Abstract
The objective of this study was to calculate the incidence, severity, and risk factors for acute kidney injury (AKI) in a tertiary care pediatric intensive care unit (PICU). Also, to assess the impact of AKI and its varying severity on mortality and length of hospital and PICU stays. A prospective observational study was performed in children between 1 month and 12 years of age admitted to the PICU between July 1, 2013, and July 31, 2014 (13 months). The change in creatinine clearance was considered to diagnose and stage AKI according to pediatric risk, injury, failure, loss, and end-stage renal disease criteria. The risk factors for AKI and its impact on PICU stay, hospital stay, and mortality were evaluated. Of the total 220 patients enrolled in the study, 161 (73.2%) developed AKI, and 59 cases without AKI served as the “no AKI” (control) group. Majority (57.1%) of children with AKI had Failure grade of AKI, whereas 26.1% had Risk grade and 16.8% had Injury grade of AKI. Infancy ( p = 0.000), hypovolemia ( p = 0.005), shock ( p = 0.008), and sepsis ( p = 0.022) were found to be significant risk factors for AKI. Mortality, PICU stay, and hospital stay were comparable in children with and without AKI as well as between the various grades of renal injury (i.e., Failure, Risk, and Injury ). An exceedingly high incidence of AKI, especially of the severe Failure grade was observed in critically ill children. Infancy and frequent PICU occurrences such as sepsis, hypovolemia, and shock predisposed to AKI.
Keywords: acute kidney injury, hospital stay, mortality, pediatric intensive care unit, pRIFLE criteria, renal, risk factors
Introduction
The term “acute kidney injury” (AKI) encompasses the smaller changes in kidney function, which do not cause overt organ failure, but are, nevertheless, associated with increased morbidity and mortality. 1 On the contrary, “acute renal failure” is now reserved for severe kidney injury requiring renal replacement therapy (RRT). 2 Numerous studies have demonstrated a high incidence of AKI in children, particularly in the pediatric intensive care unit (PICU) setting (10–82%) as well as its significant impact on mortality and hospital stay. 3 4 5 6 7 8 9 10 Furthermore, in a recent study, almost 60% of critically ill children diagnosed with AKI were observed to be either suffering from chronic kidney disease (CKD), or being at risk for CKD in the form of persistently reduced glomerular filtration rate and hypertension on long-term follow-up. 11 Most pediatric studies evaluating the incidence and outcomes of AKI are limited to the developed countries or based on retrospective analysis of case records. 5 6 7 12 In view of the paucity of Indian literature, the present study was undertaken to evaluate the incidence, severity, risk factors, and outcomes of AKI in a tertiary care PICU.
Materials and Methods
Design and Ethics
The present study was a single-center observational noninterventional study of pediatric patients who were admitted to our tertiary care medical PICU between July 1, 2013, and July 31, 2014 (over 13 months period). The study was conducted in our nine-bedded state–of-the-art PICU of an academic tertiary care hospital affiliated to a medical college (in a major metropolitan city of western India). The study was conducted after seeking approval from the institutional ethics committee. Informed consent was obtained from the parent/guardian as well as assent obtained from the patient (whenever applicable).
Study Population
The study involved consecutive pediatric patients aged 1 month to 12 years admitted to the PICU (with or without a preceding ward stay). Readmissions to the PICU were included as separate admissions for the present study purpose. Neonates younger than 1 month and children older than 12 years are not admitted in the PICU as per hospital policy and were not included in the present study. Additionally, those with preexisting chronic renal disease, those with PICU stay of less than 48 hours, and those having less than two serum creatinine estimations in the first week of PICU admission were all excluded from the study.
Sample Size Calculation
The sample size was calculated as per the average annual admissions in the PICU and was a convenience sample.
