Abstract
Neuromyelitis optica spectrum disorder (NMOSD) is an inflammatory disease of the central nervous system (CNS) associated with antibodies to aquaporin‐4 (AQP4), which has distinct clinical, radiological and pathological features, but also has some overlap with multiple sclerosis and myelin oligodendrocyte glycoprotein (MOG) antibody associated disease. Early recognition of NMOSD is important because of differing responses to both acute and preventive therapy. Magnetic resonance (MR) imaging has proved essential in this process. Key MR imaging clues to the diagnosis of NMOSD are longitudinally extensive lesions of the optic nerve (more than half the length) and spinal cord (three or more vertebral segments), bilateral optic nerve lesions and lesions of the optic chiasm, area postrema, floor of the IV ventricle, periaqueductal grey matter, hypothalamus and walls of the III ventricle. Other NMOSD‐specific lesions are denoted by their unique morphology: heterogeneous lesions of the corpus callosum, ‘cloud‐like’ gadolinium (Gd)‐enhancing white matter lesions and ‘bright spotty’ lesions of the spinal cord. Other lesions described in NMOSD, including linear periventricular peri‐ependymal lesions and patch subcortical white matter lesions, may be less specific. The use of advanced MR imaging techniques is yielding further useful information regarding focal degeneration of the thalamus and optic radiation in NMOSD and suggests that paramagnetic rim patterns and changes in normal appearing white matter are specific to MS. MR imaging is crucial in the early recognition of NMOSD and in directing testing for AQP4 antibodies and guiding immediate acute treatment decisions. Increasingly, MR imaging is playing a role in diagnosing seronegative cases of NMOSD.
Keywords: diagnosis, magnetic resonance imaging, multiple sclerosis, neuromyelitis optica
This review summarises the clinical and radiological aspects of AQP4 antibody positive NMOSD focusing on the implication for pathophysiology. Common MR imaging features of NMOSD are reviewed in detail and illustrated. The potential future role of advanced MR imaging techniques in diagnosing and characterising NMOSD are discussed.
INTRODUCTION
Historical perspective
A distinct clinical pattern of demyelination affecting optic nerves and spinal cord associated with a characteristically destructive pathological picture was first described more than 150 years ago [1, 2, 3]. What became known as Devic’s disease or neuromyelitis optica (NMO) remained ambiguous, regarded by some as an extreme variant of multiple sclerosis (MS) and by others as a separate disease state, until 2004 when Lennon and colleagues highlighted an association between cases of longitudinally extensive transverse myelitis with optic neuritis and antibodies with a specific staining pattern on suitably prepared samples of mouse brain and kidney [4]. These ‘NMO immunoglobulin (Ig)G’ antibodies were subsequently demonstrated to bind to the aquaporin‐4 (AQP4) water channel and have become sine qua non for NMO spectrum disorder (NMOSD) [5]. Interestingly, some of the cases described by Devic, particularly those with simultaneous or sequential transverse myelitis in younger adults, may have had antibodies to myelin oligodendrocyte glycoprotein (MOG) and what is now referred to as MOG antibody‐associated disease (MOGAD) [6]. The spectrum of disease associated with AQP4 antibodies has spread to encompass area postrema, hypothalamic and diencephalic syndromes, as well as encephalitic presentations [7]. In contrast, MOGAD includes acute disseminated encephalomyelitis (ADEM) presentations (particularly in paediatric populations), focal encephalitic presentations and frequent optic neuritis in a clinical picture that previously would have been difficult to distinguish from MS, as well as longitudinally extensive transverse myelitis [8]. It will be apparent that these two antibody‐mediated central nervous system (CNS) inflammatory diseases share clinical overlap with MS and other disorders that can affect the optic nerves and spinal cord, such as neurosarcoidosis [9] and chronic relapsing idiopathic optic neuritis (CRION) [10]. Isolating antibodies has become essential in diagnosing antibody‐mediated CNS inflammatory diseases. However, false‐positive and ‐negative results remain a problem. For example, if used as a screening test in all cases of CNS inflammatory disease, where in populations of European ancestry MS outnumbers antibody‐mediated disease by at least 50:1 [11], false‐positive tests may lead to inappropriate management and skew epidemiological data. Consequently, clinical features and other ancillary tests, magnetic resonance imaging (MRI) in particular, remain extremely important in raising suspicion for these diagnoses. For the purposes of this narrative review, we will concern ourselves only with MRI features of AQP4 antibody‐positive NMOSD and will refer to this as NMOSD throughout.
Clinical features
The clinical hallmarks of NMOSD are attacks of optic neuritis (severe, painless, bilateral or sequential with poor recovery), transverse myelitis (severe, bilateral and involving motor, sensory and sphincteric control pathways, sometimes with pain and pruritus), area postrema syndrome (persistent nausea, vomiting and hiccoughs), acute brain stem syndromes (cranial nerve palsies, ataxia and limb weakness), diencephalic syndromes (narcolepsy, hypothermia, daytime somnolence and obesity) and cerebral syndromes (encephalopathy and seizures) [12]. Attacks of optic neuritis tend to occur earlier in the disease course [13]. Acute myelitis (partial or complete) and optic neuritis predominate with other types of attack being less common [13]. The attacks occur more frequently and brain stem/cerebellar presentations are relatively less common compared to MS [14]. Without treatment to prevent further attacks the prognosis in NMOSD is generally worse than it is in MS [14, 15].
Serological markers
The discovery of antibodies to AQP4 in NMOSD has been integral to developing a greater understanding of the pathophysiology of NMOSD and have helped to identify distinct patterns in MR [16] and ocular computed tomographic [17] imaging that are specific to NMOSD. AQP4 antibodies in NMOSD are predominantly IgG and bind to the AQP4 protein, a water channel found abundantly in foot processes of astrocytes forming part of the blood–brain barrier in the CNS [18]. The AQP4 protein forms a tetramer of two isoforms (M1 and M23, with M1 being 22 amino acids longer) [19]. AQP4 tetramers are arranged in orthogonal arrays of multiple channels and the extent to which this occurs depends upon the proportion of the M23 isoform, which is the predominant form in the CNS [20, 21, 22]. AQP4 antibodies in NMOSD have greater avidity for orthogonal arrays of the M23 isoform than they do of the M1 or isolated tetramers, presumably due to conformational differences [23]. This led to difficulties with earlier assays being less sensitive because they used the M1 isoform, but these issues have now been resolved. The gold standard for AQP4 detection is a live‐cell based assay [24], but dried cell‐based assays are now almost as sensitive and specific [25]. Immunofluorescence methods (Figure 1a) using rat or mouse brain and kidney are less sensitive, but have good specificity in most centres [24, 25]. Enzyme‐linked immunosorbent assays (ELISA) are the least sensitive and specific, but permit ready estimation of an antibody titre when positive [24, 25]. Neurofilament light and glial fibrillary acidic protein (GFAP) are emerging as potentially useful serological biomarkers of disease activity in NMOSD [26].
FIGURE 1.
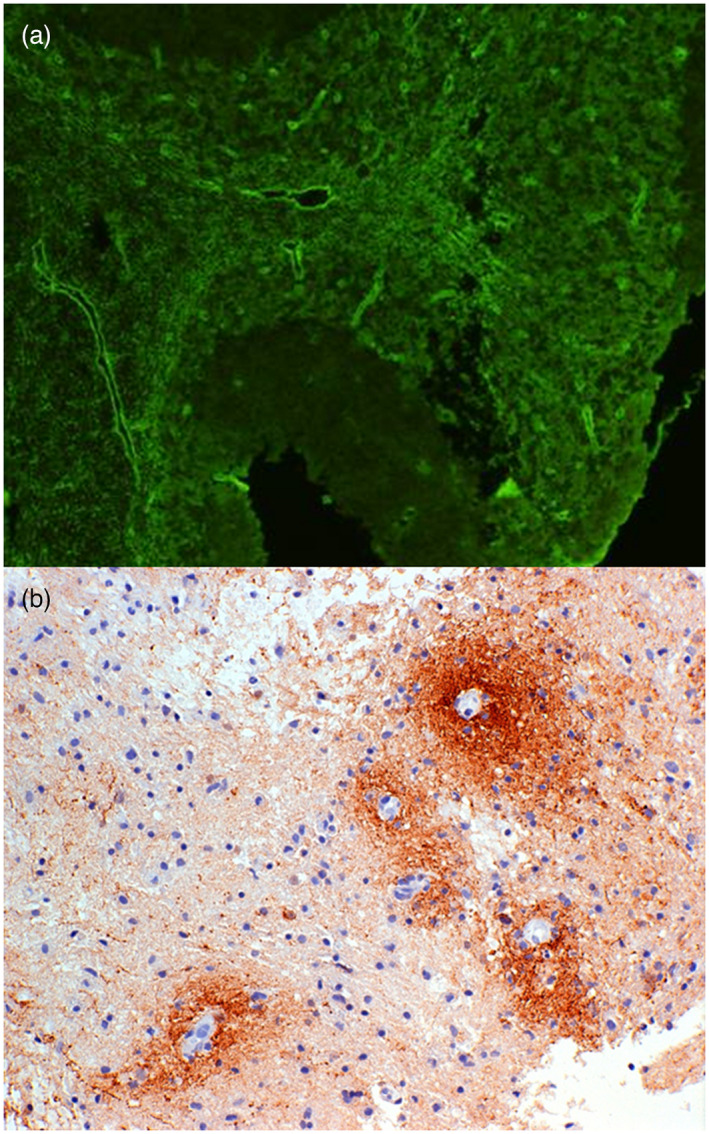
Perivascular distribution of aquaporin‐4 (AQP4) antibodies and complement in neuromyelitis optica spectrum disorder (NMOSD). AQP4 antibody positive immunofluorescence (green) in mouse cerebellum [serum dilution 1:40, goat anti‐human immunoglobulin (Ig)G F(ab)2 fluorescein isothiocyanate, ×200 magnification], showing microvessel staining of the granular layer, molecular layer and white matter (a). Section of early white matter lesion in NMOSD immunostained (brown) for Cd3 showing typical perivascular complement deposition around small blood vessels (b)
Diagnostic criteria
The 2015 IPND diagnostic criteria for NMOSD[12] are widely accepted and have good clinical utility. In patients presenting with one of the above‐listed clinical syndromes, positive AQP4 antibodies in serum is confirmatory of the diagnosis provided no better explanation exists. In situations where AQP4 antibodies are not found or testing is not available the diagnosis of NMOSD requires at least one attack of optic neuritis, transverse myelitis or area postrema syndrome and a second clinical attack of one other syndrome, including acute brain stem, diencephalic and cerebral syndromes. In addition, to fulfil seronegative criteria episodes of optic neuritis, acute myelitis, area postrema syndrome and acute brain stem syndrome must be associated with typical changes on MRI, as listed in Table 1.
