Abstract
Objective:
Loss of control over eating (LOC) is common among women, particularly those with overweight and obesity (OV/OB), and predicts weight gain. Given the importance of understanding weight and eating behaviors during pregnancy, we sought to characterize LOC across pregnancy and the postpartum period among women with pre-pregnancy OV/OB.
Methods:
Pregnant women (N = 257; 28.44 ± 5.48 years old) with self-reported OV/OB prior to pregnancy were interviewed using a pregnancy-adapted version of the Eating Disorder Examination (EDE-PV). Pre-pregnancy LOC was retrospectively assessed during the first trimester and then prospectively assessed monthly throughout pregnancy and postpartum over the course of seven assessments.
Results:
Rates of LOC were significantly higher during pregnancy compared to prior to (, p < .01) and after (, p < .01) pregnancy, with 37% (n = 95) of women reporting ≥1 LOC episode during pregnancy. LOC during pregnancy was associated with higher likelihood of LOC postpartum. Higher age (OR = 1.084, p = .04) and identifying as a minority (OR = 0.344, p = .02) was associated with greater likelihood of experiencing LOC during pregnancy only.
Discussion:
LOC during pregnancy is common among women with pre-pregnancy OV/OB, suggesting that screening and intervention for LOC during pregnancy may be warranted. Future research is necessary to examine the relationship between LOC during pregnancy and maternal and infant health outcomes.
Keywords: aberrant eating, longitudinal, loss of control eating, obesity, overweight, postpartum, pregnancy
1 ∣. INTRODUCTION
Loss of control over eating (LOC), the perception of being unable to control the amount or type of food consumed during an eating episode, is one of the most frequently reported disordered eating behaviors. Episodes of LOC are especially prevalent among women (Striegel-Moore et al., 2009) and associated with weight gain and obesity (Goldschmidt, 2017; Sonneville et al., 2013) and psychological distress (Goldschmidt, 2017; Sonneville et al., 2013), independently of the amount of food consumed (Goldschmidt et al., 2012; Meany, Conceição, & Mitchell, 2014; Tanofsky-Kraff et al., 2009). Thus, LOC is often considered the fundamental pathology of binge eating (Colles, Dixon, & O'Brien, 2008), including in non-eating disordered samples (Latner, Hildebrandt, Rosewall, Chisholm, & Hayashi, 2007). Maternal eating behaviors and weight are also important predictors of pregnancy outcomes. For instance, women who begin pregnancy with overweight or obesity (body mass index [BMI] ≥ 25 kg/m2) are at higher risk of developing gestational diabetes and pre-eclampsia (Athukorala, Rumbold, Willson, & Crowther, 2010; Doherty, Magann, Francis, Morrison, & Newnham, 2006) and are more likely to experience preterm birth or delivery via emergency cesarean section (Athukorala et al., 2010). Longitudinal evidence similarly suggests that perinatal health outcomes are compromised among women with a history of an eating disorder (Linna et al., 2014). Binge eating disorder in particular has been related to preeclampsia, higher birth weight and increased likelihood of cesarean section (Bulik et al., 2009; Linna et al., 2014). Maternal eating behaviors during pregnancy have also been linked to childhood adiposity and appetite, and may therefore impact child health and development (Brion et al., 2010; Chen et al., 2017). Despite the clear importance of maternal eating and weight patterns for maternal and infant health, there is limited longitudinal research examining the relationship between aberrant eating behaviors like LOC and perinatal health outcomes.
