophils, isolated from patients’ and controls’ blood samples. All experiments were performed in triplicate.
Neutrophil isolation
Neutrophils were isolated as previously described by Oh and colleagues [31]. Seven ml of whole blood was slowly and carefully layered on the top of 7 ml lympholyte‐poly (Cedarlane, Burlington, Ontario, Canada). Samples were centrifuged for 35 min without brakes at 500 g , 20°C. Blood was separated into six distinct bands (plasma, monocytes, isolation media, neutrophils, more isolation media and red blood cells), and the layer of neutrophils and all the isolation media below this layer were carefully transferred into new Falcon tubes. Then, cells were washed once with phosphate‐buffered saline (PBS) (without Ca2+/Mg2+) and centrifuged at 350 g for 10 min. After centrifugation, the supernatant was discarded and 6 ml of BD Pharm Lyse lysing solution (Becton Dickinson Biosciences, San Jose, California, USA) was added, gently mixed and incubated at 20°C for 8 min. Cells were then washed with PBS (without Ca2+/Mg2+) and centrifuged at 250 g for 6 min. Finally, the supernatant was discarded and the pellet was resuspended in RPMI‐1640 base cell culture medium with 2% bovine serum albumin (BSA). The purity of the neutrophils was controlled by flow cytometry by CD66b monoclonal antibody (G10F5), allophycocyanin (APC) (Thermo Fisher Scientific, Waltham, Massachusetts, USA) neutrophil‐specific marker and was found to exceed 95%. Data were analyzed using BD FACSDiva software (Becton‐Dickinson, San Jose, California, USA). Moreover, neutrophil viability was assessed by trypan blue dye exclusion and was found to exceed 96% in neutrophils isolated from HC.
NETs quantification
The ability of neutrophils to release NETs was assessed by measuring the NET‐associated elastase using NETosis assay kit (Cayman Chemical, Ann Arbor, Michigan, USA), according to the manufacturer’s protocol.
NETs microscopy
Fresh neutrophils were washed with 0.5% Triton X‐100 and 3% BSA in PBS and fixed for 15 min at room temperature (RT) with 4% paraformaldehyde (Merck Life Science S.r.l., Milano, Italy). After washing with PBS, cells were seeded on NuncLab‐Tek II CC2 Chamber Slide System (Thermo Fisher Scientific) and loaded with propidium iodide in a solution of 3% BSA, 0.5% Triton X‐100 in PBS for 5 min at RT, washing three times with PBS and analysed using a confocal Leica TCS SP8 scanning microscope (Mannheim, Germany) using a Leica Plan Apo 63X oil immersion objective [32, 33].
Intracellular ROS quantification
The methodology used in our laboratory for the assessment of intracellular ROS production has been extensively described elsewhere [6, 34]. Briefly, 100 µl of ethylenediamine tetraacetic acid (EDTA)‐anti‐coagulated blood samples were resuspended in 2 ml of BD fluorescence‐activated cell sorting (FACS) lysing solution (Becton Dickinson Biosciences), gently mixed and incubated at RT in the dark for 15 min. Cells were subsequently centrifuged (700 g for 7 min at 20°C), the supernatant was removed and cells were washed twice in PBS. Intracellular ROS levels were assayed by incubating cells with H2DCF‐DA (2.5 µM) (Invitrogen, Carlsbad, California, USA) in RPMI medium without serum and phenol red for 30 min at 37°C. Cells were then washed and resuspended in PBS and immediately analyzed by FACS cytometry (FACSCanto flow cytometer, Becton Dickinson). Data were analyzed using BD FACS Diva software (Becton Dickinson).
In‐vitro effects of colchicine on oxidation‐induced NETs production, cell viability and mitochondrial membrane potential
As a preliminary test aimed at evaluating the possible cytotoxicity of colchicine (standard treatment for BS), resazurin cell viability assay was performed (CellTiter‐Blue; Promega Corporation, Madison, Wisconsin, USA) in the presence of increasing concentrations of pure colchicine. Neutrophils were exposed to pure colchicine for 1 h at concentrations ranging between 1 ng/ml and 1 μg/ml.
Effects of colchicine on NETs production
To assess colchicine capacity in counteracting NETs production, neutrophils were plated in a black 96‐multi‐well plate (8 × 104 cells/well) and then 2,2′‐azobis (2‐amidinopropane) dihydrochloride (AAPH) (250 mM final concentration) was added to induce oxidation. After 1 h of incubation at 37°C cells were washed with PBS and then NETs production was assessed, as described above.
In the AAPH + colchicine group, cells were pretreated with pure colchicine 1 ng/ml for 1 h before incubation with AAPH (250 mM final concentration).
Cell viability assay
The effects of colchicine on cell viability was assessed by a fluorometric resazurin reduction method (CellTiter‐Blue; Promega Corporation), as previously described [35]. Neutrophils were plated in a black 96‐multi‐well plate (8 × 104 cells/well) and then AAPH (250 mM final concentration) was added. After 1 h of incubation at 37°C cells were washed with PBS. Then 100 μl of preheated resazurin diluted in RPMI was added to selected wells and the microplate was further incubated for 1 h at 37°C. Fluorescence was measured using a fluorometric microplate reader (BioTek Synergy H1; BioTek Instruments Inc., Winooski, Vermont, USA). In the AAPH + colchicine group, cells were pretreated with pure colchicine 1 ng/ml for 1 h before incubation with AAPH (250 mM final concentration).
