Abstract
Often considered a rare disease, cardiac amyloidosis is increasingly recognized by practicing clinicians. The increased rate of diagnosis is in part due the aging of the population and increasing incidence and prevalence of cardiac amyloidosis with advancing age, as well as the advent of non-invasive methods using nuclear scintigraphy to diagnose transthyretin cardiac amyloidosis due to either variant or wild type transthyretin without a biopsy. Perhaps the most important driver of the increased awareness is the elucidation of the biologic mechanisms underlying the pathogenesis of cardiac amyloidosis which have led to the development of several effective therapies with differing mechanisms of actions. In this review, the mechanisms underlying the pathogenesis of cardiac amyloidosis due to light chain (AL) or transthyretin (ATTR) amyloidosis are delineated as well as the rapidly evolving therapeutic landscape that has emerged from a better pathophysiologic understanding of disease development.
Keywords: Amyloidosis, Cardiac, Pathogenesis, Therapy, cardiomyopathy, treatment, pathophysiology, Transthyretin, immunoglobulin light chains amyloid
Subject Terms: Cardiomyopathy, Heart Failure, Pathophysiology, Treatment
The elucidation of physiologic mechanisms underlying the genesis of misfolded proteins which form amyloid fibrils that deposit in the myocardium and can cause cardiac amyloidosis has led to development of several effective therapeutic approaches1. These efforts have led to therapies that have been described as a “translational triumph”2. Among the causes of cardiac amyloidosis, the two that account for >95% of cases encountered clinically (Table 1) include: (1) immunoglobulin light chain (AL) cardiac amyloidosis, which is due to a plasma cell dyscrasia with over-production of either kappa or lambda light chains, and (2) transthyretin (TTR) cardiac amyloidosis, which results from misfolded monomers or oligomers of either wild type (ATTRwt) or variant transthyretin (ATTRv) cardiac amyloidosis3. ATTRv is inherited in an autosomal dominant fashion and is due to one of the more than 130 mutations in the transthyretin gene on chromosome #18. With the aging of the population, ATTRwt cardiac amyloidosis (CA) is anticipated to become the most common form of systemic amyloidosis. In this review, we will delineate the mechanisms underlying the pathogenesis of cardiac amyloidosis and highlight the rapidly evolving therapeutic landscape that has emerged from a better understanding of disease development.
Table 1.
Major Etiologies of Cardiac Amyloidosis
| Features | Light Chain Cardiac
Amyloidosis (AL-CA) |
Transthyretin Cardiac Amyloidosis | |
|---|---|---|---|
| Wild type (ATTRwt-CA) |
Variant / Hereditary
Transthyretin (ATTRv-CA) |
||
| Age at diagnosis | 5th to 9th decade | 7th to 10th decade | 3rd to 8th decade |
| Sex distribution | Roughly equal male:female | Very significant male predominance | Male predominance |
| Precursor protein | Light-chain | Transthyretin | Transthyretin |
| Genetic etiology | None | None | Autosomal dominant inheritance |
| Genetic modifier to therapeutic efficacy | t(11,14) presence – poor response to bortezomib but responsive to venetoclax | None | None |
| Extracardiac involvement | Nerves, kidney, liver, gastrointestinal tract, skin, tongue/soft tissue | Carpal tunnel, lumbar spine, gastrointestinal tract | Nerves, |
| Clinical Manifestations | Multi-systemic disease with cardiac and renal involvement (60–70%); liver (15%) and peripheral / autonomic neuropathy (10%) | Predominant cardiac phenotype with a restrictive cardiomyopathy, atrial and ventricular arrhythmias and HFpEF | Depends on variant. Val122Ile predominately cardiac, Thr60Ala mixed and Val30Met predominately neuropathic |
| Prognosis after diagnosis | Depends on stage. Median survival 4–6 months with advanced heart failure | Depends on stage. Median survival 2–6 years in the absence of treatment | Depends on mutation and stage. Median survival 3–12 years |
AL-CA, immunoglobulin light-chain cardiac amyloidosis; ATTRwt, wild-type transthyretin amyloidosis; ATTRv, variant (hereditary, familial) transthyretin amyloidosis; CA, cardiac amyloidosis; HFpEF, heart failure with a preserved ejection fraction.
Pathophysiology
Despite originating from different precursor proteins, the basic mechanisms underlying amyloid pathogenesis is similar in that the capability of a protein to become amyloidogenic lies in its ability to acquire more than one conformation. Amyloid formation occurs when a protein loses (or fails to acquire) its physiologic, functional fold. A number of factors may trigger protein misfolding and aggregation, such as abnormal proteolysis, point mutations and post-translational modifications such as phosphorylation, oxidation and glycation. The misfolded protein or peptide then assembles with similar proteins or peptides to form oligomers, which circulate in the blood and deposit as highly ordered fibrils in the interstitial space of target organs. In cardiac amyloidosis, the mechanisms of organ dysfunction are likely multifactorial, resulting from a combination of factors including extracellular deposition of amyloid in the parenchymal tissue leading to mechanical disruption of tissue structure, as well as proteotoxicity of the fibrils or pre-fibrillar proteins leading to inflammation, reactive oxygen species generation, apoptosis and autophagy, which can be observed even prior to fibril deposition4–7. This leads to a restrictive physiology, diastolic dysfunction and eventually manifests clinically as heart failure.
Overview of protein folding
Protein or peptide folding is a tightly regulated process. In general, proteins require specific three-dimensional conformations in order to be soluble and function correctly in the body. The process of protein folding begins after polypeptide chains are synthesized in the endoplasmic reticulum of the cell and a rapid sequence of intracellular folding consisting of conformational modifications is initiated, requiring the use of chaperones and catalysts of folding, to achieve its native structure. In this pathway, conformational intermediates become progressively more organized as they merge, resulting in the most stable native state. In this native structure, there is a minimum of free energy which results from the balance between the internal energy of the protein determined by intramolecular bonds and the level of conformational entropy, determined by the level of randomness of the polypeptide in solution8.
