Abstract
Podocyte apoptosis is a key risk factor for the progression of kidney diseases. MicroRNA (miR)-199b-5p has been shown to be involved in cell apoptosis. However, the molecular mechanisms of miR-199b-5p in podocyte apoptosis remain uncertain. Thus, the present study aimed to investigate whether miR-199b-5p participates in the regulation of podocyte apoptosis and to elucidate the involved mechanisms of this process. A podocyte apoptosis model was constructed using adriamycin (ADR) in vitro. miR-199b-5p mimic and inhibitor were transfected in podocytes to change the expression level of miR-199b-5p. RNA expression was examined by reverse transcription-quantitative PCR. Western blotting was used to measure protein expression. Apoptosis was monitored via flow cytometry and detection of apoptosis-associated proteins. The results from the present study demonstrated that miR-199b-5p was upregulated and that regulator of G-protein signaling 10 (RGS10) was downregulated in ADR-stimulated podocytes. Overexpression of miR-199b-5p could inhibit RGS10 expression and stimulate podocyte apoptosis, whereas miR-199b-5p knockdown restored the levels of RGS10 and ameliorated podocyte apoptosis in ADR-induced podocytes. Furthermore, the effects of miR-199b-5p overexpression could be significantly reversed by RGS10 overexpression. In addition, podocyte transfection of miR-199b-5p activated the AKT/mechanistic target of rapamycin (mTOR) signaling, which was blocked following RGS10 overexpression. Taken together, the present study demonstrated that miR-199b-5p upregulation could promote podocyte apoptosis by inhibiting the expression of RGS10 through the activation of AKT/mTOR signaling.
Keywords: microRNA-199b-5p, regulator of G-protein signaling 10, podocyte, apoptosis, AKT/mechanistic target of rapamycin signaling pathway
Introduction
Chronic kidney disease (CKD) is a long-term condition affecting ~10% of the global population. It is a key cause of morbidity and mortality worldwide that accounts for a large proportion of medical expenses (1,2). Among the various factors that contribute to the progression of CKD, proteinuria is relatively independent, and an important pathophysiological condition for the generation of this symptom is podocyte injury (3-5). Podocytes are highly specialized terminally differentiated epithelial cells that maintain the glomerular filtration barrier and are vulnerable to multiple injuries (6-8). One of the causes of podocyte loss and injury is apoptosis, thus it is of clinical importance to identify the potential therapeutic targets of podocyte apoptosis (9,10).
MicroRNAs (miRNAs) are small non-coding RNAs that suppress gene expression by targeting the 3'-untranslated regions (UTRs) of mRNAs, leading to mRNA degradation or transcriptional blocking (11). Previous studies reported that miRNAs are involved in numerous biological processes, including cell apoptosis, proliferation, differentiation and development, and in a variety of kidney diseases (12,13). For example, miR-24 promotes renal ischemic injury by inducing the apoptosis of endothelial and tubular epithelial cells (14). Furthermore, miR-34a promotes renal fibrosis by inhibiting klotho expression in tubular epithelial cells (15). The key role of miR-29c in promoting the progression of diabetic nephropathy is mainly achieved through the Spry1/Rho-kinase pathway (16). Recent studies demonstrated that miR-199b-5p serves a critical role in numerous diseases (17). Lai et al (18) reported that miR-199b-5p is downregulated in renal cell carcinoma and acts as a tumor suppressor. However, no study has yet revealed the role of miR-199b-5p in podocyte injury.
The present study used TargetScan and miRDB tools to analyze the interactions between miR-199b-5p and different mRNAs, and reported that miR-199b-5p had potential binding sites in the 3'-UTR of the regulator of G-protein signaling 10 (RGS10). As an important member of the RGS protein superfamily, RGS10 is involved in the regulation of certain physiological processes in various types of disease (19,20); however, its role in renal diseases remains unknown. The present study aimed therefore to investigate the effects of miR-199b-5p on RGS10 expression, cell apoptosis and injury in podocytes.
Materials and methods
Cell culture and transfection
The immortalized mouse podocytes (MPC5 cell line) were gifted by the Nephrology Research Center of The Second Affiliated Hospital of Nanjing Medical University. Podocytes were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin (Gibco; Thermo Fisher Scientific, Inc.), 100 µg/ml streptomycin and 10 U/ml IFN-γ (PeproTech, Inc.) and placed at 33˚C in an incubator containing 5% CO2 for 2-3 generations. When proliferation reached 70-80% (~2x105 cells per well), medium was replaced with IFN-γ free medium for differentiation and cells were placed in an incubator at 37˚C with 5% CO2 for 10-14 days. The miR-199b-5p mimic (sense, 5'-CCCAGU GUUUAGACUACCUGUUC-3'; antisense, 5'-ACAGGUAGU CUAAACACUGGGUU-3'; GenePharma, Shanghai, China), miR-199b-5p inhibitor (5'-GAACAGGUAGUCUAAACACU GGG-3'; Shanghai GenePharma Co., Ltd.), negative control mimic (sense, 5'-UUCUCCGAACGUGUCACGUTT-3'; antisense, 5'-ACGUGACACGUUCGGAGAATT-3'; Shanghai GenePharma Co., Ltd.), and negative control inhibitor (5'-CAG UACUUUUGUGUAGUACAA-3'; Shanghai GenePharma Co., Ltd.) were transfected for 6 h at a concentration of 100 nM using Lipofectamine 2000 transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. The medium was changed again, and 1 µg/ml ADR (MedChemExpress) was added for 24 h treatment (21). To verify the effect of AKT/mTOR pathway on ADR-induced podocyte apoptosis, the differentiated podocytes were treated with 1 mg/l rapamycin (RAPA; MedChemExpress) after ADR intervention. In addition, to verify the relationship between RGS10 and apoptosis, rescue experiments were performed in which podocytes were transfected with miR-199b-5p mimic and treated with ADR, then transfected with 1x108 PFU/ml adenoviral RGS10 (AdRGS10; Shanghai GenePharma Co., Ltd.) or adenoviral negative control (AdNC; Shanghai GenePharma Co., Ltd.) for 48 h.
