Abstract
Dental Pulp Stem Cells (DPSC) constitute a unique group of cells endowed with multipotency, self-renewal, and the capacity to regenerate the dental pulp tissue. While much has been learned about these cells in recent years, it is still unclear if each DPSC cell is multipotent or if unique sub-populations of DPSCs are “primed” to undergo specific differentiation paths. The purpose of this study was to define whether a sub-population of DPSCs is uniquely primed to undergo vasculogenic differentiation. Here, permanent tooth DPSCs or Stem cells from Human Exfoliated Deciduous teeth (SHED) were flow sorted for VEGFR1 and exposed to vasculogenic differentiation medium, i.e. Endothelial Growth Medium (EGM) 2-MV supplemented with 50 ng/ml rhVEGF165 in presence of 0 or 25 μg/ml anti-human VEGF antibody (bevacizumab; Genentech). In addition, sorted SHED (i.e. VEGFR1high or VEGFR1low) were seeded in biodegradable scaffolds and transplanted into the subcutaneous space of immunodeficient mice. Despite proliferating at a similar rate, VEGFR1high generated more in vitro sprouts than VEGFR1low cells (p<0.05). Blockade of VEGF signaling with bevacizumab inhibited VEGFR1high-derived sprouts, demonstrating specificity of responses. Similarly, VEGFR1high SHED generated more blood vessels when transplanted into murine hosts than VEGFR1low cells (p<0.05). Collectively, these data demonstrate that dental pulp stem cells contain a unique sub-population of cells defined by high VEGFR1 expression that are primed to differentiate into vascular endothelial cells. These data raise the possibility of purifying stem cells with high vasculogenic potential for regeneration of vascularized tissues or for vascular engineering in the treatment of ischemic conditions.
Keywords: stem cells, differentiation, angiogenesis, vasculogenesis, pulp biology, multipotency, tissue regeneration, vascular endothelial growth factor, endodontics
Graphical Abstract
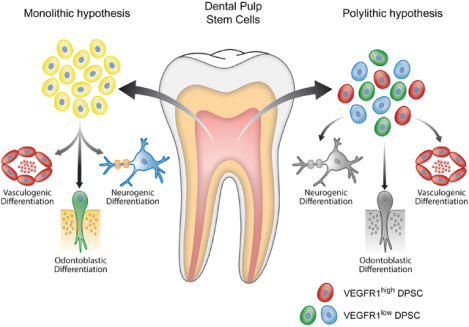
Schematic representation of the hypothesis tested here. Drawing highlights 2 working hypotheses for the multipotency of dental pulp stem cells. The monolithic hypothesis suggests that each single dental pulp stem cell is multipotent and capable of undergoing differentiation towards multiple different cell types (e.g. odontoblast, neural cell, endothelial cell). In contrast, the polylithic hypothesis proposes that dental pulp stem cells constitute a heterogeneous group of cells with different sub-populations of cells that are “primed” to a certain differentiation fate. Under this hypothesis, a certain cell sub-group is primed to become an endothelial cell while another sub-group can be primed to become an odontoblast or a neural cell.
Introduction
Dental pulp tissue regeneration requires the odontogenic and vasculogenic differentiation of resident stem cells (Sakai et al., 2010). These differentiation pathways are engaged by dental pulp stem cells (DPSC) in several clinical scenarios, such as direct pulp capping or pulpotomy (Casagrande et al., 2011; Fitzgerald et al., 1990). Interestingly, similar responses are also observed when DPSCs from permanent teeth (Gronthos et al., 2000) or stem cells from exfoliated deciduous teeth (SHED; Miura et al., 2003) are transplanted into empty root canals in attempt to engineer a new dental pulp tissue (Casagrande et al., 2010; Cordeiro et al., 2008; Rosa et al., 2013; Smith et al., 2016). While it has been clearly demonstrated that pulp stem cells differentiate into functional odontoblasts and vasculogenic endothelial cells (Sakai et al., 2010; Xu et al., 2019) that regenerate pulp-like tissues (Gan et al., 2020; Piva et al., 2017; Rosa et al., 2013), mechanisms underlying fate decisions of dental pulp stem cells remain elusive. Deep mechanistic understanding of stem cell fate decisions will allow for temporal and spatial control of differentiation events, which may further improve the success of ongoing clinical trials aiming at the engineering of dental pulps for treatment of necrotic teeth (Nakashima et al., 2017; Xuan et al., 2018).
The phenotypic hallmarks of physiological tissue-specific stem cells are self-renewal and multipotency. Recent studies showed that the presence of pulp stem cells in the perivascular niche (Oh and Nör, 2015) enables their crosstalk with vascular endothelial cells (mediated by stem cell factor, SCF) that is critical to maintain stem cell self-renewal (Cucco et al., 2020; Oh et al., 2020). It has been postulated that this process of self-renewal enables the maintenance of a population of undifferentiated (stem) cells that enable pulp regeneration throughout the life of the dentin-pulp complex (Cucco et al., 2020). The multipotency of stem cells from the dental pulp has been demonstrated unequivocally by their ability to differentiate in several cell types, including odontoblasts, osteoblasts, adipocytes, neural cells, chondrocytes, and endothelial cells (Bento et al., 2013; Cordeiro et al., 2008; D’Alimonte et al., 2011; Gronthos et al., 2000; Lambrichts et al., 2017; Miura et al., 2003; Sakai et al., 2010; Smith et al., 2016; Monache et al., 2019; Yusof et al., 2018).
Vascular Endothelial Growth Factor (VEGF) is a major regulator of angiogenesis and vasculogenesis in development, maintenance of health and in disease (Apte et al., 2019). It has been demonstrated that VEGF induces differentiation of pulp stem cells into vascular endothelial cells via signaling through VEGF receptor 1 (VEGFR1) (Sakai et al., 2010; Bento et al., 2013; Gorin et al., 2016; Zhang et al., 2016) and induces anastomosis of these stem cell-derived blood vessels with the host vasculature via VE-Cadherin (Sasaki et al., 2020). Three to 5 days after induction with VEGF, dental pulp stem cells acquire VEGFR2 expression (Sasaki et al., 2020), which then drives vessel maturation and functional angiogenesis (Janebodin et al., 2013; Monache et al., 2019; Xu et al., 2019). These pulp stem cells have been considered good candidates for bone tissue engineering, as they are able to differentiate into both, vasculogenic endothelial cells and bone-forming osteoblasts when implanted in environments conducive to bone formation (D’aquino et al., 2007; D’aquino et al., 2009; Giuliani et al., 2013; Paino et al., 2017; Yusof et al., 2018). Interestingly, VEGF can be produced by osteoblasts in response to Bone Morphogenetic Proteins (BMP) via processes that couple angiogenesis to bone formation (Wang et al., 1997; Deckers et al., 2002).
It is well known that VEGF signals through VEGFR1 to activate MEK1/ERK signaling, inhibit STAT3 transcriptional activity, and enable endothelial differentiation of pulp stem cells (Bento et al., 2013). However, it is not known if all pulp stem cells express VEGFR1 and are capable of endothelial differentiation, or if only a subpopulation of pulp stem cells express VEGFR1 and therefore are “primed” for vasculogenic differentiation. Here, we unveiled a sub-population of VEGFR1-expressing pulp stem cells that is primed to undergo vasculogenic differentiation, and began to define the polylithic nature of these tissue-specific stem cells.