Prior studies of eating psychopathology in pregnancy have demonstrated that episodes of LOC are common in the general population, with estimates suggesting that up to 36% of women experience LOC during pregnancy (Micali, Al Essimii, Field, & Treasure, 2018). Similarly, in a sample of women with pre-pregnancy overweight or obesity, we previously demonstrated that 28% report having at least one episode of LOC early in pregnancy (Kolko, Emery, Marcus, & Levine, 2017). However, important questions about whether and how LOC changes over the peripartum period remain, particularly among women at risk for poor obstetric outcomes. For instance, prior work has been limited by the use of single-item self-report assessments of LOC administered at one time point in pregnancy (Micali et al., 2018), raising questions about reliability of LOC measurement and precluding examination of longitudinal change in LOC over the course of pregnancy and postpartum. Further, Kolko et al. (2017) is the only study to date to focus on women with overweight and obesity, a population at elevated risk for eating and weight-related perinatal health complications. However, because longitudinal data from mid- and late-pregnancy and the postpartum period had not yet been collected at the time of publication, Kolko et al. (2017) were unable to examine whether and how rates of LOC change over the course of pregnancy and postpartum in this vulnerable population. Accordingly, we sought to extend our initial cross-sectional findings described in Kolko et al. (2017) by documenting rates of LOC from 3 months prior to pregnancy through 6 months postpartum in the same sample of women with overweight or obesity using a standardized eating disorders interview assessment.
2 ∣. METHOD
2.0.1 ∣. Participants
Pregnant women (N = 257) were recruited from local obstetrics clinics to participate in a longitudinal study of eating and weight patterns during the peripartum period. Eligibility criteria included having overweight or obesity prior to becoming pregnant, having a singleton pregnancy, and being ≥14 years of age at enrollment. Women were excluded if they were diagnosed with type I diabetes, taking medications or diagnosed with conditions known to influence weight, participating in a weight management program, or experiencing acute psychiatric symptoms warranting immediate intervention (e.g., suicidality). Participants 18 years and older provided written informed consent before the initiation of study procedures. Verbal assent was obtained from participants below age 18 (n = 4) and written informed consent was provided by a parent or legal guardian. Procedures were approved by the University of Pittsburgh Institutional Review Board.
2.0.2 ∣. Procedures
Eligible women attended up to seven visits over the course of the peripartum period to complete assessments of demographic, medical, and psychosocial factors and undergo structured clinical interviews evaluating psychiatric symptoms and eating patterns. The initial baseline assessment occurred when women's pregnancies were 12–20 weeks gestation (“Time 0” or T0; n = 257). At T0, participants reported current symptomatology as well as symptoms occurring in the 3 months prior to pregnancy. Subsequent assessments occurred at 18–22 weeks gestation (T1; n = 253), 23–26 weeks gestation (T2: n = 252), 27–30 weeks gestation (T3; n = 245), 31–34 weeks gestation (T4; n = 240), 35 weeks gestation through delivery (T5; n = 206), and 6 months postpartum (T6; n = 237). Data collection began in September 2012 and was completed in January 2017.
2.1 ∣. Assessments
2.1.1 ∣. Demographic and weight information
Women self-reported age, race, marital status, educational background, household income, parity, smoking status, and pre-pregnancy height and weight for estimation of pre-pregnancy BMI. To calculate BMI during pregnancy and postpartum, weight and height were objectively measured using a digital scale and a calibrated stadiometer during the T0 and T6 assessments.
2.1.2 ∣. Loss of control over eating
The pregnancy version of the Eating Disorders Examination (EDE-PV; Emery, Grace, Kolko, & Levine, 2017) was used to assess the frequency of LOC occurring prior to pregnancy, during pregnancy, and at 6 months postpartum. The EDE is a semi-structured interview that is widely used to evaluate features of disordered eating and has been shown to exhibit good reliability and validity (Fairburn, 2008). The EDE-PV has been modified to assess disordered eating cognitions and behaviors during pregnancy by accounting for normative changes across the perinatal period that may otherwise be considered pathological (Emery et al., 2017). The full EDE-PV interview was administered at T0 and at T6, while evaluations conducted at T1-T5 focused only on the Overeating section of the EDE-PV. The Overeating section includes modules to assess objective binge eating episodes (OBEs) and subjective binge eating episodes (SBEs), as well as loss of control eating occurring outside of a binge episode. At each timepoint, LOC was defined as the presence of OBEs or SBEs, or loss of control eating occurring outside of a binge episode and categorized as being present (≥1 episode) or absent (no episode) during the reporting period. We also recorded the frequency of LOC episodes at each timepoint. At baseline, interrater reliability for LOC episodes was high (intraclass correlation coefficient = 0.89; (Kolko et al., 2017). Finally, based on responses to the full EDE-PV, we documented rates of Bulimia Nervosa (BN) and Binge Eating Disorder (BED) at T0 and T6 according to established criteria (American Psychiatric Association, 2013).