Proteins must remain folded throughout their lifetimes to continue to perform their biological functions and the abundance of each of the thousands of different proteins in a cell must be tightly regulated. This state of a balanced proteome, termed protein homeostasis or proteostasis, requires an extensive network of competing pathways within cells that control the protein synthesis and folding, conformational maintenance and degradation of proteins present within and outside the cell9. The proteostasis network serves to ensure that correctly folded proteins are generated at the proper time and cellular location and in amounts allowing stoichiometric assembly in the case of oligomeric protein complexes. Additionally, it prevents proteins from misfolding and aggregating, and removes proteins that are misassembled. Normally, misfolded proteins are retained by the endoplasmic reticulum, dislocated to the cytoplasm and degraded by the proteasome10. Loss of proteostasis is the underlying cause of disease associated with protein misfolding, such as amyloidosis.
Mechanisms of amyloidogenesis.
Folded proteins structures, in most cases, are only marginally stable, meaning that a substantial proportion of protein species exist in unfolded states. The exposure to various extra-cellular denaturing stimuli causes unfolding of the polypeptide chain, an event which is normally followed by the rapid restoration of the native structure. This extra-cellular unfolding and refolding process causes the exposure of normally hidden hydrophobic residues and the protein may become the target of ubiquitous endopeptidases. Even minor proteolytic cleavage can destabilize the protein, promote its denaturation and prevent the restoration of the native structure.
Partial unfolding of the native state of the protein to less thermodynamically stable states is a required step in amyloidogenesis11. Amyloidogenic and normal protein counterparts are synthesized, but cellular quality control appears to be unable to remove misfolded proteins and they are secreted from the cell11. Outside the cell, amyloidogenic variants reach a state of equilibrium between fully folded and partially folded forms. Any factor that disrupts the normal three-dimensional protein structure, such as low pH, oxidation, increased temperature, can shift this equilibrium towards the partially folded state. A misfolded protein must then reach critical local concentration to trigger fibril formation, in conjunction with local factors including glycosaminoglycans and collagen, shear forces, endoproteases and metals that modulate aggregation and oligomer formation9, 12, 13.
In both ATTRv and ATTRwt, amyloid aggregation of TTR is preceded by destabilization of the native homotetrameric structure into its constituent monomers and dimers with an exposed hydrophobic surface, followed by misfolding and structural reorganization into amyloid aggregates (Figure 1). Physiologic TTR is a homotetramer, mainly synthesized in the liver and the choroid plexus of the brain, circulates in plasma and CSF and serves as a carrier of thyroxine and retinol bound to retinol-binding protein14. TTR consists of 2 weakly bound dimers, between which lie 2 thyroxine (T4) binding sites. It is at these sites that the dimers of TTR dissociate and the process of destabilization and unfolding occur. In ATTRv, different mutations lead to a kinetically unstable tetrameric protein with an increased propensity to dissociate into monomers leading to misfolding15. For example, the Val122Ile (p.Val142Ile), variant destabilizes the TTR tetramer by lowering the kinetic barrier for tetramer dissociation, resulting in a greater extent and faster rate of folded monomer formation that then self assembles into amyloid fibrils in vitro. Despite structural instability, mutant TTR tetramers are secreted with the same efficacy as wild type if they possess a thermodynamic and kinetic profile to escape the endoplasmic reticulum degradation pathway. In ATTRwt, which is associated with increased age, protein oxidative modifications and failures in the proteostatic machinery and repair mechanisms associated with aging, contribute to native TTR dissociation and aggregation into fibrils5.
Figure 1:
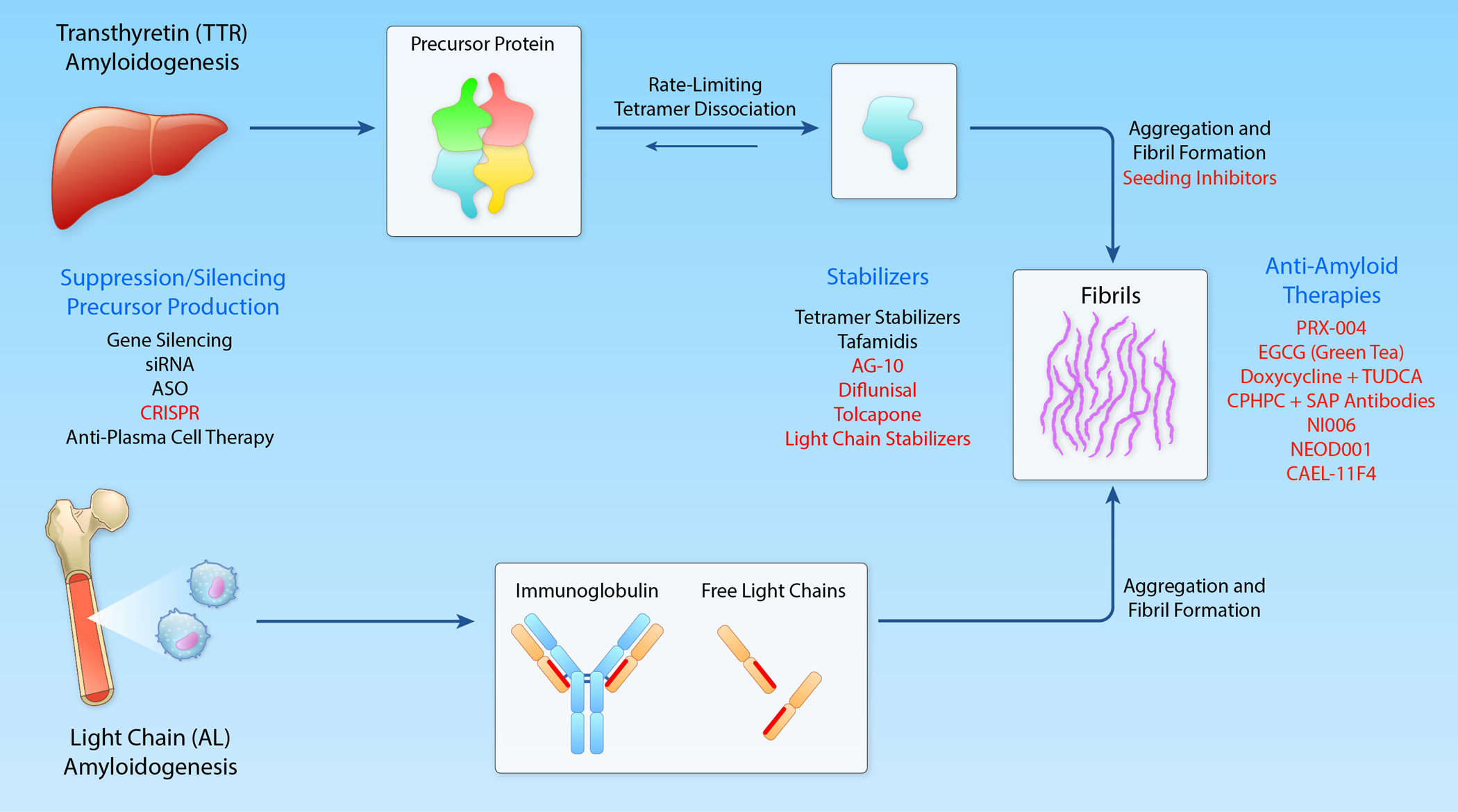
Pathogenesis of Cardiac Amyloidosis and Therapies. Mechanisms underlying formation of cardiac amyloidosis in TTR and AL. Targets for therapy are enumerated in blue. Effective therapies approved for use are shown in black and experimental therapies in a particular class are shown in red. Those listed above specific mechanism are for TTR while those listed below a specific mechanism are for AL amyloidosis. (Illustration credit: Ben Smith).