Colchicine anti‐oxidant activity assay
To assess the possible anti‐oxidant activity of colchicine, the oxygen radical absorbance capacity (ORAC) method, based on inhibition of the peroxyl radical‐induced oxidation initiated by thermal decomposition of azo compounds such as AAPH, was performed as previously described [36]. Briefly, a fluorescein solution (6 nM) prepared daily from a 4‐μM stock in 75 mM sodium phosphate buffer (pH 7.4), was used. Trolox (250 µM final concentration) was used as a standard. Seventy µl of each sample with 100 µ of fluorescein was preincubated for 30 min at 37°C in each well before rapidly adding AAPH solution (19 mM final concentration). Fluorescence was measured with excitation at 485 nm and emission at 537 nm in a fluorometric microplate reader (BioTek Synergy H1; BioTek Instruments Inc.). Results were expressed as Trolox equivalents (µM), and then normalized for mg of colchicine.
Mitochondrial membrane potential
Tetramethylrhodamine methyl ester perchlorate (TMRM) was used to assess the mitochondrial membrane potential [32]. Neutrophils were loaded for 20 min at 37°C with 100 nM TMRM (Life Technologies, Carlsbad, California, USA) in RPMI and analyzed using a FACSCanto flow cytometer (Becton Dickinson).
Statistical analysis
Categorial variables were reported as absolute numbers and percentages, and were compared between groups using the χ2 test. Continuous data were presented as median value and interquartile range (IQR) or as mean value and standard deviation (SD), according to data distribution, and were compared using the parametric Student’s t‐test or analysis of variance (ANOVA) tests or the non‐parametric Mann–Whitney or Kruskal–Wallis tests, as appropriate. Normal data distribution was checked using the Shapiro–Wilk test.
Spearman’s correlation coefficients (Rho) were calculated for the association between NETs and intracellular neutrophils ROS, separately for patients with active and inactive BS.
All tests were two‐tailed, and a p‐value of < 0.05 was considered statistically significant. Statistical analyses were performed using the Stata software version 14 (StataCorp LP, College Station, Texas, USA) and the GraphPad Prism software version 6.01 (GraphPad Software, San Diego, California, USA).
RESULTS
Clinical features of patients and HC
Eighty adult BS patients were included in this study, and were age‐ and sex‐matched to 80 HC. The demographic and clinical features of BS patients and matched controls are described in Table 1.
TABLE 1.
Demographic and clinical features of patients with Behçet’s syndrome
| BS patients (n = 80) | Active non‐vascular BS (n = 46) | Inactive BS (n = 34) | p‐value* | |
|---|---|---|---|---|
| Female sex | 41 (51.3%) | 24 (52.2%) | 17 (50.0%) | 1.000 |
| Age at onset (median, IQR), years | 31.6 (22.6–41.6) | 32.1 (27.4–36.3) | 26.7 (22.1–43.3) | 0.705 |
| Age at diagnosis (median, IQR), years | 37.1 (29.4–43.8) | 36.7 (33.8–43.5) | 39.3 (27.0–43.8) | 0.964 |
| Age at inclusion in the study (median, IQR), years | 43.0 (36.0–50.3) | 40.8 (36.2–50.3) | 43.2 (30.8–48.2) | 0.617 |
| Disease duration (median, IQR), years | 2.7 (1.0–6.2) | 2.4 (1.0–6.8) | 2.7 (1.0–5.6) | 0.782 |
| HLA‐B51 positivity | 35/75 (46.7%) | 21/43 (48.8%) | 14/32 (43.8%) | 0.815 |
| Disease involvement (whole medical history) | ||||
| Mucosal | 75 (93.8%) | 33 (97.1%) | 42 (91.3%) | 0.388 |
| Cutaneous | 53 (66.3%) | 34 (73.9%) | 19 (55.9%) | 0.102 |
| Articular | 52 (65.0%) | 34 (73.9%) | 18 (52.9%) | 0.062 |
| Ocular | 40 (50.0%) | 20 (43.5%) | 20 (58.8%) | 0.258 |
| Intestinal | 38 (47.5%) | 21 (45.7%) | 17 (50.0%) | 0.821 |
| Vascular | 32 (40.0%) | 17 (37.0%) | 15 (44.1%) | 0.645 |
| Neurological | 28 (35.0%) | 18 (39.1%) | 10 (29.4%) | 0.478 |
| Active disease at time of inclusion in the study | ||||
| BDCAF (median, IQR) | 3 (0–5) | 5 (3–7) | – | |
| Mucosal | 25 (31.3%) | 25 (54.4%) | – | |
| Articular | 21 (26.3%) | 21 (45.7%) | – | |
| Cutaneous | 10 (12.5%) | 10 (21.7%) | – | |
| Intestinal | 10 (12.5%) | 10 (21.7%) | – | |
| Neurological | 9 (11.3%) | 9 (19.6%) | – | |
| Ocular | 3 (3.8%) | 3 (6.5%) | – | |
| Ongoing treatments at time of inclusion in the study | ||||
| None | 11 (13.8%) | 10 (21.7%) | 1 (2.9%) | 0.020 |
| Active therapy | 69 (86.3%) | 36 (78.3%) | 33 (97.1%) | |
| Corticosteroids | 43 (53.8%) | 26 (56.5%) | 17 (50.0%) | 0.095 |
| Only corticosteroids | 4 (5.0%) | 2 (4.4%) | 2 (5.9%) | |
| Colchicine | 16 (20.0%) | 9 (19.6%) | 7 (20.6%) | |
| Only colchicine | ||||
| (± corticosteroids) | 10 (12.5%) | 5 (10.9%) | 5 (14.7%) | |
| Traditional/biological DMARDs (± corticosteroids and colchicine) | 55 (68.8%) | 29 (63.0%) | 26 (76.5%) | |
| Traditional DMARDs | 24 (30.0%) | 19 (41.3%) | 5 (14.7%) | 0.013 |
| Biological DMARDs | 40 (50.0%) | 18 (39.1%) | 22 (64.7%) | 0.041 |
BS = Behçet’s syndrome; BDCAF = Behçet’s disease current activity form; DMARDs = disease‐modifying anti‐rheumatic drugs; HLA = human leukocyte antigen; IQR = interquartile range.
p‐values from χ2 test or Mann–Whitney test for unpaired data.
