Abstract
Cytokines are soluble mediators of the immune response, and their evolution influences the disease outcome. Gaining knowledge on cytokines has become important, as they can constitute biomarkers allowing the diagnosis of malaria and preventing severe forms of the disease. Here, we investigated 10 cytokines and their circulating levels in asymptomatic Gabonese children with Plasmodium falciparum infection living in urban, semi‐urban and rural areas. Blood samples were collected from 273 schoolchildren (153 uninfected and 120 infected) aged 6 to 192 months. Hematological parameters were determined and P. falciparum diagnosis was performed using a rapid diagnosis test, microscopy and nested polymerase chain reaction (PCR). Plasma pro‐ [interferon (IFN)‐γ, tumor necrosis factor (TNF)‐α, interleukin (IL)‐6, IL‐12p70, IL‐17A and IL‐22] and anti‐inflammatory [IL‐10, IL‐4, IL‐13 and transforming growth factor (TGF)‐β] cytokine levels were measured by enzyme‐linked immunosorbent assay (ELISA) and compared between asymptomatic‐infected and uninfected children. Results revealed that without distinction of area, IL‐10 and IL‐6 levels were higher in infected compared to uninfected children; however, the pro‐ and anti‐inflammatory ratios (IL‐6/IL‐10 and TNF‐α/IL‐10) were similar. Furthermore, with area distinction significantly elevated levels of IL‐10 in these asymptomatic children were always accompanied by either significantly low or high levels of a proinflammatory cytokine. Also, comparison between asymptomatic‐infected children from the three areas showed significantly lower IL‐17A, IL‐22 and TGF‐β levels in urban area compared to semi‐urban and rural areas. These results suggest that asymptomatic malaria infections induce significantly high inflammatory cytokine levels without modifying the balanced between pro‐ and anti‐inflammatory cytokines and underline the higher exposure to infections of children in rural areas.
Keywords: asymptomatic Plasmodium falciparum infection, children, cytokines, rural area, semi‐urban area, urban area
This report investigated ten cytokines circulating levels in asymptomatic Gabonese children living in urban, semi‐urban and rural areas and found significantly higher IL‐10 and IL‐6 levels without modifying the balance between pro‐ and anti‐inflammatory cytokines in asymptomatic‐infected compare to uninfected children. Results suggest that IL‐10 may be a better indicator of asymptomatic infection as signicantly higher levels were associated with the asymptomatic form in all areas. Also the asymptomatic infected‐children of rural area could be subject to a higher susceptibility to infection than the asymptomatic children of the two others areas.
INTRODUCTION
Malaria is a major health problem, with an incidence of 228 million cases and 405 000 deaths reported worldwide in 2018 [1]. On the African continent, among the five species responsible for human infections, Plasmodium falciparum is responsible for one of five deaths in children [2]. According to the World Health Organization (WHO), in 2018 approximately 24 million children were estimated to be infected with P. falciparum in sub‐Saharan Africa and, among these, 1.8 million were likely to have severe anemia [1]. P. falciparum malaria infection causes a wide range of presentations of the clinical disease – asymptomatic infections that are poorly understood and slightly complicated and mild or severe forms. While efforts are increasing to achieve the millennium goals for malaria elimination, asymptomatic plasmodial infection remains widespread in endemic malaria regions and has become a major cause for concern.
Indeed, due to the absence of symptoms, asymptomatic plasmodial infections generally remain untreated and constitute a silent natural reservoir which supports the transmission of malaria and complicates malaria elimination in these regions [3]. Asymptomatic P. falciparum infection remains high, and represents approximately 96% of malaria infections in rural areas of Gabon [4, 5], meaning that almost all its population can then serve as a reservoir for malaria transmission. In Gabon, the urban area of Franceville, semi‐urban area of Makokou and rural area of the Mulundu district are characterized by their high level of malaria transmission throughout the year, as in other regions of Gabon. However, although Gabon is a hyperendemic country for malaria, endemicity levels may vary from one region to another, with significant disparities observed in the distribution of malaria in urban, semi‐urban and rural areas [6, 7, 8, 9]. According to a recent study, asymptomatic malaria is more frequent in rural (25.5%) and semi‐urban (20.5%) areas than in urban areas (4.8%) [10]. In addition to the heterogeneity of the malaria burden which has been reported in Gabon [8], the prevalence of co‐infections with P. falciparum in asymptomatic parasite carriers is 6.8%, with a predominance in helminthes infections in the rural area compared to semi‐urban and urban areas [10].
People living in malaria‐endemic areas become clinically immune after multiple reinfections over time and remain infected without apparent symptoms. Age and exposure to malaria infection are critical to the development of natural immunity to P. falciparum malaria. Therefore, the increasing numbers of multi‐resistance to anti‐malarial drugs [11] associated with vector resistance to available insecticides have increased the need to understand the immunological basis of protective immunity against malaria.
The modulation of pro‐ and anti‐inflammatory cytokines has been associated with different clinical malaria manifestations [5, 11, 12]. The evolution of cytokine balance is decisive in the outcome of the disease. The balance between pro‐ and anti‐inflammatory cytokines appears to be key in the determination of the clinical outcome of P. falciparum infection [13]. Indeed, a balance in favor of anti‐inflammatory cytokines such as interleukin (IL)‐10 suppresses the inflammatory immune response as well as parasite clearance [13], and therefore promotes the development of asymptomatic infections [14]. Finding cytokines which can constitute biomarkers would be an asset to prevent severe forms of the disease by allowing the diagnosis and indirectly the control of asymptomatic P. falciparum infections [15]. A recent study reported that in asymptomatic malaria there is a balance between pro‐ and anti‐inflammatory cytokines [16]. Also, significantly high levels of IL‐10 and IL‐4 were observed in asymptomatic cases compared to healthy individuals, whereas levels of T helper type 1 (Th1) cytokines such as IFN‐γ and tumor necrosis factor (TNF)‐α and Th17 (IL‐17, IL‐23) were higher in symptomatic patients compared to healthy controls and asymptomatic individuals [17, 18]. During asymptomatic P. vivax infection lower levels of the regulatory T cell (Treg) cytokine IL‐10 have been observed compared to controls [19], whereas in another study no significant difference in IL‐10 levels was found between asymptomatic cases and uninfected controls [16]. Conversely, an overall reduction in proinflammatory cytokines (TNF, IFN‐γ, IL‐6) and increased levels of regulatory cytokines (IL‐10 and TGF‐β) were observed when compared with those of the symptomatic groups [20]. It has also been shown that increased levels of IL‐10 were linked to asymptomatic malaria in pregnant women [21].
Although the relative balance between anti‐ and proinflammatory cytokines is thought to be a crucial factor in the outcome of malaria infection, studies on asymptomatic children living in malaria‐endemic areas with different epidemiological facies, particularly in Gabon, are relatively rare. The aim of our present study was to investigate and compare pro‐ and anti‐inflammatory cytokine responses in asymptomatic P. falciparum infections in children from urban, semi‐urban and rural areas in Gabon. We explore the hypothesis that the immune response to asymptomatic P. falciparum is mediated by anti‐inflammatory cytokines, and this response can vary according to the level of exposure to infections.
METHODS
Study sites
The study was conducted between June and July 2017 and 2019 during a voluntary screening campaign organized by the Centre Interdisciplinaire de Recherches Médicale de Franceville (CIRMF). The study took place in the primary schools of Ombellé in Franceville and Notre Dame de Victoire in Makokou and in three villages of the Mulundu district (Matsatsa, Mana‐Mana and Malende), near the city of Lastourville (Figure 1). Franceville is an urban area of south‐eastern Gabon (°41′60″S and 13°34′54″E). Demographically, it is Gabon’s third largest city after Libreville and Port‐Gentil. The city of Makokou is a semi‐urban area of north‐eastern Gabon (0°29′10″N and 12°52′4″E). Mulundu is a rural area of eastern–central Gabon (0° 49′S and 12°42′E). Although Gabon is a malaria‐endemic country, these three areas are characterized by different prevalence rates of plasmodial infection in children: 79.5% at Lastourville, 70.2% at Makokou and 21.2% at Franceville [22, 23].
FIGURE 1.