Bioinformatical analysis
Prediction of the miR-199b-5p-target gene was performed using the TargetScan (http://www.targetscan.org/vert_71/) and miRDB (http://mirdb.org/).
Western blotting
Cells were lysed using RIPA Lysis Buffer (Beyotime Institute of Biotechnology) supplemented with 1% protease inhibitor phenylmethylsulfonyl fluoride (Beyotime Institute of Biotechnology). Protein concentration was determined using the BCA method (Beyotime Institute of Biotechnology). Proteins (30 µg) were separated by 8% or 12% SDS-PAGE and were transferred onto PVFD membranes (MilliporeSigma; cat. no. HATF09025). Membranes were blocked with 5% non-fat dry milk solution prepared in 0.01 M Tris-buffered saline (pH 7.4, containing 0.01% Tween-20) at room temperature for 2 h. Membranes were incubated with primary antibodies against nephrin (1:1,000; mouse; cat. no. sc-376522; Santa Cruz Biotechnology, Inc.), podocin (1:1,000; rabbit; cat. no. ab181143; Abcam), RGS10 (1:1,000; rabbit; cat. no. DF4414; Affinity Biosciences, Ltd.), Bax (1:1,000; rabbit; cat. no. AF0120; Affinity Biosciences, Ltd.), Bcl-2 (1:1,000; rabbit; cat. no. AF6139; Affinity Biosciences, Ltd.), mTOR (1:1,000; mouse; cat. no. 66888-1-Ig; ProteinTech Group, Inc.), phosphorylated (p-) mTOR (1:1,000; rabbit; cat. no. 5536T; Cell Signaling Technology), AKT (1:1,000; rabbit; cat. no. 10176-2-AP; ProteinTech Group, Inc.), p-AKT (1:2,000; Ser473; mouse; cat. no. 66444-1-Ig; ProteinTech Group, Inc.) and GAPDH (1:1,000; rabbit; cat. no. 5174; Cell Signaling Technology, Inc.), overnight at 4˚C. Membranes were washed with TBST and incubated with horseradish peroxidase-conjugated secondary antibodies (goat anti-mouse, 1:2,000, cat. no. 91196S, Cell Signaling Technology, Inc.; or goat anti-rabbit, 1:4,000, cat. no. S0001, Affinity Biosciences, Ltd.) at room temperature for 2 h. Enhanced chemiluminescence reagent (Biosharp) and a gel imaging analysis system were used to detect the signal on the membrane. The data were analyzed via densitometry using ImageJ software 1.8.0 (National Institute of Health) and normalized to expression of the internal control (GAPDH).
Real-time quantitative PCR (RT-q)PCR
Total RNA (including mRNA and microRNA) was extracted using TRIzol® reagent (Life Technologies; Agilent, Inc.). miRNA RT-qPCR was performed using the All-in-One MicroRNA Assay Kit (iGeneBio, Inc.) and Applied Biosystem 7000 Quantitative PCR Instrument using U6 as an internal reference. mRNA RT-qPCR was performed using the HiScript III RT SuperMix (Vazyme Biotech Co., Ltd.) and GAPDH was used as an internal reference. The sequences of the primers used (Ruijie Technology) are listed in Table I. The thermocycling conditions are presented in Tables II and III. Three replicate wells were set for each sample, and the experiment was repeated at least three times. The relative expression levels were normalized to endogenous controls and were expressed as 2-ΔΔCq (22).
Table I.
Sequences of the primer sequences used for reverse transcription quantitative PCR.
| Genes | Forward primer | Reverse primer |
|---|---|---|
| miR-199b-5p | 5'-UUAUCCUAAUUGCUCCUACGGCU-3' | 5'-AUUCGGCAUCGCGCUAAACGUUA-3' |
| U6 | 5'-CTCGCTTCGGCAGCACATATACT-3' | 5'-ACGCTTCACGAATTTGCGTGTC-3' |
| GAPDH | 5'-TGGATTTGGACGCATTGGTC-3' | 5'-TTTGCACTGGTACGTTGAT-3' |
| Nephrin | 5'-ATGGGAGCTAAGGAAGCCACA-3' | 5'-GATGGAGGATTACGCTGGG-3' |
| Podocin | 5'-GCATCAAGCCCTCTGGATTAG-3' | 5'-AGACGGAGATCAACCTTGTGATA-3' |
| Bax | 5'-TGAAGACAGGGGCCTTTTTG-3' | 5'-AATTCGCCGGAGACACTCG-3' |
| Bcl-2 | 5'-GTCGCTACCGTCGTGACTTC-3' | 5'-CAGACATGCACCTACCCAGC-3' |
| RGS10 | 5'-TCCATGACGGAGATGGGAG-3' | 5'-AACAAGACATTCTCTTCGCTGAA-3' |
miR, microRNA; RGS10, regulator of G-protein signaling 10.
Table II.