Materials and Methods
Cell culture
DPSC (Lonza, Walkersville, MD, USA) and SHED (kindly provided by Songtao Shi) were cultured in Alpha-Minimal Essential Media (Alpha-MEM; Invitrogen, Carlsbad, California) supplemented with 20% Fetal Bovine Serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA), 1% Antimycotic and Antibiotic Solution (Gibco, Grand Island, NY, USA) at 37°C and 5% CO₂. Primary Human Dermal Microvascular Endothelial Cells (HDMEC; Lonza, Walkersville, MD, USA) were cultured in Endothelial Cell Growth Medium-2 (EGM2-MV; Lonza). To induce vasculogenic differentiation, SHED or DPSC were exposed to EGM2-MV supplemented with 50 ng/mL rhVEGF165 (R&D Systems, Minneapolis, MN, USA). In selected experiments, cells were exposed to 0 or 25 ug/mL anti-VEGF antibody, i.e. bevacizumab (Genentech, San Francisco, CA, USA).
Semi-quantitative RT-PCR
Total RNA from SHED or DPSC was isolated using the Trizol (Invitrogen) method, quantified by NanoDrop (Thermo Fisher Scientific), and reverse transcribed into DNA using Superscript II Reverse Transcriptase (Invitrogen) according to the manufacturer’s instructions. The PCR primers used here were: VEGFR-1(sense 5’-actcccttgaacacgagagttc-3’, antisense 5’-gatttctcagtcgcaggtaacc-3’), VEGFR-2 (sense 5’-gctgtctcagtgacaaacccat-3’, antisense 5’-ctcccacatggattggcagagg-3’), Tie-2 (sense 5’tacacctgcctcatgctcag-3’, antisense 5’-gcagagacatccttggaagc-3’), CD31 (sense 5’-tactcagtcatggccatggt-3’, antisense 5’-ttggccttggctttcctcag-3’), VE-cadherin (sense 5’-cctggtataacctgactgtg-3’, antisense 5’-tgtgatggtgaggatgcaga-3’), and GAPDH (sense 5’-gaccccttcattgacctcaact-3’, antisense 5’-caccaccttcttgatgtcatc-3’).
Cell sorting by flow cytometry
Cells were harvested into FACS tubes (Corning, Glendale, AZ, USA) at a density of 106 cells/tube, washed with PBS and incubated with 14 μL of anti-human VEGFR1 antibody (PE-conjugated; R&D Systems) in the dark at room temperature for 35 minutes. Then, cells were washed in PBS and resuspended in Stain Buffer (BD Bioscience, San Jose, CA, EUA). Cells were sorted according to VEGFR-1 expression levels (VEGFR1high or VEGFR1low). As negative controls, untreated cells or cells exposed to isotype-matched non-specific IgG antibody (R&D Systems). The analysis of the data was performed using the FlowJo Software (Ashland, OR, USA).
Sulforhodamine B (SRB) assay
Sorted VEGFR1high and VEGFR1low cells were seeded at the density 2.5×103cell/well in 96-well plates. After 24–72 hours, cells were fixed with 10% trichloroacetic acid at 4°C for 1 hour. Then, cells were washed, dried, stained with 0.4% SRB (Thermo Fisher Scientific), and incubated at room temperature for 30 minutes. To remove the unbound excess dye, cells were washed in 1% acetic acid, allowed to dry, and then the dye was solubilized with trizma-base. Plates were read in a microplate reader (GENios; Tecan, Männedorf, Switzerland) at 565 nm. Data were obtained from 8 wells per condition and time point. Here, and throughout this manuscript, experiments were performed 3 independent times to verify the reproducibility of the data.
Immunofluorescence assay
For immunofluorescence, 5×104 cells were cultured in Nunc Lab-Tek chamber slides (Millipore Sigma, Burlington, MA, USA) for 24 hours. The cells were washed and incubated overnight with rabbit anti-human VEGFR1 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Next day, excess primary antibody was washed and goat anti-rabbit antibody conjugated to Alexa Fluor 488 (Life Technologies, Eugene, OR, USA) was used to visualize VEGFR1. Nuclei were stained with Vectashield Mounting Medium containing DAPI (Vector Laboratories, Burlingame, CA, USA).
Western blot
DPSC or SHED cells were lysed in NP-40 and protein concentration was quantified at 595 nm (GENios, Tecan). Protein lysates underwent electrophoresis and transferred to nitrocellulose membranes that were blocked in 5% milk for 30 minutes. Membranes were exposed overnight at 4°C to the following primary antibodies: anti-human VEGFR-1, VEGFR-2, Tie-2, CD31, VE-Cadherin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or GAPDH (MiliporeSigma, Burlington, MA, USA) in the following dilutions 1:1000, 1:1000, 1:1000, 1:3000, 1:3000, 1:4×107, respectively. Next day, membranes were washed 2X in TBST, incubated with appropriate secondary antibodies for 2 hours, washed again, and exposed to SuperSignal West Pico chemiluminescent substrate (Thermo Fisher Scientific).
In Vitro vasculogenic differentiation assay
2×105 cells flow sorted as VEGFR1high or VEGFR1low were plated in standard tissue culture plates and cultured in alpha-MEM supplemented with 20% FBS. Next day, vasculogenic differentiation medium (EGM2-MV supplemented with 50 ng/mL rhVEGF165) was added for up to 9 days. To confirm the endothelial differentiation of the dental pulp stem cells, western blots were performed for VEGFR2, CD-31, VE-cadherin, and Tie-2, as we showed (Bento et al., 2013; Sasaki et al., 2020).
In Vitro capillary sprouting assay
After sorting for VEGFR1, 10⁴ cells/well were seeded in 12-well plates pre-coated with Growth Factor Reduced Matrigel (BD Bioscience, Bedford, MA, USA) and cultured for up to 10 days in vasculogenic differentiation medium, i.e. (EGM2-MV supplemented with 50 ng/mL rhVEGF165) in presence of 0 or 25 μg/mL anti-human VEGF antibody (bevacizumab; Genentech, CA, USA). Capillary sprouts were counted under a light microscope at 100x magnification in 12 random fields per well in 3 independent wells per experimental condition.
In vivo vasculogenic differentiation assay
Biodegradable, highly porous, poly-L-lactic acid (PLLA) scaffolds were prepared and were cut in 6 mm × 6 mm × 1 mm, as described (Nör et al., 2001). SHED cells (6×105 cells/scaffold) flow sorted for VEGFR1 were mixed with Matrigel, seeded in the scaffolds (n=6), and transplanted into the subcutaneous space of the dorsum of severe combined immunodeficient mice (CB-17 SCID; Jackson Laboratory, Bar Harbor, ME, USA). After 28 days, scaffolds were retrieved and fixed in 10% formaldehyde for 24 hours at 4°C. Histologic sections (5-μm-thick) were stained with hematoxylin-eosin or kept unstained for immunohistochemistry assay. Tissue sections were deparaffinized, antigen retrieval was performed with 1mg/ml Trypsin (Merck, Darmstadt, Germany) for 1 hour at 37° C. After incubation in 0.1% Triton-X-100 and 3% H2O2, and background Sniper (Biocare Medical, Pacheco, CA, USA) for 20 minutes at room temperature, tissue sections were incubated in 1:100 rabbit anti-human CD31 (Bethyl Laboratories, Montgomery, TX, USA) or rabbit anti-Factor VIII (Ab-1; Thermo Fisher Scientific). Next day, unbound primary antibodies were washed with Wash Buffer (Dako, Carpinteria, CA, USA) and MACH3 Rabbit/Mouse Probe (Biocare Medical), MACH 3 Rabbit/Mouse HRP-Polymer (Biocare Medical), Betazoid DAB Chromogen Kit (Biocare Medical) were added to the tissue sections for 20 minutes each, except for the DAB incubation that was performed 1–2 minutes. After the final wash, VectaMount (Vector Laboratories, Burlingame, CA, USA) was added for cover slip placement. Human microvessels (CD31-positive) were counted in 8 random fields (200x) by a calibrated researcher blinded for experimental conditions using the Image J software (NIH). The animal work included here was done under a protocol (PRO00009087) approved by the University of Michigan Animal Ethics Committee.