Both sexes were equally represented, with a median age at inclusion in the study of 43.0 years (IQR = 36.0–50.3) and a median disease duration of 2.7 years (1.0–6.2).
Approximately half the patients presented HLA‐B51 positivity. Almost all patients had history mucosal involvement. Other common disease manifestations included cutaneous, articular and ocular involvement, and 32 patients had previous history of vascular BS manifestations.
At the time of inclusion into the study, 34 patients (42.5%) of BS patients had inactive disease, while 46 (57.5%) presented active non‐vascular disease manifestations; the median BDCAF in the whole cohort was 3 (0–5). Specifically, approximately one‐third of patients presented active mucosal involvement, 26.3% articular, 12.5% cutaneous and 12.5% intestinal. Active neurological and ocular manifestations were present in nine and three patients each.
Regarding pharmacological treatments, the majority of patients (86.3%) were receiving active therapy at the time of inclusion in the study, most being treated with traditional and/or biological disease‐modifying anti‐rheumatic drugs (DMARDs).
Patients with active non‐vascular BS and with inactive disease were comparable in terms of demographic features and previous disease manifestations, but significantly differed in terms of ongoing pharmacological treatments, a higher proportion of patients with active disease being off therapy at the time of inclusion in the study (21.7 versus 2.9%, p = 0.020).
Neutrophil‐derived products in patients with Behçet’s syndrome and in HC
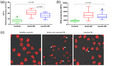
Figure 1a,b reports the levels of NETs and neutrophils ROS in the blood of BS patients with active non‐vascular manifestations and with inactive disease compared to HC.
FIGURE 1.
Neutrophil extracellular trap (NET) levels (a) and neutrophilic reactive oxygen species (ROS) production (b) in BS patients with active non‐vascular disease manifestations with inactive disease and in healthy controls (HC). (c) Representative confocal microscope images of neutrophils prone to extrude DNA in blood samples from HC, from BS patients active non‐vascular BS manifestations and with inactive disease, stained with propidium iodide. NETs can be observed as the DNA that is going to be extruded from the cell in figure (white arrows). In the microscope images of samples from BS patients with active disease (c, central image), a diffuse opacity, organic waste and cellular debris can be detected on the slide, due to the massive neutrophils cell death. Magnification ×630
NET release significantly differed among the three groups (p < 0.001): specifically, the median levels of neutrophil elastase was 7.1 mU/ml (IQR = 5.1–8.7) in HC, 16.8 mU/ml (IQR = 13.3–20.2) in patients with inactive BS and 21.2 mU/ml (IQR = 18.3–25.9) in BS patients with active non‐vascular manifestations (p < 0.001 between BS patients with active versus inactive disease). Representative confocal microscope images of NETs in blood samples from active and inactive BS patients and from HC are reported in Figure 1c.
Similarly to NETs, neutrophil ROS levels also differed in BS patients with active non‐vascular manifestations, with inactive disease and in HC (p < 0.001 among the three groups). In particular, the median intracellular ROS production was 1763 relative fluorescence units (RFU) (IQR = 1563–1945) in HC, 3607 RFU (IQR = 3161–4696) in BS patients with inactive disease and 4308 RFU (IQR = 3256–5626) in those with active non‐vascular manifestations; however, the difference between patients with active and inactive BS did not reach statistical significance (p = 0.144) (Figure 1b).
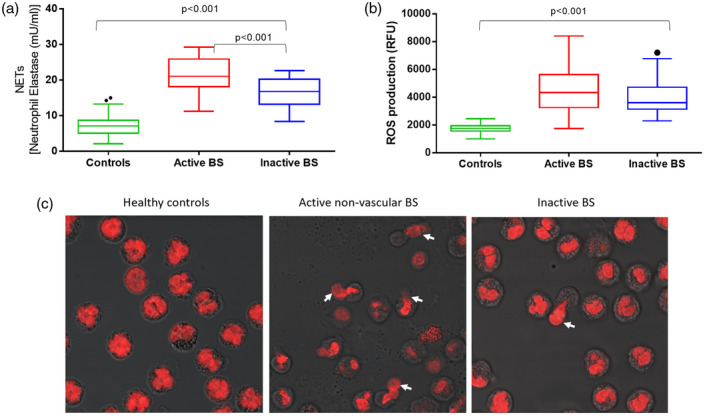
Individual levels of NETs and ROS are plotted in Figure 2, stratified according to BS disease activity.
FIGURE 2.
Correlation between neutrophil extracellular trap (NET) levels and intracellular neutrophils reactive oxygen species (ROS) production in BS patients with active non‐vascular disease manifestations with inactive disease and in healthy controls
As clearly shown in the figure, patients with non‐vascular active BS clustered around the highest NETs values. In this subgroup of patients with active disease, a statistically significant positive correlation emerged between NET release and neutrophils ROS production (Spearman’s rho = 0.563, p < 0.001). Conversely, in BS patients with inactive disease and in HC, NET release did not correlate with ROS production (for BS patients with inactive disease: Spearman’s rho = −0.220, p = 2.11; for controls: Spearman’s rho = −0.027, p = 0.816).