Thermocycling conditions used during reverse transcription quantitative PCR for microRNA expression level evaluation.
| Stage | Step | Repetition | Temperature, ˚C | Duration |
|---|---|---|---|---|
| Stage 1 | Initial denaturation | 1 | 95 | 10 min |
| Stage 2 | Amplification cycles | 40 | 95 | 10 sec |
| 60 | 20 sec | |||
| 72 | 10 sec | |||
| Stage 3 | Dissociation curve | 1 | 95 | 15 sec |
Table III.
Thermocycling conditions used during reverse transcription quantitative PCR for mRNA expression level evaluation.
| Stage | Step | Repetition | Temperature, ˚C | Duration |
|---|---|---|---|---|
| Stage 1 | Initial denaturation | 1 | 95 | 30 sec |
| Stage 2 | Amplification cycles | 40 | 95 | 10 sec |
| 60 | 30 sec | |||
| Stage 3 | Dissociation curve | 1 | 95 | 15 sec |
| 60 | 60 sec | |||
| 95 | 15 sec |
Apoptosis detection (Annexin V-APC/7-AAD)
Cells were stained with the Annexin V-APC/7-AAD apoptosis detection kit (Nanjing KeyGen Biotech Co., Ltd.) according to the manufacturer's instructions. The CytoFLEXLX Flow cytometer (Beckman Coulter, Inc.) was used for data analysis. The excitation wavelength was 633 nm. The FL4 channel was used for the red fluorescence of Annexin V-APC. The FL2 channel was used for the 7-AAD red fluorescence. Annexin V-APC was used as the x-axis, and 7-AAD was used as the y-axis. The numerical axis was the fluorescence signal intensity. A two-dimensional scatter diagram was constructed and the apoptotic rate was evaluated. Data analysis was conducted using FlowJo 7.6 (Treestar).
Statistical analysis
Data analyses were performed using GraphPad Prism 6.0 software (GraphPad Software, Inc.). Data are expressed as the means ± standard deviation. Comparisons between two groups were performed by t-test with Bonferroni correction. Comparisons between more than two groups were performed by one-way ANOVA followed by a Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
miR-199b-5p is upregulated in ADR-induced podocytes
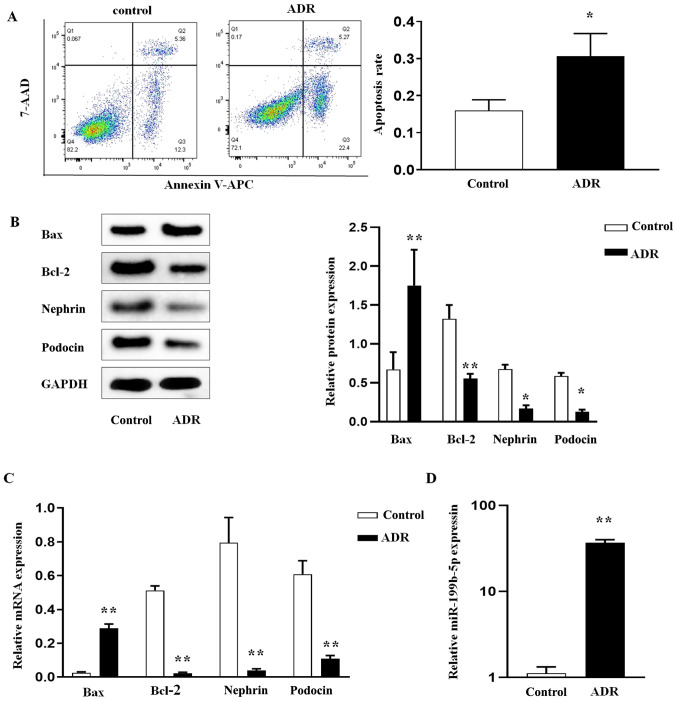
To explore the role miR-199b-5p in podocyte injury, ADR-treated podocytes were established as the in vitro experimental model. Damage of podocytes stimulated by ADR was first evaluated. To this end, podocytes were cultured and differentiated, and then treated with ADR. Following treatment, podocyte apoptosis was evaluated using Annexin V-APC/7-AAD double staining and flow cytometry. The results demonstrated that the apoptosis rate in the ADR group was significantly increased compared with the control group (normal podocytes; Fig. 1A). Subsequently, expression levels of Bax and Bcl-2, two known indicators of apoptosis (23), were evaluated. The results from western blotting (Fig. 1B) and RT-qPCR (Fig. 1C) demonstrated that, compared with the control group, ADR treatment increased the expression level of Bax, whereas it decreased the level of Bcl-2 in ADR-treated podocytes, resulting in their apoptosis. Furthermore, the expression levels of nephrin and podocin, which are markers of podocyte function, were also evaluated in the control and ADR-treated groups. The results from western blotting (Fig. 1B) and RT-qPCR (Fig. 1C) showed significant decrease in nephrin and podocin expression levels in ADR-treated podocytes compared with the control group. In addition, a significant increase in miR-199b-5p expression in ADR-treated podocytes was demonstrated compared with that in normal podocytes (Fig. 1D). These findings suggested that the elevated miR-199b-5p expression may contribute to ADR-mediated podocyte apoptosis.
Figure 1.