2.1.3 ∣. Statistical approach
Two binary logistic regression models were constructed to determine whether baseline demographic factors (age, education, income, and race) or pre-pregnancy BMI were associated with the probability of experiencing LOC (a) prior to becoming pregnant or (b) at any time across the peripartum period. We then created a nominal response variable with three different LOC patterns (LOC during pregnancy only [i.e., at any time during T0–T5]; No LOC; Other pattern of LOC) to distinguish women who experienced LOC only during pregnancy from those who reported no LOC or LOC at any other timepoint or at multiple timepoints. A multinomial logistic regression was performed to examine whether baseline characteristics related to the probability of women experiencing one of these three LOC patterns. Finally, a McNemar’s Chi Square test was conducted comparing rates of LOC during pre-pregnancy, pregnancy, and postpartum epochs to determine how the prevalence of LOC changed across the peripartum period.
3 ∣. RESULTS
3.0.1 ∣. Participant characteristics
Women completed their initial baseline pregnancy assessment visit at 15.78 (SD = 2.45) weeks gestation. Across all timepoints, including prior to pregnancy, 41% (n = 105) of women reported experiencing at least one episode of LOC. The rate of LOC during pregnancy was 37% (n = 95), with 19% (n = 50) of women endorsing LOC during pregnancy only and not during the months immediately prior or during the first 6 months postpartum. Although LOC was common across the peripartum period, rates of diagnosable eating disorders were low. Five women (1.9%) met criteria for BED at baseline during the first trimester of pregnancy (T0). Four women (1.6%) reported engaging in compensatory behaviors, such as self-induced vomiting or laxative misuse, during the first trimester, though the frequency of these behaviors was low (M episode frequency = 0.66, SD = 6.27) and they were not accompanied by a sufficient number of OBEs to meet criteria for BN. At 6 months postpartum, 2.5% (n = 6) of women met diagnostic criteria for BED, none of whom were among those meeting criteria during the first trimester of pregnancy. Similar to observed rates of compensatory behaviors early in pregnancy, few women reported engaging in such behaviors during the postpartum period (5.1%, n = 12; M episode frequency = 1.46, SD = 10.48). Additional demographic and clinical characteristics of the sample are presented in Table 1.
TABLE 1.
Demographic, pregnancy, and eating characteristics of the sample
| Total sample (n = 257) Mean (SD) |
No LOC (n = 152) |
Any LOC (n = 105) |
LOC during pregnancy only (n = 50) |
|
|---|---|---|---|---|
| Age (years) | 28.44 (5.48) | 28.33 (5.38) | 28.60 (5.65) | 28.68 (5.92) |
| Weeks gestation | 15.78 (2.45) | 15.91 (2.38) | 15.59 (2.54) | 15.77 (2.62) |
| Pre-pregnancy BMI (kg/m2) | 32.68 (6.30) | 32.12 (5.90) | 33.49 (6.79) | 33.05 (6.69) |
| BMI during early pregnancy (kg/m2; T0) | 34.19 (7.09) | 33.38 (6.31) | 35.36 (7.96) | 34.69 (7.24) |
| Parity (number of births) | 1.20 (1.35) | 1.04 (1.30) | 1.43 (1.39) | 1.56 (1.28) |