TABLE 1.
2015 IPND MR imaging criteria for diagnosing seronegative NMOSD
| Clinical attack | Additional MR imaging criteria for a seronegative diagnosis of NMOSD |
|---|---|
| Optic neuritis | (1) Normal MR imaging of the brain or non‐specific white matter changes only |
| or | |
| (2) T2 ot T1 Gd enhancement of the optic chiasm or extending over half the length of the optic nerve on MR imaging of the orbits | |
| Acute myelitis | Longitudinally extensive spinal cord lesion (or atrophy) ≥ 3 vertebral segments on MR imaging of spine |
| Area postrema syndrome | Area postrema lesion on MR imaging of brain |
| Acute brain stem syndrome | Peri‐ependymal brain stem lesion on MR imaging of brain |
IPND = International Panel for NMO diagnosis; MR = magnetic resonance; NMOSD = neuromyelitis optica spectrum disorder; Gd = gadolinium (Gd).
Epidemiology
In populations with predominantly European ancestry the prevalence of NMOSD is approximately one in 100 000, and the incidence is approximately 0.6/million [11]. Comparative studies to assess prevalence have suggested higher rates of NMOSD in East Asian [27] and black populations [28, 29]. Age of onset can range from 10 to 80 years, with a peak of approximately 30–39 years [30].
Concomitant autoimmune disease
Co‐occurrence of autoimmune disease, most notably systemic lupus erythematosus (SLE) and Sjögren’s disease, has been noted in NMOSD [31]. This complicates interpretation of MR imaging in NMOSD. In SLE, neurological features and MRI abnormalities may be a consequence of concomitant NMOSD (optic neuritis, transverse myelitis), anti‐phospholipid syndrome (stroke‐like syndromes, hemichorea, neuropsychiatric presentations) [32] and vasculitis [33]. Similarly, cases of longitudinally extensive spinal cord lesions in Sjögren’s disease may reflect co‐existent NMSOD [34]. Concomitant type I diabetes mellitus [35] and autoimmune thyroid disease [36] will increase the likelihood of CNS vascular disease, further compounding findings on MRI.
Pathophysiology
Current evidence indicates that AQP4 antibodies are pathogenic and lead to a severe astrocytopathy through complement‐mediated cell lysis (Figure 1b) [37]. Demyelination and neuronal loss are a secondary consequence of the acute inflammatory response associated with complement activation [38, 39]. This involves neutrophils, natural killer cells, macrophages, tumour necrosis factor (TNF)‐α, interleukin (IL)‐6, IL‐1β and interferon (IFN)‐γ [40]. This process is summarized in Figure 2. Much has been made of the distribution of the pathology in NMOSD being located at peri‐ependymal regions, particularly in relation to the lateral, third and fourth ventricles, cerebral aqueduct and central canal of the spinal cord [41, 42]. This may be because these regions correspond to locations of greatest AQP4 density on astrocytes; they are rich in draining venules or have increased susceptibility of the blood–brain barrier [43]. Pathologically, NMOSD leads to destructive lesions with prominent loss of astrocytes, in addition to loss of myelin and neurones [38]. Areas of necrosis, with cavitation, hyalinization of small vessels and perivascular inflammatory infiltrates, are commonly seen. Collectively, these features help to distinguish NMOSD from MS. In the majority of NMOSD cases the condition arises as a primary autoimmune disease, but a small proportion of paraneoplastic cases has been noted [44].
FIGURE 2.
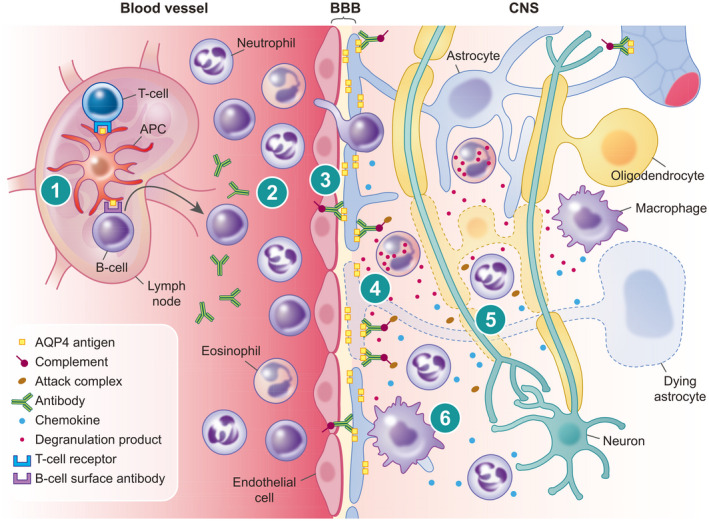
Schematic representation of the pathogenesis of neuromyelitis optica spectrum disorder (NMOSD). (1) Presentation of aquaporin‐4 (AQP4) antigen via an antigen‐presenting cell (APC) in a peripheral lymph node with promotion from T helper cell leads to activation of autoreactive B cells. (2) Maturation of antibody producing plasma cells results in free circulating autoreactive antibodies to AQP4. (3) Break‐down of the blood–brain barrier (BBB) leads to escape of autoreactive antibodies and extravasation of immune cells in response to chemokines. (4) Opsonization of autoreactive antibodies on AQP4 rafts situated on the astrocyte foot process leads to activation of complement and cell‐mediated lysis of astrocytes. (5) Indiscriminatory inflammation mediated via chemokines, activated complement and degranulation products, as well as loss of trophic support from astrocytes, leads to lysis of oligodendrocytes and neurons. (6) Macrophages attracted by chemokines released by leukocytes and astrocytes produce further proinflammatory products and phagocytose cellular debris and myelin
Treatment of NMOSD
The early and accurate recognition of NMOSD is important because optimal acute and preventive treatment is different to MS. Plasma exchange is particularly helpful, as acute treatment in addition to early intravenous high‐dose corticosteroids for NMOSD [45, 46, 47]. Treatment with IFN‐β [48], fingolimod [49] and natalizumab [50] appear to make NMOSD worse. Standard immunosuppression (azathioprine, methotrexate and mycophenolate mofetil) and B cell depletion with rituximab have been recommended to prevent future attacks of NMOSD [51]. Phases II/III clinical trials of inebilizumab (anti‐CD19 B‐cell depletory) [52], satralizumab (anti‐IL‐6) [53] and eculizumab (anti‐complement 5) [54] monoclonal antibodies have all shown positive results in NMOSD.
CONVENTIONAL MR IMAGING LESIONS
Optic nerve
Simultaneous or sequential involvement of both optic nerves on MR imaging of the orbits is highly suggestive of NMOSD (Figure 3a), with lesions sometimes being asymptomatic [55]. A recent study found bilateral optic nerve involvement in almost 50% of cases with optic nerve lesions [56]. A lesion of the optic nerve on MR imaging of the orbits that extends over at least half of the length of the optic nerve (Figure 3b) is suggestive of NMSOD [55, 57, 58]. Lesions involving the optic chiasm on MR imaging of the orbits (Figure 3c,d) seems to be quite specific for NMOSD and may be seen in a quarter of cases [42, 55, 56, 59, 60]. Any lesion of the optic nerves may be more common in NMOSD than in MS [55]. Optic perineuritis may be of particular value in distinguishing NMOSD and MS from other autoimmune causes of optic neuritis [61], including MOGAD [62].
FIGURE 3.
