Abstract
Background
Discrepant data were reported about hospital admissions for ST-segment elevation myocardial infarction (STEMI) during COVID-19 pandemic. We reviewed studies reporting STEMI hospitalizations during COVID-19 pandemic, investigating whether differences in COVID-19 epidemiology or public health-related factors could explain discrepant findings in different countries.
Methods
Search through MedLine, Embase, Scopus, Web-of-Science, Cochrane Register of Controlled Trials, of studies comparing STEMI admissions during COVID-19 pandemic with a reference period, without language restrictions, as registered in PROSPERO International Prospective Register of Systematic Reviews. Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines were followed. Data independently extracted by multiple investigators were pooled using a random-effects model. Health-related metrics were from publicly-available sources.
Results
We included 79 articles (111,557 STEMI cases, from 57 countries). During peak COVID-19 pandemic, overall incidence rate-ratio (IRR) of STEMI hospitalizations over reference period decreased (0.80; 95% CI 0.76–0.84; p < 0.05). Although wide variations and significant heterogeneity were detected among studies (I2 = 89%; p < 0.0001), no significant differences were observed by report methodology (survey vs registry), or observation/reference period. However, large differences emerged at country level not explained by COVID-related epidemiological data, nor by public health strategies. Instead, IRRs for STEMI admissions were inversely related to hospital bed availability in each country (p < 0.05).
Conclusions
During COVID-19 pandemic hospitalization for STEMI significantly decreased, although to a smaller extent than initially reported. Large variability emerged across countries, unrelated to COVID-related epidemiology or social containment measures. Disparities in healthcare organization likely contributed, indicating that proper organization of emergency medicine should be preserved during pandemics.
Keywords: COVID-19, Myocardial infarction, Acute coronary syndromes, STEMI, Healthcare organization, Sars-Cov-2
1. Introduction
Coronavirus disease (COVID)-19 syndrome is having a tremendous health impact worldwide. Being caused by a novel strain of virus (SARS-CoV-2), many issues still need to be better understood about this disease. Among them, it would be important to analyze the impact of COVID-19 on acute coronary syndromes (ACS). Several features of SARS-CoV-2 infection would lead to predict that COVID-19 might be associated with increased incidence of ACS, including: a) large-series observations [[1], [2], [3], [4], [5]], and a huge autopsy-confirmed study [6], showing a several-fold increase in the incidence of acute myocardial infarction synchronous with outbreaks of respiratory virus infections; b) evidence of intravascular coagulation, platelet activation, and arterial thrombosis in COVID-19 patients [[7], [8], [9], [10], [11]]; c) demonstration of prothrombotic autoantibodies in the serum of COVID-19 patients [12]; d) evidence of SARS-CoV-2 localization in the endothelium, and vasculitis [10,13].
However, despite these premises, early reports described a sharp decrease in hospital admissions for ST-segment elevation myocardial infarction (STEMI) during COVID-19 pandemic, around 50% or more compared to historical series [[14], [15], [16], [17], [18]], stirring much interest and concern, even among lay press [19,20]. In contrast, other studies indicated only modest (<5%) or no decrease [[21], [22], [23], [24], [25], [26]], or even substantial increase [[27], [28], [29], [30], [31], [32], [33], [34], [35]] in STEMI admissions during COVID-19 pandemic.
Being able to more accurately estimate the true effect of COVID-19 pandemic on STEMI hospitalizations across countries, and the potential role on it of differences in COVID-19 epidemiology and public health-related parameters, is a relevant and timely topic. On the one hand, it might shed light on the pathophysiology of ACS in the context of SARS-CoV-2 infection; even more importantly, understanding how a pandemic impacts STEMI hospitalizations could translate into better organization of delivery of care for this life-threatening condition, and spur optimal preparedness for possible future pandemics. Accordingly, the purpose of this study was to thoroughly review all available information with respect to the incidence of STEMI hospitalizations during COVID-19 pandemic, worldwide, and meta-analyze differences from previous years. Possible factors underlying discrepant results were also evaluated.
2. Methods
The study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines; the protocol was registered in PROSPERO International Prospective Register of Systematic Reviews (ID: CRD42020188198). Ethics Committee approval was not required as the study does not involve human participants, being based on metanalysis of aggregated published data.
2.1. Search strategy and selection of studies
A systematic search of major bibliographic databases [MedLine, Embase, Scopus, Web of Science, Cochrane Central Register of Controlled Trials (CENTRAL)] was performed independently by two researchers (F.S., M.D.) up to November 17th, 2020, without language restrictions. Comprehensive search criteria were used to identify articles addressing the impact of COVID-19 outbreak on admission for STEMI. The following terms were used for search: “covid”, “covid-19”, “sars-cov”, “sars-cov-2”, “coronavirus”, AND “ST-segment elevation myocardial infarction”, or “STEMI”, or “cardiovascular disease”, or “acute coronary syndrome”, or “myocardial infarction*” (Supplementary Table 1), following PICOS (Population, Intervention, Comparison, Outcome, Study) design format (Supplementary Table 2). Reference lists of eligible studies were also screened for additional studies. As many reports were not published as full papers, research letters on the topic were also screened. Online translation tools and language skills of colleagues were used for articles published in languages other than English.
