Abstract
Background:
Atopic dermatitis (AD) patients are often colonized with Staphylococcus aureus, and staphylococcal biofilms have been reported on adult AD skin lesions. The commensal S epidermidis can antagonize S aureus, although its role in AD is unclear. We sought to characterize S aureus and S epidermidis colonization and biofilm propensity and determine their associations with AD severity, barrier function, and epidermal gene expression in the first US early-life cohort of children with AD, the Mechanisms of Progression of Atopic Dermatitis to Asthma in Children (MPAACH).
Methods:
The biofilm propensity of staphylococcal isolates was assessed by crystal violet assays. Gene expression of filaggrin and antimicrobial alarmins S100A8 and S100A9 was measured in keratinocyte RNA extracted from skin tape strips. Staphylococcal biofilms sampled from MPAACH skin were visualized using scanning electron microscopy.
Results:
Sixty-two percent of staphylococcal isolates (sampled from 400 subjects) formed moderate/strong biofilms. Sixty-eight percent of subjects co-colonized with both staphylococcal species exhibited strains that formed cooperative mixed-species biofilms. Scanning electron microscopy verified the presence of staphylococcal biofilms on the skin of MPAACH children. Staphylococcus aureus strains showing higher relative biofilm propensity compared with S epidermidis were associated with increased AD severity (P = .03) and increased lesional and nonlesional transepidermal water loss (P = .01, P = .03).
Conclusions:
Our data suggest a pathogenic role for S aureus biofilms in AD. We found that strain-level variation in staphylococcal isolates governs the interactions between S epidermidis and S aureus and that the balance between these two species, and their biofilm propensity, has important implications for AD.
Keywords: atopic dermatitis, biofilm, filaggrin, S100A8/S100A9, Staphylococcus aureus
Graphical Abstract

Staphylococcal biofilms are observed on the skin of children with AD in the MPAACH cohort. Staphylococcus aureus strains showing higher relative biofilm propensity (compared with S epidermidis from the same subject) are associated with increased AD severity. Staphylococcus aureus strains showing higher relative biofilm propensity are associated with increased lesional and nonlesional transepidermal water loss. Abbreviations: AD, atopic dermatitis; FLG, filaggrin; MPAACH, Mechanisms of Progression of Atopic Dermatitis to Asthma in Children; SCORAD, scoring atopic dermatitis index; S100A8, S100 Calcium-Binding Protein A8; S100A9, Calcium-Binding Protein A9; TEWL, transepidermal water loss
1 |. INTRODUCTION
Atopic dermatitis (AD) is a chronic, relapsing inflammatory skin disease that affects 15%–30% of children and 2%–10% of adults.1 Although up to 70% of affected children have spontaneous remission of AD before adolescence, AD often precedes the development of allergic complications later in life. For example, more than 50% of children with AD go on to develop asthma and ~75% develop allergic rhinitis.2 A defining characteristic of AD patients is a defective skin barrier, which, in some cases, is associated with mutations in the structural skin protein filaggrin.3 This dysfunction in the skin barrier, combined with exposure to exogenous irritants or sensitization by allergens, underlies the inflammatory responses in AD skin.4–6
While the epidermis provides the first line of protection against environmental insults, the skin microbiome represents a community of microorganisms that coexist with keratinocytes and can cooperate with the host immune system to protect against pathogen colonization.7,8 For example, in healthy skin, the commensal gram-positive bacterium S epidermidis can suppress colonization by S aureus or other pathogens through a number of mechanisms.9–13 However, dysbiosis of the skin microbiome is a common feature in AD. Up to 90% of patients with AD are colonized with the gram-positive bacterium S aureus (compared with 5% or less in healthy individuals),14,15 with a marked increase in relative abundance at lesional sites, particularly during flares of severe AD.16,17 Staphylococcus aureus secretes a myriad of toxins,18–21 proteases,22,23 and molecules such as phenol-soluble modulins (PSMs)24 that may influence AD severity. In contrast, S epidermidis and some other commensal coagulase-negative staphylococci (CoNS) are not known to produce similar virulence factors that target the host; rather, they produce a range of antimicrobial agents that can inhibit S aureus growth and/or biofilm formation. These include the Esp protease,9 antimicrobial peptides,11 phenol-soluble modulins,25 and autoinducer peptides that inhibit quorum sensing by S aureus.26 In addition, S epidermidis can trigger the production of the alarmin and antimicrobial protein calprotectin (S100A8/A9) by keratinocytes in the epidermis.10 Calprotectin chelates both Zn2+ and Mn2+ 27 and inhibits microbial colonization via “nutritional immunity” by controlling the availability of these critical divalent metal nutrient ions.28–30
Bacteria that colonize surfaces under unfavorable environmental conditions (such as exposure to ultraviolet light or extremes in temperature or pH) typically grow as biofilms, which are highly cohesive and adhesive surface-adherent colonies surrounded by an extracellular matrix.31–34 The bacterial cells within biofilms are highly resistant to antibiotic action or immune responses, which can lead to recurrent, hard-to-treat infections.35–38 Both S epidermidis and S aureus can form robust biofilms, and both species have been reported to form biofilms on skin. In fact, S epidermidis typically exists on the skin as a biofilm growing between keratinocytes in the outer layers of the epidermis in healthy individuals.32 Both S aureus and S epidermidis biofilms have been observed on the surface and in eccrine ducts of lesional skin from AD patient biopsies.39–44 Given the arid, nutrient-poor environment of the skin and exposure to broad fluctuations in temperature, staphylococcal colonization of the skin is likely dependent on the ability to grow as biofilms, allowing for effective adherence and long-term persistence.32,45 Interactions between S epidermidis and S aureus in the skin environment are of increasing interest. Although certain strains of planktonic S epidermidis secrete antimicrobial peptides that inhibit S aureus growth, little is known about potential inhibitory or cooperative interactions in multi-species staphylococcal biofilms in the skin that may be relevant to AD pathogenesis.
In this study, we analyze staphylococcal colonization and biofilm propensity of the isolated staphylococcal strains sampled from the first 400 subjects in the Mechanisms of Progression of AD to Asthma in Children (MPAACH) cohort, which is the first US-based longitudinal, mechanistic cohort designed to follow the progression and severity of AD in a pediatric population and the potential progression from AD to other atopic diseases. The goal of the current study was to determine the associations between staphylococcal colonization or biofilm propensity and clinical correlates of AD severity46 and barrier dysfunction, as well as the in vivo keratinocyte gene expression of the antimicrobial alarmins S100A8 and S100A9 and the skin barrier protein filaggrin.
2 |. METHODS
2.1 |. Subjects
The MPAACH cohort is the first US-based patient cohort to exclusively enroll toddlers with AD. Inclusion criteria encompass toddlers from the greater Cincinnati, Ohio metropolitan area (≥36 weeks gestation) aged 1–2 years at enrollment with either physician-diagnosed AD or a positive response to all three questions on the Children’s Eczema Questionnaire (CEQ): (a) Does your child have or has your child had a red rash/eczema which can come and go?; (b) If yes, has this caused itching or scratching?; (c) Has this red rash/eczema affected any of the following areas: around the eyes, ears, scalp, cheeks, forehead, neck, trunk, folds of the elbows/behind the knees, wrist or ankle, outer arms/legs?47 A total of 500 children are being enrolled, with annual study visits over a 5-year period. The current study is an intermediate analysis that focuses on the first 400 MPAACH participants. Subjects provided demographic, environmental, asthma trigger information, and personal and family allergy and asthma history data. The SCORing of AD (SCORAD) scale, a composite score of objective and subjective signs and symptoms of AD and total effected area, was used to assess AD severity for each MPAACH child based on a representative lesion and subjective symptoms as reported by parents.48 While the majority of participants reported the use of a medication or cream for the treatment of eczema/AD, all were instructed to withhold the use of antihistamine medications for 7 days prior to their visit. Furthermore, children were asked to discontinue the use of other lotions, creams, ointments, and emollients the night prior to the visit.
2.2 |. Transepidermal water loss (TEWL)
Transepidermal water loss was assessed by an open-chamber TEWL machine (Tewameter, Courage + Khazaka electronic) at lesional and nonlesional sites on each MPAACH subject for 60 seconds as previously described by Gupta et al.6 Relative humidity and temperature were recorded in addition to a 60 second baseline reading prior to subject measurements being taken.