AL amyloidosis is characterized by a clonal expansion of differentiated plasma cells, that produce misfolding-prone immunoglobulin free light chains, or a fragment thereof, secreted in excess compared to heavy chains14, 16. The two classes of light chains, kappa and lambda, each consist of an N-terminal variable Ig domain attached to a C-terminal constant Ig domain. While excess free light chain production is observed in plasma cell disorders including monoclonal gammopathy of undetermined significance, multiple myeloma and Waldenstrom macroglobulinemia, only a fraction of free light chains can form amyloid deposits in vivo. Lambda light chains are observed almost twice as commonly as kappa in systemic AL amyloidosis17. DNA sequencing studies have shown presence of germline gene mutations on the variable lambda region, Vλ6a and Vλ3r, that reduce the thermodynamic stability of the protein, have a strong association with the development of amyloidosis and account for the propensity of lambda light chains to form amyloid deposits18. Amyloidogenic light chains are kinetically unstable and susceptible to endoproteolysis, which results in the release of amyloidogenic light chain fragments prone to improper aggregation19.
Other less commonly forms of cardiac amyloidosis encountered clinically include AA amyloidosis, dialyses associated amyloidosis and isolated atrial amyloidosis. AA amyloidosis is associated with auto inflammatory condition such as rheumatoid arthritis, inflammatory bowel disease and hidradenitis suppurativa, particularly when the diagnosis is delayed. Serum amyloid associated protein (SAA) is elevated and cardiac involvement in AA amyloidosis is always preceded by renal involvement. Effective control of the underlying inflammatory process can halt disease progression and even reverse organ damage.20 β2-microglobulin is the precursor protein for cardiac amyloidosis associated with long term dialysis, Cardiac amyloidosis related to β2-microglobulin occurred with low-flow dialysis membranes during dialysis of > 9 years in duration. However, newer dialysis technologies reduce serum β2-microglobulin levels in chronic dialysis patients and appear to reduce the risk of developing this form of systemic amyloidosis.21 Isolated atrial amyloidosis (IAA), due to deposits of amyloidosis from atrial natriuretic factor, is extremely common with advancing age and often seen on biopsies of the atrium obtained at the time of cardiac surgery.22 Distinguishing these forms of amyloidosis from those more commonly encountered clinically is essential.
Amyloid fibril structure
Despite originating from different precursor proteins, amyloid deposits share several structural properties as observed by electron microscopy. They are composed of rigid, non-branching fibrils with an average diameter of 7.5–10nm and a cross-ß-sheet secondary structure. Intermolecular main-chain hydrogen bonding between the amide and carbonyl groups of the main chain acts as a major stabilizing interaction between protein monomers8, 23 that allow formation of the ß-sheet. Both immunoglobulin light chains and TTR protein have extensive ß-structure in the normal folded state, but this region has to be exposed for intermolecular hydrogen bonding between monomers. Contiguous ß-sheet polypeptide chains constitute a protofilament, which are wound around one another to form an amyloid fibril, which frequently have repetitive hydrophobic or polar interactions along the fibril axis23. This ultrastructure of the fibril allows the intercalation of Congo red dye, conferring the diagnostic property to amyloid of apple-green birefringence under polarized light microscopy.
Beside the fibril core proteins, additional components are known to be part of all amyloid deposits including components of the extracellular matrix, such as lamin, entactin and collagen, and additional proteins. Serum amyloid P-component (SAP), a glycoprotein that belongs to the pentraxin family, is calcium dependently bound to amyloid fibril independently of the protein of origin24. SAP is highly protected against proteolysis25, making amyloid fibrils highly rigid, resistant to thermal and chemical denaturation and degradation. Proteoglycans or glycosaminoglycans are also common in amyloid deposits and contribute to the carbohydrate composition of amyloid, influencing the conformation of the fibril. Their role is quite complex and seem to contribute to both the genesis and the structural stabilization of amyloid fibrils. Proteoglycans are proposed to represent the initial structural scaffold that facilitates adhesion and orientation of the first nuclei of aggregated amyloid. Despite being common component of all amyloid deposits, proteoglycans show a degree of chemical and structural heterogeneity, depending on the tissue and may play a role in the localization of amyloid deposits in tissues.
Amyloid deposition
Amyloid deposition in target organs occurs by an extremely complicated aggregation process. The kinetics of fibril formation has an S-shaped growth curve and a discernable lag phase. The duration of each phase of fibril formation is protein specific and defines the rate at which amyloid deposition occurs26. First, primary nucleation occurs when soluble oligomers form from monomers. The initial nucleation process is driven by specific adhesive parts of the amyloid proteins and target organ cells may be transiently involved11. In ATTR, two adhesive segments that form the F and H ß-strands in the native TTR structure are the principal drivers of protein aggregation. Upon dissociation into monomers, these strands become exposed and enable stacking into the steric zipper spines of amyloid fibrils27. Circulating monomers can then add to this existing fibril. Secondary nucleation occurs when the surface of this existing amyloid aggregate catalyzes the formation of new small soluble aggregate and fragmentation occurs when the existing fibrils break apart, increasing the total number of fibrils. The process of amyloid deposition can be accelerated by the presence of preformed fibrils, or seeds, which can capture and catalyze the conversion of precursors, even at low concentrations, into misfolded, toxic and aggregation-prone structures28.