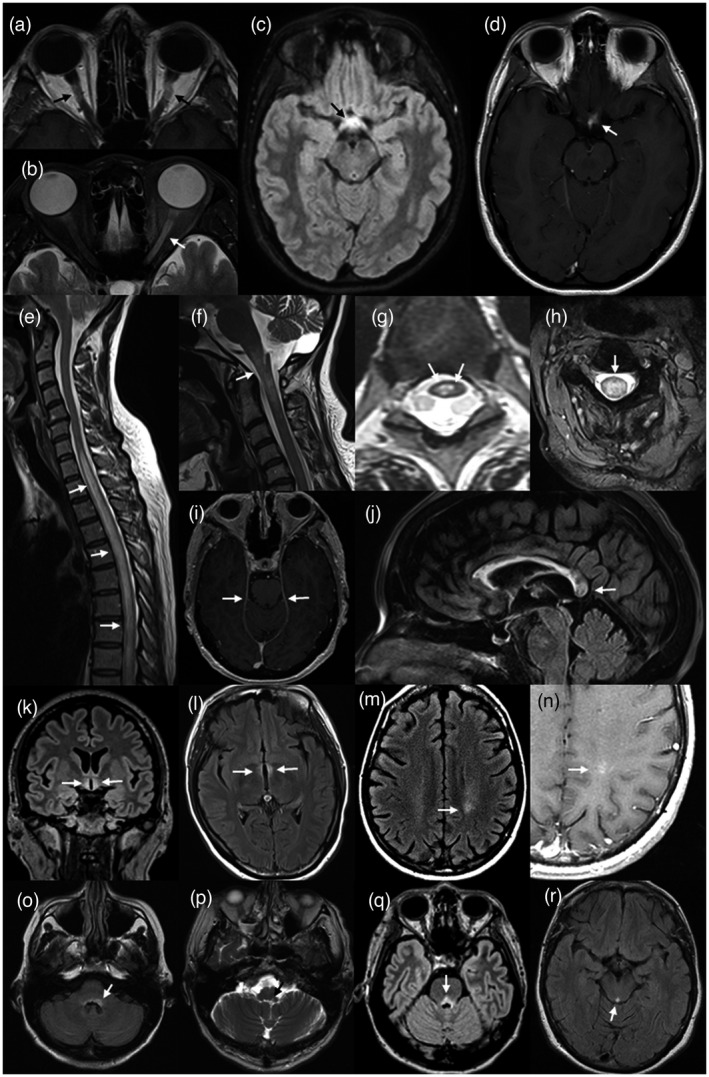
Magnetic resonance (MR) imaging of lesions (arrows) associated with aquaporin‐4 (AQP4)‐positive neuromyelitis optica spectrum disorder (NMOSD). Bilateral Gd‐enhancing retro‐orbital lesions of the optic nerves on axial T1 weighted image of the orbits (a). Longitudinally extensive (more than half the length of the optic nerve) high signal lesion of the left optic nerve on axial T2 image of the orbits (b). High signal lesion of the optic chiasm on volumetric, axial FLAIR image of the brain (c). Asymmetric gadolinium (Gd)‐enhancement of the same lesion (d). Longitudinally extensive high signal lesion of the thoracic spinal cord, associated with cord swelling, on sagittal T2 imaging of the spine (e). High cervical cord high signal lesion extending into the medulla on sagittal T2 image of the cervical cord (f). ‘Bright spotty lesions’ of the thoracic spinal cord on axial T2 imaging of the spine (g). Central, whole cord high signal lesion on axial T2 image of the cervical cord (h). Leptomeningeal Gd‐enhancement on axial T1 weighted imaging of the brain at the level of the upper pons (i). Heterogeneous rounded lesion of the corpus callosum on sagittal fluid‐attenuated inversion recovery (FLAIR) imaging of the brain (j). Hypothalamic high signal lesion on volumetric coronal FLAIR imaging of the brain (k). High signal change in the wall and adjacent parenchyma of the III ventricle on axial FLAIR imaging of the brain (l). High signal lesion of the left posterior frontal white matter on axial FLAIR imaging (m) showing ‘cloud‐like’ enhancement on axial T1 imaging of the same region (n). Bilateral area postrema lesions (more prominent on the left) on axial FLAIR image at the level of the medulla (o). Left nucleus solitarius lesion on axial T2 image at the level of the medulla (p). High signal FLAIR lesion of the pons, involving the floor of the IV ventricle (q). High signal lesion of the peri‐aqueductal grey matter on axial FLAIR image through the mid‐brain (r)
Spinal cord
Longitudinally extensive spinal cord lesions spanning three or more vertebral segments (Figure 3e) are seen in two‐thirds to three‐quarters of patients with NMOSD at some point in their disease course [63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77]. The most common sites are in the cervical region and upper to mid‐thoracic region [78]. Long lesions in the cervical region that extend up into the medulla are helpful in distinguishing NMSOD from MS [66, 79, 80, 81], but are of less value in distinguishing NMOSD from other causes of longitudinally extensive spinal cord lesions [82]. Longitudinally extensive spinal cord lesions are also seen in MOGAD [83] and monophasic (or relapsing) transverse myelitis without associated antibodies [84]. Lesions extending the whole length of the spinal cord to the conus can be seen in MOGAD, and this is uncommon in NMOSD [85, 86]. In antibody‐negative longitudinally extensive transverse myelitis the occurrence of another form of NMOSD‐associated clinical attack would suggest seronegative NMOSD. There are reports of longitudinally extensive spinal cord lesions in MS [78, 87], where the clinical picture and MR imaging is highly consistent with that disease and AQP4 antibodies are negative. However, such cases appear to be extremely rare [88]. As has been indicated previously, the possibility of NMOSD or MOGAD co‐existing with MS as another autoimmune disease needs to be considered, as does the possibility of epitope spreading in MS leading to the generation of autoreactive AQP4 antibodies over time in some cases. Superior extension of high cervical cord lesions, referred to as central medullary lesions, can be seen in NMOSD (Figure 3f) [42, 73, 79, 89, 90, 91, 92]. Short segment spinal cord lesions are also seen in approximately 15% of NMOSD cases [76].
Small areas of high signal predominantly seen on axial T2 imaging of the spinal cord in NMOSD have been described as ‘bright spotty’ lesions (Figure 3g) [93, 94, 95, 96, 97]. This picture seems to correlate with acute active inflammation, the resulting heterogeneous lesion giving rise to alternate areas of high and relatively low signals on T2 images. These bright areas do not usually show gadolinium (Gd)‐enhancement. In contrast to MS, where spinal cord lesions are usually partial and relatively peripherally situated, in NMOSD the most common axial distribution of lesions is a central location with radiation to the outer ependymal surface [95, 98]. All four quadrants are affected equally and may be associated with significant cord swelling (Figure 3h) [65]. We have noted that any form of Gd‐enhancement on T1 imaging of the spinal cord is more commonly seen in NMOSD than in MS. Others have noted that axial ‘ring’ enhancement is quite specific for NMOSD [65, 99, 100]. Heterogeneity of Gd‐enhancement in the spinal cord may be specific to NMOSD, reflecting the variable degree of destruction across lesions. Past episodes of longitudinally extensive transverse myelitis due NMOSD can sometimes be surmised due to regions of significant spinal cord atrophy spanning at least three vertebral segments [80, 101]. This appears to be extremely uncommon in MS and MOGAD.
Normal brain MRI
One of the most common findings of MR brain findings in NMOSD is no abnormality or minimal non‐specific changes. This finding is seen in approximately 20–40% of cases at presentation [102, 103, 104].
Cerebrum
Diffuse leptomeningeal enhancement on T1 MR brain imaging (Figure 3i) has been noted in NMOSD [105, 106]. This is a feature seldom seen in MS, but can be seen in neurosarcoidosis and other forms of aseptic meningitis [107]. Rounded lesions with a ‘ground glass’‐like heterogeneous appearance within the body of the corpus callosum appear to be quite specific to NMOSD (Figure 3j) [108, 109]. Corpus callosum lesions of various types are common in both NMOSD and MS. They can also be seen in vascular disease [110] and other CNS disorders [111]. Heterogeneous lesions are the only ones that appear to be specific for NMOSD, while triangulated lesions arising from the inferior margin of the corpus callosum are typically seen in MS [112].
A relative increase in signal of the ependymal lining of the hypothalamus is common on fluid‐attenuated inversion recovery (FLAIR) sequences of the brain in normal healthy brains. In NMOSD a high T2 signal, particularly on FLAIR images, can be seen to extend into the adjacent parenchyma (Figure 3k), and it is this that represents a lesion of the hypothalamus [42, 69, 80, 113, 114]. High signal lesions on T2 and FLAIR sequences in the ependymal lining and adjacent parenchyma of the walls of the III ventricle can be seen as an extension of a hypothalamic lesion or as a lesion on its own in NMOSD (Figure 3l) [42, 79, 92]. In contrast to the situation encountered in spinal cord imaging of CNS inflammatory disorders, we have found that Gd‐enhancing lesions of the brain are rare in NMOSD and less common than in MS. However, one pattern of hemispheric Gd‐enhancement has been found to be specific for NMOSD. Diffuse and heterogeneous T2/FLAIR lesions of the white matter with patchy, faint Gd‐enhancement and a wispy quality are seen in NMOSD. This pattern of enhancement has been termed as ‘cloud‐like’ (Figure 3m,n) [90, 115]. It has been noted that cerebral T2 lesions in NMOSD almost universally decrease in size over time, but that the majority persist as a residual high T2 signal [116]. This contrasts with MS, where many lesions persist, and MOGAD, where lesions are more likely to resolve.
Other brain lesions, including linear periventricular peri‐ependymal [91, 92, 109, 117, 118, 119, 120], bridging lesions of the splenium [121, 122], small subcortical lesions (punctate and patch lesions) [89, 92, 118, 119] have been seen in NMOSD, but we have noted that these lesion types are seen just as commonly in MS. The latter are very non‐specific and have been described in migraine [123]. Tumefactive [79, 121, 124] and ADEM‐like lesions [125] have also been noted in NMOSD, but these lesions are also recognized to occur in MS and MOGAD [90, 91, 92, 116, 126, 127]. It is interesting to consider the extent to which these lesions represent a common pathogenesis, whereby breakdown of the blood–brain barrier occurs in particular regions in response to some environmental factor that is ubiquitous, leading to the differing forms of immunological attack seen in MS, NMOSD and MOGAD in similar anatomical distributions. There have been several case reports of posterior reversible encephalopathy syndrome (PRES)‐like lesions in NMOSD [128, 129, 130] and it has been speculated that this could reflect dysregulation of osmotic homeostasis due to blocking of AQP4, but a similar picture was also recently reported in MOGAD [131], suggesting that a different mechanism may be responsible for this picture.
Brain stem and cerebellum
The third most common clinical presentation in NMOSD is the area postrema syndrome (persistent nausea, vomiting and hiccoughs) [13]. A high signal lesion in the posterior segment of the medulla on T2/FLAIR MR imaging (Figure 3o) is a distinctive and seemingly specific lesion for NMOSD [79, 80, 132, 133]. This area of the medulla is an intrinsic component of the autonomic nervous system that controls vomiting and hiccoughs through the vagus nerve [134]. Lesions of the adjacent nucleus tractus solitarius which extend into the pons have also been noted in NMOSD (Figure 3p) [135], as have lesions of the floor of the fourth ventricle in the pons (Figure 3q) [42, 90, 92, 105, 109, 118, 127].
Peripherally situated, crescent‐shaped, subependymal lesions of the brain stem have been noted in NMOSD [79, 119]. A particularly striking lesion of NMOSD is a T2/FLAIR high signal involving the peri‐aqueductal grey matter (Figure 3r). Sometimes these lesions of the posterior mid‐brain can be large [42, 79, 136]. Lesions of the anterior mid‐brain, both linear peri‐ependymal [42] and extensions of longitudinal corticospinal tract lesions through the cerebral peduncles, have been described [91, 92, 109, 137]. Lesions of the cerebellum are less common in NMOSD than in MS, but lesions of the dentate nuclei and cerebellar peduncles adjacent to the fourth ventricle can be seen [138].
Imaging of the peripheral nervous system
A recent case report had shown the presence of high signal lesions within lumbar nerve roots in a patient with NMOSD [139].
ADVANCED MRI TECHNIQUES IN NMOSD
High‐field MRI
Several studies have looked at the use of 7T MR imaging in NMOSD. These studies have shown that the rim‐like and nodular pattern of paramagnetic phase changes, suggestive of iron deposition which are seen in some MS brain lesions, are not seen in NMOSD [140, 141, 142]. No difference in T1 relaxation time between NMOSD and healthy controls has been observed [143]. High‐field MR imaging in NMOSD has suggested a lower rate of lesion accrual (approximately 1% per year) than is seen in MS [141].