| Number of LOC episodes prior to pregnancy | 3.42 (19.90) | 0 (0.0) | 8.37 (30.55) | 0 (0.0) |
| Number of LOC episodes during pregnancy | 4.90 (15.86) | 0 (0.0) | 11.99 (23.09) | 9.54 (16.79) |
| Number of LOC episodes at 6 months postpartum | 6.03 (37.86) | 0 (0.0) | 14.90 (58.55) | 0 (0.0) |
| n (%) | n (%) | n (%) | n (%) | |
| Yearly household income | ||||
| ≤$30,000 | 162 (65.6) | 84 (57.5) | 78 (77.2) | 40 (80.0) |
| >$30,000 | 88 (34.2) | 65 (42.8) | 23 (21.9) | 9 (18.0) |
| Education | ||||
| Grade school or some high school | 31 (12.1) | 15 (9.9) | 16 (15.2) | 7 (14.0) |
| High school graduate or GED | 55 (21.4) | 27 (17.8) | 28 (26.7) | 14 (28.0) |
| Some college or technical school | 99 (38.5) | 57 (37.5) | 42 (40.0) | 21 (42.0) |
| 4-year college graduate | 35 (13.6) | 28 (18.4) | 7 (6.7) | 3 (6.0) |
| Postgraduate degree | 37 (14.4) | 25 (16.4) | 12 (11.4) | 5 (10.0) |
| Race | ||||
| White | 117 (45.5) | 82 (53.9) | 35 (33.3) | 13 (26.0) |
| Black or African American | 113 (44.0) | 59 (38.8) | 54 (51.4) | 31 (62.0) |
| Asian | 1 (0.4) | 0 (0.0) | 1 (0.10) | 0 (0.0) |
| American Indian or Alaska native | 1 (0.4) | 1 (0.7) | 0 (0.0) | 0 (0.0) |
| Multi-racial | 23 (8.9) | 9 (5.9) | 14 (13.3) | 5 (10.0) |
| Unknown | 2 (0.8) | 1 (0.7) | 1 (1.0) | 1 (2.0) |
| Hispanic ethnicity | 7 (2.7) | 2 (1.3) | 5 (4.8) | 4 (8.0) |
Abbreviations: BED, binge eating disorder; BMI, body mass index; EDE-PV, Eating Disorders Examination—Pregnancy Version; LOC, loss of control.
3.0.2 ∣. Relationship between baseline characteristics and rates of LOC
Demographic characteristics were not associated with the probability of experiencing LOC prior to pregnancy, nor was pre-pregnancy BMI (ps > .23). Lower total household income (OR = 2.613, p = .02, 95% CI = 1.208–5.803) and older age (OR = 1.071, p = .03, 95% CI = 1.009–1.140) were associated with a higher likelihood of reporting LOC at any timepoint. Women who were of higher age (OR = 1.084, p = .04, 95% CI = 1.004–1.171) and identified as a minority (OR = 0.344, p = .02, 95% CI = 0.140–0.824) were more likely to report experiencing LOC only during pregnancy relative to those who did not experience LOC (please refer to Table 1 for descriptive information).
3.0.3 ∣. Changes in rates of LOC before, during, and after pregnancy
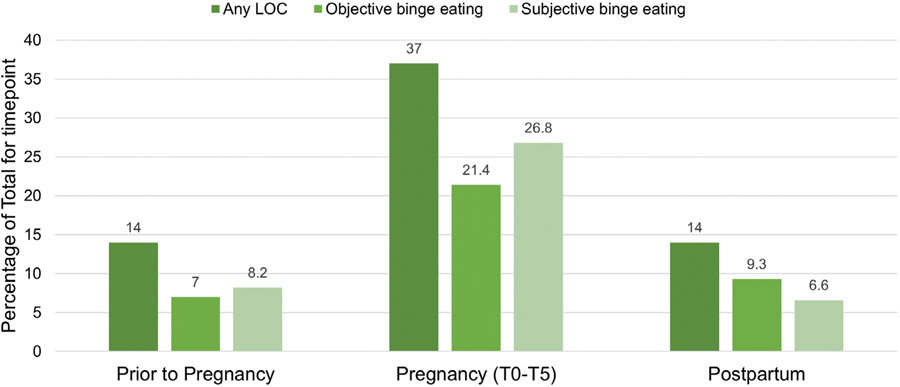
There was a significant change in LOC rates from the epoch prior-to-pregnancy to the epoch during pregnancy (, p < .01), whereby women with any LOC during the epoch prior-to-pregnancy were more likely to have LOC in the epoch during pregnancy (31/34 > 55/203). There was also significant change in LOC rates from pregnancy to postpartum (, p < .01), with women experiencing LOC during pregnancy being more likely to have LOC postpartum (28/86 > 8/151). However, there was no significant difference between reports of LOC prior-to-pregnancy and postpartum (, p = .87), indicating that women who experienced LOC prior to pregnancy were not more likely to experience LOC during the postpartum period. Rates of LOC, including objective and subjective binge episodes, at each time point are presented in more detail in Figure 1.