Procedure of the Study
Estimations of serum creatinine were performed (by an EM 200 autoanalyzer) in all critically ill children enrolled in the study for routine monitoring and early diagnosis of end-organ involvement (as required). The respective estimated creatinine clearance (eCCl) was calculated in mL/min/1.73 m 2 by the original Schwartz formula. 13 Schwartz formula: eCCl = ( k × height)/(serum creatinine), where k = 0.33 for low birth weight infants younger than 1 year; 0.45 for term infants younger than 1 year; and 0.55 for children older than 1 year . Baseline creatinine value was considered as the least value estimated within past 6 months before admission to PICU (including the value at hospital admission). If unavailable, baseline creatinine clearance was considered as 100 mL/min/1.73 m 2 . 3 The change in eCCl from baseline was considered to diagnose and stage AKI according to the pediatric risk, injury, failure, loss, and end-stage renal disease (pRIFLE) criteria 3 ( Table 1 ). The worst stage of AKI reached at any time during the first week of PICU stay was considered as pRIFLEmax for each patient. A separate case record form was devised to record various demographic and clinical details as well as investigations of the patients. Risk factors for AKI such as severe malnutrition (grade 3 or 4 malnutrition as per the Indian Academy of Pediatrics classification), mechanical ventilation, thrombocytopenia (platelets < 1.5 lakh/mm 3 ), and nephrotoxic drugs (such as amikacin, vancomycin, and amphotericin B) were also documented. Shock was defined as the presence of tachycardia, feeble pulses, cool peripheries, hypotension (blood pressure < − 2 standard deviation for age and sex), and capillary filling time > 3 seconds. 14 Hypovolemia was considered as decreased fluid volume status (decreased skin turgor, dry oral mucosa, sunken eyes, irritability, and excessive thirst) with signs (heart rate, pulse volume, urine output, and blood pressure) not amounting to shock. 15 Sepsis was defined as the presence of systemic inflammatory response syndrome with suspected or proven infection. 16 Final outcome (death/discharge), length of PICU stay, and total hospital stay were recorded.
Table 1. The pRIFLE staging system for AKI 3 .
| Grade of AKI | eCCI | Urine output |
|---|---|---|
| Risk | eCCl decrease by 25% | <0.5 mL/kg/h for 8 h |
| Injury | eCCl decrease by 50% | <0.5 mL/kg/h for 16 h |
| Failure | eCCl decrease by 75% or eCCl < 35 mL/min/1.73 m 2 | <0.3 mL/kg/h for 24 h or anuric for 12 h |
| Loss | Persistent failure > 4 wk | |
| End stage | End-stage renal disease (persistent failure > 3 mo) | |
Abbreviations: AKI, acute kidney injury; eCCl, estimated creatinine clearance; pRIFLE, pediatric risk, injury, failure, loss, and end-stage renal disease.
Statistical Analysis
Children without AKI served as the “no AKI” or the control group. Continuous variables such as age, baseline eCCl, and length of PICU stay and hospital stay in patients with and without AKI were compared using the Student's t -test. Other ordinal data such as risk factors for incidence of AKI and associated mortality in children with AKI were compared by using Pearson's chi-square test. Severity of AKI and its impact on PICU stay, hospital stay, and mortality were also assessed using the chi-square test. A p -value of less than 0.05 was considered significant for all the analyses. The statistical tests were performed using the Statistical Package for the Social Sciences (SPSS) version 22.0 for Windows (IBM Corp.; Armonk, New York, United States).
Results
A total of 511 patients were screened over a period of 13 months for eligibility of which 220 patients met the inclusion criteria and were enrolled ( Fig. 1 ).
Fig. 1.
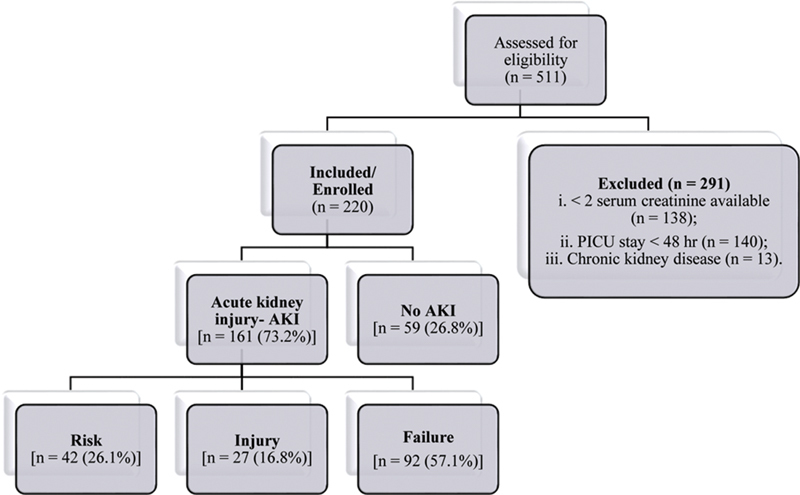
Flowchart of patients in the study. AKI, acute kidney injury; PICU, pediatric intensive care unit.
Demographic and Clinical Details
The various demographic and clinical details of the total study population are shown in Table 2 . Of the total 220 patients, 66.4% were males. The median age of the total study population was 12 months and the mean age was 33.1 ± 38.6 months. Children with AKI were observed to be significantly younger ( p = 0.000) with an average age of 25.8 ± 34.7 months than those without AKI (53.2 ± 42.2 months). A recent serum creatinine value was available in 103 patients (46.8% of all patients). The median baseline eCCL for the study group was 100 mL/min/1.73 m 2 . The average baseline eCCl was found to be higher ( p = 0.007) in the AKI group as compared with control. Associated comorbidities were present in 93 (57.8%) patients of the study population with congenital heart disease and developmental delay being most frequently observed. No significant difference was observed between the AKI and control groups with regard to gender, presence of comorbidities, length of PICU stay, and hospital stay.