Statistical analysis
The statistical analyses were performed using the GraphPad Prism software (GraphPad, San Diego, CA, USA). The Shapiro-Wilk normality test was applied in the quantitative measurements. Data were analyzed by t-tests or one-way ANOVA followed by appropriate post-hoc tests. The significance was set at p<0.05.
Results
Baseline expression of VEGFR1 and VEGFR2 in dental pulp stem cells
To evaluate the baseline level of expression of key mediators of vasculogenesis in pulp stem cells, RT-PCR (mRNA) and Western blots (protein) of untreated DPSC and SHED cells were performed, and primary human endothelial cells (HDMEC) were used as controls. While endothelial cells expressed all markers evaluated here (i.e. VEGFR1, VEGFR2, CD31 and VE-Cadherin), SHED and DPSC cells only expressed VEGFR1 at baseline (Figure 1a). An intrinsic limitation of both RT-PCR and Western blots is the fact that cells are pooled together for these assays, which does not allow for the understanding of expression levels of these markers in individual cells. To overcome this limitation, these cells were analyzed by flow cytometry for VEGFR1 or VEGFR2 (Figure 1b,c). We observed that approximately 10% and 20% of DPSCs and SHED cells express VEGFR1, respectively. In contrast, only a negligeable percentage of DPSC and SHED express VEGFR2. These data are consistent with the results obtained in the RT-PCR and Western blots, and suggest that VEGFR1 (not VEGFR2) is the receptor engaged by VEGF to induce the vasculogenic differentiation of pulp stem cells.
Fig. 1. Baseline expression of VEGFR1 and VEGFR2 in dental pulp stem cells.
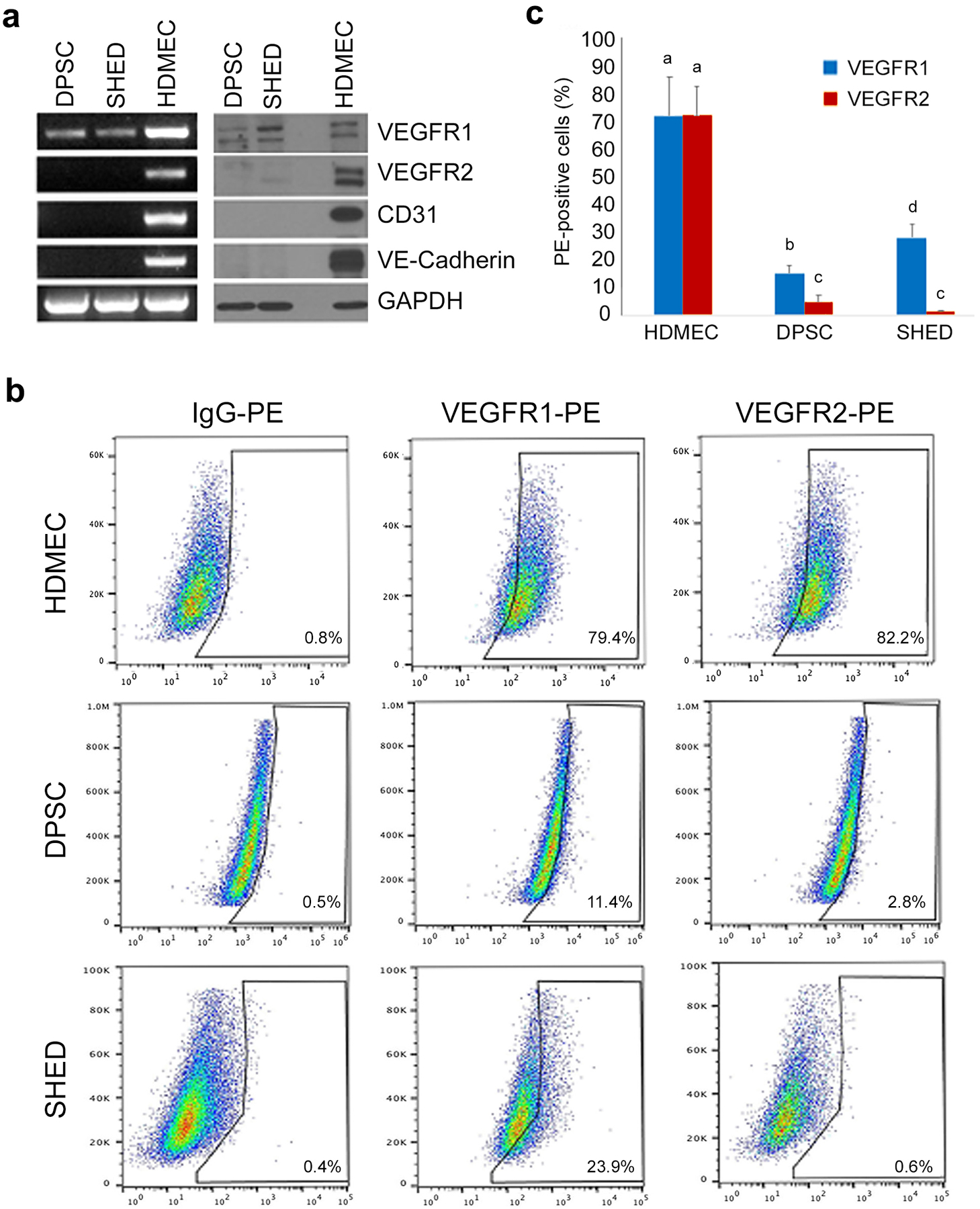
(a) RT-PCR and Western Blot analyses of VEGFR1, VEGFR2, CD-31, and VE-cadherin expression in DPSC and SHED cultured in alpha-MEM + 20% FBS. (b) Flow cytometric analyses of VEGFR1 and VEGFR2 expression in SHED, DPSC and human dermal microvascular endothelial cells (HDMEC). Cells are presented in a dot plot of Side Scatter Area (SSC-A) gating against PE fluorescence. Cells were analyzed using anti-VEGFR1 and anti-VEGFR2 PE-conjugated antibodies, and an isotype-matched IgG as control to set the gating. (c) Graph depicting the percentage of VEGFR1 and VEGFR2-positive cells in SHED, DPSC and HDMEC. Different low case letters indicate statistical significance at p<0.05.
VEGFR1 does not regulate proliferation of pulp stem cells
To examine the impact of VEGFR1 expression levels on the proliferation rate, flow sorting was used to generate a subpopulation of VEGFR1high pulp stem cells and a subpopulation of VEGFR1low pulp stem cells (Figure 2a). Immunofluorescence analysis showed that both populations, VEGFR1high and VEGFR1low cells, exhibited a homogeneous distribution of VEGFR1 expression (Figure 2b). Interestingly, the level of VEGFR1 expression (i.e. high or low) had no impact in cell density (surrogate for net effect of treatment on cell proliferation and cell survival), when DPSC cells were cultured in either basal culture medium (i.e. AlphaMEM + FBS) or in vasculogenic differentiation medium (i.e. EGM2-MV + 50 ng/ml rhVEGF165) (Figure 2c).
Fig. 2. VEGFR1 does not regulate proliferation of pulp stem cells.