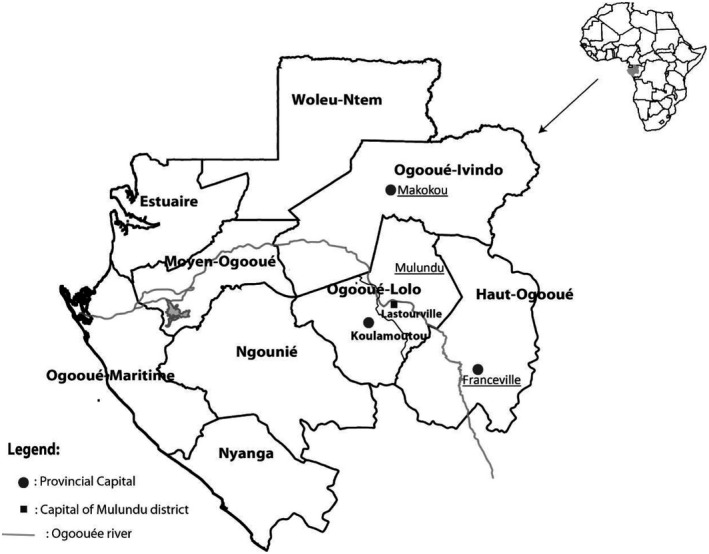
Map of Gabon. The study sites are underlined: urban (Franceville), semi‐urban (Makokou) and rural (Mulundu district) areas. The rural study site is made up of three villages (Mana‐Mana, Malende and Matsatsa) on the outskirts of Lastourville located at the south of the Mulundu district in the Ogooué‐Lolo province
Study participants and design
Two hundred and seventy‐three schoolchildren aged from 6 to 192 months were included in this study (Supporting information, Figure S1). Children were classified according to their P. falciparum infection status in two groups: asymptomatic‐infected (asymptomatic P. falciparum carriers) and uninfected (healthy controls); they were then distributed according to their living area (urban, semi‐urban and rural area) into six groups. Negative status was defined by the negativity of either the rapid diagnostic test or thick blood drop and positive status was defined by a positive rapid diagnostic test and/or thick blood drop; nested polymerase chain reaction (PCR) was then performed for species determination. Children with a febrile syndrome or a history of fever during the 2 weeks before sampling (temperature ≥ 37.5°C) and children infected with Plasmodium species other than P. falciparum were excluded. Among the uninfected children, those with high white blood cell levels above normal (>10.00 × 103 cells/mm3) were also excluded. All asymptomatic Plasmodium‐infected children were treated with artemether/lumefantrine.
Sample collection, hematological analyses and malaria diagnosis
Venous blood was collected in ethylenediamine tetraacetic acid (EDTA) tubes (2–5 ml). The hemogram (also named ‘blood count’), was performed using COBAS ABX (Roche Diagnostics, Indianapolis, Indiana, USA) counters.
For malaria diagnosis, rapid diagnostic testing (RDT) [OptiMAL‐IT® test (HRP2)] and thick drops were used for the parasite load count using the Lambarene method [24]. Thick blood smears from samples were stained with 10% Giemsa, and the number of P. falciparum parasites was determined by microscopy. The thick droplet slides were read after washing and drying by two qualified microscopists from the CIRMF, Gabon. The remainder of the whole blood was then immediately centrifuged and, after separation, the plasma was placed at −80°C and the pellet was used for the extraction of P. falciparum DNA.
Extraction of the DNA from blood was conducted using the Qiagen kit (QIAamp DNA Mini kit; Qiagen, Hilden, Germany). Then, 5 μl of extracted DNA were amplified with 1× Taq polymerase buffer (Invitrogen, San Diego, California, USA), 0.8 μM for each of the primers rPLU5 and rPLU6 for the first PCR reaction and rFAL1, rFAL2, rMAL1, rMAL2, rVIV1, rVIV2, rOVA1 and rOVA2 for the nested PCR (Eurogentec, Seraing, Belgium) [25], 0.2 mM dNTP, 2 mM MgCl2 and 0.024 U of Taq DNA polymerase (Invitrogen) using the cycling program used by Maghendji‐Nzondo et al. [22]. Primer sequences were 5′‐CCT GTT GTT GCC TTA AAC TTC‐3′ (rPLU5) and 5′‐TTAAAATTGTTGCAGTTAAAACG‐3′ (rPLU6) for the Plasmodium genus with 1100 base pairs, and for each species were rFAL1 (5′‐TTA AAC TGG TTT GGG AAA ACC AAA TAT ATT‐3′) and rFAL2 (5′‐ACA CAA TGA ACT CAA TCA TGA CTA CCC GTC‐3′) for P. falciparum with 206 base pairs, rMAL1 (5′‐ATA ACA TAG TTG TAC GTT AAG AAT AAC CGC‐3′) and rMAL2 (5′‐AAA ATT CCC ATG CAT AAA AAA TTA TAC AAA‐3′) for P. malaria with 145 base pairs, rVIV1 (5′‐CGC TTC TAG CTT AAT CCA CAT AAC TGA TAC‐3′) and rVIV2 (5′‐ACT TCC AAG CCG AAG CAA AGA AAG TCC TTA‐3′) for P. vivax with 121 base pairs and rOVA1 (5′‐ATC TCT TTT GCT ATT TTT TAG TAT TGG AGA‐3′) and rOVA2 (5′‐ATC TAA GAA TTT CAC CTC TGA CAT CTG‐3′) for P. ovale with 226 base pairs. The products of nested PCR were analyzed by electrophoresis on 2% agarose gel.
Cytokine measurements
Detection of human IL‐12 p70, TNF‐α, TGF‐β, IL‐4, IL‐6, IL‐13, IL‐22, IFN‐γ, IL‐17 and IL‐10 were determined by enzyme‐linked immunosorbent assay (ELISA) in the plasmas according to the manufacturer’s instructions (Bender MedSystems, Vienna, Austria). Optical densities (OD) were measured at 450 nm with a reference at 620 nm using an ELISA plate reader (Stat Fax 3200®; Bioblock/Fisher Scientific, Vienna, Austria). The detection limits were as follows: 2 pg/ml for IL‐6, IL‐10 and IL‐4; 4 pg/ml for IFN‐γ, TNF‐α, IL‐13, IL‐12p70 and IL‐17A; and 8 pg/ml for IL‐22 and TGF‐β. All samples were tested in duplicate, and the mean of the two OD values was used for analyses.
Statistical analysis
Variables were compared between the two subgroups (uninfected and infected) of the study participants and of each study site (urban, semi‐urban and rural areas). In addition, comparison was performed between all infected groups (infected cases of urban, semi‐urban and rural areas). Differences between the two subgroups were analyzed for statistical significance using the Mann–Whitney U non‐parametric test and between three groups using the Kruskal–Wallis non‐parametric test. Correlations between cytokines and between circulating levels of cytokines and parasite density or age were analyzed using Spearman’s rho test. The multiple t‐tests were used for ratio determination. We also performed a multivariate analysis using a generalized linear model of the Gaussian family using the stats package, and then the best model was determined using the Dredge function of the MumIn package. All statistical analyses were performed using Graph Pad Prism software version 8.4 and the R statistical software version 3.6.2. p values < 0.05 were considered statistically significant.
RESULTS
Socio‐demographic and clinical characteristics of the study participants
Among the 273 children included in this study, 120 had asymptomatic P. falciparum infection (asymptomatic‐infected group) and 153 were uninfected (uninfected group). The demographic and clinical features for children according to the study areas are presented in Table 1. The sex ratio (men/women) was slightly in favor of males in infected children from urban and semi‐urban areas (sex ratio = 1.13 and 1.38) and in uninfected children from the rural area (sex ratio = 1.05).
TABLE 1.
Demographic and clinical parameters of the participants in the study (median, interquartile range)
| Urban area (Franceville) | Semi‐urban area (Makokou) | Rural area (Mulundu) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Uninfected | Asymptomatic‐infected | p a | Uninfected | Asymptomatic‐infected | p a | Uninfected | Asymptomatic‐infected | p | |
| n = 33 | n = 17 | n = 32 | 19 | 88 | 84 | ||||
| Sex ratio | 0.83 | 1.13 | 0.68 | 1.38 | 1.05 | 0.87 | |||
| Age (IQR) months | 84 (72–102) | 108 (84–124) | 0.020 * | 96 (84–132) | 108 (72–132) | 0.759 | 92 (52–133) | 90 (60–118) | 0.921a |
| Temperature (IQR) °C | 36.5 (36.3–36.8) | 36.6 (36.6–37.2) | 0.066 | 37.0 (36.4–37.2) | 37.0 (36.6–37.1) | 0.533 | 36.6 (36.2–36.9) | 36.7 (36.1–37.1) | 0.260a |
| White blood cells (IQR) 103cells/mm3 | 5.90 (5.50–7.25) | 6.20 (5.80–6.95) | 0.696 | 7.15 (6.23–7.98) | 7.50 (5.20–9.70) | 0.527 | ND | ND | |
| Red blood cells (IQR) 103cells/mm3 | 4.57 (4.35–4.81) | 4.27 (3.95–4.49) | 0.004 * | 4.29 (4.03–4.57) | 4.42 (3.63–4.75) | 0.95 | ND | ND | |
| Hemoglobin (IQR) g/dl | 10.5 (9.70–11.2) | 10.3 (8.85–10.7) | 0.167 | 10.6 (10.0–11.0) | 9.70 (9.0–11.0) | 0.091 | ND | ND | |
| Platelets (IQR) 103cells/mm3 | 253 (206–298) | 241 (203–271) | 0.327 | 354 (276–433) | 306 (219–364) | 0.063 | ND | ND | |
| Parasitemia (IQR) parasites/µl | 1295 (560–3815) | 4590 (1820–9785) | 3500 (1260–6580) | 0.028* b | |||||
Sex ratio, age, temperature, leukocyte counts, hemoglobin concentrations and parasite densities in asymptomatic uninfected and infected children according to the study site (urban, semi‐urban and rural areas). The uninfected children were defined as Plasmodium falciparum‐exposed children, negative for parasites in thick blood smears and/or in rapid detection test kits.
Abbreviations: IQR, interquartile range; ND, not determined.