ADR induces podocyte apoptosis and the regulation of miR-199b-5p. After podocytes were treated with ADR, (A) the apoptosis rate was determined by flow cytometry. (B) Bax, Bcl-2, nephrin, and podocin protein expression was determined by western blotting. (C) RT-qPCR analysis of Bax, Bcl-2, nephrin, and podocin in podocytes. (D) RT-qPCR analysis of miR-199b-5p in podocytes. Control indicates normal podocytes. n=3 in each group. *P<0.05 and **P<0.01. ADR, adriamycin; miR, microRNA; RT-qPCR, reverse transcription quantitative PCR.
miR-199b-5p downregulation inhibits ADR-induced podocyte apoptosis and miR-199b-5p overexpression promotes apoptosis
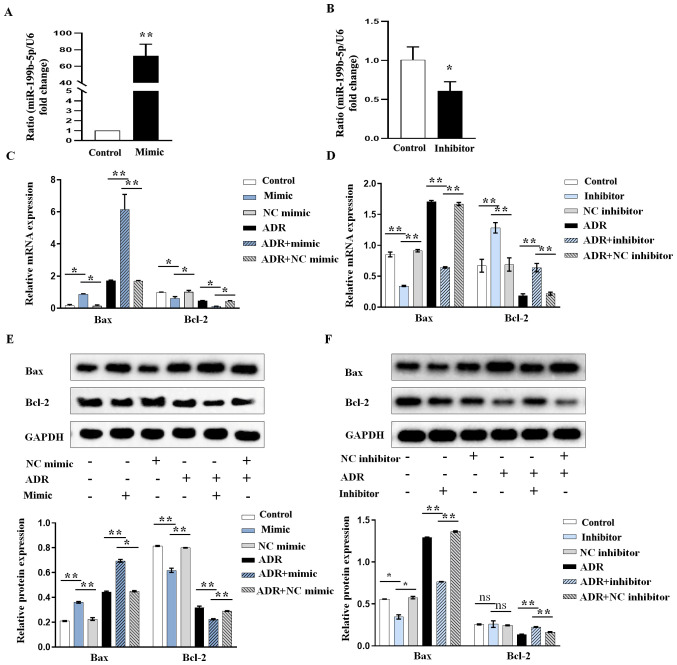
We further investigated the role of miR-199b-5p in the induction of apoptosis using miR-199b-5p overexpression or knockdown. The results from RT-qPCR confirmed that the miR-199b-5p mimic (abbreviated as mimic in all figures) could significantly increase miR-199b-5p expression (Fig. 2A) while miR-199b-5p inhibitor (abbreviated as inhibitor in all figures) significantly decreased miR-199b-5p expression in podocytes (Fig. 2B). Furthermore, according to the results from RT-qPCR and western blotting, the increase in Bax and decrease in Bcl-2 expression levels in ADR-treated podocytes were significantly aggravated by miR-199b-5p mimic (Fig. 2C and E), whereas these were significantly neutralized by miR-199b-5p inhibitor (Fig. 2D and F). These results indicated that miR-199b-5p may be considered as an important regulator in ADR-induced podocyte injury.
Figure 2.
miR-199b-5p expression affects the apoptosis of ADR-induced podocytes. (A) RT-qPCR analysis of miR-199b-5p expression in podocytes transfected with miR-199b-5p mimic. (B) RT-qPCR analysis of miR-199b-5p expression in podocytes transfected with miR-199b-5p inhibitor. (C and E) Podocytes were treated with ADR, miR-199b-5p mimic or NC mimic. ADR-treated podocytes were transfected with miR-199b-5p mimic or NC mimic. Apoptosis-related genes were detected by RT-qPCR and western blotting in podocytes with indicated treatments and transfections. (D nd F) Podocytes were treated with ADR, miR-199b-5p inhibitor or NC inhibitor. ADR-treated podocytes were transfected with miR-199b-5p inhibitor or NC inhibitor. Apoptosis-related genes were detected by RT-qPCR and western blotting in podocytes with indicated treatments and transfections. Control indicates normal podocytes. n=3 in each group. *P<0.05 and **P<0.01. ADR, adriamycin; miR, microRNA; RT-qPCR, reverse transcription quantitative PCR; NC, negative control
miR-199b-5p regulates the expression of RGS10 in podocytes
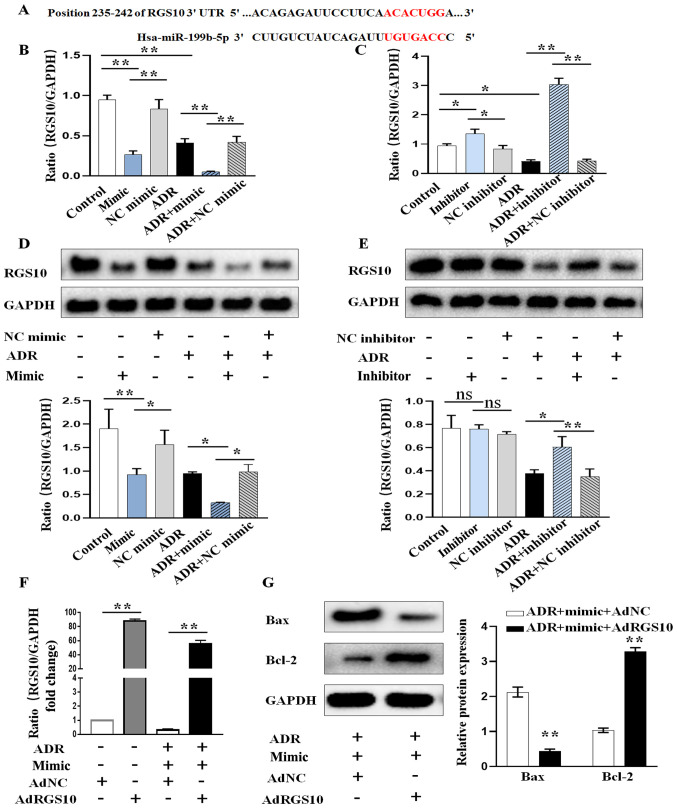
To elucidate the molecular mechanisms by which miR-199b-5p exerts its function, miR-199b-5p targets were explored using TargetScan and miRDB bioinformatics algorithms. RGS10 was predicted to be a potential target of miR-199b-5p (Fig. 3A). Thus, we further examined the effect of miR-199b-5p on the expression of RGS10. The results from RT-qPCR confirmed that miR-199b-5p overexpression significantly decreased the mRNA expression of RGS10 (Fig. 3B). Conversely, silencing miR-199b-5p significantly increased RGS10 expression level in podocytes (Fig. 3C). The results from western blotting confirmed that overexpression of miR-199b-5p also inhibited the protein expression of RGS10 (Fig. 3D), while knocking down miR-199b-5p had the opposite effect (Fig. 3E). These results suggested that miR-199b-5p may negatively regulate RGS10 expression at the post-transcriptional level in podocytes. Subsequently, we determined whether RGS10 overexpression could rescue the pro-apoptotic effect of miR-199b-5p on ADR-induced podocytes. Podocytes were transfected with AdRGS10 to increase RGS10 expression and AdNC was used as the negative control (Fig. 3F). The results from western blotting demonstrated that podocyte co-transfection with miR-199b-5p mimic and AdRGS10 significantly reversed miR-199b-5p mimic-induced cell apoptosis in ADR-induced podocytes, as evidenced by the decreased expression of Bax and increased expression of Bcl-2 (Fig. 3G). These results further confirmed that miR-199b-5p may induce apoptosis via regulating RGS10 expression in podocytes.