FIGURE 1.
Percentage of women endorsing loss of control eating (LOC), objective binge eating, or subjective binge eating at each time point. Note that LOC and binge eating were defined categorically as yes (≥1 episode for timepoint) or no (no episodes of LOC for timepoint)
4 ∣. DISCUSSION
The present study is the first to examine rates of LOC across the peripartum period among a sample of women with pre-pregnancy overweight or obesity. We documented that episodes of LOC are highly prevalent over the course of the peripartum period spanning three-months prior to pregnancy through 6-months postpartum, with 41% endorsing LOC. Further, 37% of the sample reported that they experienced at least one episode of LOC at some point during their pregnancy, and 20% of women reported LOC in pregnancy but not in the months immediately prior to pregnancy. Of note, rates of LOC during pregnancy were more than double rates prior to or after pregnancy. Moreover, incident LOC in pregnancy was associated with higher age and identifying as a member of a minority group. To our knowledge, this study is unique in identifying age and race as risk factors for the onset of LOC in pregnancy. The mechanisms by which these demographic characteristics might relate to LOC risk remain unclear. However, prior research has demonstrated that both weight-based and racial discrimination are associated with increased prevalence and frequency of disordered eating behaviors (Assari, 2018; Durso, Latner, & Hayashi, 2012; O'Brien et al., 2016; Sutin, Robinson, Daly, & Terracciano, 2016), an effect that may be exacerbated during pregnancy when women are more frequently in contact with systems of power, such as the medical system. Together, these findings suggest that pregnancy is a time of heightened vulnerability for the development of aberrant eating patterns known to be related to weight gain, psychosocial impairment, and medical comorbidity among women with pre-pregnancy overweight or obesity.
Importantly, LOC was shown to persist into the postpartum period in 5% of the women for whom LOC emerged during pregnancy. Prior research has demonstrated that longer duration of disordered eating behavior is predictive of a more severe and protracted course of illness (Reas, Williamson, Martin, & Zucker, 2000; Smink, van Hoeken, & Hoek, 2013). Thus, it is possible that pregnant women, particularly women with overweight or obesity who develop persistent LOC, may continue to engage in aberrant eating beyond the peripartum period, placing them at risk for further worsening of eating pathology and health. These findings underscore the importance of monitoring eating patterns such as LOC during pregnancy and postpartum to provide timely intervention and to prevent persistence of the behavior.
Strengths of the present study include the use of a large racially and socioeconomically diverse sample of community women, longitudinal design, and detailed assessments of LOC using a standardized eating disorders interview. However, there are several limitations that should be taken into account when interpreting our results. First, we did not evaluate lifetime history of disordered eating and relied on retrospective recall of the weeks preceding each assessment. Women were also asked to retrospectively recall eating psychopathology occurring in the 3 months prior to becoming pregnant while in their first trimester of pregnancy. It is possible, therefore, that the observed rates of LOC prior to pregnancy may be biased. Additional research is necessary to prospectively assess LOC prior to pregnancy through the postpartum period. Second, LOC data were missing for 51 women (19.8%) at T5 due to early delivery, which may have produced biased estimates of LOC rates during pregnancy, particularly if women engaging in LOC during pregnancy were more likely to deliver early. Third, we did not explore the relationship between peripartum LOC and pregnancy or obstetric outcomes. Future research is necessary to determine how maternal LOC during pregnancy affects the health and well-being of mothers and their children. It will also be important to explore whether the emergence of LOC during pregnancy increases risk for later development of diagnosable eating disorders or other psychological and medical comorbidities or increases risk for LOC in subsequent pregnancies. In addition, further longitudinal research is needed to determine whether maternal LOC during pregnancy or the postpartum period predicts offspring physical and mental health outcomes, including the development of disordered eating. Finally, given that infant feeding practices such as breastfeeding are related to maternal appetitive behavior (Brown, 2014; Perrine, Nelson, Corbelli, & Scanlon, 2016; Rodgers, O'Flynn, Bourdeau, & Zimmerman, 2018), future studies should consider examining whether and how these practices influence and are influenced by LOC and other disordered eating behaviors during the postpartum period.