Multi‐modal MRI
Multi‐modal MRI has shown fewer changes in the normal appearing white matter of NMOSD patients than is seen in MS [144, 145, 146]. One consistent pattern has been the finding of reduced fractional anisotropy on diffusion tensor imaging of the optic radiation [144, 147] and reduced functional connectivity strength in the occipital cortex [145]. An association with retinal nerve fibre layer thinning on optical coherence tomography [145] suggests that this reflects degeneration secondary to optic nerve lesions. A recent study using probability maps has indicated that lesion distribution is of little value in distinguishing NMOSD from MS [145]. However, a combination of diffusion parameters and functional connectivity strength on 3T MR imaging may have high sensitivity and specificity to distinguish NMOSD from MS. Similar differences in normal‐appearing white matter between NMOSD and MOGAD are also emerging as a useful way to distinguish these two conditions [148].
Other advanced MRI techniques
Studies have shown less marked reductions in overall brain volumes between NMOSD and MOGAD or healthy controls when compared to MS, although reduced white matter volume [149] and a trend towards reduced total brain volume [148] have been noted. The cortex seems to be relatively spared in NMOSD [150]. Pain in NMOSD is a common feature and correlates with spinal cord disease. An interesting observation was that reduced volume of the ventral posterior nucleus of the thalamus is associated with pain intensity in NMOSD [151]. Other studies have shown correlations in thalamic volume and clinical disease activity [152]. Decreased volumes of the nucleus accumbens and caudate nucleus were associated with clinical measures of disability [153]. The cross‐sectional area of the optic chiasm was found to be associated with clinical measures of visual acuity [154]. Functional MRI (fMRI) has been used to show increased recruitment of the sensorimotor network during motor tasks [155] and reduced resting state activity in the precuneus and cingulate cortex [156] in NMOSD. Increased resting fMRI activity in the inferior temporal lobes of patients with normal brain imaging may also correlate with disability [157]. Deep learning approaches have been applied to distinguish NMOSD from MS and show high accuracy [158].
DIFFERENTIAL DIAGNOSIS OF MRI APPEARANCES SUSPICIOUS FOR NMOSD
In cases presenting with typical clinical presentations of NMOSD, MRI changes consistent with NMOSD and positive AQP4 antibodies, the diagnosis of NMOSD is straightforward. However, when MRI changes are atypical or AQP4 antibodies are negative the situation is more complicated. The co‐appearance of clinical and MRI features suggestive of both NMOSD and MS within the same individual has been reported [159]. The value of NMOSD‐specific MRI features has been questioned in some non‐European ancestry populations where MS is less common [103]. In this study from Malaysia, the majority of seronegative cases had at least some features suggestive of NMOSD on MRI without necessarily meeting 2015 IPND diagnostic criteria for seronegative NMOSD.
PROGNOSTIC VALUE OF MRI IN NMOSD
Combinations of various NMOSD‐specific lesions have been shown to have high specificity and reasonable sensitivity in predicting AQP4 seropositivity and distinguishing from MS in cohorts of CNS inflammatory disease. However, it is notable that the specific features identified have varied from study to study and validation in an independent data set is lacking. The length [160, 161], degree of cord swelling [161], persistent Gd‐enhancement [162] and presence of T1 hypodensity [161] in spinal cord lesions have been found to predict both the acute nadir of disability and ultimate extent of recovery in acute myelitis associated with NMSOD.
ROLE OF ROUTINE MRI FOLLOW‐UP EXAMINATIONS IN NMOSD
As noted above, the frequency of clinically silent MR brain lesions in NMOSD is much lower than in MS, and it has therefore been argued that there is less of a role for routinely repeating MR imaging in NMOSD [163]. However, recent work has suggested that new lesions are predictive of future clinical events and may therefore play a role in monitoring disease progress and treatment response [141]. Asymptomatic Gd‐enhancing brain lesions are particularly uncommon with no enhancing lesions being identified from 708 person‐years of follow‐up in one study [164]. There is therefore little value in requesting contrast imaging of the brain in routine follow‐up of NMOSD.
CONCLUSIONS
MR imaging in cases of suspected CNS inflammatory disease is crucial in raising the possibility of NMOSD and prompting testing for AQP4 antibodies. It is also an essential component in confirming seronegative NMOSD [12]. Despite this, a significant number of suspected cases remain without a definite diagnosis and the status and treatment of these cases remains uncertain. This remains a significant dilemma in East Asian populations, where the prevalence of MS is lower and atypical forms of inflammatory CNS disease are the norm. In these cases MR imaging is of considerable potential value in determining the underlying cause [165]. However, continued work to find more precise diagnostic features on MRI is needed. Advanced MR techniques including diffusion tensor imaging may prove useful in this regard. Spinal cord lesion characteristics are of prognostic value and can indicate a need for more aggressive therapy in the setting of acute myelitis.
CONFLICT OF INTEREST
Simon A. Broadley has received honoraria for attendance at advisory boards and travel sponsorship from Bayer‐Schering, Biogen‐Idec, Merck‐Serono, Novartis and Sanofi‐Genzyme, has received speaker’s honoraria from Biogen‐Idec and Genzyme, is an investigator in clinical trials sponsored by Biogen Idec, Novartis and Genzyme and was the recipient of an unencumbered research grant from Biogen‐Idec. Laura Clarke, Simon Arnett, Kate Lilley, Jacky Liao and Sandeep Bhuta report no disclosures.
ACKNOWLEDGEMENTS
The authors are part of the Australia and New Zealand Neuromyelitis Optica (ANZ NMO) Collaboration and have been supported by funding from Multiple Sclerosis Research Australia (11‐038), the Brain Foundation, Griffith University and the Gold Coast Hospital Foundation. The authors are grateful to Ms Kerri Prain and Professor Michael Barnett for providing the images for Figure 1. The authors are grateful to Professor Dean Wingerchuk for providing permission to include Table 1.
Clarke L, Arnett S, Lilley K, Liao J, Bhuta S, Broadley SA. Magnetic resonance imaging in neuromyelitis optica spectrum disorder. Clin Exp Immunol. 2021;206:251–265. 10.1111/cei.13630
OTHER ARTICLES PUBLISHED IN THIS REVIEW SERIES
Imaging immune responses in neuroinflammatory diseases. Clinical and Experimental Immunology 2021, 206: 248–250.
Clinical and neuroimaging findings in MOGAD‐MRI and OCT. Clinical and Experimental Immunology 2021, 206: 266–281.
Towards PET imaging of the dynamic phenotypes of microglia. Clinical and Experimental Immunology 2021, 206: 282–300.
Imaging immunological processes from blood to brain in amyotrophic lateral sclerosis. Clinical and Experimental Immunology 2021, 206: 301–313.
Neuroinflammation and immunoregulation in glioblastoma and brain metastases: Recent developments in imaging approaches. Clinical and Experimental Immunology 2021, 206: 314–324.
‘A picture is worth a thousand words’: The use of microscopy for imaging neuroinflammation. Clinical and Experimental Immunology 2021, 206: 325–345.
DATA AVAILABILITY STATEMENT
Not relevant.
REFERENCES
- 1. Devic E. Myélite subaiguë compliquée de névrite optique. Bull Med. 1894;8:1033–4. [Google Scholar]
- 2. Devic E. Acute lumbar spinal myelitis with optic neuritis, autopsy. French Cong Med. 1895;1:434–9. [Google Scholar]
- 3. Albutt FC. On the ophthalmoscopic signs of spinal cord disease. Lancet 1870;i:76–8. [Google Scholar]
- 4. Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 2004;364:2106–12. [DOI] [PubMed] [Google Scholar]
- 5. Paul F, Jarius S, Aktas O, Bluthner M, Bauer O, Appelhans H, et al. Antibody to aquaporin 4 in the diagnosis of neuromyelitis optica. PLOS Med. 2007;4:e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jarius S, Wildemann B. Devic’s index case: a critical reappraisal – AQP4‐IgG‐mediated neuromyelitis optica spectrum disorder, or rather MOG encephalomyelitis? J Neurol Sci. 2019;407:116396. [DOI] [PubMed] [Google Scholar]
- 7. Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805–15. [DOI] [PubMed] [Google Scholar]
- 8. Ramanathan S, Dale RC, Brilot F. Anti‐MOG antibody: the history, clinical phenotype, and pathogenicity of a serum biomarker for demyelination. Autoimmun Rev. 2016;15:307–24. [DOI] [PubMed] [Google Scholar]
- 9. Zajicek JP, Scolding NJ, Foster O, Rovaris M, Evanson J, Moseley IF, et al. Central nervous system sarcoidosis – diagnosis and management. Q J Med. 1999;92:103–17. [DOI] [PubMed] [Google Scholar]
- 10. Kidd D, Burton B, Plant GT, Graham EM. Chronic relapsing inflammatory optic neuropathy (CRION). Brain 2003;126:276–84. [DOI] [PubMed] [Google Scholar]