Figure 3.
miR-199b-5p regulates the expression of RGS10. (A) Predicted binding sites of miR-199b-5p on the 3'UTR of RGS10. (B) RGS10 expression level after transient transfection and treatment of podocytes with miR-199b-5p mimic, NC mimic, ADR, ADR+miR-199b-5p mimic or ADR+NC mimic. (C) RGS10 expression level after the transient transfection and treatment of podocytes with miR-199b-5p inhibitor, NC inhibitor, ADR, ADR+miR-199b-5p inhibitor or ADR+NC inhibitor. (D) RGS10 protein expression in podocytes treated as in (B). (E) RGS10 protein expression in podocytes treated as in (C). (F) Expression level of RGS10 was determined by RT-qPCR in ADR-treated podocytes transfected with miR-199b-5p mimic or miR-199b-5p mimic+AdRGS10. (G) Expression of Bax and Bcl-2 was determined by western blotting in ADR-treated podocytes transfected as in (F). Control indicates normal podocytes. n=3 in each group. *P<0.05 and **P<0.01. ADR, adriamycin; miR, microRNA; RT-qPCR, reverse transcription quantitative PCR; NC, negative control; RGS10, regulator of G-protein signaling 10.
miR-199b-5p promotes the apoptosis of ADR-stimulated podocytes by inhibiting RGS10 and activating the AKT/mTOR signaling pathway
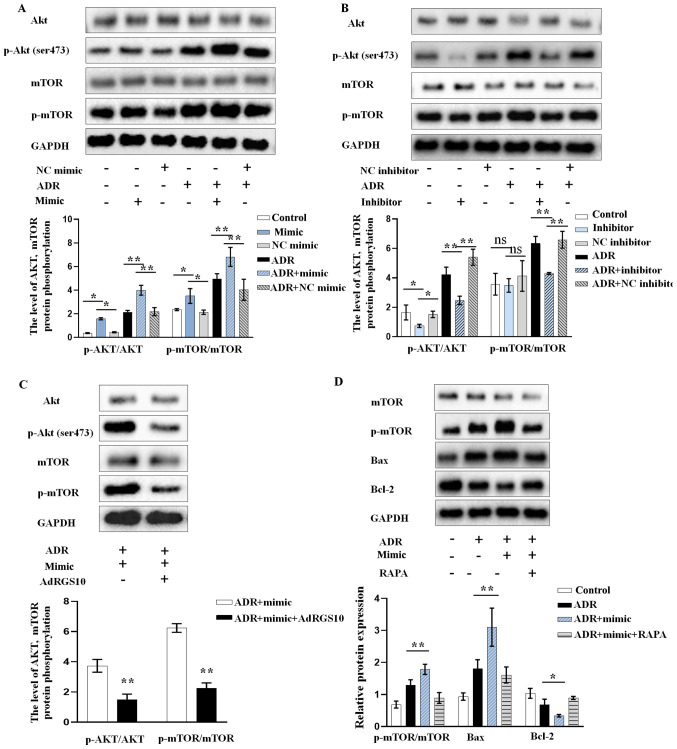
To evaluate the effects of miR-199b-5p on the downstream signaling of RGS10, we determined the expression of p-AKT and p-mTOR in ADR-induced podocytes. Podocytes were transfected with miR-199b-5p mimic, inhibitor or vector. Western blotting analysis demonstrated that miR-199b-5p mimic could promote the ADR-induced phosphorylation of AKT and mTOR in podocytes (Fig. 4A). Conversely, miR-199b-5p knockdown significantly inhibited the increase in p-AKT and p-mTOR induced by ADR (Fig. 4B). These findings indicated that the high expression of p-AKT and p-mTOR ADR-treated podocytes overexpressing miR-199b-5p was reversed by RGS10 overexpression (Fig. 4C). In addition, we confirmed that inhibition of AKT/mTOR by RAPA neutralized the pro-apoptotic effect of miR-199b-5p mimic treatment in ADR-triggered podocytes (Fig. 4D). Taken together, these results indicated that miR-199b-5p may regulate podocyte apoptosis via the RGS10/AKT/mTOR axis.