Table 2. Demographic and clinical details of the study group.
| Parameter | Total population ( n = 220) |
AKI group ( n = 161) |
No AKI group ( n = 59) |
p -Value |
|---|---|---|---|---|
| Age (mo) a | 33.1 ± 38.6 | 25.8 ± 34.7 | 53.2 ± 42.2 | 0.000 b |
| 1–24 | 137 (62.3) | 116 (72.0) | 21 (35.6) | 0.000 b |
| 25–60 | 37 (16.8) | 20 (12.4) | 17 (28.8) | |
| Above 60 | 46 (20.9) | 25 (15.5) | 21 (35.6) | |
| Male | 146 (66.4) | 105 (65.2) | 41 (69.5) | 0.552 |
| Female | 74 (33.6) | 56 (34.8) | 18 (30.5) | 0.630 |
| Available baseline creatinine | 103 (46.8) | 59 (36.7%) | 44 (74.6) | < 0.000 b |
| Baseline eCCl a (mL/min/1.73 m 2 ) | 86.3 ± 33.9 | 90.0 ± 34.8 | 76.1 ± 29.7 | 0.007 b |
| Comorbidities c | 93 (57.8) | 68 (42.2) | 25 (42.3) | 0.538 |
| PICU stay (d) a | 8.9 ± 13.9 | 9.1 ± 15.3 | 8.4 ± 9.4 | 0.742 |
| Hospital stay (d) a | 15.1 ± 15.3 | 14.7 ± 16.2 | 16.3 ± 12.7 | 0.478 |
| PICU stay > 5 d | 113 (51.4) | 85 (52.8) | 28 (47.5) | 0.109 |
| Hospital stay > 14 d | 88 (40) | 62 (38.5) | 26 (44.1) | 0.456 |
Abbreviations: AKI, acute kidney injury; eCCl, estimated creatinine clearance; PICU, pediatric intensive care unit.
Note: Numbers in parenthesis indicate percentages; continuous data are expressed as mean ± standard deviation.
Continuous variables for which statistical analysis was performed using unpaired t -test. The remaining categorical variables were analyzed using the chi-square test.
Statistically significant p -value.
Hematology/oncology: thalassemia major, aplastic anemia, intracranial space-occupying lesions and B – cell acute lymphocytic leukemia; cardiac: cyanotic and acyanotic congenital heart diseases; and Others: protein energy malnutrition, epilepsy and inborn errors of metabolism.
The systemwise distribution and the final diagnosis of children with and without AKI are enlisted in Table 3 . Congenital heart disease as well as infections, such as pneumonia and acute gastroenteritis, was diagnosed more commonly in the AKI group. Almost one-third of children with AKI was identified to have a respiratory pathology such as pneumonia, bronchiolitis, or croup. Primary renal disease was seen in 4.5% of all children in the study group and was marginally more common in the AKI group (5.6%) compared with the control group (1.7%).
Table 3. Systemwise distribution and final diagnosis of study group.
| Total population ( n = 220) |
AKI group ( n = 161) |
No AKI group ( n = 59) |
|
|---|---|---|---|
| Final diagnosis | |||
| CNS infection | 17 (7.7) | 11 (6.8) | 6 (10.2) |
| Dengue/dengue-like illness | 8 (3.6) | 6 (3.7) | 2 (3.3) |
| Congenital heart disease | 40 (18.2) | 31(19.3) | 9 (15.3) |
| Pneumonia | 38 (17.3) | 32 (19.9) | 6 (10.2) |
| Acute gastroenteritis | 8 (3.6) | 8 (5.0) | 0 (0.0) |
| Other | 109 (49.5) | 73 (45.3) | 36 (61.0) |
| System affected | |||
| CNS | 59 (26.8) | 33 (20.5) | 26 (44.1) |
| CVS | 45 (20.5) | 32 (19.9) | 13 (22.0) |
| GIT | 14 (6.4) | 13 (8.1) | 1 (1.7) |
| Infections | 13 (5.9) | 10 (6.2) | 3 (5.1) |
| Renal | 10 (4.5) | 9 (5.6) | 1 (1.7) |
| RS | 51 (23.2) | 44 (27.3) | 7 (11.9) |
| Other | 28 (12.7) | 20 (12.4) | 8 (13.6) |
Abbreviations: AKI, acute kidney injury; CNS, central nervous system; CVS, cardiovascular system; GIT, gastrointestinal system; RS, respiratory system.