- 11. Hor JY, Asgari N, Nakashima I, Broadley SA, Leite MI, Kissani N, et al. Epidemiology of neuromyelitis optica spectrum disorder and its prevalence and incidence worldwide. Front Neurol. 2020;11:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International panel for NMOD. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85:177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khalilidehkordi E, Clarke L, Arnett S, Bukhari W, Jimenez Sanchez S, O'Gorman C, et al. Relapse patterns in NMOSD: evidence for earlier occurrence of optic neuritis and possible seasonal variation. Front Neurol. 2020;11:537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bukhari W, Clarke L, O’Gorman C, Khalilidehkordi E, Arnett S, Prain KM, et al. The clinical profile of NMOSD in Australia and New Zealand. J Neurol. 2020;267:1431–43. [DOI] [PubMed] [Google Scholar]
- 15. Kleiter I, Gahlen A, Borisow N, Fischer K, Wernecke KD, Wegner B, et al. Neuromyelitis optica study G. Neuromyelitis optica: evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol. 2016;79:206–16. [DOI] [PubMed] [Google Scholar]
- 16. Kim HJ, Paul F, Lana‐Peixoto MA, Tenembaum S, Asgari N, Palace J, et al. MRI characteristics of neuromyelitis optica spectrum disorder: an international update. Neurology 2015;84:1165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bennett JL, de Seze J , Lana‐Peixoto M, Palace J, Waldman A, Schippling S, et al. Neuromyelitis optica and multiple sclerosis: Seeing differences through optical coherence tomography. Mult Sclerosis. 2015;21:678–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic‐spinal multiple sclerosis binds to the aquaporin‐4 water channel. J Exp Med. 2005;202:473–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lu M, Lee MD, Smith BL, Jung JS, Agre P, Verdijk MA, et al. The human AQP4 gene: definition of the locus encoding two water channel polypeptides in brain. Proc Natl Acad Sci USA. 1996;93:10908–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rossi A, Crane JM, Verkman AS. Aquaporin‐4 Mz isoform: brain expression, supramolecular assembly and neuromyelitis optica antibody binding. Glia 2011;59:1056–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Verkman AS, Ratelade J, Rossi A, Zhang H, Tradtrantip L. Aquaporin‐4: orthogonal array assembly, CNS functions, and role in neuromyelitis optica. Acta Pharmacol Sin. 2011;32:702–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nicchia GP, Mastrototaro M, Rossi A, Pisani F, Tortorella C, Ruggieri M, et al. Aquaporin‐4 orthogonal arrays of particles are the target for neuromyelitis optica autoantibodies. Glia 2009;57:1363–73. [DOI] [PubMed] [Google Scholar]
- 23. Crane JM, Lam C, Rossi A, Gupta T, Bennett JL, Verkman AS. Binding affinity and specificity of neuromyelitis optica autoantibodies to aquaporin‐4 M1/M23 isoforms and orthogonal arrays. J Biol Chem. 2011;286:16516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Waters PJ, Pittock SJ, Bennett JL, Jarius S, Weinshenker BG, Wingerchuk DM. Evaluation of aquaporin‐4 antibody assays. Clin Exp Neuroimmunol. 2014;5:290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prain K, Woodhall M, Vincent A, Ramanathan S, Barnett MH, Bundell C, et al.; the Australian and New Zealand NMO Collaboration . AQP4 antibody assay sensitivity comparison in the era of the diagnostic criteria for NMOSD. Front Neurol. 2015;2019:1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Watanabe M, Nakamura Y, Michalak Z, Isobe N, Barro C, Leppert D, et al. Serum GFAP and neurofilament light as biomarkers of disease activity and disability in NMOSD. Neurology 2019;93:e1299–e1311. [DOI] [PubMed] [Google Scholar]
- 27. Bukhari W, Prain KM, Waters P, Woodhall M, O'Gorman CM, Clarke L, et al. Incidence and prevalence of NMOSD in Australia and New Zealand. J Neurol Neurosurg Psychiatry. 2017;88:632–38. [DOI] [PubMed] [Google Scholar]
- 28. Flanagan EP, Cabre P, Weinshenker BG, St Sauver J, Jacobson DJ, Majed M, et al. Epidemiology of aquaporin‐4 autoimmunity and neuromyelitis optica spectrum. Ann Neurol. 2016;79:775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jacob A, Panicker J, Lythgoe D, Elsone L, Mutch K, Wilson M, et al. The epidemiology of neuromyelitis optica amongst adults in the Merseyside county of United Kingdom. J Neurol. 2013;260:2134–7. [DOI] [PubMed] [Google Scholar]
- 30. Pandit L, Asgari N, Apiwattanakul M, Palace J, Paul F, Leite MI, et al. Consortium GIC, Biorepository for neuromyelitis O . Demographic and clinical features of neuromyelitis optica: a review. Mult Sclerosis. 2015;21:845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wingerchuk DM. Evidence for humoral autoimmunity in neuromyelitis optica. Neurol Res. 2006;28:348–53. [DOI] [PubMed] [Google Scholar]
- 32. Asherson RA, Khamashta MA, Gil A, Vazquez JJ, Chan O, Baguley E, et al. Cerebrovascular disease and antiphospholipid antibodies in systemic lupus erythematosus, lupus‐like disease, and the primary antiphospholipid syndrome. Am J Med. 1989;86:391–9. [DOI] [PubMed] [Google Scholar]
- 33. Goel D, Reddy SR, Sundaram C, Prayaga AK, Rajasekhar L, Narsimulu G. Active necrotizing cerebral vasculitis in systemic lupus erythematosus. Neuropathology 2007;27:561–5. [DOI] [PubMed] [Google Scholar]
- 34. Alexander GE, Provost TT, Stevens MB, Alexander EL. Sjogren syndrome: central nervous system manifestations. Neurology 1981;31:1391–6. [DOI] [PubMed] [Google Scholar]
- 35. Raymond NT, Langley JD, Goyder E, Botha JL, Burden AC, Hearnshaw JR. Insulin treated diabetes mellitus: causes of death determined from record linkage of population based registers in Leicestershire, UK. J Epidemiol Commun Health. 1995;49:570–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Squizzato A, Gerdes VE, Brandjes DP, Buller HR, Stam J. Thyroid diseases and cerebrovascular disease. Stroke 2005;36:2302–10. [DOI] [PubMed] [Google Scholar]
- 37. Saadoun S, Waters P, Bell BA, Vincent A, Verkman AS, Papadopoulos MC. Intra‐cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain 2010;133:349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Parratt JD, Prineas JW. Neuromyelitis optica: a demyelinating disease characterized by acute destruction and regeneration of perivascular astrocytes. Mult Sclerosis. 2010;16:1156–72. [DOI] [PubMed] [Google Scholar]
- 39. Misu T, Fujihara K, Kakita A, Konno H, Nakamura M, Watanabe S, et al. Loss of aquaporin 4 in lesions of neuromyelitis optica: distinction from multiple sclerosis. Brain 2007;130:1224–34. [DOI] [PubMed] [Google Scholar]
- 40. Bukhari W, Barnett MH, Prain K, Broadley SA. Molecular pathogenesis of neuromyelitis optica. Int J Mol Sci. 2012;13:12970–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nielsen S, Nagelhus EA, Amiry‐Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high‐resolution immunogold cytochemistry of aquaporin‐4 in rat brain. J Neurosci. 1997;17:171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pittock SJ, Weinshenker BG, Lucchinetti CF, Wingerchuk DM, Corboy JR, Lennon VA. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol. 2006;63:964–8. [DOI] [PubMed] [Google Scholar]
- 43. Bradl M, Reindl M, Lassmann H. Mechanisms for lesion localization in neuromyelitis optica spectrum disorders. Curr Opin Neurol. 2018;31:325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pittock SJ, Lennon VA. Aquaporin‐4 autoantibodies in a paraneoplastic context. Arch Neurol. 2008;65:629–32. [DOI] [PubMed] [Google Scholar]
- 45. Kim SH, Kim W, Huh SY, Lee KY, Jung IJ, Kim HJ. Clinical efficacy of plasmapheresis in patients with neuromyelitis optica spectrum disorder and effects on circulating anti‐aquaporin‐4 antibody levels. J Clin Neurol. 2013;9:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kleiter I, Gahlen A, Borisow N, Fischer K, Wernecke KD, Hellwig K et al. Apheresis therapies for NMOSD attacks: A retrospective study of 207 therapeutic interventions. Neurol Neuroimmunol Neuroinflamm. 2018;5:e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Akaishi T, Takeshita T, Himori N, Takahashi T, Misu T, Ogawa R, et al. Rapid administration of high‐dose intravenous methylprednisolone improves visual outcomes after optic neuritis in patients with AQP4‐IgG‐positive NMOSD. Front Neurol. 2020;11:932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim SH, Kim W, Li XF, Jung IJ, Kim HJ. Does interferon beta treatment exacerbate neuromyelitis optica spectrum disorder? Multiple Sclerosis. 2012;18:1480–3. [DOI] [PubMed] [Google Scholar]
- 49. Yoshii F, Moriya Y, Ohnuki T, Ryo M, Takahashi W. Fingolimod‐induced leukoencephalopathy in a patient with neuromyelitis optica spectrum disorder. Mult Sclerosis Relat Disord. 2016;7:53–7. [DOI] [PubMed] [Google Scholar]
- 50. Kitley J, Evangelou N, Kuker W, Jacob A, Leite MI, Palace J. Catastrophic brain relapse in seronegative NMO after a single dose of natalizumab. J Neurol Sci. 2014;339:223–5. [DOI] [PubMed] [Google Scholar]
- 51. Sellner J, Boggild M, Clanet M, Hintzen RQ, Illes Z, Montalban X, et al. EFNS guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol. 2010;17:1019–32. [DOI] [PubMed] [Google Scholar]
- 52. Cree BAC, Bennett JL, Kim HJ, Weinshenker BG, Pittock SJ, Wingerchuk DM, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N‐MOmentum): a double‐blind, randomised placebo‐controlled phase 2/3 trial. Lancet. 2019;394:1352–63. [DOI] [PubMed] [Google Scholar]