Two investigators (F.S., M.D.) independently screened the database search for titles and abstracts. Studies that reported number of STEMI hospitalizations during the COVID-19 outbreak and number of STEMI hospitalizations during a reference period were considered eligible. Decision to include the studies was based on title, abstract (when available), and full-text screening.
2.2. Data extraction
Two investigators (M.D., F.S.) independently extracted relevant information from each eligible study using a standardized data extraction form. Disagreements were resolved by consensus, or by a third investigator (G.A.) if consensus could not be reached. Extracted data included: first author, year of publication, country of study, enrollment sites, number of days during observation period and during reference period, number of cases during observation and reference period, incidence rate (IR) per-day during observation period and during reference period, incidence rate ratio (IRR) with their 95% CIs. When multiple reference periods were reported, we chose the same period of different years as reference; in case of multiple-year comparison, the entire period was taken as reference.
2.3. Assessment of methodological quality
Two investigators (F.S., M.D.) independently assessed the methodological quality of each study using the STROBE checklist for observational studies [36]. The total number of items on the STROBE checklist was 22. If an item was not applicable due to study design, it was scored as ‘not applicable’. Disagreements were resolved by consensus, or by a third investigator (G.A.) if consensus could not be reached.
2.4. Public health-related metrics
To estimate the overall health impact of COVID-19 epidemic, for each country we retrieved (relative to the date of observation of each study) [[37], [38], [39], [40], [41]]: a) number of SARS-CoV-2 positive cases; b) number of COVID-19 deaths; c) SARS-CoV-2 reproduction rate (to estimate time-course and severity of the epidemic); d) “stringency index” of lockdown measures (based on rigorously defined parameters, measured in a comparable way across countries); e) population density; f) gross domestic product/per capita; g) cardiovascular death rate; h) health development index; i) number of hospital beds/1000 inhabitants (see also Supplement).
2.5. Statistical analysis
Publication data were analyzed using Review Manager (RevMan 5.3 for Macintosh; Copenhagen, Denmark). For each study, results were reported as IR, and IRR of IR during observation period over IR during reference period. A random-effects model (DerSimonian and Laird method), which accounts for inter-study variation and provides a more conservative effect than the fixed-effect model, was used. Pooled results were reported as IRRs, and presented with 95% confidence intervals (CIs) with 2-sided P values. A P value < 0.05 was considered significant.
The studies were also grouped per country. Statistical heterogeneity among studies was estimated using the Chi-square Cochran's Q-test with I2 statistic, which provides an estimate of the amount of variance due to heterogeneity rather than sampling error. Where I2 exceeded 50%, heterogeneity was considered substantial.
Subgroup analyses were performed to explore the source of the heterogeneity, according to several characteristics of each study [Enrolment (hospitals vs. registries); timing of observation period (February-April vs. January-May); length of observation period (<53 vs ≥53 days); reference period: inter-year (same period in different years) vs. intra-year (different period of same year, or different periods of different years); study quality (high; medium; low)]; public health-related metrics. The possibility of publication bias was explored by visual inspection of funnel plot of the effect size against the inverse of the standard error. Meta-regression models were formed to explore and identify potential effect modifiers among public-health metrics. To control for false-positive findings (type I error), when performing meta-regression with multiple covariates, we used a test based on random permutations to calculate and report multiplicity-adjusted p-values (See Supplementary Material).
3. Results
3.1. Literature search
The search strategy yielded 18,366 references. After duplicate removal, and title and abstract screening, 216 articles were selected, of which 79 were included in the analysis after full text assessment (Supplementary Fig. 1; References in Supplement).
3.2. Characteristics of studies
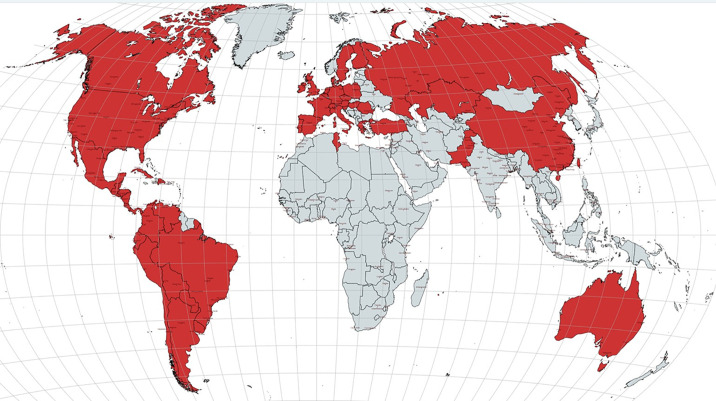
Overall, studies reported data from 57 countries (Fig. 1 ; Supplementary Table 3). Of the 79 available articles, 5 reported a drastic, >50% decrease in STEMI hospitalizations; in contrast, 6 articles reported no or modest decrease (0% to 5%), and 9 actually described an increase in STEMI hospitalizations compared with the reference period (Supplementary Table 3).
Fig. 1.
Geographical distribution of papers reporting STEMI admission data during COVID-19 pandemic.