Note: Other indicates sepsis, poisoning, inborn error of metabolism. Numbers in parenthesis indicate percentages.
Incidence and Severity of AKI
AKI was detected in 161 (73.2%) of the 220 patients enrolled using the pRIFLE criteria. Majority of the patients with AKI (92 of 161) had attained a pRIFLEmax grade as Failure during the PICU stay followed by Risk in 26.1% patients and Injury in 16.8% patients ( Fig. 2 ).
Fig. 2.
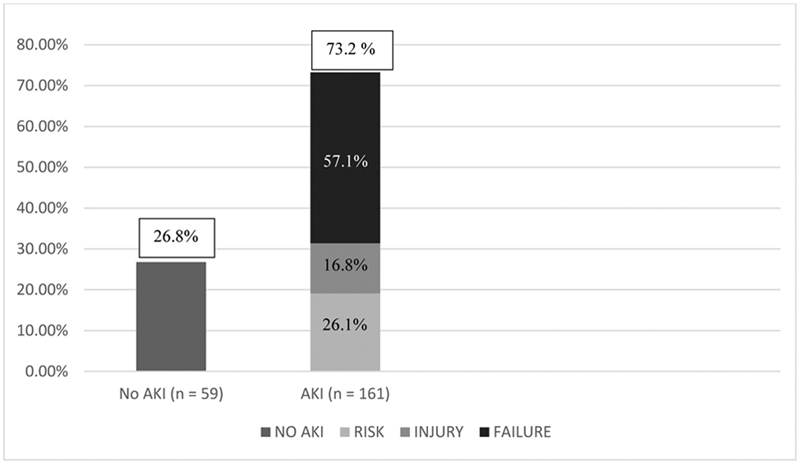
Incidence and severity of AKI (pRIFLEmax). AKI, acute kidney injury; pRIFLE, pediatric risk, injury, failure, loss, and end-stage renal disease.
Risk Factors for AKI
The various risk factors evaluated for AKI in the study population are shown in Table 4 . Infants between 1 and 24 months of age were particularly seen to be at risk for AKI ( p = 0.000) compared with older children. On univariate analysis, hypovolemia ( p = 0.005), shock ( p = 0.008), and sepsis ( p = 0.022) were also seen as significant risk factors for AKI. On the contrary, thrombocytopenia, malnutrition, need for inotropes and mechanical ventilation, and exposure to nephrotoxic drugs were found to be comparable between the AKI and no AKI groups.
Table 4. Risk factors for AKI.
| Parameter | Total population ( n = 220) |
AKI group ( n = 161) |
No AKI group ( n = 59) |
p -Value |
|---|---|---|---|---|
| Young age (1–24 mo) | 137 (62.3) | 116 (72.0) | 21 (35.6) | 0.000 a |
| Severe malnutrition | 57 (25.9) | 40 (24.8) | 17 (28.8) | 0.454 |
| Mechanical ventilation | 159 (72.3) | 119 (73.9) | 40 (67.8) | 0.369 |
| Hypovolemia | 47 (21.4) | 42 (26.1) | 5 (8.5) | 0.005 a |
| Shock | 45 (20.5) | 40 (24.8) | 5 (8.5) | 0.008 a |
| Sepsis | 54 (24.5) | 46 (28.6) | 8 (13.6) | 0.022 a |
| Thrombocytopenia | 68 (30.9) | 55 (34.2) | 13 (22.0) | 0.085 |
| Inotropes | 126 (57.3) | 91 (56.5) | 35 (59.3) | 0.710 |
| Nephrotoxic drugs | 92 (41.8) | 69 (42.9) | 23 (39.0) | 0.606 |
Abbreviation: AKI: acute kidney injury.
Statistically significant p -value.
Note: Numbers in parenthesis indicate percentages. Statistical analysis performed by the chi-square test.
Outcomes of AKI
Renal replacement therapy : Out of the total 220 patients, 4 patients required RRT. All four children were diagnosed with pRIFLEmax of Failure grade and were eventually discharged from the hospital.
Mortality : The overall PICU and hospital mortality for patients requiring intensive care was 44.5%. Seventy-five of the 161 (46.6%) children with AKI died during the PICU or hospital stay. There was no significant difference observed ( p = 0.315) in the mortality rates between children with AKI and the control groups. Also, increasing severity of renal injury was not associated with heightened mortality ( p = 0.109). Certain risk factors such as young age ( p = 0.006), hypovolemia ( p = 0.002), exposure to nephrotoxic drugs ( p = 0.005), and thrombocytopenia ( p = 0.001) were found to significantly increase mortality in children with AKI ( Table 5 ). On the contrary, mechanical ventilation, shock, sepsis, and need for inotropic support were associated with increased mortality in all children, irrespective of AKI.