2.3 |. Statistical analyses
Prior to analyses, distributional characteristics of the data were examined. SCORAD, TEWL, and gene expression data were skewed, so nonparametric statistics were used. Descriptive statistics (frequencies) were reported for categorical variables while median and the interquartile range (IQR) values were reported for continuous variables. We first tested for association between a four-level bacterial colonization variable (group 1: culture-positive for both S aureus and S epidermidis; group 2: culture-positive for S aureus but not S epidermidis; group 3: culture-positive for S epidermidis but not S aureus; and group 4: culture-positive for neither S aureus nor S epidermidis) using contingency tables (Black race and sex), and linear regression to test for associations with age.
Kruskal-Wallis test was used to test for distributional differences between the four-level bacterial colonization and clinical outcomes (SCORAD and TEWL) and gene expression data. To further evaluate potential differences, Wilcoxon rank-sum test was performed to test the association of 2-level variables (including S aureus colonization, S aureus biofilm propensity) with clinical outcomes (SCORAD and TEWL) and gene expression. Biofilm propensity was defined by the mean of the optical densities measured from the crystal violet assay of each strain. We defined a higher relative S aureus biofilm propensity as those cases in which the maximum biofilm propensity from all S aureus strains sampled per subject was greater than the maximum S epidermidis biofilm propensity from the same subject. The converse includes cases in which the subject had biofilm-forming S epidermidis but no S aureus were sampled; a separate analysis was conducted for the subset of subjects that were co-colonized with both S epidermidis and S aureus (Figure S1). To evaluate mean absolute biofilm propensity (a quantitative measure measured by the crystal violet biofilm assay) in relation to clinical outcomes and gene expression, Spearman rank correlation was used. Study data were collected and managed using REDCap electronic data capture tools hosted at Cincinnati Children’s Hospital Medical Center.49
We used α = 0.05 as the threshold for statistical significance. We recognize that this threshold does not account for the number of statistical tests performed and thus may increase the risk of false-positive findings. However, because our outcome measures are correlated with each other, application of Bonferroni’s correction would increase the risk of false-negative findings. To minimize the risk of incorrect inference, we interpret P-values close to our threshold with caution.
2.4 |. Other methods
Sampling and characterization of bacterial isolates, biofilm crystal violet assays, scanning electron microscopy, tape strip sampling, and assessment of S100A8, S100A9, and filaggrin (FLG) gene expression are described in Appendix S1.
3 |. RESULTS
3.1 |. Characteristics of study cohort
The study cohort was comprised of 51.3% (205/400) males and 48.7% (195/400) females with 61.5% (246/400) of participants’ race being parent-reported as Black (Table 1). The average age of the subjects was 2.3 years (IQR 1.7–2.5). The median SCORAD value for all children was 18.7 (IQR 11.4–29.8) (Table 1). The median TEWL value from lesional skin, 11.7 g m−2 h−1, was significantly higher than TEWL value from nonlesional skin (11.7 vs 9.4, P < .0001; Wilcoxon rank-sum test), consistent with previous reports.6,50–52
TABLE 1.
Characteristics of MPAACH study cohort
| MPAACH subjects | |
|---|---|
| No. | 400 |
| Black race (%) | 61.5 (246/400) |
| Male sex (%) | 51.3 (205/400) |
| Age (years), median (IQR) | 2.3 (1.7–2.5) |
| SCORAD, median (IQR) | 18.7 (11.4–29.8) |
| Nonlesional TEWL (g m−2 h−1) | 9.4 (7.1–13.6) |
| Lesional TEWL (g m−2 h−1) | 11.7 (8.2–19.8) |
| Presence of S aureus (%) | 26.5 (106/400) |
| Presence of S epidermidis (%) | 71.3 (285/400) |
| Co-colonization with S aureus and S epidermidis (%) | 18.8 (75/400) |
Abbreviation: IQR, interquartile range.
3.2 |. Staphylococcal colonization patterns
Among the MPAACH children in this study, 26.5% (106/400) were culture-positive for S aureus (Figure 1A). Specifically, S aureus was found at nonlesional sites in 17.3% (69/400) (Figure 1B) or at lesional sites in 18.3% (73/400) of subjects (Figure 1C); 34.0% of the subjects positive for S aureus (36/106) were culture-positive at both sites. Seventy-one percent of participants (285/400) were culture-positive for S epidermidis, with 46.3% of nonlesional sites (185/400) and 50.0% (200/400) of lesional sites being culture-positive; 36.8% (105/285) of these subjects were culture-positive for S epidermidis at both sites. Nineteen percent of MPAACH children (75/400) were culture-positive for both S aureus and S epidermidis, with 8.8% of subjects (35/400) co-colonized at lesional sites and 7.5% of subjects (30/400) co-colonized at nonlesional sites; in the remaining 2.5% of these subjects (10/400), S epidermidis and S aureus were found at distinct sites. All but 4 children (99.0%) were culture-positive for other coagulase-negative Staphylococcus spp (CoNS). Those four children yielded no culturable bacteria (1.0%) (Figure 1A–C). Forty-three percent of nonlesional sites (170/400) were colonized only with other CoNS (Figure 1B), while 37.8% of lesional sites (151/400) were colonized only with CoNS (Figure 1C). Eighty children (80/400, 20.0%) were not culture-positive for either S epidermidis or S aureus. No differences were observed in staphylococcal colonization by race (P = .27) or age of the children (P = .13). Furthermore, there were no significant differences among the four bacterial colonization groups in terms of SCORAD (P = .27), nonlesional TEWL (P = .88), or lesional TEWL (P = .34) (Table S1).
FIGURE 1.
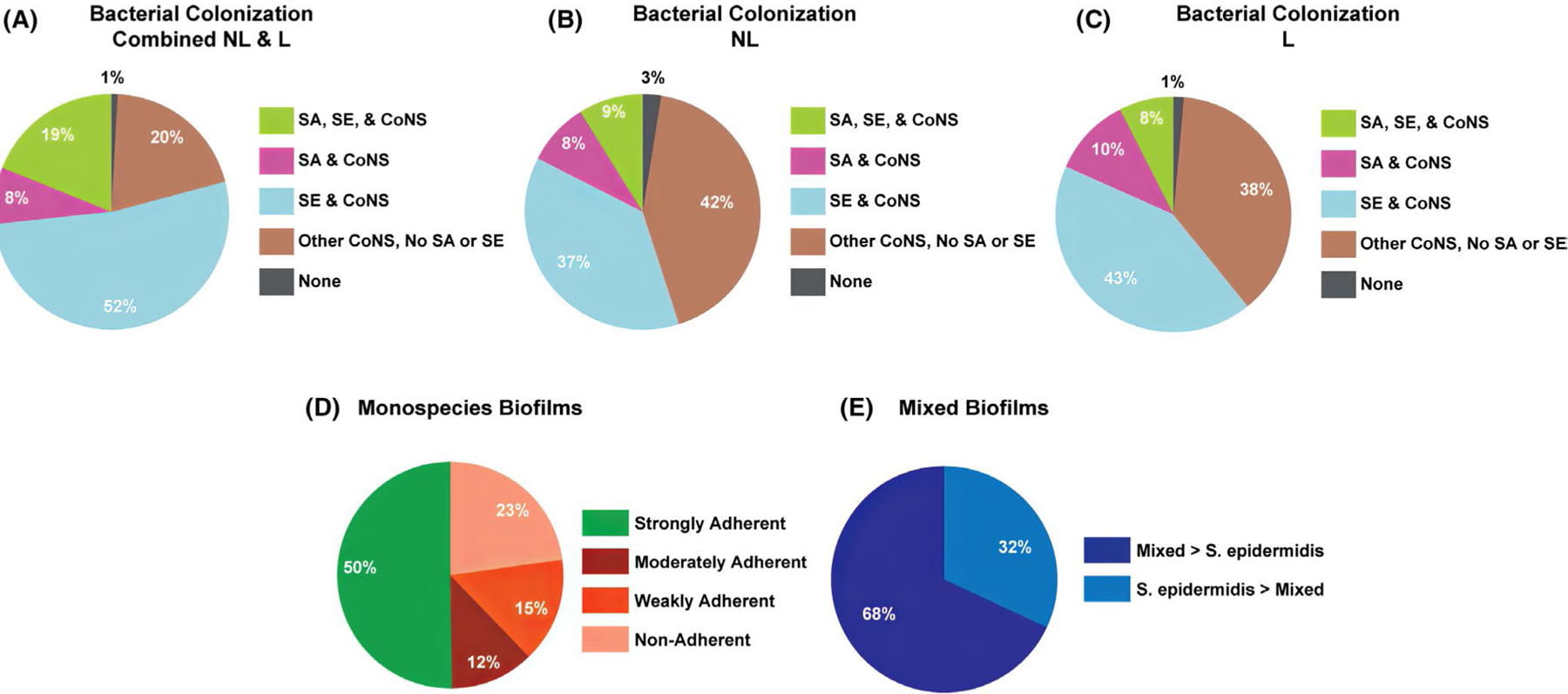
Staphylococcal colonization and biofilm propensity of clinical isolates from the MPAACH cohort. (A-C) Breakdown of children that were culture-positive for S aureus (SA), S epidermidis (SE), and Coagulase-negative Staphylococci (CoNS). (A) Overall distribution for combined nonlesional and lesional sites. (B) Distribution at nonlesional sites. (C) Distribution at lesional sites. (D) Strength of individual mono-species biofilms. (E) Breakdown of cooperative vs antagonistic mixed-species biofilms grown from isolates collected from MPAACH children that were culture-positive for both S aureus and S epidermidis