Significant levels were calculated using a Mann–Whitney rank sum analysis and bKruskal–Wallis analysis.
The bold‐type figures are to highlight the significant p‐values.
p < 0.05.
The median age in asymptomatic‐infected children was significantly higher than in uninfected children from the urban area [108 (84–124) months versus 84 (72–102) months; p = 0.02]. In the semi‐urban and rural areas, the mean age was similar between asymptomatic‐infected and uninfected children. However, comparisons between the ages of asymptomatic‐infected children in the three areas showed that even though rural children tended to have a younger age than urban and semi‐urban children, there was no statistical difference (p = 0.095, data not shown). In addition, significantly higher parasitemia loads were found in the semi‐urban and rural areas than in the urban area [4590 (1820–9785) parasites/µl, 3500 (1260–6580) parasites/µl and 1295 (560–3815) parasites/µl, respectively; p = 0.028].
Regarding red blood cells, the concentration was significantly lower in asymptomatic‐infected children compared to uninfected children in the urban area [4.27 (3.95–4.49) × 103cells/mm3 versus 4.57 (4.35–4.81) × 103cells/mm3; p = 0.004]. However, no significant difference was noted between the two subgroups in the semi‐urban area [4.42 (3.63–4.75) × 103cells/mm3 versus 4.29 (4.03–4.57) × 103cells/mm3; p = 0.95]. Moreover, although it was not significantly different, a decrease in the hemoglobin parameter was observed in asymptomatic‐infected children compared to the uninfected children from the urban and semi‐urban areas [10.3 (8.85–10.7) g/dl versus 10.5 (9.70–11.2) g/dl; p = 0.167 and 9.70 (9.0–11.0) g/dl versus 10.6 (10.0–11.0) g/dl; p = 0.091 in the urban and semi‐urban areas, respectively].
Although not significant, temperature tended to increase in asymptomatic‐infected children compared to uninfected children in the urban area [36.6 (36.6–37.2)°C versus 36.5 (36.3–36.8)°C; p = 0.066], but was similar between the two groups of children in the semi‐urban area [37.0 (36.6–37.1)°C versus 37.0 (36.4–37.2)°C; p = 0.533] and in the rural area [36.7 (36.1–37.1)°C versus 36.6 (36.2–36.9)°C; p = 0.26].
Higher IL‐6 and IL‐10 levels in children with asymptomatic P. falciparum infection compared to other cytokines
Pro‐ and anti‐inflammatory cytokines levels were assessed in the plasma samples collected in children from the Mulundu district, Makokou and Franceville. Pro‐ and anti‐inflammatory cytokines IL‐6 and IL‐10 were significantly higher in children with asymptomatic malaria than in uninfected children (Figure 2). Indeed, the IL‐6 concentration was significantly higher in asymptomatic‐infected children compared to uninfected children [0.60 (0–1.41) pg/ml versus 0.29 (0–0.88) pg/ml; p = 0.018; Figure 2a]. IL‐10 concentration was significantly higher in asymptomatic‐infected children than in uninfected children [13.14 (0–29.90) pg/ml versus 1.60 (0–7.79) pg/ml; p < 0.0001; Figure 2b]. The concentrations of TNF‐α, IL‐12p70 and IFN‐γ did not differ between asymptomatic‐infected and uninfected children [0 (0–1.88) pg/ml versus 0.39 (0–4.82) pg/ml; p = 0.106 for TNF‐α and 0 (0–0.09) pg/ml versus 0 (0–0.13) pg/ml; p = 0.713 for IL‐12p70 and 2.89 (1.28–6.03) pg/ml versus 1.84 (0.26–6.61) pg/ml; p = 0.089 for IFN‐γ; Supporting information, Figure S2]. Also, IL‐17A and IL‐22 levels in the asymptomatic‐infected children in comparison to uninfected children were similar [0 (0–1.85) pg/ml versus 0.23 (0–2.93) pg/ml; p = 0.329 for IL‐17A and 67.3 (52.3–81.3) pg/ml versus 66.1 (52–2‐85.3) pg/ml; p = 0.859 for IL‐22, respectively; Supporting information, Figure S2]. Similarly, no difference in TGF‐β, IL‐4 and IL‐13 levels was observed in asymptomatic‐infected children compared to uninfected children [0 (0–2.92) pg/ml versus 0 (0–4.62) pg/ml; p = 0.568 for TGF‐β; 0 (0–0.76) pg/ml versus 0 (0–0.70) pg/ml; p = 0.868 for IL‐4; 1.5 (0–7.0) pg/ml versus 1.5 (0–8.5) pg/ml; p = 0.957 for IL‐13, respectively; Supporting information, Figure S2].
FIGURE 2.
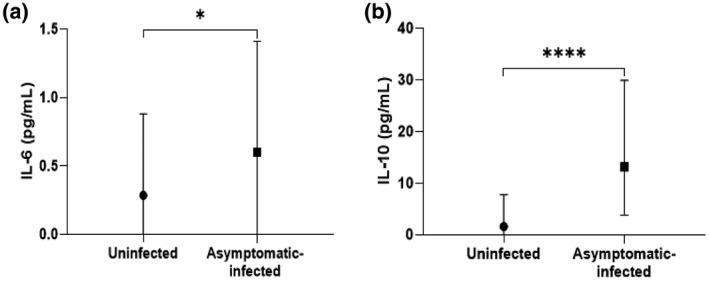
Levels of pro‐ and anti‐inflammatory cytokines in infected and uninfected children from the three study sites combined. Plasma concentration of interleukin (IL)‐6 (a) and IL‐10 (b) were quantified from asymptomatic Plasmodium falciparum‐infected and ‐uninfected children by enzyme‐linked immunosorbent assay (ELISA). Data are represented with the median of cytokine concentrations in pg/ml with interquartile range. Significant differences between infected and uninfected children were tested using Mann–Whitney’s method and are indicated. *p < 0.05; ****p < 0.0001
Pro‐/anti‐inflammatory ratio in asymptomatic malaria children
The ratios of IL‐6/IL‐10 and TNF‐α/IL‐10 were determined for evaluating whether proinflammatory cytokine or anti‐inflammatory cytokine predominates in asymptomatic P. falciparum infection. Although no significant difference was found between the pro‐/anti‐inflammatory (IL‐6 and TNF‐α to IL‐10) ratio of asymptomatic‐infected and uninfected children, data revealed that IL‐10 was nearly five‐ to four‐fold higher than TNF and IL‐6, respectively (4.793 for TNF‐α/IL‐10 ratio and 3.909 for IL‐6/IL‐10 ratio; Table 2).
TABLE 2.
Pro‐/anti‐inflammatory ratio
| Ratio | Uninfected | Infected | p‐value |
|---|---|---|---|
| IL‐6/IL‐10 | 1,367 | 3,909 | 0,333 |
| TNF‐α/IL‐10 | 0,244 | 3,858 |
Multiple t‐test ratio analysis was carried out between proinflammatory [interleukin (IL)‐6, tumor necrosis factor (TNF)‐α] and anti‐inflammatory (IL‐10) cytokines in asymptomatic Plasmodium falciparum‐infected and uninfected children. The means, standard error of the means (SEM) and n were 6.122 pg/ml, 2.541 and 117 versus 3.127 pg/ml, 1.579 and 142 for IL‐6; 6.064 pg/ml, 2.773 gp/ml and 117 versus 5.136 pg/m, 1.022 mg/ml and 149 for TNF‐α and 30.32 pg/m, 5.606 mg/ml and 119 versus 5.438 pg/m, 0.711 pg/m and 153 for IL‐10 in infected asymptomatic compared to uninfected children. The Mann–Whitney U‐test was used to compare the balance between pro‐ and anti‐inflammatory cytokines. The difference was statistically significant for p < 0.05.
Proinflammatory cytokine levels (including IL‐17A, IFN‐γ and TNF‐α) with higher IL‐10 levels in asymptomatic malaria children for each area
With the aim of characterizing the response to asymptomatic P. falciparum infection according to the level of development in the regions, the asymptomatic‐infected and uninfected groups were subdivided into three groups according to the participants’ location (urban, semi‐urban and rural area). The cytokine levels in plasma from infected and uninfected children with asymptomatic P. falciparum infection was determined and compared for each area. Interestingly, the results showed that, in each site, the levels of the anti‐inflammatory cytokine IL‐10 were statistically elevated in the asymptomatic‐infected compared to the uninfected children, making it a good marker of asymptomatic P. falciparum infection. At the same time, at least one proinflammatory cytokine (IFN‐γ, TNF‐α or IL‐17A) was significantly different between the two groups of children (Figure 3 and Supporting information, Figure S2). In the urban area, results showed that significantly lower IL‐17A levels with higher IL‐10 levels were found in the asymptomatic‐infected group compared to the uninfected group [0 (0–0.53) pg/ml versus 0.28 (0–2.44) pg/ml; p = 0.034 for IL‐17A; Figure 3c and 14.0 (1.98–22.0) pg/ml versus 1.60 (0.20–3.27) pg/ml; p < 0.001 for IL‐10; Figure 3d, respectively]. There was no difference in TNF‐α and IFN‐γ levels in urban areas [0 (0–0.26) pg/ml versus 0 (0‐0.98) pg/ml; p = 0.299 and 5.40 (4.21–8.43) pg/ml versus 4.84 (1.64–8.95) pg/ml; p = 0.379, respectively].