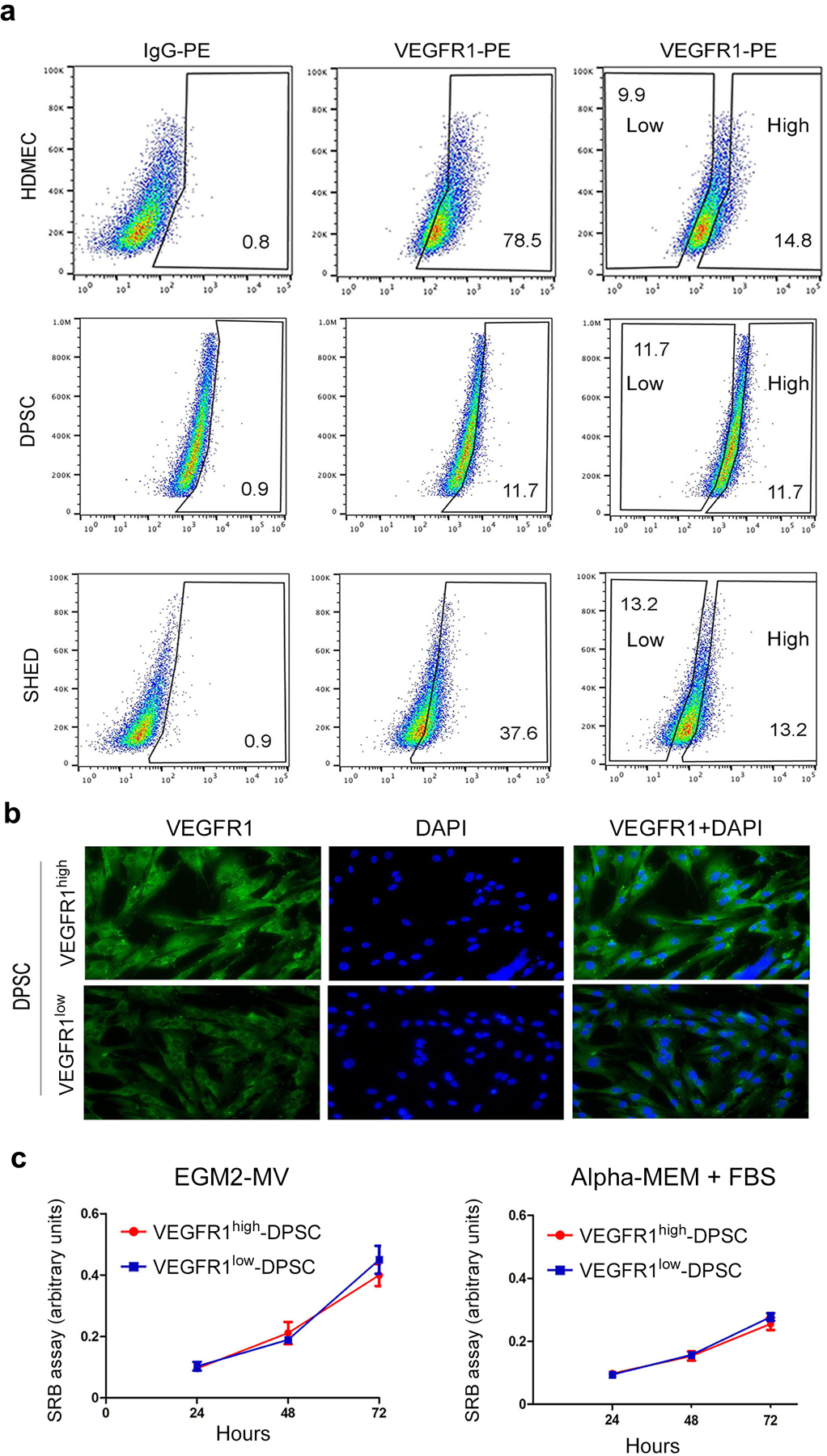
(a) Flow sorting of HDMEC, SHED, and DPSC according to VEGFR1 expression levels (i.e. high and low), using isotype-matched IgG to set the gates. For DPSC and SHED, we sorted out equivalent percentages of VEGFR1high and VEGFR1low cells. (b) Fluorescence microscopy images of VEGFR1high and VEGFR1low cells. Green depicts VEGFR1 expression while blue depicts DAPI nuclear staining. bar=20 μm. (c) Line graph depicting cell proliferation over time when VEGFR1high and VEGFR1low cells, as determined by the SRB assay. Cells were cultured in vasculogenic differentiation medium (EGM2-MV + 50 ng/ml rhVEGF165) or alpha-MEM + 20% FBS for 24 to 72 hours. Data represents average +/− s.d. in 8 wells per condition.
VEGFR1high pulp stem cells are more vasculogenic than VEGFR1low cells in vitro
To begin to evaluate the impact of VEGFR1 expression on the vasculogenic potential of dental pulp stem cells, DPSC and SHED were sorted for VEGFR1 levels, plated the cells in Matrigel-coated wells, and exposed them to vasculogenic differentiation medium for 11 days. The images depicted here (representative of 3 independent experiments) showed that VEGFR1high SHED cells are more vasculogenic than VEGFR1low SHED (Figure 3). A similar trend was observed when DPSC cells were analyzed under similar experimental conditions (Figure 3). To verify the specificity of these results, an independent set of studies in which sorted SHED and DPSC were exposed to vasculogenic differentiation medium in presence (or not) of an anti-VEGF antibody (bevacizumab) was performed (Walker et al., 2012). These experiments demonstrated that VEGFR1high DPSC cells generate more capillary sprouts than VEGFR1low DPSC cells (Figure 4a,b). The same trends were observed with VEGFR1high SHED cells versus VEGFR1low SHED cells (Figure 4c,d). Notably, blockade of VEGF with bevacizumab decreased the number of capillary sprouts generated by DPSC and SHED (Figure 4a–e), demonstrating that the responses observed here were dependent upon active VEGF signaling.
Fig. 3. VEGFR1high pulp stem cells generate more capillary sprouts than VEGFR1low cells in vitro.