Deposition of amyloid in specific organ tissues likely depends on the concurrence of several factors including high local protein concentrations, low pH and the presence of fibril seeds. Specific interactions with tissue glycosaminoglycans or cell surface receptors may be important29. In AL amyloidosis, it has been hypothesized that organ tropism may be a function of the variable region gene polymorphisms, leading to interactions between the light chain (or fragment thereof) and tissue constituents such as collagen, lipids and glycosaminoglycans19. For example, LV1–44 germ line cells favor deposition in cardiac tissue18. In ATTRv, the specific site of amino acid substitution determines the propensity for depositing primarily in the peripheral nervous system or cardiac tissue, leading to markedly different phenotypes of disease. Patients with the Val122Ile (p.Val142Ile) TTR variant present predominantly with a cardiomyopathy4 whereas other variants such as Val30Met (p.Val50Met) are associated with neuropathy. While for certain mutations the correlation between the genotype and phenotype is strong, for others clinical features can vary significantly. Furthermore, significant variability in clinical presentation is seen between patents with the same mutation. Cleavage of the TTR monomer into fragmented fibrils may play a role as well in determining the site of deposition as well as the disease penetrance30. Full length TTR fibrils are commonly seen in ATTRv caused by the Val30Met (p.Val50Met) variant with early disease and a predominant axonal polyneuropathy with rare cardiac involvement, whereas those with later onset disease often have fibrils composed of a mixture of full-length and truncated TTR. These patients also often have marked cardiac involvement at presentation with concomitant peripheral neuropathy. Similarly, in patients with ATTRwt, amyloid deposits always include both fragmented and full length TTR fibrils31. Indeed, environmental and genetic factors that have not been identified must play a role in the pathobiology of TTR amyloidosis.
Mechanisms of cardiac dysfunction (Figure 2)
Figure 2:
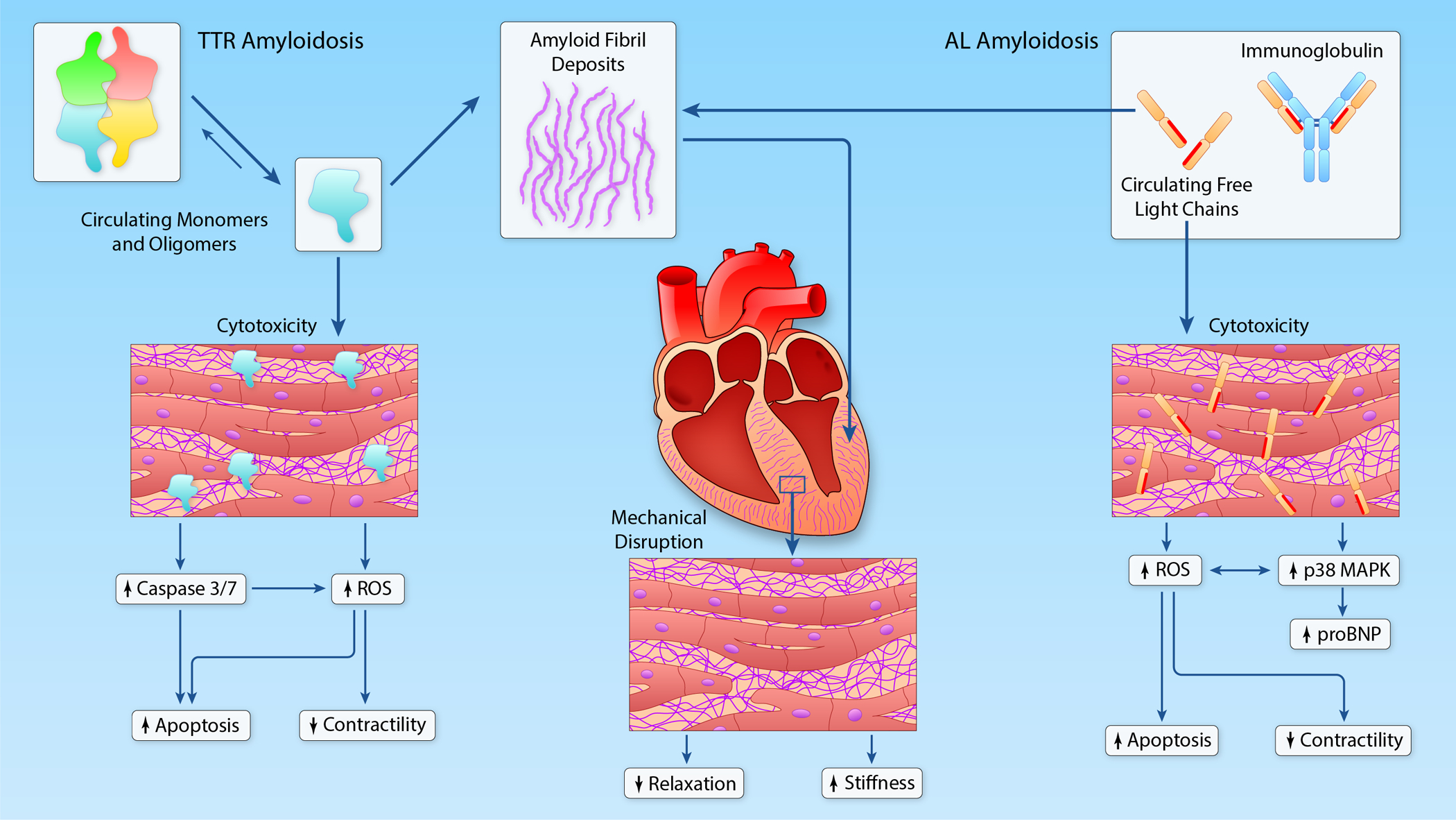
Mechanisms of myocardial dysfunction in TTR and AL amyloidosis. In both TTR and AL, extracellular deposition of amyloid fibrils causes mechanical disruption of normal tissue architecture, leading to impaired relaxation and increased ventricular stiffness. In AL amyloidosis, circulating free light chains cause cytotoxicity by increasing oxidative stress and activation of the p38 MAPK signaling pathway. Activation of the p38 MAPK pathway also leads to release of NT-proBNP. In TTR amyloidosis, circulating TTR monomers and oligomers have also been proposed to cause direct cytotoxicity. (Illustration credit: Ben Smith).