FIGURE 3.
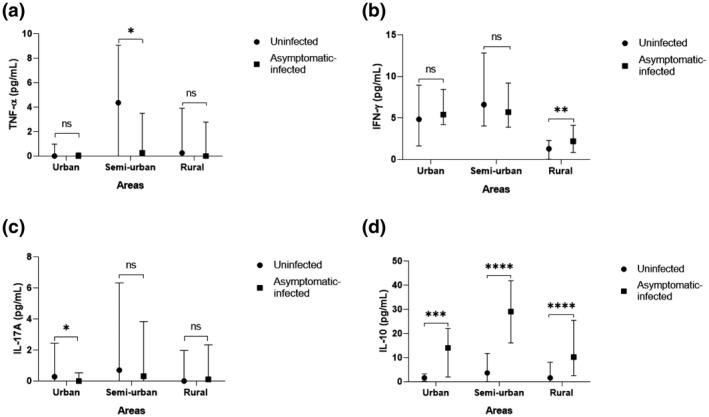
Levels of tumor necrosis factor (TNF)‐α, interferon (IFN)‐γ, interleukin (IL)‐17A and IL‐10 in plasma from infected and uninfected children according to site. Plasma concentration of TNF‐α (a), IFN‐γ (b), IL‐17A (c) and IL‐10 (d) in asymptomatic Plasmodium falciparum‐infected and ‐uninfected children according to the living area (rural, semi‐urban and urban areas) were quantified using enzyme‐linked immunosorbent assay (ELISA). Data are represented with cytokine concentration medians in pg/ml with interquartile range. Statistically significant differences between the two groups were tested using the Mann–Whitney method and are represented as *p < 0.05; **p < 0.001; ***p = 0.0001; ****p < 0.0001; NS = non‐significant difference
In the semi‐urban area there were significant lower TNF‐α levels with higher IL‐10 levels in the asymptomatic‐infected group when compared to the uninfected group [0.26 (0–3.49) pg/ml versus 4.36 (0–9.05) pg/ml; p = 0.046 for TNF‐α; Figure 3a and 29.6 (16.1–41.8) pg/ml versus 3.64 (0.05–11.7) pg/ml; p < 0.0001 for IL‐10; Figure 3d, respectively]. However, there was no difference in IL‐17A and IFN‐γ levels [0.31 (0–3.83) pg/ml versus 0.71 (0–6.32) pg/ml; p = 0.226 and 5.70 (3.88–9.20) pg/ml versus 6.61 (4.03–12.8) pg/ml; p = 0.531, respectively].
Concerning the rural area, significant higher IFN‐γ levels with higher IL‐10 levels were also observed in the asymptomatic‐infected group in comparison to the uninfected group [2.19 (0.86–4.12) pg/ml versus 1.29 (0.02–2.30) pg/ml; p = 0.001 for IFN‐γ; Figure 3b and 10.2 (2.52–25.4) pg/ml versus 1.60 (0–8.07) pg/ml; p < 0.0001 for IL‐10; Figure 3d]. However, there was no significant difference in IL‐17A and TNF‐α levels in this area [0.11 (0–2.34) pg/ml versus 0 (0–1.97) pg/ml; p = 0.713 and 0 (0–2.78) pg/ml versus 0.25 (0–3.91) pg/ml; p = 0.536, respectively].
Th1‐, Th17‐ and Th2‐circulating cytokines in asymptomatic P. falciparum‐infected children according to study area
When considering only the infected children with asymptomatic P. falciparum infection in the three areas, an overall comparison showed a significant difference between the urban, semi‐urban and rural areas in the majority of cases: all Th1 apart from TNF‐α (p < 0.0001, p = 0.009, p < 0.001 and p = 0.192 for IFN‐γ, IL‐6, IL‐12p70 and TNF‐α, respectively), Th2 (p = 0.012, p < 0.001, p = 0.007 and p = 0.006 for IL‐4, IL ‐13, TGF‐β and IL‐10, respectively) and Th17 cytokines (p < 0.0001 for IL‐22 and p = 0.101 for IL‐17A).
However, when comparing two areas at a time results showed that between asymptomatic‐infected children of urban and semi‐urban areas, there were significant differences in the Th1 cytokine response for IL‐12p70 and IL‐6 levels but not for TNF‐α and IFN‐γ levels. Indeed, higher IL‐12p70 and lower IL‐6 levels were found in the urban area than in the semi‐urban areas [0 (0–0) pg/ml versus 0.09 (0.07–0.12) pg/ml; p < 0.0001 and 0.33 (0.20–0.86) pg/ml versus 0 (0–0.67) pg/ml; p < 0.010 (Figure 4a,c), respectively]. However, TNF‐α and IFN‐γ levels were similar [0 (0–0.26) pg/ml versus 0.26 (0–3.49) pg/ml; p = 0.104 and 5.40 (4.21–8.43) pg/ml versus 5.70 (3.88–9.20) pg/ml; P > 0.999) (Figure 4b,d), respectively].
FIGURE 4.
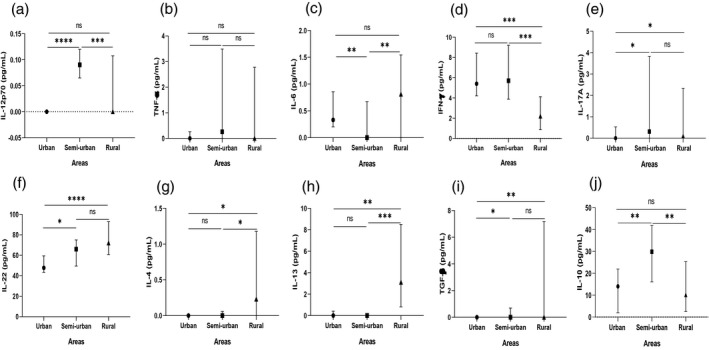
T helper type 1 (Th1), Th17 and Th2 cytokine levels (median with interquartile range) in plasma from infected children by site. Circulating levels of interleukin (IL)‐12p70 (a), tumor necrosis factor (TNF)‐α (b), interleukin (IL)‐6 (c), interferon (IFN)‐γ (d), IL‐17A (e), IL‐22 (f), IL‐4 (g), IL‐13 (h), transforming growth factor (TGF)‐β (i) and IL‐10 (j) in the plasma of asymptomatic Plasmodium falciparum‐infected children were compared in pairs according to location (urban versus rural, urban versus semi‐urban and semi‐urban versus rural). The levels of these cytokines were measured using enzyme‐linked immunosorbent assay (ELISA). Data are expressed as medians in pg/ml with the interquartile range. The Mann–Whitney method was used for comparisons and significant differences are indicated: *p < 0.05; **p = 0.001; ***p = 0.0001; ****p < 0.0001; NS = non‐significant difference
Also, there were significant differences in the Th2 cytokine response between asymptomatic‐infected children of urban and semi‐urban areas for IL‐10 and TGF‐β levels but not for IL‐4 and IL‐13 levels. IL‐10 and TGF‐β levels were lower in asymptomatic‐infected children of urban than those of semi‐urban areas [14.0 (1.98–22.0) pg/ml versus 29.9 (16.1–41.8) pg/ml; p = 0.009 for IL‐10 and 0 (0–0) pg/ml versus 0 (0–0.70) pg/ml respectively; p = 0.025 for TGF‐β (Figure 4j,i), respectively]. In contrast, levels of IL‐4 and IL‐13 were similar between the urban and the semi‐urban areas [0 (0–0) pg/ml versus 0 (0–0.06) pg/ml; p = 0.962 and 0 (0–0.40) pg/ml versus 0 (0–0) pg/ml; p = 0.339 (Figure 4g,h) respectively].
There was a significant difference in the Th17 cytokine responses (IL‐17A and IL‐22). The levels of IL‐17A and IL‐22 were lower in asymptomatic‐infected children of urban than those of semi‐urban areas [0 (0–0.53) pg/ml versus 0.31 (0–3.83) pg/ml; p = 0.042 and 47.8 (43.4–59.4) pg/ml versus 66.0 (49.5–75.0) pg/ml; p = 0.034 (Figure 4e,f) respectively].
Between asymptomatic‐infected children of urban and rural areas there was no significant difference in the Th1 cytokine responses for 12p70, TNF‐α and IL‐6 levels except for IFN‐γ. IFN‐γ levels were significantly higher in the urban than in the rural area [5.40 (4.21–8.43) pg/ml versus 2.19 (0.86–4.12) pg/ml; p < 0.001; Figure 4d], but the levels were similar [0 (0–0) pg/ml versus 0 (0–0.11) pg/ml; p = 0.432 for IL‐12p70; 0 (0–0.26) pg/ml versus 0 (0–2.78) pg/ml; p = 0.087for TNF‐α and 0.33 (0.20–0.86) pg/ml versus 0 (0–1.55) pg/ml; p = 0.186 for IL‐6, respectively; Figure 4a–c].