We performed crystal violet assays on the S aureus and S epidermidis isolates obtained from the MPAACH cohort to assess their ability to form biofilms and quantitate the extent of biofilm growth per strain. A total of 509 strains of S epidermidis and S aureus were obtained from 316 MPAACH children. The results of the crystal violet assays revealed that 50% of the clinically isolated staphylococcal strains were able to form strongly adherent biofilms (255/509), 12% were able to form moderately adherent biofilms (61/509), and 15% could only form weakly adherent biofilms (76/509). Twenty-three percent of the staphylococcal strains were not able to form biofilms (117/509) (Figure 1D). Biofilm adherence strength was defined using published cutoff values53 based on a common classification scheme.54
Nineteen percent of children (75/400) were co-colonized with S epidermidis and S aureus. From a subset of 72 co-colonized children, we tested whether S epidermidis and S aureus isolates would show cooperative or antagonistic interactions in mixed biofilms, given that some S epidermidis strains can inhibit growth and/or biofilm formation by S aureus.9–13 Strains obtained from three children (3/75) were not able to be maintained in culture. A large majority (68%, 49/72) of paired isolates from MPAACH children showed cooperative mixed biofilm formation, in which the biofilm from mixed culture showed greater biomass than the S epidermidis mono-species biofilm (Figure 1C), whereas 32% (23/72) of the paired isolates grew mixed biofilms with lesser biomass than the S epidermidis mono-species biofilm. Thus, for those MPAACH children from whom both S epidermidis and S aureus were sampled, most exhibited S epidermidis strains acting cooperatively with S aureus in terms of biofilm growth rather than inhibiting S aureus growth or biofilm formation. In fact, in 13% (9/72) of paired isolates from MPAACH children, the interaction between bacteria was synergistic, in that the mixed biofilm biomass was greater than the sum of the individual mono-species biofilms (data not shown).
3.3 |. Noninvasive tape sampling of skin reveals staphylococcal biofilms on both healthy skin and AD lesional and nonlesional sites
To confirm that staphylococcal biofilms grew on the skin of pediatric AD patients in the MPAACH cohort, we developed a noninvasive tape strip sampling protocol to remove the outer layer of corneocytes and any associated biofilm for imaging by scanning electronic microscopy (SEM). Corneocytes were collected from lesional and/or nonlesional skin sites of 26 MPAACH subjects. Fifty percent (13/26) of MPAACH participants tested were positive for clusters of bacterial cocci with diameter of approximately 0.6 μm adherent to the underside and edges of corneocytes; these clusters of cocci exactly recapitulate the morphology of staphylococcal cells in a biofilm grown in culture from MPAACH staphylococcal isolates or from laboratory strains SERP62a and SA35556 (Figure 2). Healthy adult control tape strips also showed similar clusters (Figure S2). From the 13 positive MPAACH participants, 54% (7/13) of biofilm-positive samples were from lesional sites, 38% (5/13) were from nonlesional sites, and 8% (1/13) had SEM-visible biofilms on samples from both lesional and nonlesional sites. For eight of the 13 patients from whom SEM-visible biofilm samples were observed, S aureus and/or S epidermidis were successfully cultured from contact plates; each of these staphylococcal isolates formed strong mono-species biofilms in the crystal violet assay. There were seven subjects of the 26 sampled from whom no S aureus nor S epidermidis were cultured from the contact plates, although 71% of these patients’ samples (5/7) did show visible biofilms with staphylococcal morphology by SEM.
FIGURE 2.

Noninvasive tape sampling of skin and SEM imaging reveals staphylococcal biofilms. Representative scanning electron microscopy images at increasing levels of magnification of (A) biofilms formed from mixed culture of laboratory strains SA35556 and SERP62a, (B) a biofilm grown from an S epidermidis strain isolated from an MPAACH participant, (C&D) Biofilms on corneocytes isolated using Tegaderm tape strips taken from two individual MPAACH participants. All images were taken at 5.0 kV
3.4 |. Relative biofilm propensity of S aureus strains is associated with increased AD severity and decreased skin barrier function in both lesional and nonlesional AD skin
Since our data established the presence of staphylococcal biofilms on pediatric AD skin, we next sought to investigate the role of S aureus and S epidermidis colonization and biofilm propensity in the pathogenesis of AD. We analyzed the presence of staphylococcal bacteria on subjects’ skin and quantified the total bacterial load. We also determined the mono-species biofilm propensity of MPAACH staphylococcal isolates and grew S aureus and S epidermidis isolates from the same MPAACH subject in mixed culture to investigate antagonistic vs cooperative interactions. We tested the hypotheses that staphylococcal colonization and biofilm propensity are associated with increased AD severity. The presence of culturable S aureus on the subject’s skin was associated with increased AD severity as defined by SCORAD (P = .05) but was not significantly associated with TEWL, as previously described for the MPAACH study55 (Figure 3A). The presence of S epidermidis had no significant associations with either SCORAD or TEWL (Table S1). To assess the strain-specific biofilm propensity, we defined a higher relative S aureus biofilm propensity as those cases in which the maximum biofilm propensity from all S aureus strains sampled per subject was greater than the maximum S epidermidis biofilm propensity from the same subject. Higher relative biofilm propensity of S aureus compared with S epidermidis strains sampled from the same patient was significantly associated with increased SCORAD (P = .03), nonlesional TEWL (P = .03), and lesional TEWL (P = .01) (Figure 3B–D). When comparing cooperative vs antagonistic mixed biofilms (defined as mixed biofilm biomass being greater or less than S epidermidis mono-species biofilm biomass, respectively), no significant associations with SCORAD nor TEWL were observed. Likewise, in the Spearman’s rank correlation test, the mean biofilm propensity (measured by the crystal violet biofilm assay) of neither S aureus, S epidermidis, nor mixed-species biofilms was significantly associated with SCORAD or TEWL. Collectively, these data suggest that while S aureus colonization is associated with increased AD severity, it is the ability of S aureus to form strong biofilms and the balance between biofilm-forming S aureus and S epidermidis strains that are particularly important factors affecting AD severity and barrier dysfunction.
FIGURE 3.