ACKNOWLEDGMENTS
This work was supported by NICHD (grant number R01HD068802 [PI: Michele Levine]), NHLBI (grant number T32HL007560 [PI: Karen Matthews; awardee: Shannon Donofry]), and NCATS (grant numbers TL1TR002493 [PI: Fulkerson; awardee: Rebecca Emery] and UL1TR002494 [PI: Blazar; awardee: Rebecca Emery]). The funding sources had no involvement in the study design, the collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
Footnotes
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
STATEMENT OF HUMAN RIGHTS
All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). Washington, D.C.: American Psychiatric Pub. [Google Scholar]
- Assari S. (2018). Perceived discrimination and binge eating disorder; gender difference in African Americans. Journal of Clinical Medicine, 7(5), 89. 10.3390/jcm7050089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athukorala C, Rumbold AR, Willson KJ, & Crowther CA (2010). The risk of adverse pregnancy outcomes in women who are overweight or obese. BMC Pregnancy and Childbirth, 10(1), 56. 10.1186/1471-2393-10-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brion M-JA, Ness AR, Rogers I, Emmett P, Cribb V, Davey Smith G, & Lawlor DA (2010). Maternal macronutrient and energy intakes in pregnancy and offspring intake at 10 y: Exploring parental comparisons and prenatal effects. The American Journal of Clinical Nutrition, 91(3), 748–756. 10.3945/ajcn.2009.28623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. (2014). Maternal restraint and external eating behaviour are associated with formula use or shorter breastfeeding duration. Appetite, 76, 30–35. 10.1016/j.appet.2013.12.022 [DOI] [PubMed] [Google Scholar]
- Bulik CM, Holle AV, Siega-Riz AM, Torgersen L, Lie KK, Hamer RM … Reichborn-Kjennerud T (2009). Birth outcomes in women with eating disorders in the Norwegian Mother and Child cohort study (MoBa). International Journal of Eating Disorders, 42(1), 9–18. 10.1002/eat.20578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L-W, Aris IM, Bernard JY, Tint M-T, Colega M, Gluckman PD … Lee YS (2017). Associations of maternal macronutrient intake during pregnancy with infant BMI peak characteristics and childhood BMI. The American Journal of Clinical Nutrition, 105(3), 705–713. 10.3945/ajcn.116.148270 [DOI] [PubMed] [Google Scholar]
- Colles SL, Dixon JB, & O'Brien PE (2008). Loss of control is central to psychological disturbance associated with binge eating disorder. Obesity, 16(3), 608–614. 10.1038/oby.2007.99 [DOI] [PubMed] [Google Scholar]
- Doherty DA, Magann EF, Francis J, Morrison JC, & Newnham JP (2006). Pre-pregnancy body mass index and pregnancy outcomes. International Journal of Gynecology & Obstetrics, 95(3), 242–247. 10.1016/j.ijgo.2006.06.021 [DOI] [PubMed] [Google Scholar]
- Durso LE, Latner JD, & Hayashi K (2012). Perceived discrimination is associated with binge eating in a community sample of non-overweight, overweight, and obese adults. Obesity Facts, 5(6), 869–880. 10.1159/000345931 [DOI] [PubMed] [Google Scholar]
- Emery RL, Grace JL, Kolko RP, & Levine MD (2017). Adapting the eating disorder examination for use during pregnancy: Preliminary results from a community sample of women with overweight and obesity. International Journal of Eating Disorders, 50(5), 597–601. 10.1002/eat.22646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairburn CG (2008). Cognitive behavior therapy and eating disorders. New York, NY: Guilford Press. [Google Scholar]