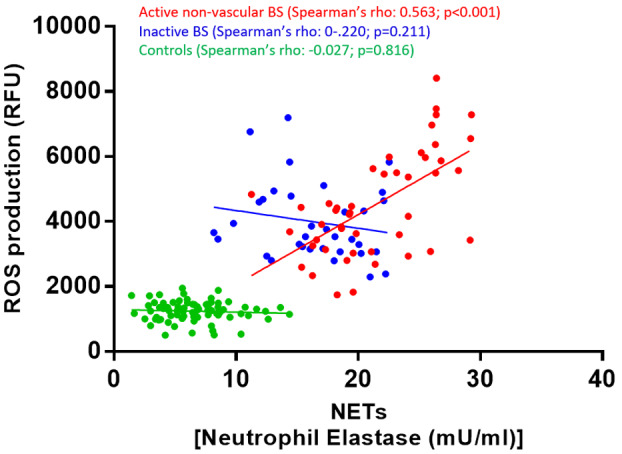
Variations in neutrophil‐derived products according to active clinical manifestations
Figure 3 and Supporting information, Table S1 report the variations in NET release and neutrophil ROS production according to the different type of active non‐vascular BS manifestations.
FIGURE 3.
Neutrophil extracellular trap (NET) levels (a–c) and neutrophils ROS production (d–f), stratified according to active BS disease manifestations
Significantly higher NET levels were found in the plasma from patients with active mucosal lesions [24.1 mU/ml in patients with (19.6–26.4) versus without 17.6 (14.7–20.6) mU/ml, respectively, p < 0.001], with active articular manifestations [22.1 (19.1–26.4) versus 18.2 (15.4–21.6) mU/ml, p = 0.004] and in those with intestinal symptoms [26.2 (21.1–26.4) versus 18.6 (15.8–22.1) mU/ml, p = 0.007]. Conversely, NET levels were comparable in patients with or without cutaneous, neurological or ocular involvement. Patients with active pharmacological therapy at time of inclusion in the study tended to present lower NET levels compared to patients off therapy [18.7 (15.8–22.1) versus 22.5 (18.3–26.4) mU/ml], although this difference did not reach statistical significance (p = 0.059).
Differently from NETs, neutrophils ROS production did not significantly vary according to the type of active non‐vascular BS manifestation, nor according to the ongoing pharmacological treatment.
Variations in intracellular lymphocytes and monocytes ROS production according to BS disease activity and disease manifestation
Similarly to neutrophils ROS, lymphocytes and monocytes ROS levels also tended to be higher in patients with active non‐vascular BS [for lymphocytes ROS = 707 (629–827) RFU in HC; 1661 (1342–2080) RFU versus 1384 (1206–1878) RFU in patients with active versus inactive disease, p < 0.001 among the three groups, p = 0.057 for active versus inactive BS; for monocytes ROS = 1314 (1142–1425) RFU versus 3322 (2488–4338) RFU versus 2998 (2440–4142) RFU, p < 0.001 among the three groups, p = 0.540 for active versus inactive BS] (Supporting information, Table S2).
Intracellular lymphocytes and monocytes ROS production were not significantly influenced by other patients’ demographic or clinical features, nor by ongoing pharmacological treatment.
In‐vitro assessment of the potential effect of colchicine in counteracting neutrophil‐mediated damage
Finally, we explored the potential capacity of colchicine—a standard treatment for BS—in counteracting neutrophil‐mediated damage.
We first tested colchicine cytotoxicity by assessing cell viability after incubation of neutrophils with pure colchicine concentrations ranging from 0 to 1000 ng/ml (Supporting information, Figure S1). Based on the results of this analysis, 100 ng/ml concentration was used for subsequent experiments.
NETosis was then induced in vitro via neutrophil incubation with AAPH (250 mM) and the potential effect of colchicine pretreatment in counteracting NETs production was tested. Representative confocal microscope images of NETs in untreated cells, incubated with AAPH or pretreated with pure colchicine before AAPH incubation, are reported in Figure 4a.
FIGURE 4.
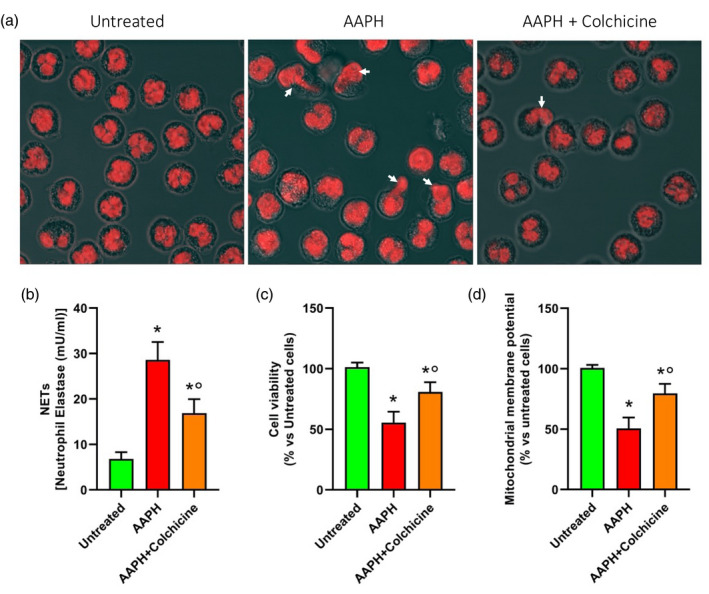
Representative confocal microscope images (a) of neutrophil extracellular traps (NETs) in neutrophils untreated, incubated with 2,2′‐azobis(2‐amidinopropane) dihydrochloride (AAPH) (250 mM) for 1 h and pretreated with colchicine (100 ng/ml) for 1 h before AAPH treatment. NET levels (b), cell viability (c) and mitochondrial polarization (d) in neutrophils untreated, incubated with AAPH or pretreated with colchicine before AAPH incubation
AAPH‐incubated cells showed a dramatic increase in NET production compared to untreated cells (28.6 ± 3.9 mU/ml versus 6.8 ± 3.9 mU/ml, respectively, p < 0.001). Conversely, colchicine pretreated cells prior to AAPH challenge showed a lower NET production (16.9 ± 3.0 mU/ml) compared to cells treated with AAPH alone (p < 0.001), although still higher compared to untreated cells (p < 0.001) (Figure 4b).