Figure 4.
miR-199b-5p activates mTOR/AKT signaling via regulating RGS10 expression. AKT, p-AKT, mTOR, and p-mTOR protein expression level after transient transfection and treatment of podocytes with (A) miR-199b-5p mimic, NC mimic, ADR, ADR+miR-199b-5p mimic or ADR+NC mimic. and (B) miR-199b-5p inhibitor, NC inhibitor, ADR, ADR+miR-199b-5p inhibitor or ADR+NC inhibitor. (C) AKT, p-AKT, mTOR, and p-mTOR protein expression level in ADR-treated podocytes transfected with miR-199b-5p mimic or miR-199b-5p mimic+AdRGS10. (D) Expression of p-mTOR, mTOR and apoptosis-related proteins in podocytes treated with ADR, ADR+miR-199b-5p mimic or ADR+miR-199b-5p mimic+RAPA evaluated by western blotting. Control indicates normal podocytes. n=3 in each group. *P<0.05 and **P<0.01. ADR, adriamycin; RAPA, rapamycin; ns, non-significant; Ad, adenovirus; RGS10, regulator of G-protein signaling 10; mTOR, mechanistic target of rapamycin; NC, negative control; p, phosphorylated.
Discussion
Podocyte apoptosis is critical in the development of CKD. To the best of our knowledge, the present study demonstrated for the first time that miR-199b-5p may serve a critical role in the pathogenesis of podocyte apoptosis. This study suggested that miR-199b-5p was upregulated in ADR-induced podocytes of mice. Furthermore, overexpression of miR-199b-5p in ADR-stimulated podocytes exacerbated podocyte apoptosis, whereas miR-199b-5p downregulation ameliorated podocyte lesions. It was also found that miR-199b-5p could negatively regulate RGS10 and activate the AKT/mTOR signaling pathway in podocytes. These findings indicated that miR-199b-5p may be considered as be a novel therapeutic target for CKD.
There is increasing evidence suggesting that miR-199b-5p can regulate apoptosis in a variety of diseases. A previous study demonstrated that miR-199b-5p inhibits cell apoptosis and promotes cell proliferation and metastasis in cervical cancer cells (24). Furthermore, in the metastasis of papillary thyroid carcinoma, miR-199b-5p was found to be upregulated and involved in the process of cancer cell apoptosis (25). In addition, Wang et al (26) reported that miR-199b-5p overexpression enhances cell apoptosis in oral cancer. So far, few studies have evaluated the role of miR-199b-5p in kidney disease; however, Kang et al (27) reported that miR-199b-5p aggravates the renal tubular injury in diabetic nephropathy by promoting apoptosis in renal tubular cells via targeting klotho. However, the role of miR-199b-5p in podocyte apoptosis remains unclear and was therefore investigated in the present study. The results from cell functional experiments indicated that miR-199b-5p overexpression promoted podocyte apoptosis, whereas miR-199b-5p knockdown had the opposite effect. Furthermore, silencing miR-199b-5p silencing rescued the aggravated impact of podocyte apoptosis induced by ADR.
Another major finding from the present study was the identification of RGS10 as a novel target gene of miR-199b-5p. RGS10 is one of the smallest members of the RGS family and it exerts various biological effects in multiple organs (19,28). It has been reported that ovarian cancer cell apoptosis is decreased following RGS10 knockdown (29). Furthermore, a previous study demonstrated that premature apoptosis occurs in osteoclasts after RGS10 downregulation (30). However, the role and underlying mechanism of RGS10 in podocyte apoptosis remain to be elucidated. The results from the present study demonstrated that RGS10 was significantly downregulated in ADR-treated podocytes. In addition, RGS10 expression was inhibited by miR-199b-5p mimic and elevated by miR-199b-5p inhibitor. These results indicated that RGS10 expression may be negatively correlated with miR-199b-5p expression. The effects of RGS10 on podocyte apoptosis in podocytes were further verified by performing cell functional experiments. Overexpression of RGS10 could reverse the promoting effects of miR-199b-5p on podocyte apoptosis. The results suggested that miR-199b-5p could stimulate podocyte apoptosis by inhibiting RGS10.
The AKT/mTOR signaling pathway is involved in cell apoptosis in a variety of kidney diseases (31,32). There is evidence that miR-199b-5p induces cell apoptosis via directly targeting mTOR in endometrial endometrioid adenocarcinoma (33) and negatively regulates cell proliferation via the AKT signaling pathway in glioma (34). These results show that miR-199b-5p might play an important role in the activation of the AKT/mTOR signaling pathway. A previous study reported that RGS10 silencing results in mTOR pathway activation in ovarian cancer cells (35) and that RGS10 overexpression inhibits AKT phosphorylation and regulates cell apoptosis in ovarian cancer cells (36). These findings indicate that RGS10 could exert biological functions by activating the AKT/mTOR signaling pathway. Subsequently, both miR-199b-5p and RGS10 may be involved in the activation of the AKT/mTOR signaling pathway. In the present study, we demonstrated that miR-199b-5p overexpression and RGS10 downregulation positively regulated the AKT/mTOR pathway, and that treating cells with RAPA, which is an AKT/mTOR inhibitor, could abolish the aggravated effects of the miR-199b-5p mimic on ADR-induced apoptosis. Thus, these findings confirmed that miR-199b-5p may activate the AKT/mTOR signaling pathway by inhibiting RGS10 expression. However, it remains to be established whether the present results could be also observed in animal experiments and other cell lines.
In summary, the present study demonstrated that miR-199b-5p may facilitate podocyte apoptosis via AKT/mTOR signaling by targeting RGS10. miR-199b-5p may therefore be considered as a potential target in the treatment of CKD in the future.