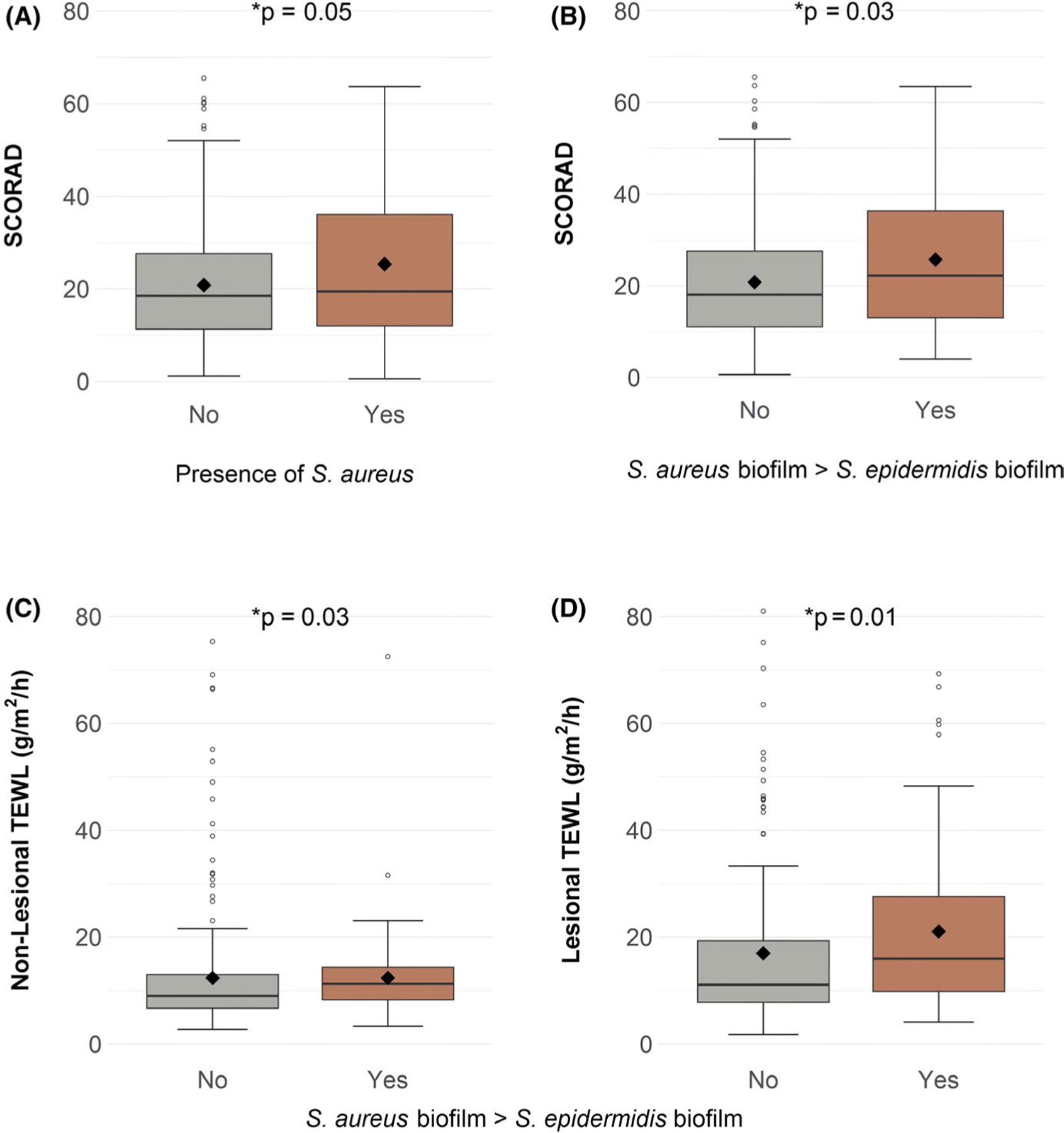
Staphylococcus aureus colonization is associated with increased SCORAD while higher relative S aureus biofilm propensity is associated with increased SCORAD and increased TEWL. (A) S aureus colonization was associated with increased AD severity as measured by SCORAD. Biofilm propensity of S aureus relative to the propensity of S epidermidis from the same MPAACH subject was assessed. If multiple isolates of S aureus or S epidermidis were sampled from the same subject, the isolate with the maximal biofilm propensity was used in the analysis. Higher relative S aureus biofilm propensity was associated with: (B) increased SCORAD, (C) increased nonlesional TEWL, and (D) increased lesional TEWL
3.5 |. Biofilm propensity of S aureus and mixed-species biofilms are associated with decreased FLG expression
Both lesional and nonlesional skin of AD patients exhibit abnormal barrier function, as evidenced by increased TEWL compared with normal healthy skin.6 The most commonly reported gene associated with barrier dysfunction in AD is FLG, which encodes filaggrin, a structural protein that plays an essential role in the formation of the cornified envelope that prevents water loss from the epidermis.3,56,57 In the MPAACH cohort, we observed that both lesional and nonlesional FLG expression levels varied significantly with staphylococcal colonization pattern (Table S1). As reported in a companion study,55 the presence of S aureus was associated with decreased FLG expression levels in nonlesional and lesional skin. Furthermore, we show here that in the Spearman’s rank correlation test, increased mean biofilm propensity of S aureus was associated with decreased nonlesional (P = .02) and lesional (P = .02) FLG expression (Table 2). Likewise, increased mean biofilm propensity of mixed biofilms (grown from co-cultured S aureus and S epidermidis sampled from the same subject) was also correlated with decreased nonlesional FLG expression (P = .01), as was mean biofilm propensity of S epidermidis (P = .02) (Table 2).
TABLE 2.
Spearman’s correlations between mean staphylococcal biofilm propensity and epidermal expression of S100A8, S100A9, and FLG
| Mean S epidermidis biofilm propensity | Mean S aureus biofilm propensity | Mean mixed biofilm propensity | ||||
|---|---|---|---|---|---|---|
| Rho | P-value | Rho | P-value | Rho | P-value | |
| Lesional S100A8 | −0.15 | .02 | −0.01 | .91 | −0.04 | .74 |
| Lesional S100A9 | −0.14 | .04 | 0.00 | .99 | −0.02 | .89 |
| Nonlesional S100A8 | −0.26 | .00006 | −0.09 | .36 | −0.17 | .16 |
| Nonlesional S100A9 | −0.24 | .0003 | −0.03 | .79 | −0.16 | .19 |
| Lesional S100A8/S100A9 | −0.06 | .37 | −0.10 | .34 | −0.12 | .33 |
| Nonlesional S100A8/S100A9 | −0.05 | .44 | −0.25 | .02 | −0.23 | .06 |
| Lesional FLG | −0.13 | .054 | −0.23 | .02 | −0.08 | .53 |
| Nonlesional FLG | −0.16 | .02 | −0.23 | .02 | −0.33 | .01 |
Bold italics are a guide to the eye to highlight significant values (p <0.05).
4 |. Staphylococcus epidermidis biofilm propensity is negatively associated with gene expression of S100A8 and S100A9
We analyzed the expression of alarmins, S100A8 and S100A9, in RNA isolated from AD skin tape strips58 and assessed potential associations with staphylococcal colonization or biofilm propensity, as commensal organisms play a role in the stimulation of alarmin expression. Surprisingly, colonization by S epidermidis per se showed no statistically significant difference in S100A8 or S100A9 expression levels. However, the Spearman’s rank correlation test revealed that the mean S epidermidis biofilm propensity was negatively associated with expression of both S100A8 and S100A9 in lesional (A8, P = .02; A9, P = .04) and nonlesional (A8, P = .00006; A9, P = .0003) skin (Table 2). Staphylococcus aureus colonization and higher relative biofilm propensity of S aureus were both associated with decreased nonlesional S100A8 expression (P = .02 and P = .04, respectively); however, neither was significantly associated with S100A9 expression, so the relevance of S aureus colonization to calprotectin expression is unclear.
5 |. DISCUSSION
In this study, we present the first large-scale analysis of staphylococcal colonization and biofilm propensity of clinical isolates sampled from a cohort of pediatric AD patients, and we directly demonstrate the presence of staphylococcal biofilms on pediatric AD skin. Interestingly, although we did observe a significant association between colonization by S aureus and higher SCORAD levels, there was no significant association between S aureus colonization per se and TEWL measurements. Instead, we found that the relative biofilm propensity of MPAACH staphylococcal isolates is associated with both increased AD severity and skin barrier dysfunction (Figure 3). Specifically, MPAACH subjects whose S aureus biofilm strains had stronger biofilm propensity compared with the S epidermidis strains from the same subject exhibited significantly higher SCORAD values and higher TEWL in both lesional and nonlesional skin (Figure 3). These data strongly suggest a pathogenic role for S aureus biofilms in AD.