In the Th2 cytokine responses there was a significant difference for IL‐4, IL‐13 and TGF‐β, apart from IL‐10. Lower levels were found in the urban area than in the rural area [0 (0–0) pg/ml versus 0.23 (0–1.18) pg/ml; p = 0.015 for IL‐4, 0 (0–0.40) pg/ml versus 3.10 (0.80–8.50) pg/ml; p = 0.002] for IL‐13 and [0 (0–0) pg/ml versus 0 (0–7.17) pg/ml; p = 0.003 for TGF‐β; Figure 4g–i, respectively]. IL‐10 levels were similar between asymptomatic‐infected children from urban and rural areas [14.0 (1.98–22.0) pg/ml versus 10.2 (2.52–25.4) pg/ml; p = 0.728; Figure 4j].
There was a significant difference in Th17 cytokine responses (IL‐17A and IL‐22). Lower levels were found in the urban area compared to the rural area [0 (0–0.53) pg/ml versus 0.11 (0–2.34) pg/ml; p = 0.045 for IL‐17A and 47.8 (43.4–59.4) pg/ml versus 72.0 (60.8–93.0) pg/ml; p < 0.0001 for IL‐22; Figure 4e,f, respectively].
Between asymptomatic‐infected children of semi‐urban and rural areas in the Th1 cytokine responses there was a significant difference for 12p70, IL‐6 and IFN‐γ levels, but no difference was found for TNF‐ α levels. IL‐12p70 and IFN‐γ levels were higher; however, IL‐IL‐6 was lower in the semi‐urban area compared to the rural area [0.09 (0.07–0.12) pg/ml versus 0 (0–0.11) pg/ml; p < 0.001 for IL‐12p70, 5.70 (3.88–9.20) pg/ml versus 2.19 (0.86–4.12) pg/ml; p < 0.001 for IFN‐γ and 0 (0–0.67) pg/ml versus 0.81 (0–1.55) pg/ml; p = 0.005 for IL‐6; Figure 4a,c,d, respectively]. TNF‐α levels were similar between asymptomatic‐infected children of these two areas [0.26 (0–3.49) pg/ml versus 0 (0–2.78) pg/ml; p = 0.806; Figure 4b].
Furthermore, in Th2 cytokine responses there were significant differences for IL‐10, IL‐4 and IL‐13 levels but not for TGF‐β levels. IL‐4 and IL‐13 levels were significantly lower in the semi‐urban area compared to the rural area [0 (0–0.06) pg/ml versus 0.23 (0–1.18) pg/ml; p = 0.043 for IL‐4; 0 (0–0) pg/ml versus 3.10 (0.80–8.50) pg/ml; p < 0.001 for IL‐13; Figure 4g,h]. However, IL‐10 was significantly higher in the semi‐urban than in the rural area [29.9 (16.1–41.8) pg/ml versus 10.2 (2.52–25.4) pg/ml; p = 0.00213; Figure 4j)], while TGF‐β levels were similar [0 (0–0.70) pg/ml versus 0 (0–7.17) pg/ml; p = 0.246; Figure 4i].
Also, no significant difference was found in the levels of IL‐17A and IL‐22, the Th17 cytokines, between asymptomatic‐infected children of the semi‐urban and rural areas [0.31 (0–3.83) pg/ml versus 0.11 (0–2.34) pg/ml; p = 0.805 and 66.0 (49.5–75.0) gp/ml versus 72.0 (60.8–93.0) pg/ml; p = 0.054; Figure 4e,f, respectively].
Association between cytokine levels and parasitemia, age, gender or residence area in asymptomatic P. falciparum infection
Associations between parasitemia and cytokines and between cytokines themselves are shown in Table 3. IL‐10 showed a moderate positive association with parasite density (r = 0.53, p < 0.0001). Statistically significant weak associations between cytokine levels and parasite density were also observed for IL‐6 (r = 0.20, p = 0.046) and IL‐12p70 (r = 0.24, p = 0.031). The correlation analysis of cytokines with each other showed that only IL‐10 and IL‐6 presented a statistically significant association trend (r = 0.21, p = 0.026). Conversely, a weak trend of negative association was observed between parasite load and IFN‐γ (r = −0.22, p = 0.027).
TABLE 3.
Correlations between plasma levels of IL‐6, IL‐10, IFN‐γ, IL‐12p70 and parasitemia and between IL‐10 and IL‐6
| Parameters | Spearman’s r (or rho) | p |
|---|---|---|
| Parasitemia versus IL‐10 | 0.53 | <0.0001**** |
| Parasitemia versus IL‐6 | 0.20 | 0.046* |
| Parasitemia versus IFN‐γ | −0.22 | 0.027* |
| Parasitemia versus IL‐12 p70 | 0.24 | 0.031* |
| IL‐10 versus IL‐6 | 0.21 | 0.026* |
Spearman’s rank correlation analysis was carried out between cytokine and parasitemia in asymptomatic Plasmodium falciparum‐infected children. Correlation coefficient (rho) is given. The correlation is strong for rho = 0.7 to 1, moderate for rho = 0.5 to 0.7 and weak for rho = 0.2 to 0.5 and for a value close to 0 there is no correlation. The correction can be positive or negative. The statistically significant difference was designated from 1.
Abbreviations: IFN, interferon; IL, interleukin.
p < 0.05
p < 0.0001.
Moreover, the effect of age, gender, parasitemia load and urbanization on cytokine levels was evaluated using a generalized linear model. None of the cytokine levels were associated with gender. Among the 10 cytokines only TGF‐β varied with age. Otherwise, data revealed that parasitemia was a good predictor of IL‐10 and the levels of three cytokines (IFN‐γ, TGF‐β and IL‐10) were influenced by urbanization or residence area (Table 4 and Supporting information, Table S1).
TABLE 4.
Association between plasma pro‐ and anti‐inflammatory cytokine levels and age, urbanization, parasitemia or gender
| Intercept | Urbanization | Parasitemia | Age | Gender | Degree of free | ΔAIC | Weight | |
|---|---|---|---|---|---|---|---|---|
| IL‐10 | 0.33 | + | 0.22 | 6 | 0 | 0.74 | ||
| IFN‐γ | −0.2 | + | 4 | 0 | 0.96 | |||
| TGF‐β | 0.54 | + | 0.003 | 5 | 0 | 0.62 |
Generalized linear model of the Gaussian family was carried out between cytokine levels, age, urbanization, parasitemia and gender in children. The ΔAIC = 0 designates the best model. The association can be positive or negative depending on the sign of intercept.
Abbreviations: AIC, Akaike information criterion; IFN, interferon; IL, interleukin; TGF, transforming growth factor.
DISCUSSION
This study analyzed and compared the levels of inflammatory cytokines of children with P. falciparum asymptomatic infection and uninfected children living in three different hyperendemic regions of Gabon.
In the urban areas, asymptomatic‐infected children were significantly older than uninfected children. Previous studies have found that malaria‐infected children were older than uninfected children in urban, but also in the semi‐urban as well as rural areas [22], and an increase in the age of the population at risk of malaria in different regions of Gabon [8]. Furthermore, an occurrence of subclinical infections in all age groups has been reported that the more age increases, the more the risk of infection and of developing the disease significantly decrease [26]. Conversely, the significant higher parasitemia in semi‐urban and rural areas in comparison with the urban area suggests that children living in these two areas are more exposed to malaria infection [10] and therefore are always asymptomatic with higher parasite loads than those living in urban areas.
There were no clinical symptoms of malaria and anemia in the children; however, the levels of red blood cells were significantly lower in infected compared to uninfected children in the urban area. Indeed, by multiplying, P. falciparum is responsible for a breakdown of red blood cells which leads to anemia in some patients [27]. During malaria, the immune system suppresses the infected erythrocytic cells and an over‐stimulation leads to excessive production of proinflammatory cytokines; this activation of immune cells can cause severe malaria symptoms such as anemia [28].
In this study we found a significantly higher level of cytokines such as IL‐6 and IL‐10 in the plasma of infected children with asymptomatic P. falciparum parasitemia compared to uninfected children, while IFN‐γ, TNF‐α, IL‐12 p70, IL‐17A, IL‐22, IL‐4, IL‐13 and TGF‐β were similar.
IL‐6 is a pleiotropic cytokine with pro‐ and anti‐inflammatory properties [29, 30]. It is produced by various innate and adaptive cells including macrophages, dendritic cells, T and B cells. This cytokine is involved in the production of antibody differentiation of monocytes to macrophage and can exert regulatory role upon CD4+ T cells to become Th1 or Th2 effector cells [31]. It has been suggested that the cytokine network as a whole (not just one cytokine) may contribute to serious illness [32]. Moreover, a recent study has showed that asymptomatic malaria among children is maintained by a homeostasis between pro‐ and anti‐inflammatory cytokine responses [16]. This can explain the minimally but significantly increased levels of IL‐6 and IL‐10 observed in our asymptomatic P. falciparum‐infected children. This result suggests that IL‐6 plays a role in parasite control. A similar study in Gabonese children by Guiyedi et al. has reported a high plasma level of IFN‐γ and IL‐10 associated with high antigen‐specific antibody responses in asymptomatic P. falciparum infection [33]. These authors suggested that the antibody response may have a protective immune effect. A study in Indonesia also found that levels of TNF‐α increased in adult subjects with asymptomatic malaria [34].