Acknowledgements
Not applicable.
Funding Statement
Funding: This study was supported by the National Natural Science Foundation of China (grant no. 81970664), the Natural Science Foundation of Jiangsu Province (grant no. BK20191083), the 789 Outstanding Talent Program of SAHNMU (grant nos. 789ZYRC202080119 and 789ZYRC202090251) and the Science and Technology Development Foundation of Nanjing Medical University (grant no. NMUB2020052).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
AZ and WG designed and managed the study. GQ, TH and AD performed the experiments. GQ, TH, AD, YZ, SL, HS and DG contributed to the data collection and analysis. GQ, TH, AZ, AD and WG drafted and finalized the manuscript. DG, SL, HS and AD contributed to revision of the manuscript. All authors have read and approved the final manuscript. GQ, TH and AZ confirm the authenticity of all the raw data.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kota SK, Kota SB. Noncoding RNA and epigenetic gene regulation in renal diseases. Drug Discov Today. 2017;22:1112–1122. doi: 10.1016/j.drudis.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L, Yang T, Lu D-W, Zhao H, Feng Y-L, Chen H, Chen DQ, Vaziri ND, Zhao YY. Central role of dysregulation of TGF-β/Smad in CKD progression and potential targets of its treatment. Biomed Pharmacother. 2018;101:670–681. doi: 10.1016/j.biopha.2018.02.090. [DOI] [PubMed] [Google Scholar]
- 3.Webster AC, Nagler EV, Morton RL, Masson P. Chronic Kidney Disease. Lancet. 2017;389:1238–1252. doi: 10.1016/S0140-6736(16)32064-5. [DOI] [PubMed] [Google Scholar]
- 4.Regeniter A, Freidank H, Dickenmann M, Boesken WH, Siede WH. Evaluation of proteinuria and GFR to diagnose and classify kidney disease: Systematic review and proof of concept. Eur J Intern Med. 2009;20:556–561. doi: 10.1016/j.ejim.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Zhou L, Chen X, Lu M, Wu Q, Yuan Q, Hu C, Miao J, Zhang Y, Li H, Hou FF, et al. Wnt/β-catenin links oxidative stress to podocyte injury and proteinuria. Kidney Int. 2019;95:830–845. doi: 10.1016/j.kint.2018.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagata M. Podocyte injury and its consequences. Kidney Int. 2016;89:1221–1230. doi: 10.1016/j.kint.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Liu M, Liang K, Zhen J, Zhou M, Wang X, Wang Z, Wei X, Zhang Y, Sun Y, Zhou Z, et al. Sirt6 deficiency exacerbates podocyte injury and proteinuria through targeting Notch signaling. Nat Commun. 2017;8(413) doi: 10.1038/s41467-017-00498-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assady S, Wanner N, Skorecki KL, Huber TB. New Insights into Podocyte Biology in Glomerular Health and Disease. J Am Soc Nephrol. 2017;28:1707–1715. doi: 10.1681/ASN.2017010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang T, Gao Y, Wang X, Shi Y, Xu J, Wu B, He J, Li Y. Calpain-10 drives podocyte apoptosis and renal injury in diabetic nephropathy. Diabetes Metab Syndr Obes. 2019;12:1811–1820. doi: 10.2147/DMSO.S217924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiffer M, Bitzer M, Roberts IS, Kopp JB, ten Dijke P, Mundel P, Böttinger EP. Apoptosis in podocytes induced by TGF-β and Smad7. J Clin Invest. 2001;108:807–816. doi: 10.1172/JCI12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hannon GJ. RNA interference. Nature. 2002;418:244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 12.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaswani P, Prakash S, Dhar A, Sharma RK, Prasad N, Agrawal S. MicroRNAs involvement in renal pathophysiology: A bird's eye view. Indian J Nephrol. 2017;27:337–341. doi: 10.4103/ijn.IJN_264_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lorenzen JM, Kaucsar T, Schauerte C, Schmitt R, Rong S, Hübner A, Scherf K, Fiedler J, Martino F, Kumarswamy R, et al. MicroRNA-24 antagonism prevents renal ischemia reperfusion injury. J Am Soc Nephrol. 2014;25:2717–2729. doi: 10.1681/ASN.2013121329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Bi X, Xiong J, Han W, Xiao T, Xu X, Yang K, Liu C, Jiang W, He T, et al. MicroRNA-34a promotes renal fibrosis by downregulation of Klotho in tubular epithelial cells. Mol Ther. 2019;27:1051–1065. doi: 10.1016/j.ymthe.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long J, Wang Y, Wang W, Chang BH, Danesh FR. MicroRNA-29c is a signature microRNA under high glucose conditions that targets Sprouty homolog 1, and its in vivo knockdown prevents progression of diabetic nephropathy. J Biol Chem. 2011;286:11837–11848. doi: 10.1074/jbc.M110.194969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pang Q, Wang Y, Bi D, Lu H. LRRC75A-AS1 targets miR-199b-5p/PDCD4 axis to repress multiple myeloma. Cancer Biol Ther. 2020;21:1051–1059. doi: 10.1080/15384047.2020.1831373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai Y, Quan J, Lin C, Li H, Hu J, Chen P, Xu J, Guan X, Xu W, Lai Y, et al. miR-199b-5p serves as a tumor suppressor in renal cell carcinoma. Exp Ther Med. 2018;16:436–444. doi: 10.3892/etm.2018.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miao R, Lu Y, Xing X, Li Y, Huang Z, Zhong H, Huang Y, Chen AF, Tang X, Li H, et al. Regulator of G-Protein signaling 10 negatively regulates cardiac remodeling by blocking mitogen-activated protein kinase–extracellular signal-regulated protein kinase 1/2 signaling. Hypertension. 2016;67:86–98. doi: 10.1161/HYPERTENSIONAHA.115.05957. [DOI] [PubMed] [Google Scholar]