- 53. Traboulsee A, Greenberg BM, Bennett JL, Szczechowski L, Fox E, Shkrobot S, et al. Safety and efficacy of satralizumab monotherapy in neuromyelitis optica spectrum disorder: a randomised, double‐blind, multicentre, placebo‐controlled phase 3 trial. Lancet Neurol. 2020;19:402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pittock SJ, Berthele A, Fujihara K, Kim HJ, Levy M, Palace J, et al. Eculizumab in aquaporin‐4‐positive neuromyelitis optica spectrum disorder. N Engl J Med. 2019;381:614–25. [DOI] [PubMed] [Google Scholar]
- 55. Buch D, Savatovsky J, Gout O, Vignal C, Deschamps R. Combined brain and anterior visual pathways’ MRIs assist in early identification of neuromyelitis optica spectrum disorder at onset of optic neuritis. Acta Neurol Belg. 2017;117:67–74. [DOI] [PubMed] [Google Scholar]
- 56. Carnero Contentti E, Delgado‐García G, López PA, Criniti J, Pettinicchi JP, Correa‐Díaz EP, et al. Acute optic nerve lesions in first‐ever NMOSD‐related optic neuritis using conventional brain MRI: a Latin American multicenter study. Mult Sclerosis Relat Disord. 2020;46:102558. [DOI] [PubMed] [Google Scholar]
- 57. Mealy MA, Whetstone A, Orman G, Izbudak I, Calabresi PA, Levy M. Longitudinally extensive optic neuritis as an MRI biomarker distinguishes neuromyelitis optica from multiple sclerosis. J Neurol Sci. 2015;355:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pula JH, Kattah JC, Keung B, Wang H, Daily J. Longitudinally extensive optic neuritis in neuromyelitis optica spectrum disorder. J Neurol Sci. 2014;345:209–12. [DOI] [PubMed] [Google Scholar]
- 59. Khanna S, Sharma A, Huecker J, Gordon M, Naismith RT, Van Stavern GP. Magnetic resonance imaging of optic neuritis in patients with neuromyelitis optica versus multiple sclerosis. J Neuro Ophthalmol. 2012;32:216–20. [DOI] [PubMed] [Google Scholar]
- 60. Lim YM, Pyun SY, Lim HT, Jeong IH, Kim KK. First‐ever optic neuritis: distinguishing subsequent neuromyelitis optica from multiple sclerosis. Neurol Sci. 2014;35:781–3. [DOI] [PubMed] [Google Scholar]
- 61. Li H, Zhou H, Sun J, Wang H, Wang Y, Wang Z, et al. Optic perineuritis and its association with autoimmune diseases. Front Neurol. 2020;11:627077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ramanathan S, Fraser C, Curnow SR, Ghaly M, Leventer RJ, Lechner‐Scott J, et al. Uveitis and optic perineuritis in the context of myelin oligodendrocyte glycoprotein antibody seropositivity. Eur J Neurol. 2019;26:1137–e75. [DOI] [PubMed] [Google Scholar]
- 63. Nakashima I, Fujihara K, Miyazawa I, Misu T, Narikawa K, Nakamura M, et al. Clinical and MRI features of Japanese patients with multiple sclerosis positive for NMO‐IgG. J Neurol Neurosurg Psychiatry. 2006;77:1073–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chan KH, Tsang KL, Fong GC, Ho SL, Cheung RT, Mak W. Idiopathic inflammatory demyelinating disorders after acute transverse myelitis. Eur J Neurol. 2006;13:862–8. [DOI] [PubMed] [Google Scholar]
- 65. Cassinotto C, Deramond H, Olindo S, Aveillan M, Smadja D, Cabre P. MRI of the spinal cord in neuromyelitis optica and recurrent longitudinal extensive myelitis. J Neuroradiol. 2009;36:199–205. [DOI] [PubMed] [Google Scholar]
- 66. Lu Z, Qiu W, Zou Y, Lv K, Long Y, You W, et al. Characteristic linear lesions and longitudinally extensive spinal cord lesions in Chinese patients with neuromyelitis optica. J Neurol Sci. 2010;293:92–6. [DOI] [PubMed] [Google Scholar]
- 67. Li R, Qiu W, Lu Z, Dai Y, Wu A, Long Y, et al. Acute transverse myelitis in demyelinating diseases among the Chinese. J Neurol. 2011;258:2206–13. [DOI] [PubMed] [Google Scholar]
- 68. Wang KC, Tsai CP, Lee CL, Chen SY, Chen SJ. The prevalence of long spinal cord lesions and anti‐aquaporin 4 antibodies in neuromyelitis optica patients in Taiwan. Eur Neurol. 2011;65:99–104. [DOI] [PubMed] [Google Scholar]
- 69. Downer JJ, Leite MI, Carter R, Palace J, Kuker W, Quaghebeur G. Diagnosis of neuromyelitis optica (NMO) spectrum disorders: is MRI obsolete? Neuroradiology 2012;54:279–85. [DOI] [PubMed] [Google Scholar]
- 70. Lalan S, Khan M, Schlakman B, Penman A, Gatlin J, Herndon R. Differentiation of neuromyelitis optica from multiple sclerosis on spinal magnetic resonance imaging. Int J MS Care. 2012;14:209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chan KH, Lee R, Lee JC, Tse AC, Pang SY, Lau GK, et al. Central nervous system inflammatory demyelinating disorders among Hong Kong Chinese. J Neuroimmunol. 2013;262:100–5. [DOI] [PubMed] [Google Scholar]
- 72. Kiyat‐Atamer A, Ekizoglu E, Tuzun E, Kurtuncu M, Shugaiv E, Akman‐Demir G, et al. Long‐term MRI findings in neuromyelitis optica: seropositive versus seronegative patients. Eur J Neurol. 2013;20:781–7. [DOI] [PubMed] [Google Scholar]
- 73. Barhate KS, Ganeshan M, Singhal BS. A clinical and radiological profile of neuromyelitis optica and spectrum disorders in an Indian cohort. Ann Indian Acad Neurol. 2014;17:77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lin A, Zhu J, Yao X, Lin S, Murong S, Li Z. Clinical manifestations and spinal cord magnetic resonance imaging findings in Chinese neuromyelitis optica patients. Eur Neurol. 2014;71:35–41. [DOI] [PubMed] [Google Scholar]
- 75. Liu Y, Zhao G, Yu H, Lyu C, Li Z, Wu Z. Differentiation of neuromyelitis optica from multiple sclerosis in a cohort from the mainland of China. Chin Med J. 2014;127:3213–8. [PubMed] [Google Scholar]
- 76. Flanagan EP, Weinshenker BG, Krecke KN, Lennon VA, Lucchinetti CF, McKeon A, et al. Short myelitis lesions in aquaporin‐4‐IgG‐positive neuromyelitis optica spectrum disorders. JAMA Neurol. 2015;72:81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cai W, Tan S, Zhang L, Shan Y, Wang Y, Lin Y, et al. Linear lesions may assist early diagnosis of neuromyelitis optica and longitudinally extensive transverse myelitis, two subtypes of NMOSD. J Neurol Sci. 2016;360:88–93. [DOI] [PubMed] [Google Scholar]
- 78. Bigaut K, Lambert C, Kremer L, Lebrun C, Cohen M, Ciron J, et al. Atypical myelitis in patients with multiple sclerosis: characterization and comparison with typical multiple sclerosis and neuromyelitis optica spectrum disorders. Mult Sclerosis. 2021;27:232–8. [DOI] [PubMed] [Google Scholar]
- 79. Pittock SJ, Lennon VA, Krecke K, Wingerchuk DM, Lucchinetti CF, Weinshenker BG. Brain abnormalities in neuromyelitis optica. Arch Neurol. 2006;63:390–6. [DOI] [PubMed] [Google Scholar]
- 80. Asgari N, Skejoe HP, Lillevang ST, Steenstrup T, Stenager E, Kyvik KO. Modifications of longitudinally extensive transverse myelitis and brainstem lesions in the course of neuromyelitis optica (NMO): a population‐based, descriptive study. BMC Neurol. 2013;13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Iorio R, Damato V, Mirabella M, Evoli A, Marti A, Plantone D, et al. Distinctive clinical and neuroimaging characteristics of longitudinally extensive transverse myelitis associated with aquaporin‐4 autoantibodies. J Neurol. 2013;260:2396–402. [DOI] [PubMed] [Google Scholar]
- 82. Dubey D, Pittock SJ, Krecke KN, Flanagan EP. Association of extension of cervical cord lesion and area postrema syndrome with neuromyelitis optica spectrum disorder. JAMA Neurol. 2017;74:359–61. [DOI] [PubMed] [Google Scholar]
- 83. Cobo‐Calvo A, Sepulveda M, Bernard‐Valnet R, Ruiz A, Brassat D, Martinez‐Yelamos S, et al. Antibodies to myelin oligodendrocyte glycoprotein in aquaporin 4 antibody seronegative longitudinally extensive transverse myelitis: clinical and prognostic implications. Mult Sclerosis. 2016;22:312–9. [DOI] [PubMed] [Google Scholar]
- 84. Hyun JW, Kim SH, Huh SY, Kim W, Yun J, Joung A, et al. Idiopathic aquaporin‐4 antibody negative longitudinally extensive transverse myelitis. Mult Sclerosis. 2015;21:710–7. [DOI] [PubMed] [Google Scholar]
- 85. Rempe T, Tarhan B, Rodriguez E, Viswanathan VT, Gyang TV, Carlson A, et al. Anti‐MOG associated disorder‐Clinical and radiological characteristics compared to AQP4‐IgG+ NMOSD‐A single‐center experience. Mult Sclerosis Relat Disord. 2021;48:102718. [DOI] [PubMed] [Google Scholar]
- 86. Shahriari M, Sotirchos ES, Newsome SD, Yousem DM. MOGAD: how it differs from and resembles other neuroinflammatory disorders. Am J Roentgenol. 2021;216:1031–9. [DOI] [PubMed] [Google Scholar]
- 87. Komatsu J, Sakai K, Nakada M, Iwasa K, Yamada M. Long spinal cord lesions in a patient with pathologically proven multiple sclerosis. J Clin Neurosci. 2017;42:106–8. [DOI] [PubMed] [Google Scholar]
- 88. Asnafi S, Morris PP, Sechi E, Pittock SJ, Weinshenker BG, Palace J, et al. The frequency of longitudinally extensive transverse myelitis in MS: a population‐based study. Mult Sclerosis Relat Disord. 2020;37:101487. [DOI] [PubMed] [Google Scholar]
- 89. Li Y, Xie P, Lv F, Mu J, Li Q, Yang Q, et al. Brain magnetic resonance imaging abnormalities in neuromyelitis optica. Acta Neurol Scand. 2008;118:218–25. [DOI] [PubMed] [Google Scholar]
- 90. Chan KH, Tse CT, Chung CP, Lee RL, Kwan JS, Ho PW, et al. Brain involvement in neuromyelitis optica spectrum disorders. Arch Neurol. 2011;68:1432–9. [DOI] [PubMed] [Google Scholar]