Colchicine pretreatment also counteracted AAPH‐induced cell death (cell viability being of 55.5 ± 9.0% versus 80.8 ± 7.9% in AAPH + colchicine and AAPH alone groups, respectively, p < 0.001), although cell viability was still lower compared to that of control cells (p = 0.002) (Figure 4c).
Accordingly, when we analyzed mitochondrial membrane potential (Figure 4d), flow cytometry analysis revealed a remarkable decrease in the proportion of cells presenting mitochondrial polarization following AAPH treatment (50.7 ± 9.0% versus 100.1 ± 2.6% in control cells, p < 0.001); this proportion was instead significantly higher in cells receiving colchicine pretreatment (79.5 ± 7.9%, p = 0.002), although still lower compared to untreated cells (p < 0.001).
We finally investigated whether these colchicine effects could be mediated by anti‐oxidant activity.
However, the assay revealed that pure colchicine lacks anti‐oxidant activity, its anti‐oxidant capacity being approximately 27 000‐fold lower compared to Trolox.
DISCUSSION
BS offers a peculiar model to unveil the role of neutrophil‐mediated mechanisms in the pathophysiology of systemic vasculitis. Neutrophils and neutrophil‐derived products (NETs and ROS) have been identified as key mediators of the thrombo‐inflammatory processes related to BS [7, 8, 19, 21, 22, 23]; conversely, little is known on their etiopathogenetic role in non‐vascular BS manifestations, including mucosal, cutaneous, articular, gastrointestinal, neurological and ocular involvement.
In this study we deepened the association between neutrophil‐derived products (NETs and ROS) and non‐vascular BS involvement. Our results demonstrate that BS patients with active non‐vascular manifestations show increased NETs levels compared to healthy subjects and to BS patients with inactive disease. Intracellular ROS production (sustained by neutrophils, as well as by lymphocytes and monocytes) appeared slightly, but not significantly, increased in patients with active non‐vascular BS. Importantly, we found that in patients with active non‐vascular BS NET release significantly correlated with intracellular neutrophil ROS, while no association was found in patients with inactive disease. Focusing upon the different non‐vascular BS manifestations, we found that NET levels were significantly increased in cases of active mucosal, articular or gastrointestinal involvement, while neutrophil ROS were not.
The association between BS disease activity and neutrophil‐derived products is not surprising [12, 21]. Neutrophil hyperactivation is known to promote ROS generation and to sustain BS disease activity [15], and protein oxidation parameters have been proposed as markers of disease progression and activity in BS [37, 38].
Our findings suggest that NETs, more than intracellular ROS, might represent a key mechanism of damage in non‐vascular BS manifestations.
A previous study by Le Joncour and colleagues [21] on 73 BS patients showed that markers of NETs were significantly increased in the serum of active BS patients compared with inactive patients, and highlighted a clear association between NETs and vascular BS. NETs are known to promote thrombogenesis [39], as they can activate coagulation via both the intrinsic and extrinsic pathway and can induce thrombin production in plasma, probably via histone/polyphosphate triggering [6, 39]. Accordingly, Chen and colleagues further showed that LDGs—a proinflammatory neutrophil subset which represents a major source of NETs—significantly correlate with BS disease activity and in particular with vascular disease involvement [23].
In our study, we also found that NETs are directly involved in non‐vascular BS manifestations, particularly in mucosal, articular and gastrointestinal involvement. Neutrophil infiltration has been consistently reported in BS patients at sites of disease involvement, such as oral and general ulcers [40], synovia [25], intestinal mucosa and vessels [27]. Regarding NETs, their association with articular damage has been described in different systemic autoimmune conditions, including rheumatoid arthritis (RA) and systemic lupus erythematosus [41]. NETs and NET‐derived elastase have been shown to directly induce cartilage damage and synovial inflammation in RA [42], and the titer of antibodies against carbamylate/citrullinated proteins extruded by synovial NETs has been found to correlate with radiographic bone erosion in these subjects [42, 43].
At intestinal level, aberrant NET formation has been shown to modulate intestinal barrier integrity and to drive the progression of gut inflammation in ulcerative colitis, inflammatory bowel disease and Crohn disease [44, 45, 46]. In a study on a mouse model of experimental colitis, NETs resulted in impaired gut permeability, enabling the initiation of luminal bacterial translocation and inflammation [47]. BS patients are known to present an increased gastrointestinal permeability and gut microbiome dysbiosis [48], irrespective of the presence of specific gastrointestinal BS manifestations [49, 50], and we could speculate that NETs might be directly involved in these alterations.
Regarding oral mucosa, localized NETosis has been described as a physiological mechanism of anti‐microbial defense. Mohanty and colleagues [51] found that saliva from BS patients had a reduced ability to form NETs compared to that from healthy individuals, because components of saliva prevented mucins from inducing NETs. We might suppose that a disequilibrium in the physiological mechanisms of NET formation might occur in these patients, with site‐specific ‘protective’ NETosis being impaired and systemic NET‐driven inflammatory mechanisms being increased instead.
Notably, in our study, systemic NET levels were measured in the peripheral blood and no assessment of NET release at local sites of disease was performed. Further studies assessing NET levels on biopsies from active sites of BS involvement might help to gain further knowledge on the mechanisms sustaining this multi‐faceted syndrome.