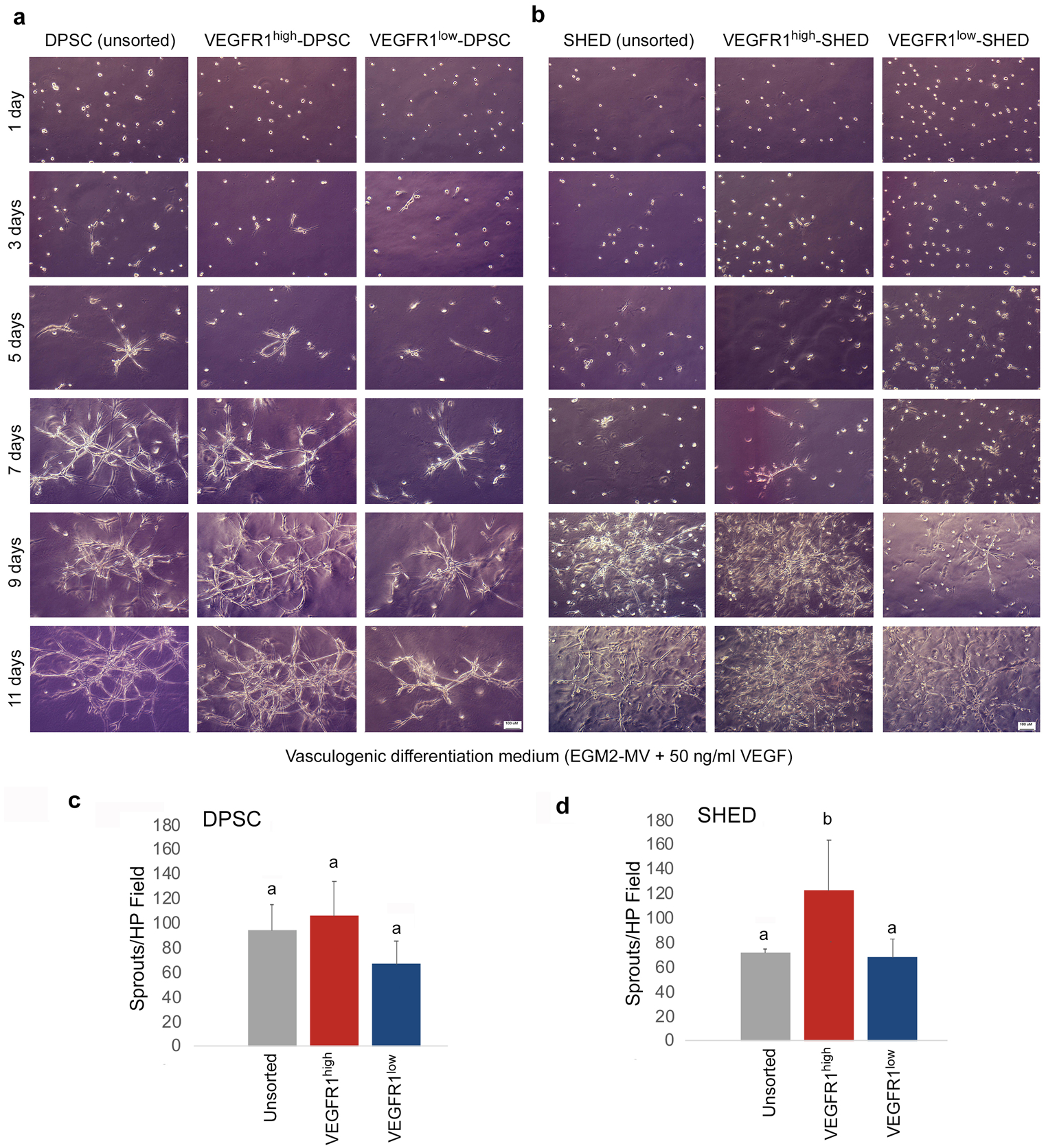
Cells were sorted for VEGFR1 expression levels and then plated in plates coated with growth factor reduced Matrigel. (a,b) Representative photomicrographs (bar: 100 μm) of capillary sprouts generated by VEGFR1high, VEGFR1low, or unsorted DPSC and SHED cells cultured in vasculogenic differentiation medium for up to 11 days. (c,d) Bar graphs showing the number of capillary-like sprouts at the end of the experimental period (i.e. 11 days). Different low case letters indicate statistical significance at p<0.05. Number of capillary sprouts (average +/− s.d.) is representative of 12 random microscopic fields from triplicate wells per condition.
Fig. 4. VEGF blockade inhibits the vasculogenic potential of VEGFR1high cells in vitro.
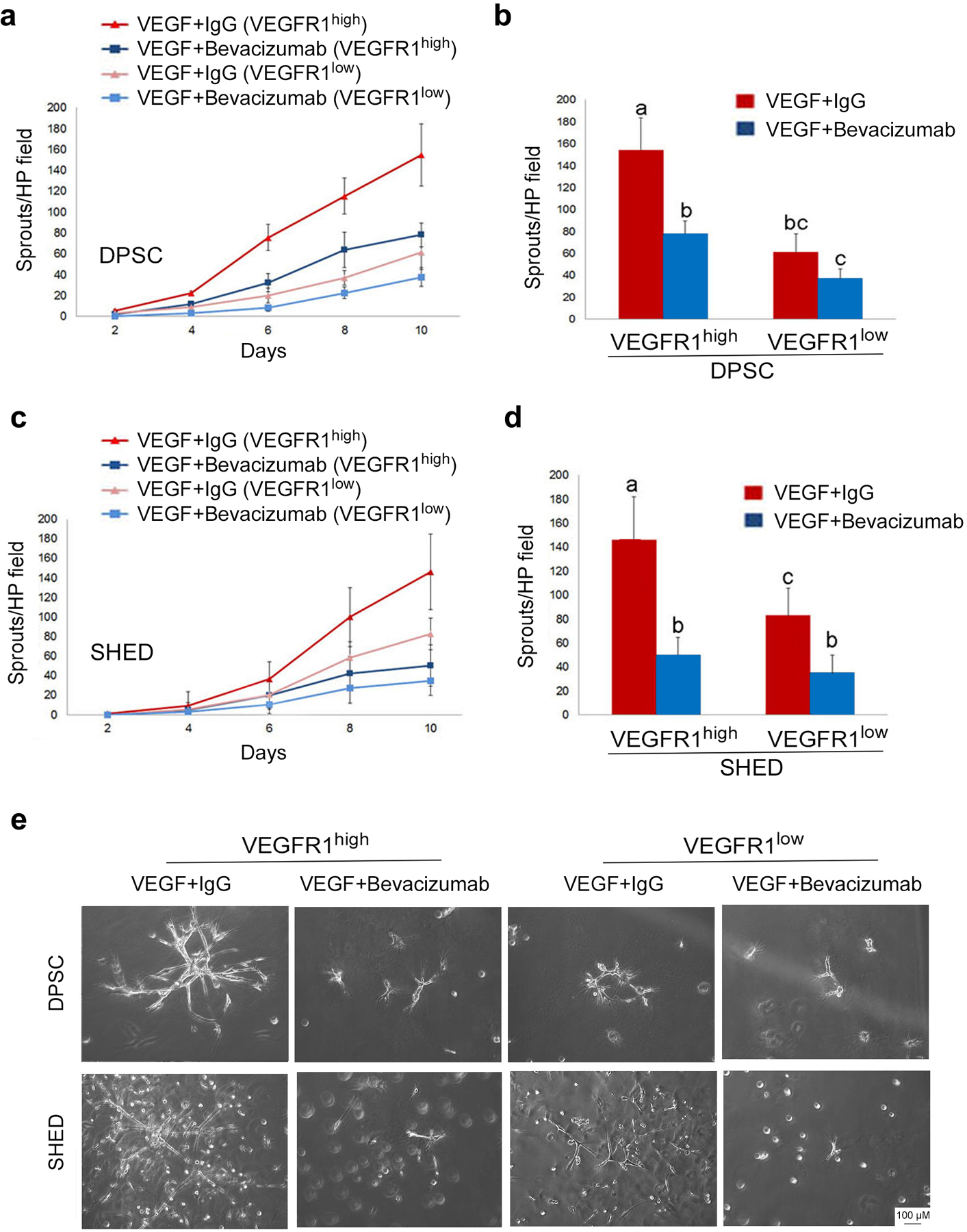
(a,c) Line graphs depicting the number of sprouts per high power field generated by DPSC or SHED. (b,d) Bar graphs showing the number of capillary-like sprouts at the end of the experimental period (i.e. 10 days). VEGFR1high and VEGFR1low DPSC or SHED cells were cultured in wells pre-coated with growth factor reduced Matrigel and stimulated with vasculogenic differentiation medium in presence of 0 or 25 μg/ml bevacizumab (anti-VEGF antibody). Different low case letters indicate statistical significance at p<0.05. Number of capillary sprouts (average +/− s.d.) is representative of 12 random microscopic fields from triplicate wells per condition. (e) Representative photomicrographs of the capillary sprouts observed after 10 days under the experimental conditions described above (bar: 100 μm).