World map highlighting (in red) all 57 countries for which STEMI admission data during COVID-19 pandemic peak were reported and utilized for this meta-analysis. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
For 69 reports, data were collected through “ad hoc” surveys of selected hospitals, whereas 10 studies reported data obtained from already ongoing registries or health-insurance funds. Fifty-five studies chose a narrower observation timing (Mid-February-April 2020), whereas 24 included January and/or May 2020, observation period ranging from 7 to 152 days (mean 53 days). Regarding the reference period, different selection methods were found: a) same period of year 2019; b) a period immediately preceding observation period; c) average of same period of previous years. Mean duration of reference period was 147 days.
Overall analysis includes a total of 48,396 STEMI cases during observation period, and 63,161 cases during reference period. Based on STROBE checklist, quality was high in 43 studies (54.4%), moderate in 8 studies (10.1%) and low in 28 studies (35.4%) (Supplementary Table 3).
3.3. Meta-analytic results
The IRR under a random-effects model reported a significant reduction in the number of hospitalizations for STEMI (0.80, 95% CI 0.76–0.84; p < 0.0001) during peak COVID-19 epidemic compared with the reference period (Supplementary Fig. 2). However, substantial degree of heterogeneity was found (I 2 = 89%; p < 0.0001).
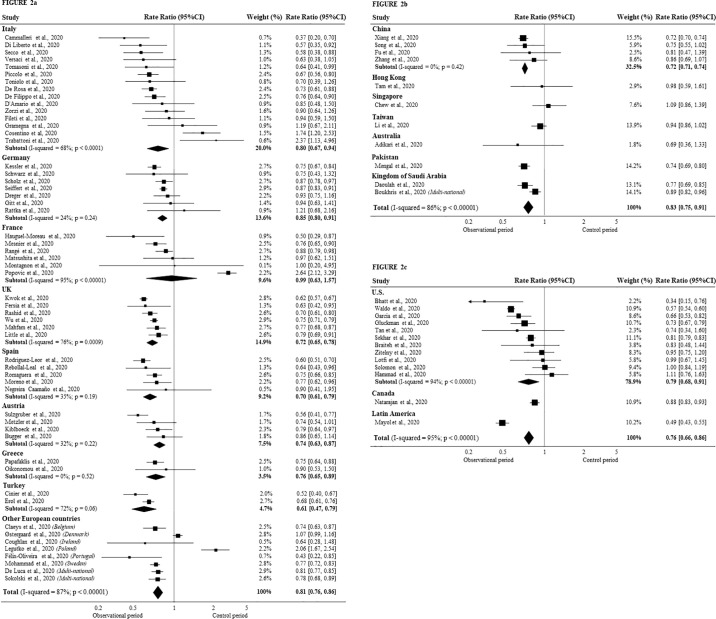
Country specific analyses are shown in Fig. 2a-c. Overall, around 20% reduction in STEMI hospitalizations was reported for Europe (0.81, 95% CI 0.76–0.86; p < 0.0001), Asia-Pacific and Middle East (0.83, 95% CI 0.75–0.91), and North, Central, and South America (NCSA) region (0.76, 95% CI 0.66–0.86) (all p < 0.0001). Latin America countries reported the largest decrease in STEMI hospitalizations (>50%), whereas France, Denmark, and South-East Asia reported no appreciable decrease. Country-wise, low or no heterogeneity (I 2 < 50%) was observed for Germany, Spain, Austria, Greece, China.
Fig. 2.
Meta-analysis of all papers reporting STEMI admission data during COVID-19 pandemic, grouped by geographic areas.
Each panel shows Forest plot of studies reporting STEMI hospitalizations during the COVID-19 peak compared to the control period in: European countries (2a); Asia-Pacific and Middle East (2b); North-Central-South America (NCSA) (2c).
Note: a) variability of results within the same geographical area; b) marked differences among different countries.
3.4. Subgroup analyses and publication bias
Stratifying studies on the basis of several variables to detect possible sources of heterogeneity, revealed no significant differences according to: type of reporting (“ad hoc” surveys or registries), observation or reference period, study quality; importantly, similar decrease in STEMI admissions was observed when 55 studies reporting narrower observation periods, bracketing Mid-February-April were analyzed (IRR 0.77, 0.72–0.81; Supplementary Table 4). Possible presence of publication bias among studies was assessed through funnel plot estimate of effect size vs. standard error, which reported an asymmetrical visual examination (Supplementary Fig. 3).
3.5. Public health-related metrics
Possible effects of various public health metrics during peak COVID-19 pandemic on STEMI admissions in the various countries were also investigated. We found no statistical evidence supporting an effect of: SARS-CoV-2 positive cases or deaths, SARS-CoV-2 reproduction rate, “stringency index” of lockdown measures, population density, gross domestic product per capita, health development index, or cardiovascular death rate (Table 1a ).
Table 1a.