Finally, this study provides novel in‐vitro data on the role of colchicine—a well‐known anti‐neutrophilic activity standard treatment for BS [1, 2]—in counteracting neutrophil‐mediated mechanisms of damage. Namely, our findings clearly show that colchicine, despite the lack of an anti‐oxidant power, exerts a protective effect on oxidation‐induced NETs production and oxidation‐induced neutrophil apoptosis. Recently, Vaidya and colleagues [52] showed that, in patients with acute coronary syndrome, colchicine is capable of inhibiting NETs production post‐percutaneous coronary intervention by stabilizing neutrophil cytoskeleton, thereby inhibiting NET release. Whether the well‐established therapeutic action of colchicine in BS is mediated by its anti‐inflammatory activity and/or by its preventive role on neutrophil‐mediated mechanisms of damage deserves further investigation.
The results from this study are strengthened by the fact that neutrophil‐mediated mechanisms of damage were assessed in purified neutrophils, directly isolated from blood of BS patients and controls. Moreover, importantly, NETs production was confirmed by microscopic evaluation.
Taken together, our data support the hypothesis that neutrophil‐mediated mechanisms might be directly involved in non‐vascular BS, and that NETs, more than an impaired redox status, might play a central role in the pathogenesis of mucosal, articular and intestinal BS manifestations. Colchicine might be effective in counteracting neutrophil‐mediated damage in BS via the inhibition of NETs release, although further studies are needed to strengthen these findings.
ETHICS APPROVAL STATEMENT
This study involving human participants was reviewed and approved by the local ethics committee (Comitato Etico Area Vasta Centro—CEAVC, AOU Careggi, Florence, Italy). All participants provided their written informed consent to participate in this study.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
A.B., M.B., E.S., N.T., G.E., D.P. and C.F. conceived and designed the work. M.B., F.R.A., E.F., S.G. and A.M. performed the experiments. E.S., I.M., M.L.U., D.M., A.P. and G.E. enrolled patients and collected clinical data. A.B. performed the statistical analysis. A.B., M.B., E.S. and G.E. wrote the manuscript, assisted by I.M. and M.L.U. All authors contributed to the article, critically revised the manuscript and approved the submitted version. All contributors to this study are listed as co‐authors.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
This study was partly supported by a grant by Associazione Italiana Sindrome e Malattia di Behçet e Behçet‐like o.d.v. (SIMBA). Open Access Funding provided by Universita degli Studi di Firenze within the CRUI‐CARE Agreement.
Bettiol A, Becatti M, Silvestri E, Argento FR, Fini E, Mannucci A, et al. Neutrophil‐mediated mechanisms of damage and in‐vitro protective effect of colchicine in non‐vascular Behçet’s syndrome. Clin Exp Immunol. 2021;206:410–421. 10.1111/cei.13664
Alessandra Bettiol, Matteo Becatti and Elena Silvestri contributed equally to this work.
Domenico Prisco and Claudia Fiorillo share senior authorship.
DATA AVAILABILITY STATEMENT
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
REFERENCES
- 1. Yazici H, Ugurlu S, Seyahi E. Behçet syndrome: is it one condition? Clin Rev Allergy Immunol. 2012;43:275–80. 10.1007/s12016-012-8319-x [DOI] [PubMed] [Google Scholar]
- 2. Bettiol A, Prisco D, Emmi G. Behçet: the syndrome. Rheumatology. 2020;59:iii101–iii107. 10.1093/rheumatology/kez626 [DOI] [PubMed] [Google Scholar]
- 3. Greco A, De Virgilio A, Ralli M, Ciofalo A, Mancini P, Attanasio G, et al. Behçet’s disease: new insights into pathophysiology, clinical features and treatment options. Autoimmun Rev. 2018;17:567–75. [DOI] [PubMed] [Google Scholar]
- 4. Emmi G, Bettiol A, Silvestri E, Di Scala G, Becatti M, Fiorillo C, et al. Vascular Behçet’s syndrome: an update. Intern Emerg Med. 2018. 10.1007/s11739-018-1991-y [DOI] [PubMed] [Google Scholar]
- 5. Eksioglu‐Demiralp E, Direskeneli H, Kibaroglu A, Yavuz S, Ergun T, Akoglu T. Neutrophil activation in Behçet’s disease. Clin Exp Rheumatol. 2001;19:S19‐24. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11760393 (accessed 29 December 2018). [PubMed] [Google Scholar]
- 6. Becatti M, Emmi G, Silvestri E, Bruschi G, Ciucciarelli L, Squatrito D, et al. Neutrophil activation promotes fibrinogen oxidation and thrombus formation in Behçet disease. Circulation. 2016;133:302–11. [DOI] [PubMed] [Google Scholar]
- 7. Becatti M, Emmi G, Bettiol A, Silvestri E, Di Scala G, Taddei N, et al. Behçet’s syndrome as a tool to dissect the mechanisms of thrombo‐inflammation: clinical and pathogenetic aspects. Clin Exp Immunol. 2018. 10.1111/cei.13243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Emmi G, Becatti M, Bettiol A, Hatemi G, Prisco D, Fiorillo C. Behçet’s syndrome as a model of thrombo‐inflammation: the role of neutrophils. Front Immunol. 2019;10:1085. 10.3389/fimmu.2019.01085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Emmi G, Mannucci A, Argento FR, Silvestri E, Vaglio A, Bettiol A, et al. Stem‐cell‐derived circulating progenitors dysfunction in Behçet’s syndrome patients correlates with oxidative stress. Front Immunol. 2019;10:2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Emmi G, Bettiol A, Niccolai E, Ramazzotti M, Amedei A, Pagliai G, et al. Butyrate‐rich diets improve redox status and fibrin lysis in Behçet’s syndrome. Circ Res. 2021;128:278–80. [DOI] [PubMed] [Google Scholar]