Elevated levels of IL‐10 in asymptomatic P. falciparum infection in pregnant women, adult subjects and children have been reported in different studies from Uganda, Ghana, Indonesia, Mali and Gabon [17, 21, 33, 34, 35, 36]. IL‐10 is an anti‐inflammatory cytokine that exerts an immunoregulatory role. Its presence in these asymptomatic‐infected children suggests that IL‐10 suppresses parasite clearance, thus preventing the development of anti‐parasitic immunity and the progression to clinical forms of malaria [15]. Thus, IL‐10 promotes the preservation of asymptomatic infections [17]. Many studies have reported that repeated exposures to infection lead to the development of anti‐inflammatory immunity (by producing IL‐10) [17, 37, 38, 39]. During a Plasmodium infection, IL‐10 is produced by a wide variety of innate and adaptive immune cells, including macrophages, dendritic cells (DCs), Th1 cells, Treg cells, IL‐17‐producing CD4+ T (Th17) cells, Tfh cells, CD8+ T cells, regulatory B cells, natural killer (NK) cells and γδ T cells [15]. Through its action on different cells, IL‐10 promotes the tolerance of the pathogen and inhibits the production of IL‐4, IL‐5 and TNF‐α in addition to IL‐2 and IFN‐γ, thus promoting the development of parasites [40, 41, 42, 43].
Moreover, in endemic areas, repeated infections due to Plasmodium in children lead to the development of asymptomatic forms to the detriment of serious or severe forms. Monunill et al. have found that the number of P. falciparum malaria infections does not affect the levels of IL‐5, IL‐6 and IL‐10, unlike other cytokines [37]. Depending on the number of malaria episodes, no difference has been found between three groups of patients in both their IL‐6 and IL‐10 levels [44]. All this is consistent with our current results.
In addition, the positive association between cytokines IL‐10, IL‐6, IL‐12p70 and parasitemia in asymptomatic P. falciparum infection suggests that these three cytokines are produced concurrently. IL‐6 and IL‐12p70 display a proinflammatory cytokine role promoting parasite suppression, whereas IL‐10 plays an anti‐inflammatory role. However, the absence of significant differences in IL‐12p70 between asymptomatic P. falciparum‐infected children revealed that this cytokine is minimally produced during asymptomatic malaria. The negative association of IFN‐γ with parasitemia shows that this cytokine is not produced during asymptomatic parasitemia and highlights the regulatory role of IL‐10 in the inhibition of the effects of IFN‐γ, as already described above.
Frimpong et al. have found significant increased levels of TNF‐α, IL‐6, IFN‐γ, IL‐17 and IL‐4 in asymptomatic malaria infection, although a lack of difference was observed in IL‐10 levels and the ratios of IFN‐γ/IL‐10, TNF‐α/IL‐10 and IL‐6/IL‐10 when compared to controls, suggesting the maintenance of the balance between pro‐ and anti‐inflammatory cytokines [16]. Indeed, the latter is crucial in the outcome of the disease. Here, data demonstrated that even though asymptomatic P. falciparum infection seems to be up‐regulated by anti‐inflammatory cytokine there is a balance between pro‐ and anti‐inflammatory cytokines. In fact, a balance in favor of anti‐inflammatory cytokines such as IL‐10 or maintenance of the balance between pro‐ and anti‐inflammatory responses suppresses parasite clearance and contributes to parasite growth in increasing parasitemia [13, 16], and therefore promotes the development of asymptomatic infection. Due to the absence of clinical signs, as asymptomatic carriers remain untreated they constitute a silent natural reservoir which supports the transmission of malaria. All this has led to postulating that people with asymptomatic malaria infection can be either in acute, mild or late stages of infection, as in the case of symptomatic infection. Indeed, it has been observed that in symptomatic malaria infection the acute phase may reflect an early and effective immune response regulated by proinflammatory cytokines for controlling the parasite load, whereas the mid‐stage might be down‐regulated by anti‐inflammatory cytokines that is put in place for suppressing the proinflammatory effect and a very unbalanced response leads to severe disease [45, 46].
The significant lower levels of IL‐17A and TNF‐α found, respectively, in the infected group from the urban and semi‐urban areas with higher IL‐10 levels suggest that asymptomatic P. falciparum infection does not induce these proinflammatory cytokines. These results were close to previous reports. In Uganda, Jagannathan et al. found a low level of IFN‐γ and TNF‐α in asymptomatic children with malaria and concluded that repeated exposure to malaria infection leads to a decrease in the frequency of TNF‐producing CD4+ T cells and autoregulating Th1 CD4+ cells that co‐produce IFN‐γ together with IL‐10 [35]. Production of Th1 and Th17 cytokines (such as TNF‐α and IL‐17A, respectively) was shown to decrease in asymptomatic P. falciparum infection, while IL‐10 levels were high [36]. This is consistent with the suppressive activity that IL‐10 would play upon production of proinflammatory cytokines in our asymptomatic children.
However, there are conflicting reports on IFN‐γ and TNF‐α levels resulting from asymptomatic P. falciparum infection in Gabonese children and Indonesian adults. In these studies, the authors report that high IFN‐γ and TNF‐α levels were associated with asymptomatic forms of the disease [33, 34]. Interestingly enough, these results corroborate with the significantly elevated levels of IFN‐γ and IL‐6 with higher IL‐10 levels found in our asymptomatic children from both the rural area and the general study population, and re‐emphasize the possible existence of a balance between pro‐ and anti‐inflammatory cytokines in asymptomatic P. falciparum infection. Given that the urbanization was predictive of IFN‐γ and IL‐10 levels, especially in the urban and semi‐urban areas and in the semi‐urban area, all the above suggests that IL‐10 in asymptomatic P. falciparum carriers could counter‐regulate the IFN‐γ, TNF‐α and IL‐17A effects to maintain tissue integrity.
The following comparisons relate only to the asymptomatic P. falciparum‐infected children between areas. Significantly elevated differences in cytokine levels found in the rural area when compared to the semi‐urban and/or urban area but also observed in the semi‐urban area when compared to the urban area may highlight the effect of immune activation due to repeated exposures to infections observed in high endemicity regions. Indeed, it has been shown that infection frequencies are higher in rural than in urban and semi‐urban areas [10]. Another study stipulated that chronic exposure to infection and immune activation may affect the development and preservation of natural acquired immunity [47]. An association between repeated malaria infection in children and the loss or decrease of cytokine production has been observed; this dysfunction was correlated with clinical tolerance to malaria [48, 49, 50]. After repeated malaria infections children lost their ability to produce strong levels of cytokines [38]; this is reflected by the elevated cytokine levels observed in our asymptomatic rural children and accordingly accounts for their exposure to infections compared to the urban area.
In particular, IL‐17 and IL‐22 are known as Th17 cytokines [39, 51], and their significant difference between infected children from urban and semi‐urban areas and between children from urban and rural areas to the disadvantage of the urban area may imply that the Th17 response is also modified by repeated exposition to infections. The Th17 pathway appears to be playing an extremely important role in immunity against P. falciparum [52]. It has been stipulated that IL‐17 could play a dual role in malaria in the early phase of infection through the expression of a high production of IL‐17, which can prevent severe malaria, and during the acute phase by over‐production of IL‐17, which induces severe malaria anemia [12].
In addition, similar to that of IL‐17A and IL‐22, the profile observed by TGF‐β would bring to light the role played by this regulatory cytokine upon Th17 response. Development of Th17 cells in humans depends upon cytokine microenvironments rich in IL‐1β, IL‐6 and TGF‐β that initiate the differentiation process of the Th17 cells [52]. A study has shown that after many exposures to infection the standard baseline level of cytokines of people living in hyperendemic regions remained more elevated compared to people living in hypo‐endemic areas [53]. This underlines the higher level of exposure to infections of children in rural than in semi‐urban and urban areas.
Moreover, that IL‐4 and IL‐13 levels were significantly lower in the urban and semi‐urban compared to the rural area suggests that Th2 immune response is modulated differently in these two areas. It has been established that these Th2 cytokines are involved in anti‐helminth responses [54]. A study conducted in Gabon found 6.8% polyparasitism among asymptomatic parasite carriers living in urban, semi‐urban and rural areas, and that the prevalence of helminthic infections in rural areas was higher than in semi‐urban and urban areas [10].
The significantly higher IFN‐γ levels in the urban and semi‐urban areas compared to the rural area suggest a probable inhibition of IFN‐γ production in the rural area, the most hyperendemic region. In a hyperendemic context, the higher regulatory cytokine (TGF‐β and IL‐10) levels can inhibit IFN‐γ production and thus suppress proinflammatory responses and promote the preservation of parasites through asymptomatic forms of infection.
In contrast, the significant difference between urban and semi‐urban values of IL‐12p70 and IL‐10 levels in favor of the semi‐urban area supports the idea that the semi‐urban area is more exposed than the urban area.
The significantly elevated levels of IL‐10 in the semi‐urban area and IL‐6 in the urban area compared, respectively, to the rural and urban areas suggest a probable inhibition of their secretion. However, the similarity between the urban and rural areas for the IL‐12p70, IL‐6 and IL‐10 levels could be due to the difference in sample size observed.