Public health variables in the various countries that did not contribute to the variance of IRR for STEMI explained by the meta-regression model.
| Variable | IRR | 95% CIs | Unadjusted p-value | Multiplicity adjusted p-value |
|---|---|---|---|---|
| Total SARS-Cov-2 positive cases | 1.000 | 1.000–1.000 | 0.374 | 0.550 |
| Total SARS-Cov-2 deaths | 1.000 | 1.000–1.000 | 0.407 | 0.743 |
| SARS-Cov-2 reproduction rate | 0.839 | 0.691–1.018 | 0.063 | 0.075 |
| Stringency index | 1.001 | 0.995–1.007 | 0.140 | 0.802 |
| Population density | 1.000 | 1.000–1.000 | 0.597 | 0.696 |
| Gross domestic product per capita | 1.000 | 1.000–1.000 | 0.576 | 0.688 |
| Cardiovascular death rate | 1.000 | 0.999–1.001 | 0.607 | 0.942 |
| Human development index | 1.370 | 0.428–4.383 | 0.331 | 0.591 |
IRR: Incidence rate ratio; CI: Confidence interval.
However, IRR was inversely and significantly related to hospital bed availability in the various countries (Table 1b ; Fig. 3 ).
Table 1b.
IRR for STEMI in Relation to Explanatory Variables in the various countries explained by the meta-regression model (see also Fig. 3).
| Variable | IRR | 95% CIs | Unadjusted p-value | Multiplicity adjusted p-value |
|---|---|---|---|---|
| Intercept | 0.662 | 0.563–0.779 | <0.001 | <0.001 |
| Hospital beds/1000 inhabitants | 1.046 | 1.008–1.085 | 0.009 | 0.017 |
IRR: Incidence rate ratio; CI: Confidence interval.
Test of Moderators effect: F = 6.0450, p = 0.0165.
Proportion of heterogeneity accounted for: 12.89%.
Test for Residual Heterogeneity: Q = 569.698, p < 0.0001.
Fig. 3.
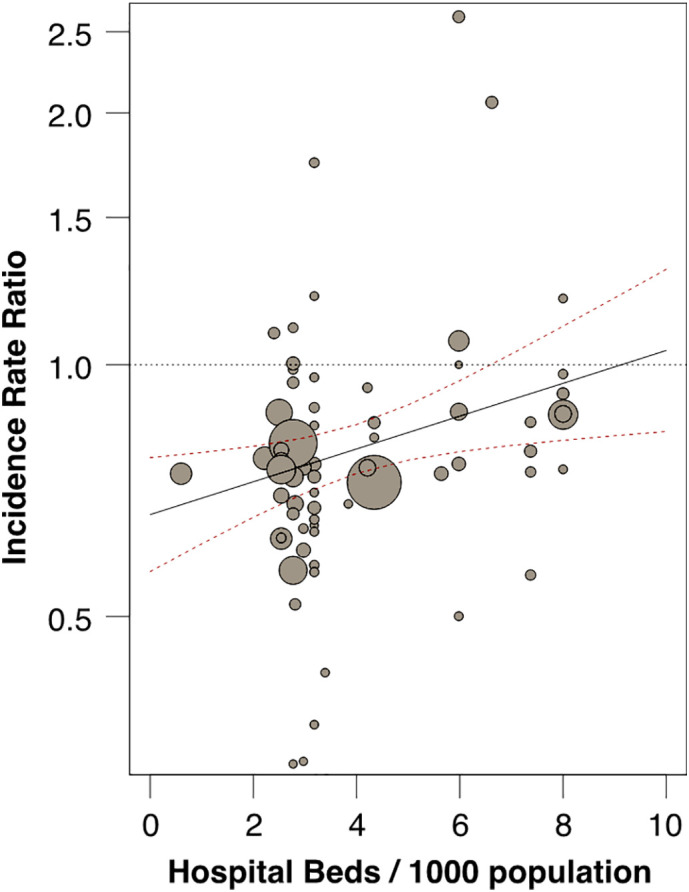
Impact of hospital bed availability per country on STEMI hospitalizations during COVID-19 pandemic.
Meta-regression of number of hospital beds/1000 inhabitants (X-axis) vs incidence rate ratio (IRR) of STEMI hospitalizations in each country during peak COVID-19 pandemic, relative to reference period (Y-axis). Circles represent individual studies; diameter is proportional to the inverse of the variance of the IRRs (p < 0.017). See Table 2 for details.
Note the inverse relationship between bed availability and STEMI hospitalization, which stayed around historical levels in countries with greater bed availability, while it sharply decreased along with bed availability, suggesting an important role of health organization.
4. Discussion
This study documents that during the first peak of COVID-19 pandemic there has been a significant decrease in STEMI hospitalizations worldwide. However, the magnitude of decrease was of a lesser extent than initially described. Most importantly, substantial differences emerged among different studies and countries. Through a meta-analytical approach of a large number of reports, including >100,000 cases from 57 countries, and systematic assessment of various health-related metrics, a clearer picture emerges of an issue that has remarkable implications, both with respect to clinical medicine and to health care organization.
In the aftermath of the spread of SARS-CoV-2 infection, reports emerged of a dramatic decrease in hospital admissions for STEMI. These observations generated enormous interest in the medical community, and also in the lay press [19,20], yielding a number of reports which however widely differ with respect to the methodology employed to collect information, ranging from solidly-structured national/multihospital registries, to quick surveys of a few hospitals, to even Twitter polls [42]. Perhaps not surprisingly, this approach yielded uncertainty, admission rates for STEMI ranging from an appalling >50% decrease [[14], [15], [16], [17], [18]], to modest changes or even a substantial increase [[21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35]].