The deposition of amyloid fibrils results in cellular injury, tissue damage and finally organ dysfunction. Although the type of cardiac amyloidosis cannot be distinguished based on patterns of deposition, it appears there is a predominance of diffuse, peri-cellular, endocardial and arterial or arteriolar deposits in AL amyloidosis and nodular deposits in TTR amyloidosis32. In both AL and TTR cardiac amyloidosis, large deposits of amyloid in the extracellular space of the myocardium leads to loss of normal tissue architecture and function, progressive biventricular wall thickening and stiffness without compensatory ventricular dilation, leading to a restrictive myopathy and low cardiac output11. Early disease is marked by isolated diastolic dysfunction with normal systolic function but as the disease progressive restrictive physiology becomes apparent, atrial infiltration is present and frequently causes contractile dysfunction. Insights from non-invasive pressure-volume analysis in patients with both wild-type and Val122Ile associated TTR-CA demonstrate a complex cascade of events occurring overtime marked by decreasing ventricular capacitance and chamber contractility leading to reduced stroke volume, alterations in ventricular-vascular coupling and progressive pump dysfunction not simply due to impairment in diastolic dysfunction but systolic derangements as well33 (Figure 3).
Figure 3:
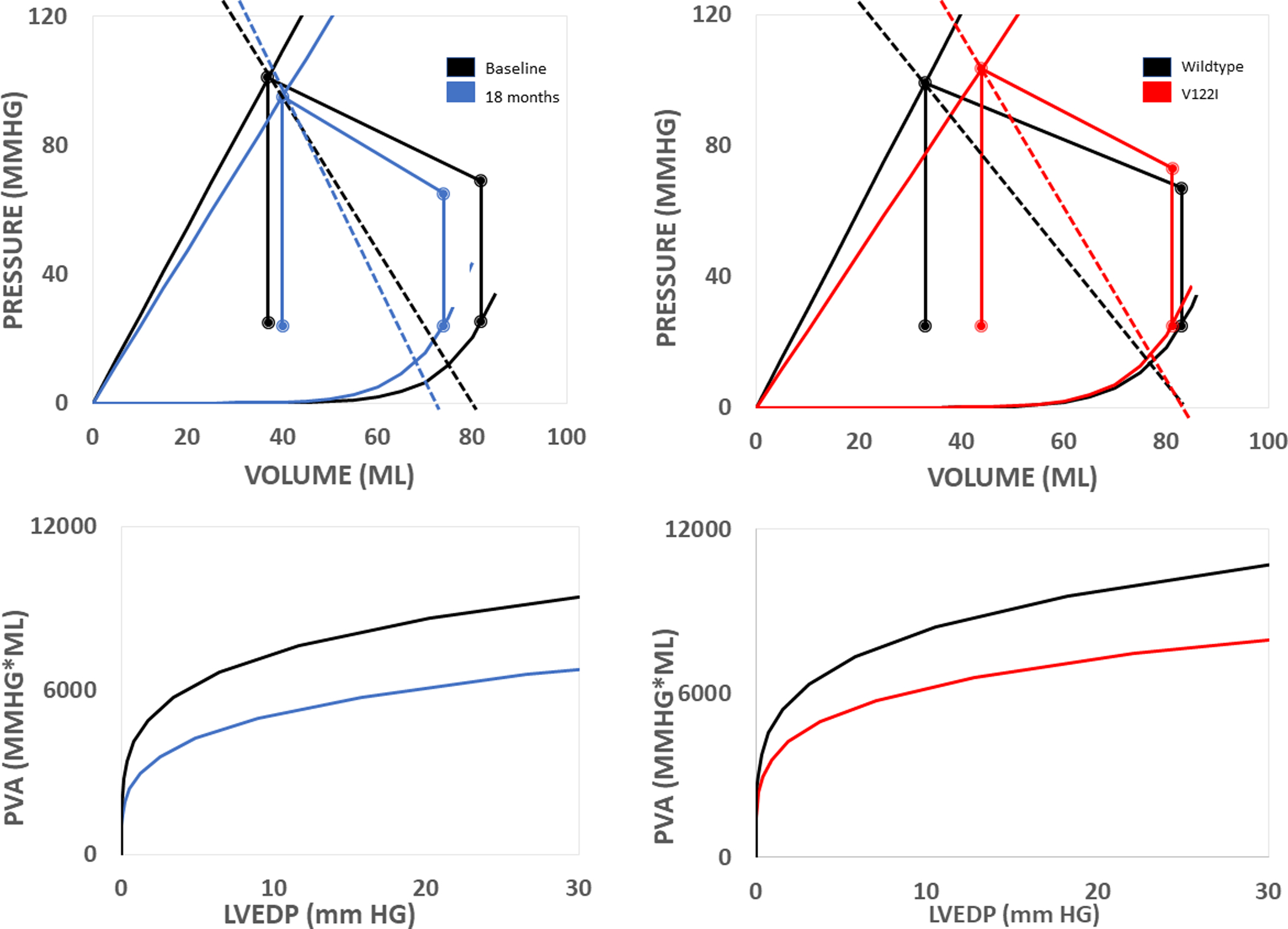
Cardiac mechanics in TTR amyloidosis. Non-invasive pressure-volume loops (top) and isovolumetric pressure volume area (bottom).
(Left) Progression of cardiac chamber dysfunction overtime marked by reduced ventricular capacitance and impaired contractility. Collectively, both abnormalities lead to reduced stroke volume and isovolumetric pressure volume area overtime.
(Right) Compared to WT, patients with V122I associated ATTR-CA have more impaired cardiac function at baseline. Patients with both WT and V122I associated ATTR-CA have reduced ventricular capacitance. However, patients with V122I associated ATTR-CA also have impaired contractility, leading to lower stroke volume and isovolumetric pressure-volume area in V122I associated ATTR-CA compared to WT.