Consistent with their important role in AD, we directly observed staphylococcal biofilms around and under corneocytes sampled from the skin of young children with AD. A small number of previous studies observed staphylococcal biofilms on AD skin biopsies.39,41,44 However, these studies primarily focused on biopsies from adults; our analysis is the first demonstration that staphylococcal biofilms are present on AD skin from a number of young pediatric subjects. Our observations are consistent with the proposed role of bacterial biofilms as a near-ubiquitous growth mode under harsh environmental conditions such as the arid, nutrient-poor milieu of the skin.31,32,34,36 Our results agree in part with those of Simpson et al,59 who reported similar levels of S aureus colonization and found that the colonization was associated with increased AD severity; however, they also found that S aureus colonization was associated with increased nonlesional TEWL. In contrast, we observed that higher relative biofilm propensity of S aureus rather than colonization per se was associated with higher TEWL. However, the Simpson et al study enrolled European-American adult AD patients including a large percentage with severe to very severe AD, whereas MPAACH is a pediatric cohort with 61.5% Black representation that present with mild to moderate AD. Our group has recently published a study that suggests that Black subjects display different phenotypes of allergic disease and the atopic march, having an increased risk of developing asthma without preceding AD.60 Our data are also consistent with the report by Di Domenico et al61 that increased biofilm propensity by S aureus isolates was correlated with increased AD lesion severity.
Our study does have a few limitations; although we requested that MPAACH participants discontinue AD treatments 7 days prior and emollients 24 hours before a visit, concurrent treatments could influence our results in terms of detected bacterial colonization. Thirty-five percent of MPAACH subjects are treated with both over-the-counter and prescribed medications. Furthermore, our current study has focused on culture-based techniques, which allowed us to assess the biofilm propensity of clinical strains but may have undersampled the full range of bacterial strains present on the skin, particularly those that form strongly cohesive biofilms and are not efficiently sampled by contact plates. Future studies using shotgun metagenomic sequencing and whole-genome functional analyses could further assess the roles of S aureus, S epidermidis, and other potentially important species within the skin microbiome of MPAACH subjects.
In AD, mutations in FLG or in genes encoding proteins involved in the normal processing of filaggrin can lead to altered ultrastructure of the epidermis. Furthermore, FLG expression by keratinocytes is reduced in AD lesional skin, likely due to inflammatory cytokines including IL-4, IL-13, and IL-31.62,63 Colonization by S aureus is associated with decreased FLG expression in both nonlesional and lesional skin,55 similar to the report by Clausen et al64 that increased S aureus colonization was observed in AD patients with FLG mutations. These findings are consistent with results from a tissue culture model of human epidermis, in which knockdown of FLG expression led to increased colonization by S aureus.63 Thus, in children with AD, relative deficiency in FLG expression in nonlesional skin likely precedes colonization by S aureus strains that may be more pathogenic, that is, posess higher biofilm propensity. Furthermore, breakdown products of filaggrin found in healthy skin are reduced in AD patients with food allergy65; these products have been shown to inhibit growth of S aureus in vitro66 and in vivo.65 Taken together, these data further support the idea that deficiencies in the skin barrier due to mutations in FLG or lower expression of FLG predispose these individuals to colonization by S aureus. Interestingly, we also observed that decreased FLG expression in both nonlesional and lesional skin was associated with S aureus strains that formed more extensive mono-species and mixed-species biofilms, raising the possibility that cooperative interactions with some S epidermidis strains may promote S aureus biofilm growth and persistent colonization in the context of decreased FLG expression.
Our data suggest that perturbations in skin barrier function arising from genetic (eg FLG mutations), environmental, or other factors predispose infants to colonization by S aureus, which then drives increased severity of AD lesions and further degradation of barrier function (Figure 4).
FIGURE 4.
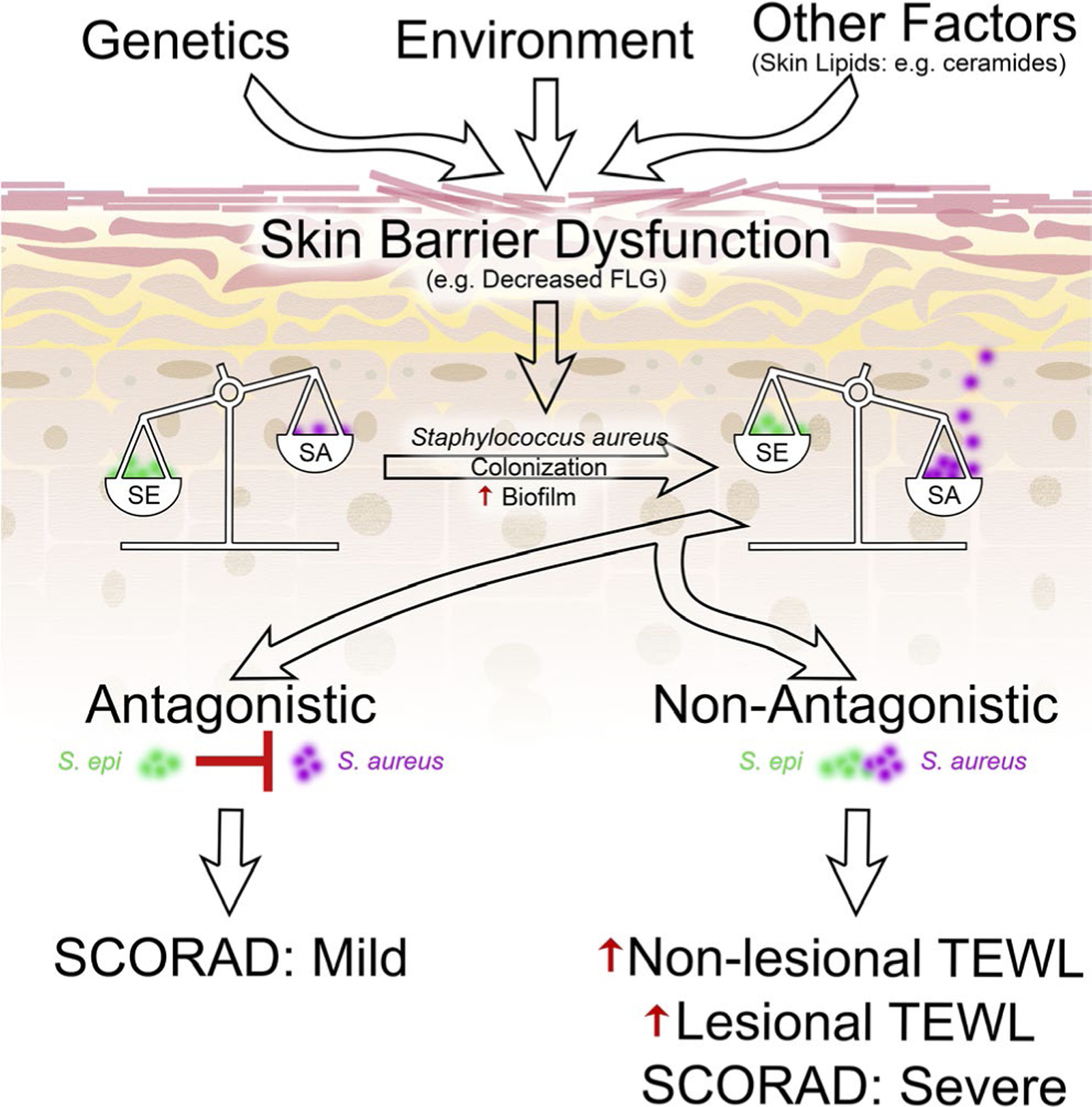
Proposed model relating skin barrier dysfunction, staphylococcal colonization and biofilm formation, and clinical outcomes in AD
The premise that dysregulation in the skin barrier function is upstream of microbial dysbiosis is consistent with the observation that infants that developed AD by age 1 were significantly more likely to show elevated TEWL as early as 2 days postbirth.67 Meylan et al found that infants that went on to develop AD exhibited a significant increase in the prevalence of S aureus colonization by 3 months after birth, which preceded the development of AD symptoms by 2 months on average.68 Likewise, a mouse model of AD using tissue-specific ADAM17 knockout revealed the initial development of eczematous inflammatory lesions followed by dysbiosis, with sequential steps of predominant colonization by Corynebacterium mastitidis, S aureus, and Corynebacterium bovis.69 Introduction of antibiotics specific for these microbial species could reverse inflammatory symptoms.69 Colonization by S aureus is favored by lower expression levels of filaggrin63 and the resulting reduction in normal filaggrin breakdown products.65,66 We propose that these factors favoring S aureus colonization will disrupt the balance between S aureus and protective commensal microbes including certain S epidermidis strains. Colonization of the skin with commensal staphylococcal species in infants has been shown to be associated with decreased rate of developing AD by 1 year of age.70 However, it is becoming clear that protective effects from S epidermidis vary in a strain-specific manner.