- 91. Huh SY, Min JH, Kim W, Kim SH, Kim HJ, Kim BJ, et al. The usefulness of brain MRI at onset in the differentiation of multiple sclerosis and seropositive neuromyelitis optica spectrum disorders. Mult Sclerosis. 2014;20:695–704. [DOI] [PubMed] [Google Scholar]
- 92. Kim W, Park MS, Lee SH, Kim SH, Jung IJ, Takahashi T, et al. Characteristic brain magnetic resonance imaging abnormalities in central nervous system aquaporin‐4 autoimmunity. Mult Sclerosis. 2010;16:1229–36. [DOI] [PubMed] [Google Scholar]
- 93. Yonezu T, Ito S, Mori M, Ogawa Y, Makino T, Uzawa A, et al. ‘Bright spotty lesions’ on spinal magnetic resonance imaging differentiate neuromyelitis optica from multiple sclerosis. Mult Sclerosis. 2014;20:331–7. [DOI] [PubMed] [Google Scholar]
- 94. Kister I, Johnson E, Raz E, Babb J, Loh J, Shepherd TM. Specific MRI findings help distinguish acute transverse myelitis of neuromyelitis optica from spinal cord infarction. Mult Sclerosis Relat Disord. 2016;9:62–7. [DOI] [PubMed] [Google Scholar]
- 95. Pekcevik Y, Mitchell CH, Mealy MA, Orman G, Lee IH, Newsome SD, et al. Differentiating neuromyelitis optica from other causes of longitudinally extensive transverse myelitis on spinal magnetic resonance imaging. Mult Sclerosis. 2016;22:302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Salama S, Levy M. Bright spotty lesions as an imaging marker for neuromyelitis optica spectrum disorder. Mult Sclerosis J. 2021. doi: 10.1177/1352458521994259. [DOI] [PubMed] [Google Scholar]
- 97. Rabasté S, Cobo‐Calvo A, Nistiriuc‐Muntean V, Vukusic S, Marignier R, Cotton F, et al. Diagnostic value of bright spotty lesions on MRI after a first episode of acute myelopathy. J Neuroradiol. 2021;48:28–36. [DOI] [PubMed] [Google Scholar]
- 98. Adoni T, Lino AM, da Gama PD , Apostolos‐Pereira SL, Marchiori PE, Kok F, et al. Recurrent neuromyelitis optica in Brazilian patients: clinical, immunological, and neuroimaging characteristics. Mult Sclerosis. 2010;16:81–6. [DOI] [PubMed] [Google Scholar]
- 99. Yokote H, Nose Y, Ishibashi S, Tanaka K, Takahashi T, Fujihara K, et al. Spinal cord ring enhancement in patients with neuromyelitis optica. Acta Neurol Scand. 2015;132:37–41. [DOI] [PubMed] [Google Scholar]
- 100. Zalewski NL, Morris PP, Weinshenker BG, Lucchinetti CF, Guo Y, Pittock SJ, et al. Ring‐enhancing spinal cord lesions in neuromyelitis optica spectrum disorders. J Neurol Neurosurg Psychiatry. 2017;88:218–25. [DOI] [PubMed] [Google Scholar]
- 101. Dumrikarnlert C, Siritho S, Chulapimphan P, Ngamsombat C, Satukijchai C, Prayoonwiwat N. The characteristics of spinal imaging in different types of demyelinating diseases. J Neurol Sci. 2017;372:138–43. [DOI] [PubMed] [Google Scholar]
- 102. Carnero Contentti E, Daccach Marques V, Soto de Castillo I, Tkachuk V, Antunes Barreira A, Armas E, et al. Frequency of brain MRI abnormalities in neuromyelitis optica spectrum disorder at presentation: a cohort of Latin American patients. Mult Sclerosis Relat Disord. 2018;19:73‐8. [DOI] [PubMed] [Google Scholar]
- 103. Abdullah S, Fadzli F, Ramli N, Tan CT. There is less MRI brain lesions and no characteristic MRI Brain findings in IIDDs patients with positive AQP4 serology among Malaysians. Mult Sclerosis Relat Disord. 2017;12:34–8. [DOI] [PubMed] [Google Scholar]
- 104. Cao G, Duan Y, Zhang N, Sun J, Li H, Li Y, et al. Brain MRI characteristics in neuromyelitis optica spectrum disorders: a large multi‐center retrospective study in China. Mult Sclerosis Relat Disord. 2020;46:102475. [DOI] [PubMed] [Google Scholar]
- 105. Long Y, Chen M, Zhang B, Gao C, Zheng Y, Xie L, et al. Brain gadolinium enhancement along the ventricular and leptomeningeal regions in patients with aquaporin‐4 antibodies in cerebral spinal fluid. J Neuroimmunol. 2014;269:62–7. [DOI] [PubMed] [Google Scholar]
- 106. Kim W, Lee JE, Kim SH, Huh SY, Hyun JW, Jeong IH, et al. Cerebral cortex involvement in neuromyelitis optica spectrum disorder. J Clin Neurol. 2016;12:188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wengert O, Rothenfusser‐Korber E, Vollrath B, Bohner G, Scheibe F, Otto C, et al. Neurosarcoidosis: correlation of cerebrospinal fluid findings with diffuse leptomeningeal gadolinium enhancement on MRI and clinical disease activity. J Neurol Sci. 2013;335:124–30. [DOI] [PubMed] [Google Scholar]
- 108. Nakamura M, Misu T, Fujihara K, Miyazawa I, Nakashima I, Takahashi T, et al. Occurrence of acute large and edematous callosal lesions in neuromyelitis optica. Mult Sclerosis. 2009;15:695–700. [DOI] [PubMed] [Google Scholar]
- 109. Kim SH, Hyun JW, Joung A, Lee SH, Kim HJ. Occurrence of asymptomatic acute neuromyelitis optica spectrum disorder‐typical brain lesions during an attack of optic neuritis or myelitis. PLOS ONE. 2016;11:e0167783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wilson CA, Mullen MT, Jackson BP, Ishida K, Messe SR. Etiology of corpus callosum lesions with restricted diffusion. Clin Neuroradiol. 2017;27:31–7. [DOI] [PubMed] [Google Scholar]
- 111. Oliveira P, Mayeux J. Teaching neuroimages: snowball‐like lesions with sudden hearing loss. Neurology 2014;82:e100. [DOI] [PubMed] [Google Scholar]
- 112. Renard D, Castelnovo G, Bousquet PJ, de Champfleur N , de Seze J , Vermersch P, et al. Brain MRI findings in long‐standing and disabling multiple sclerosis in 84 patients. Clin Neurol Neurosurg. 2010;112:286–90. [DOI] [PubMed] [Google Scholar]
- 113. Wang F, Liu Y, Duan Y, Li K. Brain MRI abnormalities in neuromyelitis optica. Eur J Radiol. 2011;80:445–9. [DOI] [PubMed] [Google Scholar]
- 114. Zhang L, Wu A, Zhang B, Chen S, Men X, Lin Y, et al. Comparison of deep gray matter lesions on magnetic resonance imaging among adults with acute disseminated encephalomyelitis, multiple sclerosis, and neuromyelitis optica. Mult Sclerosis. 2014;20:418–23. [DOI] [PubMed] [Google Scholar]
- 115. Ito S, Mori M, Makino T, Hayakawa S, Kuwabara S. ‘Cloud‐like enhancement’ is a magnetic resonance imaging abnormality specific to neuromyelitis optica. Ann Neurol. 2009;66:425–8. [DOI] [PubMed] [Google Scholar]
- 116. Kim SH, Huh SY, Hyun JW, Jeong IH, Lee SH, Joung A, et al. A longitudinal brain magnetic resonance imaging study of neuromyelitis optica spectrum disorder. PLOS ONE. 2014;9:e108320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Cabrera‐Gomez JA, Quevedo‐Sotolongo L, Gonzalez‐Quevedo A, Lima S, Real‐Gonzalez Y, Cristofol‐Corominas M, et al. Brain magnetic resonance imaging findings in relapsing neuromyelitis optica. Mult Sclerosis. 2007;13:186–92. [DOI] [PubMed] [Google Scholar]
- 118. Pires CE, Silva CM, Lopes FC, Malfetano FR, Pereira VC, Kubo T, et al. Brain MRI abnormalities in Brazilian patients with neuromyelitis optica. J Clin Neurosci. 2012;19:969–74. [DOI] [PubMed] [Google Scholar]
- 119. Liao MF, Chang KH, Lyu RK, Huang CC, Chang HS, Wu YR, et al. Comparison between the cranial magnetic resonance imaging features of neuromyelitis optica spectrum disorder versus multiple sclerosis in Taiwanese patients. BMC Neurol. 2014;14:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Banker P, Sonni S, Kister I, Loh JP, Lui YW. Pencil‐thin ependymal enhancement in neuromyelitis optica spectrum disorders. Mult Sclerosis. 2012;18:1050–3. [DOI] [PubMed] [Google Scholar]
- 121. Cabrera‐Gomez J, Saiz‐Hinarejos A, Graus F, Gonzalez‐Quevedo A, Rodriguez‐Rojas R, Quevedo‐Sotolongo L, et al. Brain magnetic resonance imaging findings in acute relapses of neuromyelitis optica spectrum disorders. Mult Sclerosis. 2008;14:248–51. [DOI] [PubMed] [Google Scholar]
- 122. Makino T, Ito S, Mori M, Yonezu T, Ogawa Y, Kuwabara S. Diffuse and heterogeneous T2‐hyperintense lesions in the splenium are characteristic of neuromyelitis optica. Mult Sclerosis. 2013;19:308–15. [DOI] [PubMed] [Google Scholar]
- 123. Kruit MC, van Buchem MA , Launer LJ, Terwindt GM, Ferrari MD. Migraine is associated with an increased risk of deep white matter lesions, subclinical posterior circulation infarcts and brain iron accumulation: the population‐based MRI CAMERA study. Cephalalgia 2010;30:129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Matsushita T, Isobe N, Piao H, Matsuoka T, Ishizu T, Doi H, et al. Reappraisal of brain MRI features in patients with multiple sclerosis and neuromyelitis optica according to anti‐aquaporin‐4 antibody status. J Neurol Sci. 2010;291:37‐43. [DOI] [PubMed] [Google Scholar]
- 125. Absoud M, Lim MJ, Appleton R, Jacob A, Kitley J, Leite MI, et al. Paediatric neuromyelitis optica: clinical, MRI of the brain and prognostic features. J Neurol Neurosurg Psychiatry. 2015;86:470–2. [DOI] [PubMed] [Google Scholar]
- 126. Ikeda K, Ito H, Hidaka T, Takazawa T, Sekine T, Yoshii Y, et al. Repeated non‐enhancing tumefactive lesions in a patient with a neuromyelitis optica spectrum disorder. Intern Med. 2011;50:1061–4. [DOI] [PubMed] [Google Scholar]
- 127. Matthews L, Marasco R, Jenkinson M, Kuker W, Luppe S, Leite MI, et al. Distinction of seropositive NMO spectrum disorder and MS brain lesion distribution. Neurology 2013;80:1330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Magana SM, Matiello M, Pittock SJ, McKeon A, Lennon VA, Rabinstein AA, et al. Posterior reversible encephalopathy syndrome in neuromyelitis optica spectrum disorders. Neurology 2009;72:712–7. [DOI] [PubMed] [Google Scholar]