Mechanical displacement of normal parenchymal tissue by amyloid deposits is insufficient to fully explain organ dysfunction associated with both AL and TTR amyloidosis (Figure 2). In addition to the mechanical problems imposed by amyloid fibril deposition, small soluble monomers and oligomers are extremely toxic and believed to play a major role in cell and tissue toxicity. The direct toxic effect of circulating light chains in AL amyloidosis has been postulated to explain discrepancies between myocardial amyloid fibril burden, cardiac dysfunction and the more aggressive disease trajectory in those with AL compared with TTR cardiac amyloidoses34. Notably, not only amyloid deposition, but light chain proteotoxicity exhibits specific organ tropism7. Pre-fibrillar cardiotropic light chains alter cellular redox state in cardiomyocytes, marked by an increase in intracellular reactive oxygen species (ROS), increased apoptosis and cause oxidative stress7. Oxidant stress imposed by the light chains result in direct impairment of cardiomyocyte contractility and relaxation with associated alterations in intracellular calcium handling4, 35. The activation of p38 mitogen-activated protein kinase (MAPK) is one of the molecular mechanisms responsible for cardiotoxicity by increasing oxidative stress and apoptosis. This pathway also mediates type B natriuretic peptide transcription, supporting the association between cardiotoxic light chain effects with induced MAPK signaling and elevated brain natriuretic peptide (BNP) levels36. Indeed, the degree of circulating light chain abnormality is clinically prognostic in patients with AL amyloidosis37, and correlates with cardiac biomarker elevations. Furthermore, reduction in circulating amyloidogenic free light chain concentrations by chemotherapy translates into reductions in BNP, despite unaltered amyloid deposition in the myocardium38.
In TTR amyloidosis, an accumulating body of evidence suggests that tissue dysfunction precedes TTR fibril deposition, suggesting as well that circulating pre-fibrillar proteins cause toxicity. In vitro, TTR monomers and oligomeric intermediates smaller than 100kDa, but not large aggregates or amyloid fibrils, induce cytotoxicity through interactions with membrane proteins and cholesterol7. Apoptotic mechanisms are activated through cleavage of caspase 3/7 and superoxide formation4, 6. However, the relevance of these short-term in vitro findings to disease that manifest clinically over months to years remains unclear6.
Presentation and diagnostic evaluation
Systemic manifestations of TTR deposition, such as carpal tunnel syndrome, lumbar spine stenosis or biceps tendon rupture, may precede cardiac diagnosis of ATTR-CA by several years, survival after which is approximately 2–5 years without treatment. In contrast, AL-CA is a more rapidly progressing disease, with a median survival of 6 months from the onset of heart failure if untreated. Although a detailed description of the presentation and diagnostic evaluation of cardiac amyloidosis is outside of the scope of this review, table 2 outlines the cardinal manifestations, approach to diagnostic testing and characteristic findings for both AL- and ATTR-CA.
Table 2:
Clinical manifestations and diagnostic evaluation of cardiac amyloidosis
| AL (Light Chain Amyloidosis) | TTR (Transthyretin Amyloidosis) | |
|---|---|---|
| Clinical features | Heart failure with a preserved
ejection fraction Right heart failure (JVP, hepatomegaly and edema) Atrial arrhythmias (atrial fibrillation and flutter) Intolerance of neurohormonal blockade |
|
| Periorbital purpura and
macroglossia Proteinuria and nephrotic syndrome Peripheral neuropathy or autonomic dysfunction Gastrointestinal motility disorder |
Men affected more than females in
ATTRwt Peripheral neuropathy or autonomic dysfunction especially in ATTRv Orthopedic manifestations including bilateral carpal tunnel, lumbar spinal stenosis and biceps tendon rupture Conduction disease |
|
| ECG | Low voltage to mass
ratio Pseudoinfarct pattern Low QRS voltage (late phase phenomenon, not sensitive but specific) |
|
| Low voltage more common due to toxic light chains | Conduction disease Left Ventricular Hypertrophy in 15% |
|
| Echocardiogram | Left ventricular wall thickness
>12mm Low tissue doppler velocities Restrictive filling pattern Apical sparing Atrial septal thickening |
|
| LV wall thickness usually < 15 mm | LV wall thickness usually > 15
mm Concomitant aortic stenosis |
|
| Cardiac Magnetic Resonance Imaging | Late gadolinium enhancement (in
any pattern) Elevated native T1 and ECV |
|
| ECV higher in AL than ATTR | Native T1 lower in ATTR than AL | |
| 99mTechnetium- pyrophosphate scintigraphy | Myocardial uptake < grade 2 | Myocardial uptake ≥ grade 2 |
| Endomyocardial or extracardiac biopsy | Positive congo red
staining Apple-green birefringence under polarized light |
|
| Fat pad biopsy positive >
50% Renal involvement Carpal tunnel |
Fat pad biopsy positive <
50% Lumbar spine and carpal tunnel |
|
| Serum Biomarkers | Elevated troponin and natriuretic peptides based on stage of disease | |
| Abnormal free light chain
ratio Monoclonal protein present on immunofixation |
Normal free light chain ratio No monoclonal protein on immunofixation |
|
AL, immunoglobulin light-chain amyloidosis; ATTRwt, wild-type transthyretin amyloidosis; ATTRv, variant transthyretin amyloidosis; ECV, extracellular volume; JVP, jugular venous pressure; LV, left ventricle.
Therapeutic targets
Emerging from the basic molecular mechanisms of the genesis of amyloid fibrils in the myocardium that underlie the development of cardiac amyloidosis, there are several therapeutic strategies that have either been shown to be effective or are actively being explored in human clinical trials. These approaches broadly include one of four strategies including:
knocking down production of the precursor protein with either gene silencing techniques for TTR that leverage small interfering RNA or anti-sense oligonucleotides (both of which include approved compounds for ATTRv disease with or without a concomitant cardiomyopathy) and CRISPR based approaches; for AL amyloidosis there is a large and growing armamentarium of anti-plasma cell therapies.
Stabilization of the precursor protein in order to maintain its normal conformational structure which has led to the development of tafamidis for TTR cardiac amyloidosis39 and the investigation of other TTR stabilizers (e.g. AG10), as well the identification of small molecules that kinetically stabilize light chains.40
Degradation/Disruption of amyloid fibrils with use of monoclonal antibodies targeted at particular epitopes on misfolded and/or aggregated proteins that either induce macrophage medicated dissolution or disruption of amyloid formation41–43
Anti-seeding therapies that involve peptides designed as inhibitors to cap fibril growth.44, 45
Effective and Emerging Therapies Based on Biologic Mechanisms
Although ATTR and AL amyloidosis both result in the deposition of amyloid fibrils and damage to the involved organs, treatment regimens are distinct. Therapies are generally more effective if administered before significant cardiac dysfunction has ensued (Figure 4). The following sections will describe:
Reducing the precursor protein or stabilization of TTR amyloid in cardiac amyloidosis.