Several studies have reported that S epidermidis and other coagulase-negative staphylococcal species can directly inhibit S aureus growth or biofilm formation.9,11,25,26 In contrast, it was recently reported that peptidoglycans from S epidermidis and other commensals can actually potentiate S aureus virulence.71 In this study, we analyzed not only colonization by S epidermidis and S aureus, but also the biofilm propensity of staphylococcal isolates. We found that when matched pairs of S epidermidis and S aureus strains from the same subject were co-cultured, the combinations produced synergistic or cooperative biofilm growth in mixed culture for 68% of the co-colonized MPAACH subjects, whereas 32% showed strong antagonism by S epidermidis. In 13% of subjects, their S epidermidis acted synergistically with S aureus, forming mixed-species biofilms that that exceeded the sum of the two mono-species biofilms from the same isolates. These results demonstrate that S epidermidis can either inhibit, coexist with, or even potentiate S aureus biofilms. This highlights the importance of tracking strain-level variation in S epidermidis, consistent with the report by Nakatsuji et al11 that antimicrobial peptide-expressing, coagulase-negative staphylococcal strains that inhibited S aureus were common on healthy skin but rare on AD skin.
Furthermore, S epidermidis can also have context-dependent effects on immune responses and other microbes that may depend on skin barrier integrity or local inflammation.72,73 One interesting example from this study is that the growth mode of S epidermidis (biofilm vs planktonic) appears to differentially influence immune responses in the skin. Naik et al10 demonstrated using a mouse model system that S epidermidis can induce commensal-specific T cells that home to the epidermis and trigger secretion of calprotectin (S100A8/S100A9) by keratinocytes in an IL-17A-dependent manner. However, we found that S epidermidis biofilm propensity was negatively associated with S100A8 and S100A9 expression levels in both nonlesional and lesional skin (Table 2), suggesting that S epidermidis growing as a biofilm is unable to be taken up by skin-resident dendritic cells for presentation to commensal-specific T cells. This is plausible, given that staphylococcal biofilms inhibit phagocytosis by macrophages.74,75 This reduction in expression of S100A8 and S100A9 could lead to a feedback loop that further promotes colonization by biofilm-forming S aureus strains and growth of mixed biofilms by S aureus and S epidermidis, since calprotectin sequesters both Mn2+ (which is required for staphylococcal growth27,29,30) and Zn2+ (which is required for intercellular adhesion in S epidermidis and S aureus biofilms76–81).
Biofilms are highly adhesive and cohesive and will promote long-term persistence of staphylococcal colonization on the epidermis.31–34,61 Although staphylococci growing in the biofilm mode generally express lower levels of proteases or toxins compared with planktonic cells, S aureus and S epidermidis biofilms utilize the accessory gene regulator (Agr) quorum-sensing machinery to undergo periodic cycles of biofilm remodeling and release of planktonic bacteria that can secrete increased levels of toxins and proteases.82,83 Among these are S aureus δ-toxin that activates mast cells in allergic skin disease19; S aureus exotoxins that act as superantigens in AD84; and a number of S aureus proteases that can cleave desmoglein-1, an important skin structural protein.85–89 Thus, S aureus biofilms on AD skin will provide a persistent reservoir of staphylococcal cells in the epidermis. This may be especially pertinent in children with AD as it provides a recurring source of proteases and toxins that will trigger inflammation and further degrade skin barrier function.
Taken together, the results presented herein suggest that the relative balance of biofilm-forming S aureus and S epidermidis strains is implicated in both severity of AD and barrier function in pediatric patients, and that S epidermidis plays a mixed role depending on strain-level variation in antagonism or cooperation with S aureus strains in co-colonized patients. This highlights the complexity of the interactions between species in the commensal skin biome and supports a recent, more nuanced view of S epidermidis, in which this nearly ubiquitous commensal plays multiple roles in skin immune responses and can either inhibit or coexist with S aureus, depending on strain-level variability. Therapeutic approaches that specifically target biofilm formation by S aureus or that shift the balance from cooperative to antagonistic (ie protective) strains of S epidermidis could help to mitigate the inflammation and barrier dysfunction characteristic of severe AD.
Supplementary Material
ACKNOWLEDGMENTS
We thank all the children and their families who participated in the MPAACH cohort and in this study. We thank Angela Sadler for administrative assistance. This work was supported by National Institutes of Health grant U19 AI070235 (GKH, JBM, LJM, and ABH), the corresponding Infrastructure and Opportunity Fund U19 AI070235-140323 (JBM), T32 GM063483-17 (TG), and the Center for Pediatric Genomics at Cincinnati Children’s Hospital Medical Center (ABH). The project was also supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1 TR001425.
Funding information
National Center for Advancing Translational Sciences, Grant/Award Number: UL1 TR001425; National Institute of Allergy and Infectious Diseases, Grant/Award Number: U19 AI070235 and U19 AI070235-140323; Cincinnati Children’s Hospital Medical Center, Grant/Award Number: Center for Pediatric Genomics Pilot Grant; National Institute of General Medical Sciences, Grant/Award Number: T32 GM063483
CONFLICTS OF INTEREST
T Gonzalez, Dr Stevens, A Baatrebek kyzy, R. Alarcon, H He, JW Kroner, D Spagna, B Grashel, and E Sidler have nothing to disclose. Dr Martin reports grants from National Institutes of Health, during the conduct of the study. Dr Biagini Myers reports grants from National Institutes of Health, during the conduct of the study; in addition, Dr Biagini Myers has a patent “Noninvasive methods for skin sample collection and analysis” pending. Dr Khurana Hershey reports grants from National Institutes of Health, during the conduct of the study; equity ownership in Hoth Therapeutics, outside the submitted work; in addition, Dr Khurana Hershey has a patent “NonInvasive Methods for Skin Sample Collection and Analysis” pending to Cincinnati Children’s Hospital Medical Center; Dr Khurana Hershey serves on the Scientific Advisory Board for Hoth Therapeutics, Inc. Dr Herr reports grants from National Institutes of Health, during the conduct of the study; equity ownership in Chelexa BioSciences, equity ownership and personal fees from Hoth Therapeutics, outside the submitted work; In addition, Dr Herr has a patent “Use of Zinc Chelators Comprising DTPA to Inhibit Biofilm Formation” licensed to Chelexa BioSciences and sub-licensed to Hoth Therapeutics, and a patent “Antimicrobial Compositions Of Aminoglycosidic Antibiotics And Zinc Ion Chelators Specifically Formulated For Enhanced Inhibition Of Bacterial Colonization And Antibacterial Efficacy” licensed to Chelexa BioSciences and sub-licensed to Hoth Therapeutics; Dr Herr serves on the Scientific Advisory Board for Hoth Therapeutics, Inc.