Several factors may have contributed to the uncertainties about STEMI hospitalization data during COVID-19 pandemic. First, in the wake of the pandemic countless articles on COVID-19 appeared, raising concerns about lack of in-depth review; indeed, that in such a short time we retrieved as many as 79 reports, many of whom brief research letters, hints to this potential problem. Secondly, surveys launched “on the heat of the moment” might lack the depth and rigor found in solidly-structured, long-running registries. Thirdly, while registries have a stable referral base including most sites with certain characteristics, surveys only provide data on those hospitals that were asked, and agreed, to contribute. Fourthly, while the observation period was obviously centered around peak COVID-19, its width spanned differently across reports, while time periods chosen as reference could also differ. Finally, of even greater relevance, differences across countries in STEMI hospitalization might be traced to differences in the epidemiology of COVID-19 epidemic, lockdown measures, health organization policies.
In light of largely discrepant reports, these various factors indicate the need for a thorough, systematic assessment of the true impact of COVID-19 pandemic on STEMI hospitalizations. This information would have important implications in terms of understanding the consequences of SARS-CoV-2 infection not only at the level of individual patients, but also in terms of overall health care organization strategies, and ability to cope with farther-reaching consequences of a pandemic.
We specifically focused on STEMIs, for several reasons. First, it is a simple, unequivocal diagnosis, which can be easily reached “on the field”. Secondly, most countries had a centralized hub-and-spoke organization for STEMI management, which allows to keep track of cases, while changes in the organization of emergency medicine as a consequence of the pandemic are easily detectable. Finally, several long-running registries were already in place for STEMIs.
Through a large meta-analysis, we document a 20% decrease in STEMI hospitalizations during the first peak of COVID-19 pandemic, worldwide. While significant, this is a sobering finding compared to reports of dramatic, >50% decrease. At the same time, it curbs the relevance of reports showing instead no impact of COVID-19 on STEMI hospitalizations. Importantly, our meta-analysis may allay concerns attributable to data reporting. From our sub-analyses, around 20% decrease in STEMI hospitalizations was consistently seen regardless of data type (surveys vs. registries), timing of observation period (narrower or broader), or reference period. Furthermore, through STROBE approach we estimated the quality of reports to be high in 54.4% of publications; although it was moderate or low in 45.6% of publications, this did not have an impact on overall results.
At the same time, our analysis also shows some unexpected results. A major finding is the notable differences in STEMI admission rate in some countries. For example, >50% decrease was reported for Latin America; although this finding comes from a single report [15], it should not be dismissed lightly, as it is based on 1211 cases accrued from 79 hospitals in 19 countries. In contrast, other countries (e.g., France, Denmark, Hong-Kong, Singapore, Taiwan) report no appreciable decrease in STEMI hospitalizations, which distinguishes them from their immediate neighboring countries, suggesting that differences in admissions could not be traced to geography or ethnicity. Furthermore, contrary to what might have been anticipated, COVID-related epidemiological metrics, and social policy parameters (e.g., lockdown measures), were not associated with different incidence of STEMI hospitalization across countries. Instead, different organization of emergency medicine programs and/or of hospital operation during the pandemic might have played a role.
For example, the hub-and-spoke organization dealing with STEMIs may have been subverted to address the exceptional hospital bed needs imposed by the COVID-19 pandemic. In some countries, many hospitals had been designated “COVID-only”, admitting only COVID-positive patients and shunting away all other patients, whereas other hospitals had been granted “COVID-free” status, thus admitting also patients usually referred to other hospitals. Consequently, data from either type of hospital may have shown disproportionately lower, or higher, admissions for STEMI than previously, just because of this change in admission strategy. This is reflected in the papers by Cosentino et al. [29], Trabattoni et al. [30] (from Italy), and Legutko et al. from Poland [35]; while reporting a large increase in STEMI admissions over the corresponding reference period (74%, 137%, and 106%, respectively), those authors specify that their emergency departments had been re-fitted to accept STEMIs from hospitals outside their network. Refitting, or conversely preclusion to accept STEMIs, may have occurred also in other hospitals, either way affecting historical comparison of STEMI admissions. Different implementation of this policy across countries, or variable sampling of such hospitals across reports, may have affected surveys.
In principle, it cannot be excluded that a real decrease in STEMI incidence occurred, as a consequence of in-home-bound subjects being less exposed to external triggers of acute coronary events (pollution, work-place stress), or achieving lower blood pressure or better medication compliance [43]. Conversely, it has been suggested that some STEMI patients simply did not present at the hospital. There has been much speculation about unintended consequences of “stay-at-home” orders which may have inadvertently resulted in patients not seeking medical care, or of COVID-19 health warnings making patients fearful of infection, thus avoiding hospital help [44,45]. Yet, our data indicate that COVID infection rate, or degree of lockdown measures did not influence STEMI hospitalization in the various countries.