Anti-plasma cell therapies for the treatment of AL amyloidosis.
Agents targeting the degradation and/or extraction of TTR or AL amyloid fibrils.
Emerging therapies for ATTR and AL amyloidosis.
Figure 4:
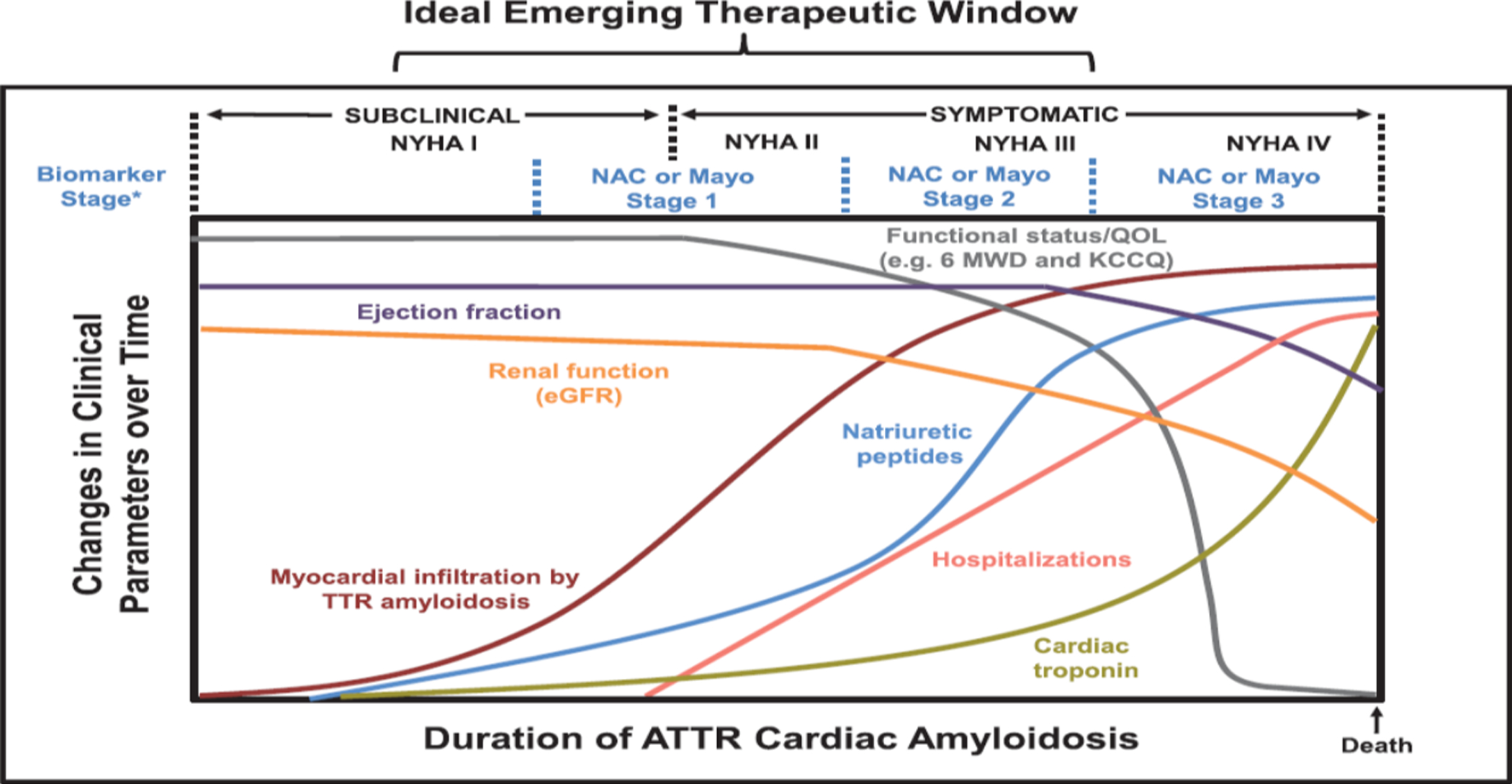
Model of ATTR-CA progression over time. Myocardial amyloid infiltration occurs before clinically manifested changes in ejection fraction, cardiac biomarkers, and renal function. The ideal emerging therapeutic window for novel therapies is hypothesized to be before significant organ dysfunction has occurred and before rapid and potentially irreversible declines in functional capacity. The relative scale specific to each factor and time course are not proportional. (From Circulation. 2019 Jul 2;140(1):27–30).
Knocking Down or Reducing Precursor Production (Table 3).