Table 5. Risk factors for mortality in the AKI and control groups.
| Risk factor | AKI group p -value |
No AKI group p -value |
|---|---|---|
| Young age a | 0.006 b | 0.888 |
| Female gender | 0.103 | 0.021 b |
| Severe malnutrition | 0.462 | 0.017 b |
| Mechanical ventilation | 0.000 b | 0.000 b |
| Hypovolemia c | 0.002 b | 0.314 |
| Shock c | 0.000 b | 0.049 b |
| Sepsis | 0.000 b | 0.025 b |
| Thrombocytopenia c | 0.001 b | 0.965 |
| Inotropes | 0.000 b | 0.001 b |
| Nephrotoxic drugs | 0.005 b | 0.097 |
Abbreviation: AKI, acute kidney injury.
Note: Statistical analysis performed using chi-square test.
Age between 1 and 24 months.
Statistically significant p -value.
p -Values by Fisher's exact test in no AKI group for hypovolemia ( p = 0.369); shock ( p = 0.070); and thrombocytopenia ( p = 0.100).
Length of PICU and hospital stays : The median lengths of PICU and hospital stays were 6 and 12 days, respectively. As shown in Table 2 , the number of children requiring a PICU stay for more than 5 days ( p = 0.109) or a hospital stay of more than 14 days ( p = 0.456) was similar between the AKI and the control groups. Additionally, no difference in the duration of PICU ( p = 0.481) and hospital stays ( p = 0.650) was observed between children with varying severity of AKI.
Discussion
A high incidence of AKI (73.2%) was observed in critically ill children admitted to the PICU in our study. On univariate analysis, infancy, hypovolemia, shock, and sepsis were found to be significant risk factors for AKI. Mortality, PICU stay, and hospital stay were comparable in children with and without AKI as well as between the various grades of renal injury (i.e., Risk , Injury , and Failure ).Young age, administration of nephrotoxic drugs, and presence of thrombocytopenia were identified to significantly increase mortality in children with AKI compared with the control group.
Variations in the definition of AKI used (Acute Kidney Injury Network vs. the pRIFLE vs. the Kidney Disease: Improving Global Outcomes [KDIGO]), the study population setting (hospital ward vs. PICU), and the type of study performed (prospective vs. retrospective) have led to greatly varying rates of AKI incidence (0.3–82%) 3 4 5 6 7 8 9 10 17 and associated mortality (11–47.5%) 6 7 8 9 10 in children. Previous studies from varying pockets of India have reported the occurrence of AKI in the PICU setting to be between 14 and 43% with a mortality rate of 36 to 48% in such children. 8 9 10 18 In comparison, our study found a much higher incidence of AKI, although the related mortality was similar (46.6%).
Unlike the past, primary renal diseases are now seen to be infrequent causes of AKI. 19 20 21 22 Likewise, in our study, only 6% of children with AKI had a renal etiology such as glomerulonephritis or nephrotic syndrome. In the west, postcardiac surgery, solid organ transplantation, and sepsis are common settings for AKI. 19 20 21 23 24 In contrast, a vast majority of AKI in Indian PICUs have been seen to develop secondary to infections such as pneumonia, central nervous system infections, sepsis, and acute gastroenteritis. 4 25 26 Secondary to infection, congenital heart disease was seen to be the most common diagnosis in children with AKI in our study ( Table 3 ).
Majority (57.1%) of the children diagnosed with AKI in our study were observed to be in the Failure grade of the pRIFLE staging system. Although most previous reports state the Risk grade to be the commonest class of AKI observed, 3 4 8 9 10 a similar finding was reported from a study performed in Pakistan by Tresa et al. 27 One possible explanation for this finding may be the increased severity of illness in children admitted to our PICU center. The present study institute being a nodal referral center for Mumbai and almost the entire state, typically receives patients who have a prolonged illness and complicated treatment history prior to admission.
Another interesting finding in our study was the baseline eCCl being significantly higher in children with AKI. This may be attributed to nonavailability of a recent creatinine (therefore assumption of baseline eCCl as 100 mL/min/1.73 m 2 ) in a significantly larger proportion of children with AKI than without kidney injury ( Table 2 ). An assumption of baseline eCCl of 120 mL/min/1.73 m 2 has been reported to be more accurate in assessing AKI than 100 mL/min/1.73 m 2 . 28 This raises a question whether even the chosen, lower baseline eCCl value holds true for children in developing nations, whose overall growth and muscle mass are lesser than their western counterparts, and therefore the possibility of overestimation of AKI.