- 11. Kessenbrock K, Krumbholz M, Schönermarck U, Back W, Gross WL, Werb Z, et al. Netting neutrophils in autoimmune small‐vessel vasculitis. Nat Med. 2009;15:623–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Safi R, Kallas R, Bardawil T, Mehanna CJ, Abbas O, Hamam R, et al. Neutrophils contribute to vasculitis by increased release of neutrophil extracellular traps in Behçet’s disease. J Dermatol Sci. 2018;92:143–50. [DOI] [PubMed] [Google Scholar]
- 13. Delgado‐Rizo V, Martínez‐Guzmán MA, Iñiguez‐Gutierrez L, García‐Orozco A, Alvarado‐Navarro A, Fafutis‐Morris M. Neutrophil extracellular traps and its implications in inflammation: an overview. Front Immunol. 2017;8:81. 10.3389/fimmu.2017.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sørensen OE, Borregaard N. Neutrophil extracellular traps ‐ The dark side of neutrophils. J Clin Invest. 2016;126:1612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Denny MF, Yalavarthi S, Zhao W, Thacker SG, Anderson M, Sandy AR, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol. 2010;184:3284–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu Y, Xia C, Chen J, Fan C, He J. Elevated circulating pro‐inflammatory low‐density granulocytes in adult‐onset Still’s disease. Rheumatology. 2021;60:297–303. [DOI] [PubMed] [Google Scholar]
- 17. Liu Y, Kaplan MJ. Neutrophils in the pathogenesis of rheumatic diseases: fueling the fire. Clin Rev Allergy Immunol. 2021;60:1–16. [DOI] [PubMed] [Google Scholar]
- 18. Stoiber W, Obermayer A, Steinbacher P, Krautgartner WD. The role of reactive oxygen species (ROS) in the formation of extracellular traps (ETs) in humans. Biomolecules. 2015;5:702–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li L, Yu X, Liu J, Wang Z, Li C, Shi J, et al. Neutrophil extracellular traps promote aberrant macrophages activation in Behçet’s disease. Front Immunol. 2021;11:590622. 10.3389/fimmu.2020.590622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakazawa D, Shida H, Kusunoki Y, Miyoshi A, Nishio S, Tomaru U, et al. The responses of macrophages in interaction with neutrophils that undergo NETosis. J Autoimmun. 2016;67:19–28. [DOI] [PubMed] [Google Scholar]
- 21. Le Joncour A, Martos R, Loyau S, Lelay N, Dossier A, Cazes A, et al. Critical role of neutrophil extracellular traps (NETs) in patients with Behcet’s disease. Ann Rheum Dis. 2019;78:1274–82. [DOI] [PubMed] [Google Scholar]
- 22. Michailidou D, Mustelin T, Lood C. Role of neutrophils in systemic vasculitides. Front Immunol. 2020;11:619705. 10.3389/fimmu.2020.619705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen J, Liu T, He J, Liu Y. Correspondence on ‘Critical role of neutrophil extracellular traps (NETs) in patients with Behcet’s disease. Ann Rheum Dis. 2020. 10.1136/annrheumdis-2020-219472 [DOI] [PubMed] [Google Scholar]
- 24. Kobayashi M, Ito M, Nakagawa A, Matsushita M, Nishikimi N, Sakurai T, et al. Neutrophil and endothelial cell activation in the vasa vasorum in vasculo‐Behcet disease. Histopathology. 2000;36:362–71. [DOI] [PubMed] [Google Scholar]
- 25. Cañete JD, Celis R, Noordenbos T, Moll C, Gómez‐Puerta JA, Pizcueta P, et al. Distinct synovial immunopathology in Behçet disease and psoriatic arthritis. Arthritis Res Ther. 2009;11:R17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matsuo T, Itami M, Nakagawa H, Nagayama M. The incidence and pathology of conjunctival ulceration in Behçet’s syndrome. Br J Ophthalmol. 2002;86:140–3. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11815335. (accessed 29 December 2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hayasaki N, Ito M, Suzuki T, Ina K, Ando T, Kusugami K, et al. Neutrophilic phlebitis is characteristic of intestinal Behçet’s disease and simple ulcer syndrome. Histopathology. 2004;45:377–83. [DOI] [PubMed] [Google Scholar]
- 28. Borhani Haghighi A, Sharifzad HR, Matin S, Rezaee S. The pathological presentations of neuro‐Behçet disease: a case report and review of the literature. Neurologist. 2007;13:209–14. [DOI] [PubMed] [Google Scholar]
- 29. International Team for the Revision of the International Criteria for Behçet’s Disease (ITR‐ICBD) , Davatchi F, Assaad‐Khalil S, Calamia KT, Crook JE, Sadeghi‐Abdollahi B, Schirmer M, et al. The International Criteria for Behçet’s Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol. 2014;28:338–47. [DOI] [PubMed] [Google Scholar]
- 30. Lawton G, Bhakta BB, Chamberlain MA, Tennant A. The Behçet’s disease activity index. Rheumatology. 2004;43:73–8. 10.1093/rheumatology/keg453 [DOI] [PubMed] [Google Scholar]