- Goldschmidt AB (2017). Are loss of control while eating and overeating valid constructs? A critical review of the literature. Obesity Reviews: An Official Journal of the International Association for the Study of Obesity, 18(4), 412–449. 10.1111/obr.12491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt AB, Engel SG, Wonderlich SA, Crosby RD, Peterson CB, Grange DL … Mitchell JE (2012). Momentary affect surrounding loss of control and overeating in obese adults with and without binge eating disorder. Obesity, 20(6), 1206–1211. 10.1038/oby.2011.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolko RP, Emery RL, Marcus MD, & Levine MD (2017). Loss of control over eating before and during early pregnancy among community women with overweight and obesity. International Journal of Eating Disorders, 50(5), 582–586. 10.1002/eat.22630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latner JD, Hildebrandt T, Rosewall JK, Chisholm AM, & Hayashi K (2007). Loss of control over eating reflects eating disturbances and general psychopathology. Behaviour Research and Therapy, 45(9), 2203–2211. 10.1016/j.brat.2006.12.002 [DOI] [PubMed] [Google Scholar]
- Linna MS, Raevuori A, Haukka J, Suvisaari JM, Suokas JT, & Gissler M (2014). Pregnancy, obstetric, and perinatal health outcomes in eating disorders. American Journal of Obstetrics and Gynecology, 211 (4), 392.e1–392.e8. 10.1016/j.ajog.2014.03.067 [DOI] [PubMed] [Google Scholar]
- Meany G, Conceição E, & Mitchell JE (2014). Binge eating, binge eating disorder and loss of control eating: Effects on weight outcomes after bariatric surgery. European Eating Disorders Review, 22(2), 87–91. 10.1002/erv.2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micali N, Al Essimii H, Field AE, & Treasure J (2018). Pregnancy loss of control over eating: A longitudinal study of maternal and child outcomes. The American Journal of Clinical Nutrition, 108(1), 101–107. 10.1093/ajcn/nqy040 [DOI] [PubMed] [Google Scholar]
- O'Brien KS, Latner JD, Puhl RM, Vartanian LR, Giles C, Griva K, & Carter A (2016). The relationship between weight stigma and eating behavior is explained by weight bias internalization and psychological distress. Appetite, 102, 70–76. 10.1016/j.appet.2016.02.032 [DOI] [PubMed] [Google Scholar]
- Perrine CG, Nelson JM, Corbelli J, & Scanlon KS (2016). Lactation and maternal cardio-metabolic health. Annual Review of Nutrition, 36(1), 627–645. 10.1146/annurev-nutr-071715-051213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reas DL, Williamson DA, Martin CK, & Zucker NL (2000). Duration of illness predicts outcome for bulimia nervosa: A long-term follow-up study. International Journal of Eating Disorders, 27(4), 428–434. [DOI] [PubMed] [Google Scholar]
- Rodgers RF, O'Flynn JL, Bourdeau A, & Zimmerman E (2018). A biopsychosocial model of body image, disordered eating, and breastfeeding among postpartum women. Appetite, 126, 163–168. 10.1016/j.appet.2018.04.007 [DOI] [PubMed] [Google Scholar]
- Smink FRE, van Hoeken D, & Hoek HW (2013). Epidemiology, course, and outcome of eating disorders. Current Opinion in Psychiatry, 26(6), 543–548. 10.1097/YCO.0b013e328365a24f [DOI] [PubMed] [Google Scholar]
- Sonneville KR, Horton NJ, Micali N, Crosby RD, Swanson SA, Solmi F, & Field AE (2013). Longitudinal associations between binge eating and overeating and adverse outcomes among adolescents and young adults: Does loss of control matter? JAMA Pediatrics, 167(2), 149–155. 10.1001/2013.jamapediatrics.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striegel-Moore RH, Rosselli F, Perrin N, DeBar L, Wilson GT, May A, & Kraemer HC (2009). Gender difference in the prevalence of eating disorder symptoms. International Journal of Eating Disorders, 42(5), 471–474. 10.1002/eat.20625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin A, Robinson E, Daly M, & Terracciano A (2016). Weight discrimination and unhealthy eating-related behaviors. Appetite, 102, 83–89. 10.1016/j.appet.2016.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Yanovski SZ, Schvey NA, Olsen CH, Gustafson J, & Yanovski JA (2009). A prospective study of loss of control eating for body weight gain in children at high risk for adult obesity. International Journal of Eating Disorders, 42(1), 26–30. 10.1002/eat.20580 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.