VEGFR1high pulp stem cells are more vasculogenic than VEGFR1low cells in vivo
Considering that SHED and DPSC presented similar results in the in vitro studies performed here (cell proliferation, capillary-like sprouting and response to therapeutic blockade of VEGF signaling with bevacizumab), a decision was made to focus on the use of SHED as model pulp stem cells for the in vivo studies. To understand the impact of VEGFR1 expression on the vasculogenic potential of dental pulp stem cells, SHED cells were sorted for VEGFR1, seeded in biodegradable scaffolds, and transplanted into SCID mice, as shown (Bento et al., 2013; Nör et al., 2001; Sasaki et al., 2020). Similar to in vitro experiments, VEGFR1high SHED are more vasculogenic than VEGFR1low cells in 8 randomly selected high-power fields per scaffold (n=6) (Figure 5). Using the anti-human CD31 antibody, which is specific to human endothelial cells (Nör et al., 2001), we observed that scaffolds seeded with VEGFR1high SHED contained approximately twice as many blood vessels as scaffolds seeded with VEGFR1low SHED cells (Figure 5a,b). Notably, immunohistochemistry with anti-Factor VIII antibody confirmed the results obtained with anti-CD31 (Figure 5c,d), despite the fact that the anti-Factor VIII antibody used here crossreact with both human and mouse endothelial cells. These findings confirm our previous reports that transplantation of human endothelial cells or human dental pulp stem cells result in the engineering of human blood vessels in murine hosts (Bento et al., 2013; Nör et al., 2001; Sakai et al., 2010; Sasaki et al., 2020).
Fig. 5. VEGFR1high SHED cells are more vasculogenic than VEGFR1low SHED in vivo.
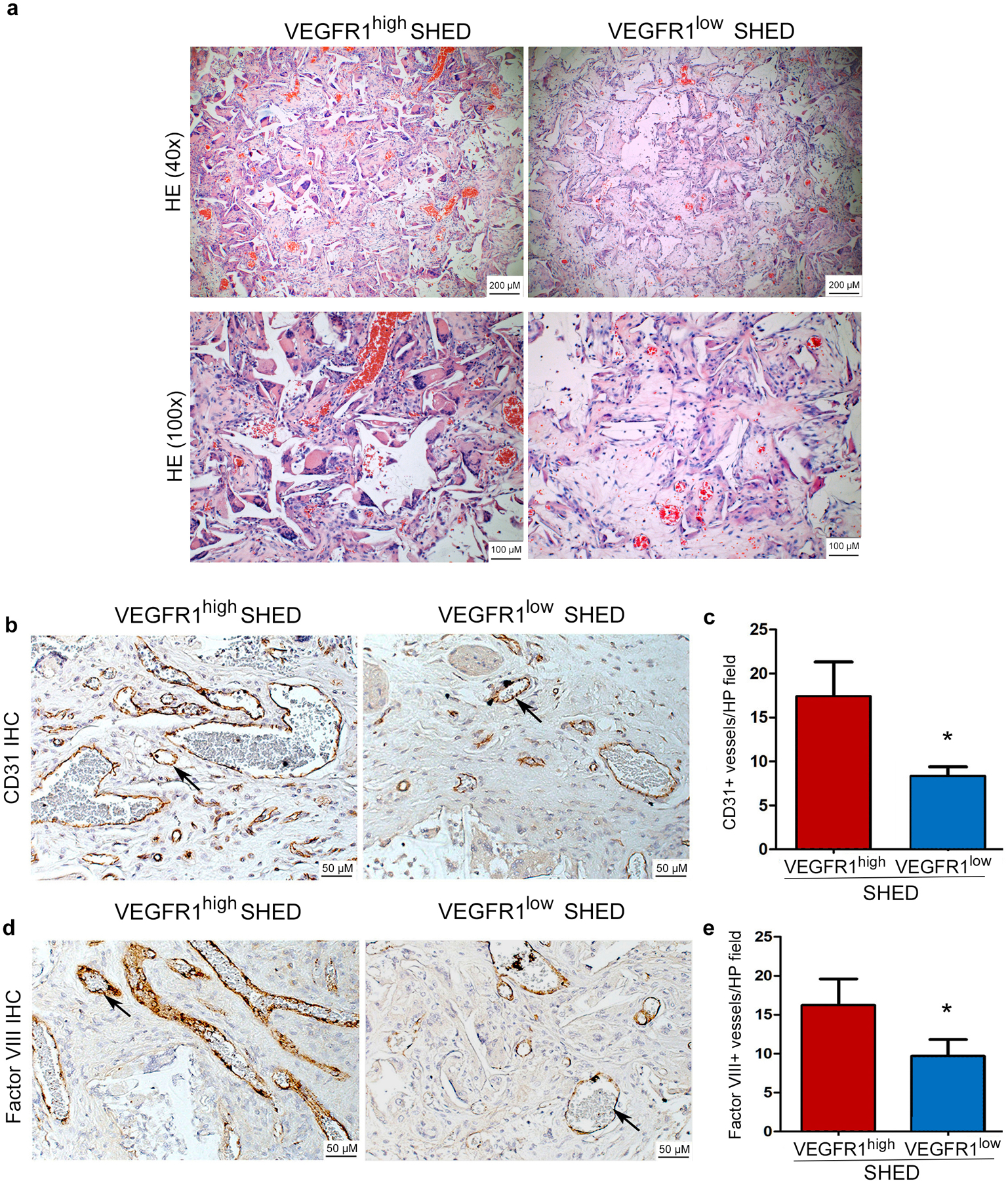
(a,c) Human VEGFR1high and VEGFR1low SHED were seeded in biodegradable scaffolds (n=6 per experimental condition) and transplanted into the subcutaneous space of immunodeficient mice. Four weeks after transplantation, the scaffolds were retrieved, fixed, and paraffin embedded. (a) representative images of sections stained with Hematoxylin and eosin at low and high magnification (bar: 100 μm/ 200 μm) and (b,d) Immunohistochemistry with anti-human CD-31 or anti-Factor VIII antibody to identify blood vessels (brown color). Representative vessels are highlighted with black arrows (bar: 50 μm). (c,e) Graphs depicting the number of CD31-positive or Factor VIII-positive blood vessels inside the scaffolds. Data represent analysis of 8 randomly selected microscopic fields from each scaffold (n=6) at 200x.
Discussion
Dental pulps stem cells are rather unique stem cells developmentally derived from the neural crest (REF). The 2 major hallmarks of physiological stemness (multipotency and self-renewal) have been extensively characterized in these pulp stem cells (Cucco et al., 2020; Gronthos et al., 2000; Lambrichts et al., 2017; Miura et al., 2003; Oh et al., 2020; Sakai et al., 2010). While it is well-known that dental pulp stem cells can differentiate into multiple cell types, it is unclear whether every single stem cell is multipotent or if dental pulp stem cells are a heterogeneous cell type containing smaller sub-groups of cells that are “primed” to undergo diverse differentiation pathways. Here, we began to explore this question by hypothesizing that stem cells of dental pulp origin contain a subgroup of cells that are primed to undergo a vasculogenic differentiation pathway.
VEGFR1 and VEGFR2 are constitutively expressed in endothelial cells and function as the primary regulators of VEGF signaling in blood vessels (Karaman et al., 2018; Trapiella-Alfonso et al., 2018). While VEGFR1 signaling is required for the survival of vascular endothelial cells, VEGFR2 regulates blood vessel sprouting and neovascularization (Zhang et al., 2010). Interestingly, the receptor that fine tune angiogenesis and vascular remodeling is VEGFR2, but VEGF binds to VEGFR1 (soluble or membrane bound) with more affinity than to VEGFR2. In this way, the number of VEGF molecules available to bind to VEGFR2 is modulated and the angiogenic process is regulated (Balsera et al., 2017; Millauer et al., 1993; Trapiella-Alfonso et al., 2018). We observed here that dental pulp stem cells express VEGFR1 constitutively, but not VEGFR2. However, VEGFR2 expression can be induced upon exposure of dental pulp stem cells to vasculogenic differentiation medium containing VEGF165 (Bento et al., 2013; Sasaki et al., 2020). We also observed that expression of CD31 and VE-Cadherin follow upregulation of VEGFR2 expression in dental pulp stem cells (Sasaki et al., 2020). Indeed, VEGF induces activation of MEK/ERK signaling and induction of ERG transcriptional activity that results in expression of VE-Cadherin (Sasaki et al., 2020). Collectively, these data suggest that VEGF binding to VEGFR1 initiates the vasculogenic differentiation of dental pulp stem cells. Once these cells begin to express VEGFR2, then they acquire the capacity of becoming a differentiated vascular endothelial cell expressing CD31 that is able of forming functional vascular networks that anastomize with existing vessels through the function of VE-Cadherin (Sasaki et al., 2020).