Abbreviations:
- AD
atopic dermatitis
- FLG
filaggrin
- MPAACH
mechanisms of progression of atopic dermatitis to asthma in children
- S100A8
S100 calcium-binding protein A8
- S100A9
calcium-binding protein A9
- SCORAD
scoring atopic dermatitis index
- TEWL
transepidermal water loss
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Bieber T Atopic dermatitis. N Engl J Med. 2008;358:1483–1494. [DOI] [PubMed] [Google Scholar]
- 2.Bantz SK, Zhu Z, Zheng T. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. J Clin Cell Immunol. 2014;5:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal R, Woodfolk JA. Skin barrier defects in atopic dermatitis. Curr Allergy Asthma Rep. 2014;14:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawasaki H, Nagao K, Kubo A, et al. Altered stratum corneum barrier and enhanced percutaneous immune responses in filaggrin-null mice. J Allergy Clin Immunol. 2012;129(6):1538. [DOI] [PubMed] [Google Scholar]
- 5.Oyoshi MK, Murphy GF, Geha RS. Filaggrin-deficient mice exhibit TH17-dominated skin inflammation and permissiveness to epicutaneous sensitization with protein antigen. J Allergy Clin Immunol. 2009;124(3):485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta J, Grube E, Ericksen MB, et al. Intrinsically defective skin barrier function in children with atopic dermatitis correlates with disease severity. J Allergy Clin Immunol. 2008;121(3):725. [DOI] [PubMed] [Google Scholar]
- 7.Belkaid Y, Tamoutounour S. The influence of skin microorganisms on cutaneous immunity. Nat Rev Immunol. 2016;16:353–366. [DOI] [PubMed] [Google Scholar]
- 8.Belkaid Y, Segre JA. Dialogue between skin microbiota and immunity. Science. 2014;346:954–959. [DOI] [PubMed] [Google Scholar]
- 9.Iwase T, Uehara Y, Shinji H, et al. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature. 2010;465:346–349. [DOI] [PubMed] [Google Scholar]
- 10.Naik S, Bouladoux N, Linehan JL, et al. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature. 2015;520:104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakatsuji T, Chen TH, Narala S, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med. 2017;9(378):eaah4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olson ME, Todd DA, Schaeffer CR, et al. Staphylococcus epidermidis agr quorum-sensing system: signal identification, cross talk, and importance in colonization. J Bacteriol. 2014;196:3482–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otto M, Echner H, Voelter W, Gotz F. Pheromone cross-inhibition between Staphylococcus aureus and Staphylococcus epidermidis. Infect Immun. 2001;69:1957–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leung DY. Infection in atopic dermatitis. Curr Opin Pediatr. 2003;15:399–404. [DOI] [PubMed] [Google Scholar]
- 15.Lin YT, Wang CT, Chiang BL. Role of bacterial pathogens in atopic dermatitis. Clin Rev Allergy Immunol. 2007;33:167–177. [DOI] [PubMed] [Google Scholar]
- 16.Kong HH, Oh J, Deming C, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22:850–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altunbulakli C, Reiger M, Neumann AU, et al. Relations between epidermal barrier dysregulation and Staphylococcus species-dominated microbiome dysbiosis in patients with atopic dermatitis. J Allergy Clin Immunol. 2018;142(5):1643. [DOI] [PubMed] [Google Scholar]
- 18.Brauweiler AM, Goleva E, Leung DYM. Th2 cytokines increase Staphylococcus aureus alpha toxin-induced keratinocyte death through the signal transducer and activator of transcription 6 (STAT6). J Invest Dermatol. 2014;134:2114–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura Y, Oscherwitz J, Cease KB, et al. Staphylococcus delta-toxin induces allergic skin disease by activating mast cells. Nature. 2013;503:397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park KD, Pak SC, Park KK. The pathogenetic effect of natural and bacterial toxins on atopic dermatitis. Toxins (Basel). 2016;9(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wichmann K, Uter W, Weiss J, et al. Isolation of alpha-toxin-producing Staphylococcus aureus from the skin of highly sensitized adult patients with severe atopic dermatitis. Br J Dermatol. 2009;161:300–305. [DOI] [PubMed] [Google Scholar]
- 22.de Veer SJ, Furio L, Harris JM, Hovnanian A. Proteases: common culprits in human skin disorders. Trends Mol Med. 2014;20:166–178. [DOI] [PubMed] [Google Scholar]
- 23.Williams MR, Nakatsuji T, Sanford JA, Vrbanac AF, Gallo RL. Staphylococcus aureus induces increased serine protease activity in keratinocytes. J Invest Dermatol. 2017;137:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Syed AK, Reed TJ, Clark KL, Boles BR, Kahlenberg JM. Staphlyococcus aureus phenol-soluble modulins stimulate the release of proinflammatory cytokines from keratinocytes and are required for induction of skin inflammation. Infect Immun. 2015;83:3428–3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cogen AL, Yamasaki K, Sanchez KM, et al. Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J Invest Dermatol. 2010;130:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paharik AE, Parlet CP, Chung N, et al. Coagulase-negative staphylococcal strain prevents staphylococcus aureus colonization and skin infection by blocking quorum sensing. Cell Host Microbe. 2017;22(6):746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damo SM, Kehl-Fie TE, Sugitani N, et al. Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proc Natl Acad Sci USA. 2013;110:3841–3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark HL, Jhingran A, Sun Y, et al. Zinc and manganese chelation by neutrophil S100A8/A9 (Calprotectin) limits extracellular Aspergillus fumigatus hyphal growth and corneal infection. J Immunol. 2016;196:336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corbin BD, Seeley EH, Raab A, et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science. 2008;319:962–965. [DOI] [PubMed] [Google Scholar]
- 30.Zackular JP, Chazin WJ, Skaar EP. Nutritional immunity: S100 proteins at the host-pathogen interface. J Biol Chem. 2015;290:18991–18998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. [DOI] [PubMed] [Google Scholar]
- 32.Costerton W, Veeh R, Shirtliff M, Pasmore M, Post C, Ehrlich G. The application of biofilm science to the study and control of chronic bacterial infections. J Clin Invest. 2003;112:1466–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. [DOI] [PubMed] [Google Scholar]
- 35.Patel R Biofilms and antimicrobial resistance. Clin Orthop Relat Res. 2005;437:41–47. [DOI] [PubMed] [Google Scholar]
- 36.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–138. [DOI] [PubMed] [Google Scholar]
- 37.Vuong C, Voyich JM, Fischer ER, et al. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiol. 2004;6:269–275. [DOI] [PubMed] [Google Scholar]
- 38.Scherr TD, Heim CE, Morrison JM, Kielian T. Hiding in plain sight: interplay between Staphylococcal biofilms and host immunity. Front Immunol. 2014;5:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akiyama H, Hamada T, Huh WK, et al. Confocal laser scanning microscopic observation of glycocalyx production by Staphylococcus aureus in skin lesions of bullous impetigo, atopic dermatitis and pemphigus foliaceus. Br J Dermatol. 2003;148:526–532. [DOI] [PubMed] [Google Scholar]
- 40.Allen HB, Mueller JL. A novel finding in atopic dermatitis: film-producing Staphylococcus epidermidis as an etiology. Int J Dermatol. 2011;50:992–993. [DOI] [PubMed] [Google Scholar]
- 41.Allen HB, Vaze ND, Choi C, et al. The presence and impact of biofilm-producing staphylococci in atopic dermatitis. JAMA Dermatol. 2014;150:260–265. [DOI] [PubMed] [Google Scholar]
- 42.Katsuyama M, Ichikawa H, Ogawa S, Ikezawa Z. A novel method to control the balance of skin microflora. Part 1. Attack on biofilm of Staphylococcus aureus without antibiotics. J Dermatol Sci. 2005;38:197–205. [DOI] [PubMed] [Google Scholar]
- 43.Katsuyama M, Kobayashi Y, Ichikawa H, et al. A novel method to control the balance of skin microflora Part 2. A study to assess the effect of a cream containing farnesol and xylitol on atopic dry skin. J Dermatol Sci. 2005;38:207–213. [DOI] [PubMed] [Google Scholar]
- 44.Sonesson A, Przybyszewska K, Eriksson S, et al. Identification of bacterial biofilm and the Staphylococcus aureus derived protease, staphopain, on the skin surface of patients with atopic dermatitis. Sci Rep. 2017;7:8689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akiyama H, Tada J, Toi J, Kanzaki H, Arata J. Changes in Staphylococcus aureus density and lesion severity after topical application of povidone-iodine in cases of atopic dermatitis. J Dermatol Sci. 1997;16:23–30. [DOI] [PubMed] [Google Scholar]
- 46.European Task Force on Atopic Dermatitis. Severity scoring of atopic dermatitis: the SCORAD index. consensus report of the European task force on atopic dermatitis. Dermatology. 1993;186:23–31. [DOI] [PubMed] [Google Scholar]
- 47.von Kobyletzki LB, Berner A, Carlstedt F, Hasselgren M, Bornehag CG, Svensson A. Validation of a parental questionnaire to identify atopic dermatitis in a population-based sample of children up to 2 years of age. Dermatology. 2013;226:222–226. [DOI] [PubMed] [Google Scholar]