Young age, especially infancy, was demonstrated as a risk factor for AKI analogous to previous studies, probably reflecting a physiological lower creatinine clearance and immature kidney function in younger children. 8 9 10 Akin to our study, a male preponderance in PICU admissions has been previously documented by Mehta et al. 9 This observation probably reflects the tendency of health care assistance being sought for male children than females in developing nations. Comparable to prior studies, hypovolemia, shock, and sepsis were seen as risk factors for AKI. 3 4 9 29 30 It is known that prerenal AKI results from inadequate blood flow to the kidneys due to hypovolemia and shock. 31 As compared with previous studies, our study did not demonstrate exposure to nephrotoxic drugs, need for inotropes and mechanical ventilation, and thrombocytopenia as risk factors for AKI. 7 8 9 10 Severe malnutrition as a risk factor for AKI, though not significant in this study, has not been evaluated previously.
An increased mortality in patients with AKI had been reported in the past. 6 7 8 9 10 However, as was the case in our study, this association may be less evident in a PICU setting where an overall higher mortality prevails. 3 32 The duration of PICU stay as well as hospital stay was also not found to differ between patients with and without AKI, in contrast to findings from similar studies. 4 5 7 10 Few studies have evaluated risk factors for increased mortality in children with AKI. 4 9 18 33 As seen in our study, infants with AKI have been previously reported to have a decreased survival compared with older children with AKI. 18 34 In addition, our study revealed thrombocytopenia and exposure to nephrotoxic drugs to significantly increase the mortality in the AKI group. Furthermore, in contrast to previous studies, increasing severity of AKI in our study did not correlate with increased mortality, PICU stay or hospital stay duration. 3 6 7 8 9 10
Although it may appear as though AKI and its varying severity had little influence on the length of PICU stay, morbidity, and mortality of children admitted to the PICU, the true association may have been masked due to the presence of confounding factors such as an overall increased rate of mechanical ventilation (72.3%), inotropic support (57.3%), and associated comorbidities (57.8%). Additionally, the sheer high incidence of AKI in the PICU may translate to a greater number of children being at risk for developing CKD in the future, thus posing a cause for concern. 11 It remains to be seen whether an early diagnosis of AKI with facilitation of optimized fluid protocols has a positive impact on the morbidity and mortality in our PICU. The patients' severity of AKI was graded for better description of the population and for analysis purpose.
Kaddourah et al conducted the largest worldwide study (Assessment of Worldwide Acute Kidney Injury, Renal Angina, Epidemiology) in 12 countries, including India, to observe the epidemiology and risk factors of AKI in critically ill children. 33 In comparison to our study, a much lower incidence of AKI (27%) and mortality (3.5%) was observed. In addition, severe AKI (KDIGO stage 2 or 3), shock, and requirement of mechanical ventilation and RRT were observed as significant risk factors for death in children with AKI.
One of the limitations of our study was the inability to perform serum creatinine estimations for all patients on a daily basis. To compensate for this, only those patients who had at least two serum creatinine values in the first week of PICU stay or till discharge (whichever was lesser) were included. This may have resulted in a bias with a higher incidence of AKI being observed in the present study. The absence of daily serum creatinine estimation also precluded us from determining the time of onset of AKI and its sequential outcome. Another limitation was the lack of follow-up of patients diagnosed with AKI to evaluate the persistence of renal involvement and their possible progression to CKD.
A rise in serum creatinine is now considered to be a late marker of renal injury. 35 Comparing the early period of AKI to cardiac angina, a scoring system known as renal angina index has been proposed as a sensitive tool to detect children at risk of severe AKI ( grade Injury and above). 36 The sensitivity increases when used in combination with biochemical markers of kidney injury such as neutrophil gelatinase-associated lipocalin and neutrophil elastase-2. 37 Utilizing biomarkers ensures the assessment of only those children who are truly at risk for AKI. 38
Conclusion
In summary, AKI was found to be exceedingly common in critically ill children in our study. This may be attributed to an overall delayed presentation and prolonged duration of illness prior to referral to our tertiary care center. Young infants and those with hypovolemia, shock, and sepsis were observed to be at an increased risk of AKI. Moreover, majority of children being diagnosed with the severe Failure grade of AKI necessitate the formation of PICU protocols which can detect AKI early and facilitate timely corrective measures. Performing daily serum creatinine estimations for the first 3 days of PICU stay, during which the risk of AKI is the highest, can be employed in the PICUs to address this concern.
Acknowledgment
The authors thank Dr. Hemant Deshmukh, Dean, Seth G.S. Medical College and King Edward Memorial Hospital for granting permission to submit this manuscript for publication.