An important issue to consider here is the scope of the impact of VEGF signaling through VEGFR1 on vasculogenic responses mediated by dental pulp stem cells. It is known that VEGF induces proliferation, migration and survivals of endothelial cells, but these cells express both VEGFR1 and VEGFR2 (Apte et al., 2019; Karaman et al., 2018). However, the full impact of VEGF on dental pulp stem cells (expressing only VEGFR1 at baseline) was unclear. The results presented here demonstrated that VEGFR1 levels (i.e. high or low) had no impact on DPSC proliferation when cells were exposed to vasculogenic medium (containing 50 ng/ml VEGF165) or regular medium (containing trace levels of VEGF present in bovine serum). As such, the increased number of capillary sprouts observed here with high VEGFR1 cells is not simply a consequence of increased number of cells. Our in vitro data also suggest that in unsorted conditions the VEGFR1high population “takes over” and exhibit a predominant effect on overall capillary sprouting, as the number of sprouts generated by unsorted cells is lower than that of sorted VEGFR1high cells (particularly with SHED cells).
Western blots and flow cytometric analyses demonstrated that a higher percentage of SHED cells exhibit high levels of VEGFR1, when compared to DPSC cells. This is consistent with the observation that SHED cells are more angiogenic than DPSC in response to VEGF (Xu et al., 2018), and with the results of the capillary sprout assays performed here with unsorted cells. However, once we sorted out the VEGFR1high cells from both DPSC and SHED, the sorted cells from both cell types generated similar numbers of capillary sprouts in vitro. As such, one concludes that the vasculogenic potential of each individual VEGFR1high SHED is similar to the vasculogenic potential of each individual VEGFR1high DPSC. But, in aggregate SHED are more vasculogenic because they contain about twice as many VEGFR1high cells as DPSC from permanent teeth.
For many years, our laboratory worked under the assumption that dental pulp stem cells were monolithic, i.e. they consisted of a homogeneous cell population in which multipotency was a consequence of the possibility of each stem cell to differentiate into several different cell types (Graphical Abstract). However, a series of observations contradict this hypothesis, at least in regards to vasculogenic differentiation, as follows. Studies performed several years ago demonstrated that global (shRNA-mediated) silencing of VEGFR1 expression inhibits vasculogenic differentiation of dental pulp stem cells (Bento et al., 2013; Sakai et al., 2010). However, at that time we did not know if every single dental pulp stem cell expressed VEGFR1, or if only a sub-population of these cells expressed VEGFR1 (and was capable of responding to VEGF stimulation). Here, we observed that only 10–15% DPSC (permanent teeth) and 20–25% SHED (primary teeth) express constitutive VEGFR1, while the remaining cells (i.e. the majority of these cells) do not express this receptor. This finding gave rise to the hypothesis that dental pulp stem cells constitute of polylithic (i.e. heterogeneous) cells containing one small sub-population of cells that are primed to respond to VEGF stimulation and undergo vasculogenic differentiation (as they express VEGFR1), while the remaining cells cannot respond to VEGF (as they do not express VEGFR1). This raises the intriguing possibility that other sub-populations of dental pulp stem cells are primed to undergo alternative differentiation pathways, such as odontoblastic or neurogenic fates (Graphical Abstract). We are currently pursuing studies that aim at expanding the understanding of the polylithic hypothesis, through identification of signaling events and characterization of dental pulp stem cells that undergo non-vasculogenic differentiation pathways.
A limitation inherent to our study design is that we do not know the stability of VEGFR1 expression levels after transplantation of the cells into murine hosts. It is possible that cells that were initially sorted as VEGFR1high cells do not maintain a high VEGFR1 expression level after several weeks in the mouse, and conversely if the VEGFR1low cells remain exhibiting low expression levels of this receptor. These expression levels cannot be accurately quantified in the SHED-derived blood vessels in vivo. Notably, this perceived limitation may explain the observation that SHED-derived blood vessels were also found in scaffolds seeded with VEGFR1low cells, albeit in significantly lower numbers.
In conclusion, this work demonstrates the critical role of VEGF signaling through VEGFR1 for the vasculogenic differentiation of dental pulp stem cells. Perhaps more importantly, it demonstrates that dental pulp stem cells are polylithic and contain at least one unique subset of stem cells characterized by high VEGR1 expression that are primed for vasculogenic differentiation. These results suggest the possibility of purifying specific subpopulations of pulp stem cells according to specific needs. This discovery raises the possibility of sorting for, or specifically engaging, VEGFR1high dental pulp stem cells for vascular engineering and treatment of ischemic conditions.
Acknowledgments:
The authors thank Dr. Songtao Shi for providing us with the SHED cells used in this study. We also thank Dr. Maria A. Machado for all the support, mentorship and guidance throughout this research project. This work was funded by grant #RO1-DE021410 from the National Institutes of Health (N.I.H.) and grant #2018/13675-0 from São Paulo Research Foundation (FAPESP). The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Financial support:
This work was funded by grant #RO1-DE021410 from the National Institutes of Health (NIH/NIDCR) to JEN, and grant #2018/13675-0 from São Paulo Research Foundation (FAPESP) to MTOPB
Footnotes
Competing interests: The authors declare no conflicts of interest related to this work
References
- Apte RS, Chen DS, Ferrara N (2019) VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 176(6):1248–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsera B, Bonache MA, Reille-Seroussi M, Gagey-Eilstein N, Vidal M, Gonzalez-Muniz R, Perez de Vega MJ (2017) Disrupting vegf-vegfr1 interaction: De novo designed linear helical peptides to mimic the vegf13–25 fragment. Molecules 22:1846–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bento LW, Zhang Z, Imai A, Nor F, Dong Z, Shi S, Araujo FB, Nör JE (2013) Endothelial differentiation of shed requires mek1/erk signaling. J Dent Res 92:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande L, Cordeiro MM, Nor SA, Nör JE (2011) Dental pulp stem cells in regenerative dentistry. Odontology 99:1–7. [DOI] [PubMed] [Google Scholar]
- Casagrande L, Demarco FF, Zhang Z, Araujo FB, Shi S, Nor JE (2010) Dentin-derived BMP2 and odontoblast differentiation. J Dent Res 89:603–608. [DOI] [PubMed] [Google Scholar]
- Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith AJ, Nör JE (2008) Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 34:962–969. [DOI] [PubMed] [Google Scholar]
- Cucco C, Zhang Z, Botero TM, Chiego DJ, Castilho RM, Nör JE (2020) SCF/C-Kit Signaling Induces Self-Renewal of Dental Pulp Stem Cells. J Endod 46(9S):S56–S62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alimonte I, Nargi E, Mastrangelo F, Falco G, Lanuti P, Marchisio M, Miscia S, Robuffo I, Capogreco M, Buccella S, Caputi S, Caciagli F, Tetè S, Ciccarelli R (2011) Vascular endothelial growth factor enhances in vitro proliferation and osteogenic differentiation of human dental pulp stem cells. J Biol Regul Homeost Agents 25:57–69. [PubMed] [Google Scholar]