- 48.Kunz B, Oranje AP, Labreze L, Stalder JF, Ring J, Taieb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European task force on atopic dermatitis. Dermatology. 1997;195:10–19. [DOI] [PubMed] [Google Scholar]
- 49.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berardesca E, Fideli D, Borroni G, Rabbiosi G, Maibach H. In vivo hydration and water-retention capacity of stratum corneum in clinically uninvolved skin in atopic and psoriatic patients. Acta Derm Venereol. 1990;70:400–404. [PubMed] [Google Scholar]
- 51.Choi SJ, Song MG, Sung WT, et al. Comparison of transepidermal water loss, capacitance and pH values in the skin between intrinsic and extrinsic atopic dermatitis patients. J Korean Med Sci. 2003;18:93–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suehiro M, Hirano S, Ikenaga K, Katoh N, Yasuno H, Kishimoto S. Characteristics of skin surface morphology and transepidermal water loss in clinically normal-appearing skin of patients with atopic dermatitis: a video-microscopy study. J Dermatol. 2004;31:78–85. [DOI] [PubMed] [Google Scholar]
- 53.Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods. 2000;40:175–179. [DOI] [PubMed] [Google Scholar]
- 54.Christensen GD, Simpson WA, Younger JJ, et al. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985;22:996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biagini Myers JM, Sherenian MG, Baatyrbek Kyzy A, et al. Events in normal skin promote early-life atopic dermatitis - the MPAACH Cohort. J Allergy Clin Immunol Pract. 2020;8(7):2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gonzalez T, Biagini Myers JM, Herr AB, Khurana Hershey GK. Staphylococcal biofilms in atopic dermatitis. Curr Allergy Asthma Rep. 2017;17:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ong PY, Leung DY. Bacterial and viral infections in atopic dermatitis: a comprehensive review. Clin Rev Allergy Immunol. 2016;51:329–337. [DOI] [PubMed] [Google Scholar]
- 58.Stevens ML, Gonzalez T, Schauberger E, et al. Simultaneous skin biome and keratinocyte genomic capture reveals microbiome differences by depth of sampling. J Allergy Clin Immunol. 2020. 10.1016/j.jaci.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simpson EL, Villarreal M, Jepson B, et al. Patients with atopic dermatitis colonized with Staphylococcus aureus have a distinct phenotype and endotype. J Invest Dermatol. 2018;138:2224–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johansson E, Biagini Myers JM, Martin LJ, et al. Identification of two early life eczema and non-eczema phenotypes with high risk for asthma development. Clin Exp Allergy. 2019;49:829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Di Domenico EG, Cavallo I, Bordignon V, et al. Inflammatory cytokines and biofilm production sustain Staphylococcus aureus outgrowth and persistence: a pivotal interplay in the pathogenesis of Atopic Dermatitis. Sci Rep. 2018;8:9573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Howell MD, Kim BE, Gao P, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2007;120:150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Drongelen V, Haisma EM, Out-Luiting JJ, Nibbering PH, El Ghalbzouri A. Reduced filaggrin expression is accompanied by increased Staphylococcus aureus colonization of epidermal skin models. Clin Exp Allergy. 2014;44:1515–1524. [DOI] [PubMed] [Google Scholar]
- 64.Clausen ML, Edslev SM, Andersen PS, Clemmensen K, Krogfelt KA, Agner T. Staphylococcus aureus colonization in atopic eczema and its association with filaggrin gene mutations. Br J Dermatol. 2017;177:1394–1400. [DOI] [PubMed] [Google Scholar]
- 65.Leung DYM, Calatroni A, Zaramela LS, et al. The nonlesional skin surface distinguishes atopic dermatitis with food allergy as a unique endotype. Sci Transl Med. 2019;11(480):eaav2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miajlovic H, Fallon PG, Irvine AD, Foster TJ. Effect of filaggrin breakdown products on growth of and protein expression by Staphylococcus aureus. J Allergy Clin Immunol. 2010;126(6):1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kelleher M, Dunn-Galvin A, Hourihane JO, et al. Skin barrier dysfunction measured by transepidermal water loss at 2 days and 2 months predates and predicts atopic dermatitis at 1 year. J Allergy Clin Immunol. 2015;135(4):930. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Meylan P, Lang C, Mermoud S, et al. Skin colonization by Staphylococcus aureus precedes the clinical diagnosis of atopic dermatitis in infancy. J Invest Dermatol. 2017;137:2497–2504. [DOI] [PubMed] [Google Scholar]
- 69.Kobayashi T, Glatz M, Horiuchi K, et al. Dysbiosis and Staphylococcus aureus colonization drives inflammation in atopic dermatitis. Immunity. 2015;42:756–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kennedy EA, Connolly J, Hourihane JO, et al. Skin microbiome before development of atopic dermatitis: early colonization with commensal staphylococci at 2 months is associated with a lower risk of atopic dermatitis at 1 year. J Allergy Clin Immunol. 2017;139:166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boldock E, Surewaard BGJ, Shamarina D, et al. Human skin commensals augment Staphylococcus aureus pathogenesis. Nat Microbiol. 2018;3:881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stacy A, Belkaid Y. Microbial guardians of skin health. Science. 2019;363:227–228. [DOI] [PubMed] [Google Scholar]
- 73.Harrison OJ, Linehan JL, Shih HY, et al. Commensal-specific T cell plasticity promotes rapid tissue adaptation to injury. Science. 2019;363(6422):eaat6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scherr TD, Hanke ML, Huang O, et al. Staphylococcus aureus biofilms induce macrophage dysfunction through leukocidin AB and alpha-toxin. MBio 2015;6:e01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thurlow LR, Hanke ML, Fritz T, et al. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J Immunol. 2011;186:6585–6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chaton CT, Herr AB. Defining the metal specificity of a multifunctional biofilm adhesion protein. Protein Sci. 2017;26(10):1964–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Conrady DG, Brescia CC, Horii K, Weiss AA, Hassett DJ, Herr AB. A zinc-dependent adhesion module is responsible for intercellular adhesion in staphylococcal biofilms. Proc Natl Acad Sci USA. 2008;105:19456–19461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Conrady DG, Wilson JJ, Herr AB. Structural basis for Zn2+-dependent intercellular adhesion in staphylococcal biofilms. Proc Natl Acad Sci USA. 2013;110:E202–E211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Formosa-Dague C, Speziale P, Foster TJ, Geoghegan JA, Dufrene YF. Zinc-dependent mechanical properties of Staphylococcus aureus biofilm-forming surface protein SasG. Proc Natl Acad Sci USA. 2016;113:410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Geoghegan JA, Corrigan RM, Gruszka DT, et al. Role of surface protein SasG in biofilm formation by Staphylococcus aureus. J Bacteriol. 2010;192:5663–5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shelton CL, Conrady DG, Herr AB. Functional consequences of B-repeat sequence variation in the staphylococcal biofilm protein Aap: deciphering the assembly code. Biochem J. 2017;474:427–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boles BR, Horswill AR. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 2008;4:e1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Periasamy S, Joo HS, Duong AC, et al. How Staphylococcus aureus biofilms develop their characteristic structure. Proc Natl Acad Sci USA. 2012;109:1281–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leung DY, Walsh P, Giorno R, Norris DA. A potential role for superantigens in the pathogenesis of psoriasis. J Invest Dermatol. 1993;100:225–228. [DOI] [PubMed] [Google Scholar]
- 85.Amagai M, Matsuyoshi N, Wang ZH, Andl C, Stanley JR. Toxin in bullous impetigo and staphylococcal scalded-skin syndrome targets desmoglein 1. Nat Med. 2000;6:1275–1277. [DOI] [PubMed] [Google Scholar]
- 86.Amagai M, Yamaguchi T, Hanakawa Y, Nishifuji K, Sugai M, Stanley JR. Staphylococcal exfoliative toxin B specifically cleaves desmoglein 1. J Invest Dermatol. 2002;118:845–850. [DOI] [PubMed] [Google Scholar]
- 87.Hanakawa Y, Schechter NM, Lin C, Nishifuji K, Amagai M, Stanley JR. Enzymatic and molecular characteristics of the efficiency and specificity of exfoliative toxin cleavage of desmoglein 1. J Biol Chem. 2004;279:5268–5277. [DOI] [PubMed] [Google Scholar]
- 88.Williams MR, Gallo RL. The role of the skin microbiome in atopic dermatitis. Curr Allergy Asthma Rep. 2015;15:65. [DOI] [PubMed] [Google Scholar]
- 89.Miedzobrodzki J, Kaszycki P, Bialecka A, Kasprowicz A. Proteolytic activity of Staphylococcus aureus strains isolated from the colonized skin of patients with acute-phase atopic dermatitis. Eur J Clin Microbiol Infect Dis. 2002;21:269–276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