This study has some limitations. First, some participants in the uninfected group might have been wrongly classified due, on one hand, to the existence of submicroscopic parasitemia load that only a PCR‐based method can detect, and on the other hand to the fact that the uninfected group was classified based on the presence of infection as determined by either a rapid diagnostic test or a thick blood smear. Secondly, the participants were not followed to determine if any of them progressed to symptomatic infection and therefore note the cytokine variations in the onset of these clinical signs. A final limitation could be the lack of detection of other infections such as intestinal parasites, which are common in children. Nevertheless, this study was able to show that asymptomatic P. falciparum infection results in increased pro‐ and anti‐inflammatory cytokines levels without modifying the balance between pro‐ and anti‐inflammatory cytokines.
In conclusion, this study demonstrates that higher levels of IL‐6 and IL‐10 among the 10 cytokines evaluated in the Gabonese children in this study are associated with asymptomatic P. falciparum infection. IL‐10 may be a clearer indicator of the asymptomatic malaria, as significantly higher levels were associated with the asymptomatic form in all three distinct areas and with parasitemia load, in contrast to IL‐6. Our studies reinforce recent studies reporting that the asymptomatic malaria infection results in maintenance of the balance between pro‐ and anti‐inflammatory cytokines [16], and underline the higher exposure to infections of asymptomatic‐infected children in rural areas than in the two other areas.
CONFLICTS OF INTEREST
The authors have no financial conflicts of interest.
AUTHOR CONTRIBUTIONS
C.N.M.M.N. co‐ordinated the study, performed the cytokine measurements and all analyses and wrote the manuscript. S.L.O.L. co‐ordinated the study and contributed to the cytokine measurements and drafting of the manuscript. C.L.K. and R.K.I.L. contributed to the collection of samples and data and performed the P. falciparum diagnosis. APO contributed to the sample and data collections and performed P. falciparum diagnosis and with S.S.O. contributed the cytokine measurements. N.P.T.T. contributed to the sample collection and prescribed the treatment to the asymptomatic malaria participants. J.B.L.D. conceived and designed the study, contributed to the drafting of the manuscript and will be responsible for communication with the journal during the manuscript submission, peer review, publication process and after publication. All authors critically reviewed and approved the final version of the manuscript.
ETHICAL STATEMENT
This study was approved by the Gabonese National Research Ethics Committee (no. 0023/2013/SG/CNE). Written informed consent was obtained from the parents or guardians before each child’s participation in the study.
Supporting information
Fig S1
Fig S2
Fig S3
Table S1
ACKNOWLEDGEMENTS
This study was supported by the European and Developing Countries Clinical Trials Partnership (EDCTP): Central Africa Clinical Research Network (CANTAM), Grant no. EDCTP‐RegNet2015‐1045. We thank all the children and their parents or guardians who consented to participate in this study. We are also grateful for the support, advice and reviews of all the clinicians and to Dr Franck Mounioko and Mr Neil Michel Longo Pendy for their guidance, advice and help on statistical analysis.
Mbani Mpega Ntigui CN, Oyegue‐Liabagui SL, Kouna LC, Imboumy KR, Tsafack Tegomo NP, Okouga AP, et al. Inflammatory cytokine responses in children with asymptomatic malaria infection living in rural, semi‐urban and urban areas in south‐eastern Gabon. Clin Exp Immunol. 2021;206:395–409. 10.1111/cei.13653
Chérone Nancy Mbani Mpega Ntigui and Sandrine Lydie Oyegue‐Liabagui contributed equally to this work.
DATA AVAILABILITY STATEMENT
Due to the confidentiality of participants’ data, the data associated with this study are not directly accessible via a link, but readers or researchers can access it by contacting the corresponding author.
REFERENCES
- 1. World Health Organization . The ‘World Malaria Report 2019’ at a glance. [Cited 2021 Apr 13]. Available from: https://www.who.int/news‐room/feature‐stories/detail/world‐malaria‐report‐2019
- 2. World Health Organization . World Malaria Report 2015. Geneva: WHO; 2016. [Google Scholar]
- 3. Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L. The silent threat: asymptomatic parasitemia and malaria transmission. Exp Rev Anti Infect Ther. 2013;11:623–39. [DOI] [PubMed] [Google Scholar]
- 4. Nkoghe D, Akue J‐P, Gonzalez J‐P, Leroy EM. Prevalence of Plasmodium falciparum infection in asymptomatic rural Gabonese populations. Malaria J. 2011;10:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pegha Moukandja I, Biteghe Bi Essone JC, Sagara I, Kassa Kassa RF, Ondzaga J, Lékana Douki J‐B, et al. Marked rise in the prevalence of asymptomatic Plasmodium falciparum infection in rural Gabon. PLOS ONE. 2016;11:e0153899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lekana‐Douki J, Pontarollo J, Zatra R, Touré‐Ndouo F. Malaria in Gabon: results of a clinical and laboratory study at the Chinese–Gabonese Friendship Hospital of Franceville. Santé. 2012;21:193–8. [DOI] [PubMed] [Google Scholar]
- 7. Jiram AI, Ooi CH, Rubio JM, Hisam S, Karnan G, Sukor NM, et al. Evidence of asymptomatic submicroscopic malaria in low transmission areas in Belaga district, Kapit division, Sarawak, Malaysia. Malaria J. 2019;18:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mawili‐Mboumba DP, Akotet MKB, Kendjo E, Nzamba J, Medang MO, Mbina J‐RM, et al. Increase in malaria prevalence and age of at risk population in different areas of Gabon. Malaria J. 2013:12. 10.1186/1475-2875-12-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maghendji‐Nzondo S, Nzoughe H, Lemamy GJ, Kouna LC, Pegha‐Moukandja I, Lekoulou F, et al. Prevalence of malaria, prevention measures, and main clinical features in febrile children admitted to the Franceville Regional Hospital, Gabon. Parasite. 2016;23:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moutongo Mouandza R, M'bondoukwe NP, Obiang Ndong GP, Nzaou Nziengui A, Batchy Ognagosso FB, Nziengui Tirogo C, et al. Anaemia in asymptomatic parasite carriers living in urban, rural and peri‐urban settings of Gabon. Trans R Soc Trop Med Hyg. 2020;114:618–26. [DOI] [PubMed] [Google Scholar]
- 11. Antony HA, Parija SC. Antimalarial drug resistance: an overview. Trop Parasitol. 2016;6:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oyegue‐Liabagui SL, Bouopda‐Tuedom AG, Kouna LC, Maghendji‐Nzondo S, Nzoughe H, Tchitoula‐Makaya N, et al. Pro‐ and anti‐inflammatory cytokines in children with malaria in Franceville, Gabon. Am J Clin Exp Immunol. 2017;6:9–20. [PMC free article] [PubMed] [Google Scholar]
- 13. Andrade BB, Barral‐Netto M. Biomarkers for susceptibility to infection and disease severity in human malaria. Mem Inst Oswaldo Cruz. 2011;106:70–8. [DOI] [PubMed] [Google Scholar]
- 14. Punnath K, Dayanand K, Chandrashekhar V, Achur R, Kakkilaya S, Ghosh S, et al. Association between inflammatory cytokine levels and anemia during Plasmodium falciparum and Plasmodium vivax infections in Mangaluru: a southwestern coastal region of India. Trop Parasitol. 2019;9:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kumar R, Ng S, Engwerda C. The role of IL‐10 in malaria: a double edged sword. Front Immunol. 2019;10. [Cited 2020 Mar 26]. Available from: https://www.frontiersin.org/articles/ 10.3389/fimmu.2019.00229/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frimpong A, Amponsah J, Adjokatseh AS, Agyemang D, Bentum‐Ennin L, Ofori EA, Kyei‐Baafour E, et al. Asymptomatic malaria infection is maintained by a balanced pro‐ and anti‐inflammatory response. Front Microbiol 2020;11. [Cited 2021 Jan 20]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7705202/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Portugal S, Moebius J, Skinner J, Doumbo S, Doumtabe D, Kone Y, et al. Exposure‐dependent control of malaria‐induced inflammation in children. PLOS Pathog. 2014;10:e1004079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grau GE, Piguet P‐F, Pointaire P, Bieler G, Taylor TE, Molyneux ME, et al. TNF and other cytokines in malaria: dual role in pathology and protection. Tumor necrosis factor: structure–function relationship and clinical application. Presented at the 3rd International Conference on Tumor Necrosis Factor and Related Cytokines, Makuhari, Chiba, November 1990. Basel: Karger; 1992;197–214. [Google Scholar]