- 31. Oh H, Siano B, Diamond S. Neutrophil isolation protocol. J Vis Exp. 2008;17:745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Becatti M, Barygina V, Mannucci A, Emmi G, Prisco D, Lotti T, et al. Sirt1 protects against oxidative stress‐induced apoptosis in fibroblasts from psoriatic patients: a new insight into the pathogenetic mechanisms of psoriasis. Int J Mol Sci. 2018;19:1572. 10.3390/ijms19061572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pensalfini A, Cecchi C, Zampagni M, Becatti M, Favilli F, Paoli P, et al. Protective effect of new S‐acylglutathione derivatives against amyloid‐induced oxidative stress. Free Radic Biol Med. 2008;44:1624–36. [DOI] [PubMed] [Google Scholar]
- 34. Barygina VV, Becatti M, Soldi G, Prignano F, Lotti T, Nassi P, et al. Altered redox status in the blood of psoriatic patients: involvement of NADPH oxidase and role of anti‐TNF‐α therapy. Redox Rep. 2013;18:100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barygina V, Becatti M, Lotti T, Moretti S, Taddei N, Fiorillo C. ROS‐challenged keratinocytes as a new model for oxidative stress‐mediated skin diseases. J Cell Biochem. 2019;120:28–36. [DOI] [PubMed] [Google Scholar]
- 36. Becatti M, Fucci R, Mannucci A, Barygina V, Mugnaini M, Criscuoli L, et al. A biochemical approach to detect oxidative stress in infertile women undergoing assisted reproductive technology procedures. Int J Mol Sci. 2018;19:592. 10.3390/ijms19020592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yazici C, Köse K, Çaliş M, Demir M, Kirnap M, Ateş F. Increased advanced oxidation protein products in Behçet’s disease: a new activity marker? Br J Dermatol. 2004;151:105–11. [DOI] [PubMed] [Google Scholar]
- 38. Ozyazgan S, Andican G, Erman H, Tuzcu A, Uzun H, Onal B, Ozyazgan Y. Relation of protein oxidation parameters and disease activity in patients with Behçet’s disease. Clin Lab. 2013;59:819–25. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24133911 (accessed 29 December 2018). [DOI] [PubMed] [Google Scholar]
- 39. Folco EJ, Mawson TL, Vromman A, Bernardes‐Souza B, Franck G, Persson O, et al. Neutrophil extracellular traps induce endothelial cell activation and tissue factor production through interleukin‐1α and cathepsin G. Arterioscler Thromb Vasc Biol. 2018;38:1901–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gündüz Ö. Histopathological evaluation of Behçet’s disease and identification of new skin lesions. Patholog Res Int. 2012;2012:1–7. 10.1155/2012/209316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chapman EA, Lyon M, Simpson D, Mason D, Beynon RJ, Moots RJ, et al. Caught in a trap? Proteomic analysis of neutrophil extracellular traps in rheumatoid arthritis and systemic lupus erythematosus. Front Immunol. 2019;10:423. 10.3389/fimmu.2019.00423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carmona‐Rivera C, Carlucci PM, Goel RR, James E, Brooks SR, Rims C, et al. Neutrophil extracellular traps mediate articular cartilage damage and enhance cartilage component immunogenicity in rheumatoid arthritis. JCI Insight. 2020;5:e139388. 10.1172/jci.insight.139388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. O’Neil LJ, Barrera‐Vargas A, Sandoval‐Heglund D, Merayo‐Chalico J, Aguirre‐Aguilar E, Aponte AM, et al. Neutrophil‐mediated carbamylation promotes articular damage in rheumatoid arthritis. Sci Adv. 2020;6:eabd2688. 10.1126/sciadv.abd2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gottlieb Y, Elhasid R, Berger‐Achituv S, Brazowski E, Yerushalmy‐Feler A, Cohen S. Neutrophil extracellular traps in pediatric inflammatory bowel disease. Pathol Int. 2018;68:517–23. [DOI] [PubMed] [Google Scholar]
- 45. Dinallo V, Marafini I, Di Fusco D, Laudisi F, Franzè E, Di Grazia A, et al. Neutrophil extracellulartraps sustain inflammatory signals in ulcerative colitis. J Crohn’s Colitis. 2019;13:772–84. [DOI] [PubMed] [Google Scholar]
- 46. He Z, Si YU, Jiang T, Ma R, Zhang Y, Cao M, et al. Phosphotidylserine exposure and neutrophil extracellular traps enhance procoagulant activity in patients with inflammatory bowel disease. Thromb Haemost. 2016;115:738–51. [DOI] [PubMed] [Google Scholar]
- 47. Lin EYH, Lai HJ, Cheng YK, Leong KQ, Cheng LC, Chou YC, et al. Neutrophil extracellular traps impair intestinal barrier function during experimental colitis. Biomedicines. 2020;8:275. 10.3390/BIOMEDICINES8080275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Consolandi C, Turroni S, Emmi G, Severgnini M, Fiori J, Peano C, et al. Behçet’s syndrome patients exhibit specific microbiome signature. Autoimmun Rev. 2015;14:269–76. [DOI] [PubMed] [Google Scholar]
- 49. Koc B, Aymelek S, Sonmez A, Yilmaz MI, Kocar H. Increased sucrose permeability in Behçet’s disease. Rheumatol Int. 2004;24:347–50. [DOI] [PubMed] [Google Scholar]
- 50. Fresko I, Hamuryudan V, Demir M, Hizli N, Sayman H, Melikoǧlu M, et al. Intestinal permeability in Behçet’s syndrome. Ann Rheum Dis. 2001;60:65–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mohanty T, Sjögren J, Kahn F, Abu‐Humaidan AHA, Fisker N, Assing K, et al. A novel mechanism for NETosis provides antimicrobial defense at the oral mucosa. Blood. 2015;126:2128–37. [DOI] [PubMed] [Google Scholar]
- 52. Vaidya K, Tucker B, Kurup R, Khandkar C, Pandzic E, Barraclough J, et al. Colchicine inhibits neutrophil extracellular trap formation in patients with acute coronary syndrome after percutaneous coronary intervention. J Am Heart Assoc. 2021;10:e018993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.