Our data, instead, reveal that STEMI hospitalizations plummeted in countries with lower hospital bed availability, whereas they stayed around historical levels in countries with much greater bed availability, suggesting that hospital beds had been overwhelmed with COVID-19 patients, and/or that intensive/coronary care units had been converted into COVID intensive care beds. This interpretation is supported by recent data documenting -among possible causes for discrepant COVID-19 mortality across countries- that case-fatality rate associated with COVID-19 was negatively associated with number of hospital beds ×1000 inhabitants, similar to our findings [46,47]. Higher cases of out-of-hospital cardiac arrest during COVID-19 pandemic [48,49] lend further credence to the concerns about logistic hurdles (e.g., ambulance services overloaded with COVID-19 cases) being a factor behind reduced STEMI admission in some areas.
Collectively, these considerations indicate that public health strategies to cope with a pandemic, and overall health care organization, may be an issue of concern deserving further investigation.
5. Limitations
Our meta-analysis has limitations, inherent in data collected in the midst of a pandemic; however, through separate sub-analysis we could ascertain that findings from ad-hoc surveys were comparable to those obtained from solid registries. Also, differences in length or timing of observation period did not affect the results, as most (55 out of 79) studies investigated a narrow time-window (Mid-February-April), which bracketed the first peak of COVID-19 pandemic. Finally, we analyzed bed availability data at country level, not at hospital level.
6. Conclusions
This large meta-analysis shows that STEMI hospitalizations decreased significantly during COVID-19 pandemic. However, the magnitude of the phenomenon was decidedly less than initially feared, and sometimes substantially different among countries. Proper functioning of hospital services, and of hub-and-spoke approach to STEMI, along with adequate public information, should be pursued to effectively deal with acute coronary events during a pandemic.
Sources of funding
Supported in part by Italian Ministry of Health “Ricerca Corrente” funds to IRCCS MultiMedica, #01:02:00 “Diagnostica e terapia delle sindromi coronariche acute”, and #3.11 RCR 2020- 23670065 “Clinical and Imaging Biomarkers Associated With Plasma and Cellular Determinants of Cardiovascular Disease at the Time of Covid-19”.
Author statement file
Francesco Sofi
Substantial contributions to the design of the work;
Drafting the work
Final approval of the version to be published;
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Monica Dinu
Substantial contributions to the acquisition of data for the work;
Revising the work critically for important intellectual content;
Final approval of the version to be published;
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
GianPaolo Reboldi
Substantial contributions to the analysis, or interpretation of data for the work;
Drafting revising the work critically for important intellectual content;
Final approval of the version to be published;
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Fabrizio Stracci
Substantial contributions to the analysis of data for the work;
Revising the work critically for important intellectual content;
Final approval of the version to be published;
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Roberto FE Pedretti
Substantial contributions to the interpretation of data for the work;
Revising the work critically for important intellectual content;
Final approval of the version to be published;
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Serafina Valente
Substantial contributions to the interpretation of data for the work;
Revising the work critically for important intellectual content;
Final approval of the version to be published;
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
GianFranco Gensini
Substantial contributions to the interpretation of data for the work;
Revising the work critically for important intellectual content;
Final approval of the version to be published;
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
C Michael Gibson
Substantial contributions to the interpretation of data for the work;
Revising the work critically for important intellectual content;
Final approval of the version to be published;
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Giuseppe Ambrosio
Substantial contributions to the conception and design of the work, and interpretation of data.
Drafting the work.
Final approval of the version to be published;
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of Competing Interest
All authors declare: no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Footnotes
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Authors report no conflicts of interest potentially related to this work.
Partly supported by Italian Ministry of Health “Ricerca Corrente” funds to IRCCS MultiMedica, #01:02:00 “Diagnostica e terapia delle sindromi coronariche acute”, and #3.11 RCR 2020- 23670065 “Clinical and Imaging Biomarkers Associated With Plasma and Cellular Determinants of Cardiovascular Disease at the Time of Covid-19.”
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcard.2021.10.156.