Funding Statement
Funding None.
Conflict of Interest None declared.
Authors' Contributions
Akanksha C. Parikh and Milind S. Tullu were both involved in conceptualization of the manuscript and the project, collecting patient data and data analysis, conducting literature search, and drafting the manuscript. Both the authors are designated as First Authors of this manuscript. Milind S. Tullu will act as the corresponding author and the guarantor for the manuscript.
References
- 1.Hoste E A, Kellum J A. Incidence, classification, and outcomes of acute kidney injury. Contrib Nephrol. 2007;156:32–38. doi: 10.1159/000102013. [DOI] [PubMed] [Google Scholar]
- 2.Van Biesen W, Vanholder R, Lameire N. Defining acute renal failure: RIFLE and beyond. Clin J Am Soc Nephrol. 2006;1(06):1314–1319. doi: 10.2215/CJN.02070606. [DOI] [PubMed] [Google Scholar]
- 3.Akcan-Arikan A, Zappitelli M, Loftis L L, Washburn K K, Jefferson L S, Goldstein S L. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71(10):1028–1035. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
- 4.Naik S, Sharma J, Yengkom R, Kalrao V, Mulay A. Acute kidney injury in critically ill children: risk factors and outcomes. Indian J Crit Care Med. 2014;18(03):129–133. doi: 10.4103/0972-5229.128701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider J, Khemani R, Grushkin C, Bart R. Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med. 2010;38(03):933–939. doi: 10.1097/CCM.0b013e3181cd12e1. [DOI] [PubMed] [Google Scholar]
- 6.Hui W F, Chan W K, Miu T Y. Acute kidney injury in the paediatric intensive care unit: identification by modified RIFLE criteria. Hong Kong Med J. 2013;19(01):13–19. [PubMed] [Google Scholar]
- 7.Alkandari O, Eddington K A, Hyder A. Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: a two-center retrospective cohort study. Crit Care. 2011;15(03):R146. doi: 10.1186/cc10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta S, Sengar G S, Meti P K, Lahoti A, Beniwal M, Kumawat M. Acute kidney injury in pediatric intensive care unit: incidence, risk factors, and outcome. Indian J Crit Care Med. 2016;20(09):526–529. doi: 10.4103/0972-5229.190368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta P, Sinha A, Sami A. Incidence of acute kidney injury in hospitalized children. Indian Pediatr. 2012;49(07):537–542. doi: 10.1007/s13312-012-0121-6. [DOI] [PubMed] [Google Scholar]
- 10.Rustagi R S, Arora K, Das R R, Pooni P A, Singh D. Incidence, risk factors and outcome of acute kidney injury in critically ill children - a developing country perspective. Paediatr Int Child Health. 2017;37(01):35–41. doi: 10.1080/20469047.2015.1120409. [DOI] [PubMed] [Google Scholar]
- 11.Mammen C, Al Abbas A, Skippen P. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis. 2012;59(04):523–530. doi: 10.1053/j.ajkd.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 12.Palmieri T, Lavrentieva A, Greenhalgh D. An assessment of acute kidney injury with modified RIFLE criteria in pediatric patients with severe burns. Intensive Care Med. 2009;35(12):2125–2129. doi: 10.1007/s00134-009-1638-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz G J, Brion L P, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34(03):571–590. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 14.Carcillo J A, Fields A I. Task force committee members: clinical practice variables for hemodynamic support of pediatric and neonatal patients in septic shock. Crit Care Med. 2002;30:1365–1378. doi: 10.1097/00003246-200206000-00040. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization ; Department of Child, Adolescent Health, World Health Organization, UNICEF . World Health Organization; Geneva, Switzerland: 2005. Handbook IMCI: Integrated Management of Childhood Illness. [Google Scholar]
- 16.International Consensus Conference on Pediatric Sepsis . Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(01):2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 17.Cao Y, Yi Z W, Zhang H, Dang X Q, Wu X C, Huang A W. Etiology and outcomes of acute kidney injury in Chinese children: a prospective multicentre investigation. BMC Urol. 2013;13(01):41–48. doi: 10.1186/1471-2490-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnamurthy S, Mondal N, Narayanan P, Biswal N, Srinivasan S, Soundravally R. Incidence and etiology of acute kidney injury in southern India. Indian J Pediatr. 2013;80(03):183–189. doi: 10.1007/s12098-012-0791-z. [DOI] [PubMed] [Google Scholar]