- 129. Berger JR, Neltner J, Smith C, Cambi F. Posterior reversible encephalopathy syndrome masquerading as progressive multifocal leukoencephalopathy in rituximab treated neuromyelitis optica. Mult Sclerosis Relat Disord. 2014;3:728–31. [DOI] [PubMed] [Google Scholar]
- 130. Igel C, Garretto D, Robbins MS, Swerdlow M, Judge N, Dayal A. Neuromyelitis optica in pregnancy complicated by posterior reversible encephalopathy syndrome, eclampsia and fetal death. J Clin Med Res. 2015;7:193–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Corbett J, Bhuta S, Prain K, Brilot F, Sabet A, Broadley SA. PRES‐like presentation in MOG antibody‐related demyelination (MARD). J Clin Neurosci. 2020;72:453–5. [DOI] [PubMed] [Google Scholar]
- 132. Kulkarni GB, Kallollimath P, Subasree R, Veerendrakumar M. Intractable vomiting and hiccups as the presenting symptom of neuromyelitis optica. Ann Ind Acad Neurol. 2014;17:117–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Popescu BF, Lennon VA, Parisi JE, Howe CL, Weigand SD, Cabrera‐Gomez JA, et al. Neuromyelitis optica unique area postrema lesions: nausea, vomiting, and pathogenic implications. Neurology 2011;76:1229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Pedigo NW Jr, Brizzee KR. Muscarinic cholinergic receptors in area postrema and brainstem areas regulating emesis. Brain Res Bull. 1985;14:169–77. [DOI] [PubMed] [Google Scholar]
- 135. Misu T, Fujihara K, Nakashima I, Sato S, Itoyama Y. Intractable hiccup and nausea with periaqueductal lesions in neuromyelitis optica. Neurology 2005;65:1479–82. [DOI] [PubMed] [Google Scholar]
- 136. Etemadifar M, Sabeti F, Ebrahimian S, Momeni F. Dorsal midbrain involvement in MRI as a core clinical manifestation for NMOSD diagnosis. Mult Sclerosis Relat Disord. 2020;43:102150. [DOI] [PubMed] [Google Scholar]
- 137. Lemos MD, Carvalho GB, Carvalho RS, Bichuetti DB, de Oliveira EM , Abdala N. Neuromyelitis optica spectrum disorders: beyond longitudinally extensive transverse myelitis. Clin Radiol. 2015;70:630–7. [DOI] [PubMed] [Google Scholar]
- 138. Kim JE, Kim SM, Ahn SW, Lim BC, Chae JH, Hong YH, et al. Brain abnormalities in neuromyelitis optica. J Neurol Sci. 2011;302:43–8. [DOI] [PubMed] [Google Scholar]
- 139. Toru S, Soejima I, Katayama Y, Saito K, Yokote H. A case of anti‐AQP4 antibody‐positive neuromyelitis optica spectrum disorder with MRI‐proven lesions in lumbar nerve roots. Mult Sclerosis Relat Disord. 2020;46:102557. [DOI] [PubMed] [Google Scholar]
- 140. Sinnecker T, Schumacher S, Mueller K, Pache F, Dusek P, Harms L, et al. MRI phase changes in multiple sclerosis vs neuromyelitis optica lesions at 7T. Neurol Neuroimmunol Neuroinflamm. 2016;3:e259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Chawla S, Ge Y, Wuerfel J, Asadollahi S, Mohan S, Paul F, et al. Longitudinal ultra‐high field MRI of brain lesions in neuromyelitis optica spectrum disorders. Mult Sclerosis Relat Disord. 2020;42:102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Jang J, Nam Y, Choi Y, Shin NY, An JY, Ahn KJ, et al. Paramagnetic rims in multiple sclerosis and neuromyelitis optica spectrum disorder: a quantitative susceptibility mapping study with 3‐T MRI. J Clin Neurol. 2020;16:562–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Pasquier B, Borisow N, Rasche L, Bellmann‐Strobl J, Ruprecht K, Niendorf T, et al. Quantitative 7T MRI does not detect occult brain damage in neuromyelitis optica. Neurol Neuroimmunol Neuroinflamm. 2019;6:e541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Pache F, Zimmermann H, Finke C, Lacheta A, Papazoglou S, Kuchling J, et al. Brain parenchymal damage in neuromyelitis optica spectrum disorder – a multimodal MRI study. Eur Radiol. 2016;26:4413–22. [DOI] [PubMed] [Google Scholar]
- 145. Zhang N, Sun J, Wang Q, Qin W, Zhang X, Qi Y, et al. Differentiate aquaporin‐4 antibody negative neuromyelitis optica spectrum disorders from multiple sclerosis by multimodal advanced MRI techniques. Mult Sclerosis Relat Disord. 2020;41:102035. [DOI] [PubMed] [Google Scholar]
- 146. Aboul‐Enein F, Krssak M, Hoftberger R, Prayer D, Kristoferitsch W. Diffuse white matter damage is absent in neuromyelitis optica. Am J Neuroradiol. 2010;31:76–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Pichiecchio A, Tavazzi E, Poloni G, Ponzio M, Palesi F, Pasin M, et al. Advanced magnetic resonance imaging of neuromyelitis optica: a multiparametric approach. Mult Sclerosis. 2012;18:817–24. [DOI] [PubMed] [Google Scholar]
- 148. Schmidt FA, Chien C, Kuchling J, Bellmann‐Strobl J, Ruprecht K, Siebert N, et al. Differences in advanced magnetic resonance imaging in MOG‐IgG and AQP4‐IgG seropositive neuromyelitis optica spectrum disorders: a comparative study. Front Neurol. 2020;11. doi: 10.3389/fneur.2020.499910499910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Chanson JB, Lamy J, Rousseau F, Blanc F, Collongues N, Fleury M, et al. White matter volume is decreased in the brain of patients with neuromyelitis optica. Eur J Neurol. 2013;20:361–7. [DOI] [PubMed] [Google Scholar]
- 150. Calabrese M, Oh MS, Favaretto A, Rinaldi F, Poretto V, Alessio S, et al. No MRI evidence of cortical lesions in neuromyelitis optica. Neurology 2012;79:1671–6. [DOI] [PubMed] [Google Scholar]
- 151. Asseyer S, Kuchling J, Gaetano L, Komnenic D, Siebert N, Chien C, et al. Ventral posterior nucleus volume is associated with neuropathic pain intensity in neuromyelitis optica spectrum disorders. Mult Sclerosis Relat Disord. 2020;46. doi: 10.1016/j.msard.2020.102579102579. [DOI] [PubMed] [Google Scholar]
- 152. Papadopoulou A, Oertel FC, Gaetano L, Kuchling J, Zimmermann H, Chien C, et al. Attack‐related damage of thalamic nuclei in neuromyelitis optica spectrum disorders. J Neurol Neurosurg Psychiatry. 2019;90:1156–64. [DOI] [PubMed] [Google Scholar]
- 153. Chien C, Oertel FC, Siebert N, Zimmermann H, Asseyer S, Kuchling J, et al. Imaging markers of disability in aquaporin‐4 immunoglobulin G seropositive neuromyelitis optica: a graph theory study. Brain Commun. 2019;1. doi: 10.1093/braincomms/fcz026fcz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Juenger V, Cooper G, Chien C, Chikermane M, Oertel FC, Zimmermann H, et al. Optic chiasm measurements may be useful markers of anterior optic pathway degeneration in neuromyelitis optica spectrum disorders. Eur Radiol. 2020;30:5048–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Rocca MA, Agosta F, Mezzapesa DM, Falini A, Martinelli V, Salvi F, et al. A functional MRI study of movement‐associated cortical changes in patients with Devic’s neuromyelitis optica. NeuroImage 2004;21:1061–8. [DOI] [PubMed] [Google Scholar]
- 156. Liu Y, Liang P, Duan Y, Jia X, Wang F, Yu C, et al. Abnormal baseline brain activity in patients with neuromyelitis optica: a resting‐state fMRI study. Eur J Radiol. 2011;80:407–11. [DOI] [PubMed] [Google Scholar]
- 157. Liu Y, Xiong H, Li X, Zhang D, Yang C, Yu J, et al. Abnormal baseline brain activity in neuromyelitis optica patients without brain lesion detected by resting‐state functional magnetic resonance imaging. Neuropsychiatr Dis Treat. 2020;16:71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kim H, Lee Y, Kim YH, Lim YM, Lee JS, Woo J, et al. Deep Learning‐based method to differentiate neuromyelitis optica spectrum disorder from multiple sclerosis. Front Neurol. 2020;11:599042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Maillart E, Lippi A, Lubetzki C, Louapre C, Papeix C. Early relapse after RTX initiation in a patient with NMO/MS overlap syndrome: How long to conclude to a failure treatment? Mult Sclerosis Relat Disord. 2018;20:220–2. [DOI] [PubMed] [Google Scholar]
- 160. Murchison A, Kitley J, Leite MI, Kuker W, Palace J. Predictive value of MRI parameters in severity and recovery of first‐episode myelitis in aquaporin‐4 antibody disease. J Neurol Sci. 2015;355:49–53. [DOI] [PubMed] [Google Scholar]
- 161. Bonnan M, Debeugny S, Mejdoubi M, Cabre P. Predictive value of conventional MRI parameters in first spinal attacks of neuromyelitis optica spectrum disorder. Mult Sclerosis. 2020;26:468–75. [DOI] [PubMed] [Google Scholar]
- 162. Xu Y, Ren Y, Li X, Xu W, Wang X, Duan Y, et al. Persistently gadolinium‐enhancing lesion is a predictor of poor prognosis in NMOSD attack: a clinical trial. Neurotherapeutics 2021. doi: 10.1007/s13311-020-00973-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Jurynczyk M, Messina S, Palace J. Spinal cord and brain MRI should be routinely performed during follow‐up in patients with NMOSD – no. Mult Sclerosis. 2021;27:15–6. [DOI] [PubMed] [Google Scholar]
- 164. Lee MY, Yong KP, Hyun JW, Kim SH, Lee SH, Kim HJ. Incidence of interattack asymptomatic brain lesions in NMO spectrum disorder. Neurology 2020;95:e3124–e8. [DOI] [PubMed] [Google Scholar]
- 165. Cai MT, Zheng Y, Shen CH, Yang F, Fang W, Zhang YX, et al. Evaluation of brain and spinal cord lesion distribution criteria at disease onset in distinguishing NMOSD from MS and MOG antibody‐associated disorder. Mult Sclerosis. 2020;1352458520939008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not relevant.