- 19. Jangpatarapongsa K, Chootong P, Sattabongkot J, Chotivanich K, Sirichaisinthop J, Tungpradabkul S, et al. Plasmodium vivax parasites alter the balance of myeloid and plasmacytoid dendritic cells and the induction of regulatory T cells. Eur J Immunol. 2008;38:2697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Monastero RN, Pentyala S. Cytokines as biomarkers and their respective clinical cutoff levels. Int J Inflamm. 2017;2017:e4309485. [Cited 2020 July 24]. Available from: https://www.hindawi.com/journals/iji/2017/4309485/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilson NO, Bythwood T, Solomon W, Jolly P, Yatich N, Jiang Y, Shuaib F, et al. Elevated levels of IL‐10 and G‐CSF associated with asymptomatic malaria in pregnant women. Infect Dis Obstet Gynecol 2010;2010:e317430. [Cited 2020 Mar 26]. Available from: https://www.hindawi.com/journals/idog/2010/317430/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maghendji‐Nzondo S, Kouna L‐C, Mourembou G, Boundenga L, Imboumy‐Limoukou R‐K, Matsiegui P‐B, et al. Malaria in urban, semi‐urban and rural areas of southern of Gabon: comparison of the Pfmdr 1 and Pfcrt genotypes from symptomatic children. Malaria J. 2016;15:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23. Assele V, Ndoh GE, Nkoghe D, Fandeur T. No evidence of decline in malaria burden from 2006 to 2013 in a rural province of Gabon: implications for public health policy. BMC Public Health. 2015;15:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Planche T, Krishna S, Kombila M, Engel K, Faucher JF, Ngou‐Milama E, et al. Comparison of methods for the rapid laboratory assessment of children with malaria. Am J Trop Med Hyg. 2001;65:599–602. [DOI] [PubMed] [Google Scholar]
- 25. Snounou G, Singh B. Nested PCR analysis of Plasmodium parasites. Methods Mol Med. 2002;72:189–203. [DOI] [PubMed] [Google Scholar]
- 26. Ladeia‐Andrade S, Ferreira MU, de Carvalho ME, Curado I, Coura JR. Age‐dependent acquisition of protective immunity to malaria in riverine populations of the Amazon basin of Brazil. Am J Trop Med Hyg. 2009;80:452–9. [PubMed] [Google Scholar]
- 27. Anstey NM, Granger DL, Hassanali MY, Mwaikambo ED, Duffy PE, Weinberg JB. Nitric oxide, malaria, and anemia: inverse relationship between nitric oxide production and hemoglobin concentration in asymptomatic, malaria‐exposed children. Am J Trop Med Hyg. 1999;61:249–52. [DOI] [PubMed] [Google Scholar]
- 28. Lyke KE, Burges R, Cissoko Y, Sangare L, Dao M, Diarra I, et al. Serum levels of the proinflammatory cytokines interleukin‐1 beta (IL‐1beta), IL‐6, IL‐8, IL‐10, tumor necrosis factor alpha, and IL‐12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun. 2004;72:5630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kishimoto T. Interleukin‐6: discovery of a pleiotropic cytokine. Arthritis Res Ther. 2006;8:S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scheller J, Chalaris A, Schmidt‐Arras D, Rose‐John S. The pro‐ and anti‐inflammatory properties of the cytokine interleukin‐6. Biochim Biophys Acta Mol Cell Res. 2011;1813:878–88. [DOI] [PubMed] [Google Scholar]
- 31. Diehl S, Rincón M. The two faces of IL‐6 on Th1/Th2 differentiation. Mol Immunol. 2002;39:531–6. [DOI] [PubMed] [Google Scholar]
- 32. Mandala WL, Msefula CL, Gondwe EN, Drayson MT, Molyneux ME, MacLennan CA. Cytokine profiles in Malawian children presenting with uncomplicated malaria, severe malarial anemia, and cerebral malaria. Clin Vaccine Immunol. 2017;24. [Cited 2020 Nov 24]. Available from: https://cvi.asm.org/content/24/4/e00533‐16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guiyedi V, Bécavin C, Herbert F, Gray J, Cazenave P‐A, Kombila M, et al. Asymptomatic Plasmodium falciparum infection in children is associated with increased auto‐antibody production, high IL‐10 plasma levels and antibodies to merozoite surface protein 3. Malaria J 2015;14. [Cited 2020 Aug 21]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4419484/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nurdianto AR, Arwati H, Dachlan YP, Febiyanti DA. The relationship of hemoglobin, interleukin‐10 and tumor necrosis factor alpha levels in asymptomatic malaria patients in Trenggalek, Jawa Timur, Indonesia. Mol Cell Biomed Sci. 2019;3:13–6. [Google Scholar]
- 35. Jagannathan P, Eccles‐James I, Bowen K, Nankya F, Auma A, Wamala S, et al. IFNγ/IL‐10 Co‐producing cells dominate the CD4 Response to malaria in highly exposed children. PLOS Pathog. 2014;10:e1003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Metenou S, Dembele B, Konate S, Dolo H, Coulibaly YI, Diallo AA, et al. Filarial infection suppresses malaria‐specific multifunctional Th1 and Th17 responses in malaria and filarial coinfections. J Immunol 1950. 2011;186:4725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moncunill G, Mayor A, Jiménez A, Nhabomba A, Puyol L, Manaca MN, et al. Cytokine and antibody responses to Plasmodium falciparum in naïve individuals during a first malaria episode: effect of age and malaria exposure. PLOS ONE. 2013;8. [Cited 2020 Aug 5]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3578867/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bediako Y, Adams R, Reid AJ, Valletta JJ, Ndungu FM, Sodenkamp J, et al. Repeated clinical malaria episodes are associated with modification of the immune system in children. BMC Med. 2019;17. [Cited 2020 Sep 29]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6415347/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chaves YO, da Costa AG, Pereira MLM, de Lacerda MVG, Coelho‐dos‐Reis JG, Martins‐Filho OA, et al. Immune response pattern in recurrent Plasmodium vivax malaria. Malaria J. 2016;15:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Macatonia SE, Doherty TM, Knight SC, O’Garra A. Differential effect of IL‐10 on dendritic cell‐induced T cell proliferation and IFN‐gamma production. J Immunol. 1950;1993:3755–65. [PubMed] [Google Scholar]
- 41. McBride JM, Jung T, de Vries JE, Aversa G. IL‐10 alters DC function via modulation of cell surface molecules resulting in impaired T‐cell responses. Cell Immunol. 2002;215:162–72. [DOI] [PubMed] [Google Scholar]
- 42. Joss A, Akdis M, Faith A, Blaser K, Akdis CA. IL‐10 directly acts on T cells by specifically altering the CD28 co‐stimulation pathway. Eur J Immunol. 2000;30:1683–90. [DOI] [PubMed] [Google Scholar]
- 43. Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM. IL‐10 inhibits macrophage costimulatory activity by selectively inhibiting the up‐regulation of B7 expression. J Immunol 1950. 1993;151:1224–34. [PubMed] [Google Scholar]
- 44. Guimarães da Costa A, do Valle Antonelli LR, Augusto Carvalho Costa P, Paulo Diniz Pimentel J, Garcia NP, Monteiro Tarragô A, et al. The robust and modulated biomarker network elicited by the Plasmodium vivax infection is mainly mediated by the IL‐6/IL‐10 axis and is associated with the parasite load. J Immunol Res 2014. [Cited 2020 Jan 6]. Available from: https://new.hindawi.com/journals/jir/2014/318250/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Torre D, Speranza F, Giola M, Matteelli A, Tambini R, Biondi G. Role of Th1 and Th2 cytokines in immune response to uncomplicated Plasmodium falciparum malaria. Clin Vaccine Immunol. 2002;9:348–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Malaguarnera L, Musumeci S. The immune response to Plasmodium falciparum malaria. Lancet Infect Dis. 2002;2:472–8. [DOI] [PubMed] [Google Scholar]
- 47. Dobaño C, Moncunill G. Naturally acquired immunity (NAI). In: Kremsner PG, Krishna S, eds. Encyclopedia of malaria. New York, NY: Springer; 2018:1–15. [Cited 2020 Aug 28]. Available from: https://doi.org/ 10.1007/978-1-4614-8757-9_131-1. [DOI] [Google Scholar]
- 48. Jagannathan P, Kim CC, Greenhouse B, Nankya F, Bowen K, Eccles‐James I, et al. Loss and dysfunction of Vδ2+ γδ T cells are associated with clinical tolerance to malaria. Sci Transl Med. 2014;6:251ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jagannathan P, Lutwama F, Boyle MJ, Nankya F, Farrington LA, McIntyre TI, et al. Vδ2+ T cell response to malaria correlates with protection from infection but is attenuated with repeated exposure. Sci Rep. 2017;7:11487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Farrington LA, Jagannathan P, McIntyre TI, Vance HM, Bowen K, Boyle MJ, et al. Frequent malaria drives progressive Vδ2 T‐cell loss, dysfunction, and CD16 up‐regulation during early childhood. J Infect Dis. 2016;213:1483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hu W‐C‐J, Bourgeois AL, Wolfe N, Singh S, Jedlicka A, Scott A. Human immune responses to Plasmodium falciparum infection: molecular evidence for a suboptimal THαβ(TH9) and TH17 bias over ideal and effective traditional TH1 immunity. Nat Prec. 2011:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009;2:403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Labuda LA, de Jong SE, Meurs L, Amoah AS, Mbow M, Ateba‐Ngoa U, et al. Differences in innate cytokine responses between European and African children. PLOS ONE. 2014;9:e95241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Turner J, Faulkner H, Kamgno J, Cormont F, Van Snick J, Else K, et al. Th2 cytokines are associated with reduced worm burdens in a human intestinal helminth infection. J Infect Dis. 2003;188:1768–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Table S1
Data Availability Statement
Due to the confidentiality of participants’ data, the data associated with this study are not directly accessible via a link, but readers or researchers can access it by contacting the corresponding author.