- D’Aquino R, Graziano A, Sampaolesi M, Laino G, Pirozzi G, Rosa AD, Papaccio G (2007) Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ 14:1162–1171. [DOI] [PubMed] [Google Scholar]
- D’Aquino R, Rosa AD, Lanza V, Tirino V, Laino L, Graziano A, Desiderio V, Laino G, Papaccio G (2009) Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater 18:75–83. [DOI] [PubMed] [Google Scholar]
- Deckers MM, van Bezooijen RL, van der Horst G, Hoogendam J, van Der Bent C, Papapoulos SE, Löwik CW (2002) Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology 143:1545–1553. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Chiego DJ, Heys DR (1990) Autoradiographic analysis of odontoblast replacement following pulp exposure in primate teeth. Arch Oral Biol 35:707–15. [DOI] [PubMed] [Google Scholar]
- Gan L, Liu Y, Cui D, Pan Y, Zheng L, Wan M (2020) Dental tissue-derived human mesenchymal stem cells and their potential in therapeutic application. Stem Cells Int 2020:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani A, Manescu A, Langer M, Rustichelli F, Desiderio V, Paino F, Rosa AD, Laino L, D’aquino R, Tirino V, Papaccio G (2013) Three years after transplants in human mandibles, histological and in-line holotomography revealed that stem cells regenerated a compact rather than a spongy bone: biological and clinical implications. Stem Cells Transl Med 2:316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin C, Rochefort GY, Bascetin R, Ying H, Lesieur J, Sadoine J, Beckouche N, Berndt S, Novais A, Lesage M, Hosten B,, Vercellino L, Merlet P, Le-Denmat D, Marchiol C, Letourneur D, Nicoletti A, Vital SO, Poliard A, Salmon B, Muller L, Chaussain C, Germain S (2016) Priming dental pulp stem cells with fibroblast growth factor-2 increases angiogenesis of implanted tissue-engineered constructs through hepatocyte growth factor and vascular endothelial growth factor secretion. Stem Cells Transl Med 5:392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (dpscs) in vitro and in vivo. Proc Natl Acad Sci U S A 97:13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janebodin K, Zeng Y, Buranaphatthana W, Ieronimakis N, Reyes M (2013) VEGFR2-dependent angiogenic capacity of pericyte-like dental pulp stem cells. J Dent Res 92:524–531. [DOI] [PubMed] [Google Scholar]
- Karaman S, Leppänen VM, Alitalo K (2018) Vascular endothelial growth factor signaling in development and disease. Development 145:dev151019. [DOI] [PubMed] [Google Scholar]
- Lambrichts I, Driesen RB, Dillen Y, Gervois P, Ratajczak J, Vangansewinkel T, Wolfs E, Bronckaers A, Hilkens P (2017) Dental pulp stem cells: Their potential in reinnervation and angiogenesis by using scaffolds. J Endod 43:S12–S16. [DOI] [PubMed] [Google Scholar]
- Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NP, Risau W, Ullrich A (1993) High affinity vegf binding and developmental expression suggest flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell 72:835–846. [DOI] [PubMed] [Google Scholar]
- Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S (2003) Shed: Stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A 100:5807–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monache SD, Martellucci S, Clementi L, Pulcini F, Santilli F, Mei C, Piccoli L, Angelucci A, Mattei V (2019) In Vitro conditioning determines the capacity of dental pulp stem cells to function as pericyte-like cells. Stem Cells Dev 28:695–706 [DOI] [PubMed] [Google Scholar]
- Nakashima M, Iohara K, Murakami M, Nakamura H, Sato Y, Ariji Y, Matsushita K (2017) Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: a pilot clinical study. Stem Cell Res Ther 8:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nör JE, Peters MC, Christensen JB, Sutorik MM, Linn S, Khan MK, Addison CL, Mooney DJ, Polverini PJ (2001) Engineering and characterization of functional human microvessels in immunodeficient mice. Lab Invest 81:453–463. [DOI] [PubMed] [Google Scholar]
- Oh M, Nör JE (2015) The Perivascular Niche and Self-Renewal of Stem Cells. Front Physiol 6:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh M, Zhang Z, Mantesso A, Oklejas AE, Nör JE (2020) Endothelial-Initiated Crosstalk Regulates Dental Pulp Stem Cell Self-Renewal. J Dent Res 99:1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paino F, Noce ML, Giuliani A, Rosa AD, Mazzoni S, Laino L, Amler E, Papaccio G, Desiderio V, Tirino V (2017) Human DPSCs fabricate vascularized woven bone tissue: a new tool in bone tissue engineering. Clin Sci 131:699–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piva E, Tarle SA, Nör JE, Zou D, Hatfield E, Guinn T, Eubanks EJ, Kaigler D (2017) Dental pulp tissue regeneration using dental pulp stem cells isolated and expanded in human serum. J Endod 43:568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa V, Zhang Z, Grande RH, Nör JE (2013) Dental pulp tissue engineering in full-length human root canals. J Dent Res 92:970–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai VT, Zhang Z, Dong Z, Neiva KG, Machado MA, Shi S, Santos CF, Nör JE (2010) SHED differentiate into functional odontoblasts and endothelium. J Dent Res 89:791–796. [DOI] [PubMed] [Google Scholar]
- Sasaki JI, Zhang Z, Oh M, Pobocik AM, Imazato S, Shi S, Nör JE (2020) VE-Cadherin and Anastomosis of Blood Vessels Formed by Dental Stem Cells. J Dent Res 99:437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AJ, Duncan HF, Diogenes A, Simon S, Cooper PR (2016) Exploiting the bioactive properties of the dentin-pulp complex in regenerative endodontics. J Endod 42:47–56. [DOI] [PubMed] [Google Scholar]
- Trapiella-Alfonso L, Broussy S, Liu WQ, Vidal M, Lecarpentier E, Tsatsaris V, Gagey-Eilstein N (2018) Colorimetric immunoassays for the screening and specificity evaluation of molecules disturbing vegfs/vegfrs interactions. Anal Biochem 544:114–120. [DOI] [PubMed] [Google Scholar]
- Walker EJ, Su H, Shen F, Degos V, Amend G, Jun K, Young WL (2012) Bevacizumab attenuates vegf-induced angiogenesis and vascular malformations in the adult mouse brain. Stroke 43:1925–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DS, Miura M, Demura H, Sato K (1997) Anabolic effects of 1,25-dihydroxyvitamin D3 on osteoblasts are enhanced by vascular endothelial growth factor produced by osteoblasts and by growth factors produced by endothelial cells. Endocrinology 138:2953–2962. [DOI] [PubMed] [Google Scholar]
- Xu JG, Gong T, Wang YY, Zou T, Heng BC, Yang YQ, Zhang CF (2018) Inhibition of tgf-beta signaling in shed enhances endothelial differentiation. J Dent Res 97:218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, He J, Zhang C, Xu J, Wang Y (2019) Strategies for derivation of endothelial lineages from human stem cells. Stem Cell Res Ther 10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan K, Li B, Guo H, Sun W, Kou X, He X, Zhang Y, Sun J, Liu A, Liao L, Liu S, Liu W, Hu C, Shi S, Jin Y (2018) Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci Transl Med 10:eaaf3227. [DOI] [PubMed] [Google Scholar]
- Yusof MFH, Zahari W, Hashim SNM, Osman ZF, Chandra H, Kannan TP, Noordin KBAA, Azlina A (2018) Angiogenic and osteogenic potentials of dental stem cells in bone tissue engineering. J Oral Biol Craniofac Res 8:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Neiva KG, Lingen MW, Ellis LM, Nör JE (2010) VEGF-dependent tumor angiogenesis requires inverse and reciprocal regulation of VEGFR1 and VEGFR2. Cell Death Diff 17:499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Nor F, Lingen MW, Oh M, Cucco C, Shi S, Nör JE (2016) Wnt-B-catenin signaling determines the vasculogenic fate of postnatal mesenchymal stem cells. Stem Cells 34:1576–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]