Appendix A. Supplementary data
Supplementary material
References
- 1.Ohland J., Warren-Gash C., Blackburn R., et al. Acute myocardial infarctions and stroke triggered by laboratory-confirmed respiratory infections in Denmark, 2010 to 2016. Euro Surveill. 2020;25(17):1900199. doi: 10.2807/1560-7917.ES.2020.25.17.1900199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes M., Heywood A.E., Mahimbo A., Rahman B., Newall A.T., Macintyre C.R. Acute myocardial infarction and influenza: a meta-analysis of case-control studies. Heart. 2015;101(21):1738–1747. doi: 10.1136/heartjnl-2015-307691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwong J.C., Schwartz K.L., Campitelli M.A., et al. Acute myocardial infarction after laboratory-confirmed influenza infection. N. Engl. J. Med. 2018;378(4):345–353. doi: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 4.Blackburn R., Zhao H., Pebody R., Hayward A., Warren-Gash C. Laboratory-confirmed respiratory infections as predictors of hospital admission for myocardial infarction and stroke: time- series analysis of english data for 2004–2015. Clin. Infect. Dis. 2018;67(1):8–17. doi: 10.1093/cid/cix1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warren-Gash C., Blackburn R., Whitaker H., McMenamin J., Hayward A.C. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self- controlled case series analysis of national linked datasets from Scotland. Eur. Respir. J. 2018;51(3):1701794. doi: 10.1183/13993003.01794-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madjid M., Miller C.C., Zarubaev V.V., et al. Influenza epidemics and acute respiratory disease activity are associated with a surge in autopsy-confirmed coronary heart disease death: results from 8 years of autopsies in 34,892 subjects. Eur. Heart J. 2007;28(10):1205–1210. doi: 10.1093/eurheartj/ehm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowles L., Platton S., Yartey N., et al. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. N. Engl. J. Med. 2020;383(3):288–290. doi: 10.1056/NEJMc2013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J. Am. Coll. Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu S.X., Tyagi T., Jain K., et al. Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation. Nat. Rev. Cardiol. 2021;18(3):194–209. doi: 10.1038/s41569-020-00469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blasco A., Coronado M.J., Hernández-Terciado F., et al. Assessment of neutrophil extracellular traps in coronary thrombus of a case series of patients with COVID-19 and myocardial infarction. JAMA Cardiol. 2020 Dec;29 doi: 10.1001/jamacardio.2020.7308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Case Rep. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhatt A.S., Moscone A., McElrath E.E., et al. Fewer hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J. Am. Coll. Cardiol. 2020;76(3):280–288. doi: 10.1016/j.jacc.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayol J., Artucio C., Batista I., et al. An international survey in Latin America on the practice of interventional cardiology during the COVID-19 pandemic, with a particular focus on myocardial infarction. Neth. Heart J. 2020;28(7–8):424–430. doi: 10.1007/s12471-020-01440-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauguel-Moreau M., Pillière R., Prati G., et al. Impact of Coronavirus Disease 2019 outbreak on acute coronary syndrome admissions: four weeks to reverse the trend. J. Thromb. Thrombolysis. 2021;51(1):31–32. doi: 10.1007/s11239-020-02201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cammalleri V., Muscoli S., Benedetto D., et al. Who has seen patients with ST-segment–elevation myocardial infarction? First results from italian real-world coronavirus disease 2019. J. Am. Heart Assoc. 2020;9(19) doi: 10.1161/JAHA.120.017126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Félix-Oliveira A., de Sousa Almeida M., Ferreira J., et al. Caring for cardiac patients amidst the SARS-CoV-2 pandemic: the scrambled pieces of the puzzle. Rev. Port. Cardiol. 2020;39(5):299–301. doi: 10.1016/j.repc.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krumholz H.M. The New York Times; April 6, 2020. Where Have All the Heart Attacks Gone? [Google Scholar]
- 20.Wood S. The Mystery of the Missing STEMIs During the COVID-19 Pandemic. April 02, 2020. https://www.tctmd.com/news
- 21.Matsushita K., Hess S., Marchandot B., et al. Clinical features of patients with acute coronary syndrome during the COVID-19 pandemic. J. Thromb. Thrombolysis. 2020:1–10. doi: 10.1007/s11239-020-02340-z. published online ahead of print, 2020 Nov 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solomon M.D., McNulty E.J., Rana J.S., et al. The Covid-19 pandemic and the incidence of acute myocardial infarction. N. Engl. J. Med. 2020;383(7):691–693. doi: 10.1056/NEJMc2015630. [DOI] [PubMed] [Google Scholar]
- 23.Tam C.C.F., Cheung K.S., Lam S., et al. Impact of coronavirus disease 2019 (COVID-19) outbreak on outcome of myocardial infarction in Hong Kong, China. Catheter. Cardiovasc. Interv. 2021;97(2):E194–E197. doi: 10.1002/ccd.28943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zitelny E., Newman N., Zhao D. STEMI during the COVID-19 pandemic – an evaluation of incidence. Cardiovasc. Pathol. 2020;48:107232. doi: 10.1016/j.carpath.2020.107232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montagnon R., Rouffilange L., Agard G., Benner P., Cazes N., Renard A. Impact of the COVID-19 pandemic on emergency department use: focus on patients requiring urgent revascularization. J. Emerg. Med. 2021;60(2):229–236. doi: 10.1016/j.jemermed.2020.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lotfi A.S., Capatina A., Kugelmass A.D. Assessment of ST-segment elevation myocardial infarction volume trends during the COVID-19 pandemic. Am. J. Cardiol. 2020;131:132–133. doi: 10.1016/j.amjcard.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rattka M., Baumhardt M., Dreyhaupt J., et al. 31 days of COVID-19—cardiac events during restriction of public life—a comparative study. Clin. Res. Cardiol. 2020;109(12):1476–1482. doi: 10.1007/s00392-020-01681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gramegna M., Baldetti L., Beneduce A., et al. ST-segment-elevation myocardial infarction during COVID-19 pandemic: insights from a regional public service healthcare hub. Circ. Cardiovasc. Interv. 2020;13(8) doi: 10.1161/CIRCINTERVENTIONS.120.009413. [DOI] [PubMed] [Google Scholar]