- 20.Almutairi F, Lee J-K, Rada B. Regulator of G protein signaling 10: Structure, expression and functions in cellular physiology and diseases. Cell Signal. 2020;75(109765) doi: 10.1016/j.cellsig.2020.109765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li S, Liu Y, He X, Luo X, Shi H, Qu G, Wen X, Gan W, Wang J, Zhang A. tRNA-Derived Fragments in Podocytes with Adriamycin-Induced Injury Reveal the Potential Mechanism of Idiopathic Nephrotic Syndrome. BioMed Res Int. 2020;2020(7826763) doi: 10.1155/2020/7826763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Peng B, Hu Q, Liu X, Wang L, Chang Q, Li J, Tang J, Wang N, Wang Y. Duchesnea phenolic fraction inhibits in vitro and in vivo growth of cervical cancer through induction of apoptosis and cell cycle arrest. Exp Biol Med (Maywood) 2009;234:74–83. doi: 10.3181/0806-RM-204. [DOI] [PubMed] [Google Scholar]
- 24.Xu LJ, Duan Y, Wang P, Yin HQ. miR-199b-5p promotes tumor growth and metastasis in cervical cancer by down-regulating KLK10. Biochem Biophys Res Commun. 2018;503:556–563. doi: 10.1016/j.bbrc.2018.05.165. [DOI] [PubMed] [Google Scholar]
- 25.Ren L, Xu Y, Qin G, Liu C, Yan Y, Zhang H. miR-199b-5p-Stonin 2 axis regulates metastases and epithelial-to-mesenchymal transition of papillary thyroid carcinoma. IUBMB Life. 2019;71:28–40. doi: 10.1002/iub.1889. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Guo Y, Mi N, Zhou L. miR-101-3p and miR-199b-5p promote cell apoptosis in oral cancer by targeting BICC1. Mol Cell Probes. 2020;52(101567) doi: 10.1016/j.mcp.2020.101567. [DOI] [PubMed] [Google Scholar]
- 27.Kang WL, Xu GS. Atrasentan increased the expression of klotho by mediating miR-199b-5p and prevented renal tubular injury in diabetic nephropathy. Sci Rep. 2016;6(19979) doi: 10.1038/srep19979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alqinyah M, Almutairi F, Wendimu MY, Hooks SB. RGS10 regulates the expression of cyclooxygenase-2 and tumor necrosis factor alpha through a G protein-independent mechanism. Mol Pharmacol. 2018;94:1103–1113. doi: 10.1124/mol.118.111674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cacan E, Ali MW, Boyd NH, Hooks SB, Greer SF. Inhibition of HDAC1 and DNMT1 Modulate RGS10 Expression and Decrease Ovarian Cancer Chemoresistance. PLoS One. 2014;9(e87455) doi: 10.1371/journal.pone.0087455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang S, Chen W, Stashenko P, Li Y-P. Specificity of RGS10A as a key component in the RANKL signaling mechanism for osteoclast differentiation. J Cell Sci. 2007;120:3362–3371. doi: 10.1242/jcs.008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J, Deng Y, Wang Y, Sun X, Chen S, Fu G. SPAG5-AS1 inhibited autophagy and aggravated apoptosis of podocytes via SPAG5/AKT/mTOR pathway. Cell Prolif. 2020;53(e12738) doi: 10.1111/cpr.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yingjie K, Haihong Y, Lingwei C, Sen Z, Yuanting D, Shasha C, Liutong P, Ying W, Min Z. Apoptosis repressor with caspase recruitment domain deficiency accelerates ischemia/reperfusion (I/R)-induced acute kidney injury by suppressing inflammation and apoptosis: The role of AKT/mTOR signaling. Biomed Pharmacother. 2019;112(108681) doi: 10.1016/j.biopha.2019.108681. [DOI] [PubMed] [Google Scholar]
- 33.Cai J, Zhang Y, Huang S, Yan M, Li J, Jin T, Bao S. miR-100-5p, miR-199a-3p and miR-199b-5p induce autophagic death of endometrial carcinoma cell through targeting mTOR. Int J Clin Exp Pathol. 2017;10:9262–9272. [PMC free article] [PubMed] [Google Scholar]
- 34.Yang R, Yi L, Dong Z, Ouyang Q, Zhou J, Pang Y, Wu Y, Xu L, Cui H. Tigecycline inhibits glioma growth by regulating miRNA-199b-5p-HES1-AKT pathway. Mol Cancer Ther. 2016;15:421–429. doi: 10.1158/1535-7163.MCT-15-0709. [DOI] [PubMed] [Google Scholar]
- 35.Altman MK, Alshamrani AA, Jia W, Nguyen HT, Fambrough JM, Tran SK, Patel MB, Hoseinzadeh P, Beedle AM, Murph MM. Suppression of the GTPase-activating protein RGS10 increases Rheb-GTP and mTOR signaling in ovarian cancer cells. Cancer Lett. 2015;369:175–183. doi: 10.1016/j.canlet.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shelley B. Hooks, Phillip Callihan, Molly K Altman, Jillian H Hurst, Mourad W Ali, Mandi M Murph. Regulators of G-Protein signaling RGS10 and RGS17 regulate chemoresistance in ovarian cancer cells. Mol Cancer. 2010;9(289) doi: 10.1186/1476-4598-9-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.