- 19.Kwiatkowski D M, Sutherland S M. Acute kidney injury in pediatric patients. Best Pract Res Clin Anaesthesiol. 2017;31(03):427–439. doi: 10.1016/j.bpa.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Sutherland S M, Kwiatkowski D M. Acute kidney injury in children. Adv Chronic Kidney Dis. 2017;24(06):380–387. doi: 10.1053/j.ackd.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Hui-Stickle S, Brewer E D, Goldstein S L. Pediatric ARF epidemiology at a tertiary care center from 1999 to 2001. Am J Kidney Dis. 2005;45(01):96–101. doi: 10.1053/j.ajkd.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein S L. Acute kidney injury in children: prevention, treatment and rehabilitation. Contrib Nephrol. 2011;174:163–172. doi: 10.1159/000329394. [DOI] [PubMed] [Google Scholar]
- 23.Williams D M, Sreedhar S S, Mickell J J, Chan J C. Acute kidney failure: a pediatric experience over 20 years. Arch Pediatr Adolesc Med. 2002;156(09):893–900. doi: 10.1001/archpedi.156.9.893. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein S L, Kirkendall E, Nguyen H. Electronic health record identification of nephrotoxin exposure and associated acute kidney injury. Pediatrics. 2013;132(03):e756–e767. doi: 10.1542/peds.2013-0794. [DOI] [PubMed] [Google Scholar]
- 25.Maqbool J, Mir A A, Bhat N A, Moona W Q. Incidence and etiology of acute kidney injury in children admitted to PICU using pRIFLE criteria. Int J Contemp Pediatr. 2018;5(03):917–921. [Google Scholar]
- 26.Bharat A, Mehta A, Tiwari H C, Sharma B, Singh A, Singh V. Spectrum and immediate outcome of acute kidney injury in a pediatric intensive care unit: a snapshot study from Indian subcontinent. Indian J Crit Care Med. 2019;23(08):352–355. doi: 10.5005/jp-journals-10071-23217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tresa V, Yaseen A, Lanewala A A. Etiology, clinical profile and short-term outcome of acute kidney injury in children at a tertiary care pediatric nephrology center in Pakistan. Ren Fail. 2017;39(01):26–31. doi: 10.1080/0886022X.2016.1244074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zappitelli M, Parikh C R, Akcan-Arikan A, Washburn K K, Moffett B S, Goldstein S L. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol. 2008;3(04):948–954. doi: 10.2215/CJN.05431207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freire K M, Bresolin N L, Farah A C, Carvalho F L, Góes J E. Acute kidney injury in children: incidence and prognostic factors in critical ill patients [in Portuguese] Rev Bras Ter Intensiva. 2010;22(02):166–174. [PubMed] [Google Scholar]
- 30.Nawaz S, Afzal K. Pediatric acute kidney injury in North India: a prospective hospital-based study. Saudi J Kidney Dis Transpl. 2018;29(03):689–697. doi: 10.4103/1319-2442.235172. [DOI] [PubMed] [Google Scholar]
- 31.Srivastava R, Bagga A. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd.; 2011. Pediatric Nephrology, 5th ed; pp. 235–260. [Google Scholar]
- 32.Soler Y A, Nieves-Plaza M, Prieto M, García-De Jesús R, Suárez-Rivera M. Pediatric risk, injury, failure, loss, end-stage renal disease score identifies acute kidney injury and predicts mortality in critically ill children: a prospective study. Pediatr Crit Care Med. 2013;14(04):e189–e195. doi: 10.1097/PCC.0b013e3182745675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.AWARE Investigators . Kaddourah A, Basu R K, Bagshaw S M, Goldstein S L. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017;376(01):11–20. doi: 10.1056/NEJMoa1611391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang J W, Jeng M J, Yang L Y. The epidemiology and prognostic factors of mortality in critically ill children with acute kidney injury in Taiwan. Kidney Int. 2015;87(03):632–639. doi: 10.1038/ki.2014.299. [DOI] [PubMed] [Google Scholar]
- 35.Goldstein S L, Chawla L S. Renal angina. Clin J Am Soc Nephrol. 2010;5(05):943–949. doi: 10.2215/CJN.07201009. [DOI] [PubMed] [Google Scholar]
- 36.Basu R K, Zappitelli M, Brunner L. Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children. Kidney Int. 2014;85(03):659–667. doi: 10.1038/ki.2013.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basu R K, Wang Y, Wong H R, Chawla L S, Wheeler D S, Goldstein S L. Incorporation of biomarkers with the renal angina index for prediction of severe AKI in critically ill children. Clin J Am Soc Nephrol. 2014;9(04):654–662. doi: 10.2215/CJN.09720913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldstein S L. Pediatric acute kidney injury—the time for nihilism is over. Front Pediatr. 2020;8:16. doi: 10.3389/fped.2020.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]