- 29.Cosentino N., Bartorelli A.L., Marenzi G. Time to treatment still matters in ST-elevation myocardial infarction: a call to maintain treatment effectiveness during the COVID-19 pandemic. Eur. Heart J. 2020;6(6):408–409. doi: 10.1093/ehjcvp/pvaa054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trabattoni D., Montorsi P., Merlino L. Late STEMI and NSTEMI patients’ emergency calling in COVID-19 outbreak. Can. J. Cardiol. 2020;36(7):1161.e7–1161.e8. doi: 10.1016/j.cjca.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Popovic B., Varlot J., Metzdorf P.A., Jeulin H., Goehringer F., Camenzind E. Changes in characteristics and management among patients with ST-elevation myocardial infarction due to COVID-19 infection. Catheter. Cardiovasc. Interv. 2020 doi: 10.1002/ccd.29114. published online ahead of print, 2020 Jul 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Østergaard L., Butt J.H., Kragholm K., et al. Incidence of acute coronary syndrome during national lock-down: insights from nationwide data during the Coronavirus disease 2019 (COVID-19) pandemic. Am. Heart J. 2021;232:146–153. doi: 10.1016/j.ahj.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chew N.W., Sia C.-H., Wee H.-L., et al. Impact of the COVID-19 pandemic on door-to-balloon time for primary percutaneous coronary intervention — results from the Singapore Western STEMI Network. Circ. J. 2021;85(2):139–149. doi: 10.1253/circj.CJ-20-0800. [DOI] [PubMed] [Google Scholar]
- 34.Hammad T.A., Parikh M., Tashtish N., et al. Impact of COVID-19 pandemic on ST-elevation myocardial infarction in a non-COVID-19 epicenter. Catheter. Cardiovasc. Interv. 2021;97(2):208–214. doi: 10.1002/ccd.28997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Legutko J., Niewiara Ł., Bartuś S., et al. Decline in the number of coronary angiography and percutaneous coronary intervention procedures in patients with acute myocardial infarction in Poland during the coronavirus disease 2019 pandemic. Kardiol. Pol. 2020;78(6):574–576. doi: 10.33963/KP.15393. [DOI] [PubMed] [Google Scholar]
- 36.von Elm E., Altman D.G., Egger M., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Our World in Data, available at https://ourworldindata.org/.
- 38.World Bank database, available at https://datacatalog.worldbank.org/.
- 39.Human Development Reports Statistical Update Technical Notes. 2018. http://hdr.undp.org/sites/default/files/hdr2018_technical_notes.pdf Available at.
- 40.Hale T., Angrist N., Goldszmidt R., et al. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker) Nat. Hum. Behav. 2021;5:529–538. doi: 10.1038/s41562-021-01079-8. [DOI] [PubMed] [Google Scholar]
- 41.Achilleos S., Quattrocchi A., Gabel J., Heraclides A., Kolokotroni O., Constantinou C., et al. Excess all-cause mortality and COVID-19 related mortality: a temporal analysis in 22 countries, from January until August 2020. Int. J. Epidemiol. 2021 Jul 20 doi: 10.1093/ije/dyab123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ashraf S., Ilyas S., Alraies M.C. Acute coronary syndrome in the time of the COVID-19 pandemic. Eur. Heart J. 2020;41:2089–2091. doi: 10.1093/eurheartj/ehaa454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanner R., MacDaragh Ryan P., Caplice N.M. COVID-19: where have all the STEMIs gone? Can. J. Cardiol. 2020;36(7) doi: 10.1016/j.cjca.2020.04.032. (1161.e9e1161.e10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katsoulis M., Gomes M., Lai A.G., et al. Estimating the effect of reduced attendance at emergency departments for suspected cardiac conditions on cardiac mortality during the COVID-19 pandemic. Circ. Cardiovasc. Qual. Outcomes. 2021;14(1) doi: 10.1161/CIRCOUTCOMES.120.007085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Onder G., Olimpieri P.P., Celant C., on behalf of AIFA Monitoring Registries Group, et al. Under-prescription of direct oral anticoagulants for treatment of non-valvular atrial fibrillation and venous thromboembolism in the COVID-19 lockdown period. Eur. J. Prev. Cardiol. 2021 doi: 10.1093/eurjpc/zwab096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorci G., Faivre B., Morand S. The Lancet Infectious Diseases. 2021. Why does COVID-19 case fatality rate vary among countries? Available at SSRN: [Google Scholar]
- 47.Sorci G., Faivre B., Morand S. Explaining among-country variation in COVID-19 case fatality rate. Sci Rep. 2020 Nov 3;10(1):18909. doi: 10.1038/s41598-020-75848-2. (PMID: 33144595; PMCID: PMC7609641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baldi E., Sechi G.M., Mare C., et al. Lombardia CARe researchers. Out-of-hospital cardiac arrest during the Covid-19 outbreak in Italy. N. Engl. J. Med. 2020;383(5):496–498. doi: 10.1056/NEJMc2010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lai P.H., Lancet E.A., Weiden M.D., et al. Characteristics associated with out-of-hospital cardiac arrests and resuscitations during the novel coronavirus disease 2019 pandemic in New York City. JAMA Cardiol. 2020;5(10):1154–1163. doi: 10.1001/jamacardio.2020.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material