Table 3:
Therapies for Transthyretin Cardiac Amyloidosis
| Drug | Phase of study | Indication by Amyloid Type | Mechanism of action | Dose | Adverse Effects | Concomitant Therapy & Monitoring | Annual Cost | Primary Endpoints | |
|---|---|---|---|---|---|---|---|---|---|
| KNOCK-DOWN | Inotersen (Tegsedi) | Phase 2 NCT03702829 |
ATTRv & ATTRwt-CA NYHA I-III |
2’-O-methoxyethyl-modified ASO, binds to nuclear target mRNA in the liver and via RNase H2 initiates mRNA degradation | 300 mg SC per week |
|
Vitamin A supplementation CBC, BMP & UA every 2 weeks |
~$450,000 | Systolic strain imaging on echo compared to baseline at month 6 |
| Patisiran (Onpattro) | Phase 3 APOLLO-B NCT03997383 |
ATTRv & ATTRwt-CA NYHA I-III |
siRNA which targets the 3’ untranslated region of the TTR mRNA, forming the RISC and subsequent mRNA degradation | 0.3 mg/kg IV infusion q 3 weeks (max dose 30mg) |
|
Steroid IV, APAP, H1 & H2 blocker IV & Vitamin A Supplement | ~$450,000 | Change from baseline at month 12 in 6-MWT | |
| Vutrisirian | Phase 3 HELIOS-B NCT04153149 |
ATTRv & ATTRwt-CA NYHA I-III |
siRNA conjugated to GalNAc, binds to TTR mRNA in the nucleus and initiates mRNA degradation via RNase H2 | 25mg SC every 3 months | Unknown | Vitamin A supplement | Unknown | Composite outcome of all-cause mortality and recurrent CV hospitalizations at 30–36 months | |
| AKCEA-TTR-LRx / ION 682884 | Phase 3 Cardio- TTRansform NCT04136171 |
ATTRv & ATTRwt-CA NYHA I-III |
ASO conjugated to GalNAc, ASO portion shares the same base sequence as Inotersen, thus same mechanism of action | 45mg SC every 4 weeks | Unknown | Vitamin A supplement Platelets every week BMP, LFTs and UPCR every 2 weeks |
Unknown | Composite of CV mortality and frequency of CV clinical events at 120 weeks | |
| CRISPR (NTLA-2001) | Phase 1 Open label and Single Dose Expansion Study to Evaluate Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics |
ATTRv-FAP | Cas9/CRISPR genome editing | IV dose escalation study | Unknown | None | Unknown |
|
|
| STABILIZERS | Tafamidis meglumine60
(Vyndaqel) Tafamidis free salt (Vyndamax) |
Approved | ATTRv & ATTRwt-CA NYHA I-III |
Benzoxazole derivative without NSAID activity which binds to T4 site on TTR | 80mg po daily 61mg po daily |
No safety signals of potential clinical concern | None | $225,000 |
|
| AG-10 (Acoramidis) | Phase 3 ATTRIBUTE-CM NCT03860935 |
ATTRv & ATTRwt-CA NYHA I-III |
Mimics super-stabilizing activity of T119M, forms hydrogen bonds between neighboring serine residues at position 117 of each monomer | 800 mg po twice daily | Unknown | None | Unknown |
|
|
| Diflunisal57 | Phase 2 | ATTRv & ATTRwt-CA (off-label use) | NSAID, binds to T4 binding site on serum TTR | 250mg BID po |
|
Proton pump inhibitor Monitor CBC and BMP q 3–6 months |
$420 | Safety and efficacy | |
| Tolcapone66 | Phase 1 In-vitro and ex-vivo |
ATTRv & ATTRwt-CA (V122I & V30M) | Cathecol-O-methyltransferase (COMT) inhibitor with a high affinity for T4 binding site on serum TTR | Unknown | Acute liver failure | LFTs at baseline and q 2–4 weeks for the first 6 months, | Unknown | No phase 3 trial to date | |
| DEGRADATION / EXTRACTION | Doxycycline +/− TUDCA | Phase 2 | AL-CA*111 & ATTR-CA112 | Doxycycline – inhibition of
MMP TUDCA – antiamyloid fibril activity Synergistic activity to reduce amyloid fiber burden |
Oral | Dermatologic Gastrointestinal | None | Unknown | AL-CA: 20% 1-yr mortality and 60% ASCT
utilization with CyBorD (no comparison arm)111 ATTR-CA: No significant changes in NYHA class, cardiac biomarkers or echocardiographic parameters over 22 months112 |
| ANTI-SEEDING | Tab FH2 | Pre-clinical | ATTRv | Peptide inhibitor which binds to the amyloid driving F- and H-stands of fragmented fibrils, thereby impeding self-recognition and seeding. | Pre-clinical | Unknown | Unknown | Unknown | Pre-clinical |
ASO, antisense oligonucleotide; ATTRv, variant transthyretin amyloid; ATTRwt, wild-type ATTR; ATTR-FAP, ATTR familial amyloid polyneuropathy; BMP, basic metabolic panel; CRISPR, clustered regularly interspaced short palindromic repeats; CV, cardiovascular; LFT, liver function test; MMP, matrix metalloproteinase; NSAID, non-steroidal anti-inflammatory; NYHA, New York Heart Association heart failure class; RISC, RNA-induced silencing complex; TUDCA, tauroursodeoxycholic acid; UPCR, urine protein-creatinine ratio.
doxycycline alone
There are 2 classes of gene silencer therapy for TTR amyloidosis currently commercially available or in late phase clinical trials. The first, small interfering RNA (siRNA) molecules and the second, antisense oligonucleotides (ASOs). These molecules knockdown or reduce TTR production, but with slightly different mechanisms. Vitamin A supplementation must be provided in those receiving silencer therapy given that a major function of TTR is to transport retinol, the major circulating form of vitamin A, via retinol binding protein 4. Thyroxine supplementation is not necessary given the fact that the majority (~85%) of thyroxine is bound to thyroxine binding globulin or albumin.
First Generation Therapies:
Inotersen (Tegsedi, Akcea Therapeutics, Inc), an ASO, is a 2’-O-methoxyethyl-modified antisense oligonucleotide inhibitor of TTR production in the liver. It is currently approved in the US for treatment of stage 1 or 2 polyneuropathy due to ATTRv. ASOs46 are single stranded, amphipathic DNA sequences which have a high binding affinity for proteins, thus enhancing distribution in the body. ASOs are taken up into the liver by binding with hepatocyte surface proteins, mainly clathrin- or caveolin-mediated uptake46, and subsequently transported to the nucleus by chaperone proteins. It is here in the nucleus that the ASO binds to the target mRNA protein and via the endonuclease, RNase H2, initiates mRNA degradation. The 2’-O-methoxyethyl modification provides resistance against endogenous degradation of the ASO. The NEURO-TTR trial randomized 172 patients with familial amyloid polyneuropathy with or without ATTR-CA to inotersen 300mg delivered subcutaneously weekly or placebo for 64 weeks. Subjects with New York Heart Association (NYHA) class III or greater were excluded from participation. During the NEURO-TTR trial47, reduction in serum TTR reached steady-state levels by 13 weeks, with a mean reduction in serum TTR of 74% and median of 79%. Serious adverse events included glomerulonephritis (3%) and thrombocytopenia, with 3 patients having a platelet count < 25,000 (3%) and one death from intracranial hemorrhage (platelet count <10,000). These adverse effects have led to FDA approval of inotersen with a Risk Evaluation and Mitigation (REMS) Program that requires weekly monitoring of platelet counts and biweekly assessments of eGFR, urinalysis and urine creatinine protein ratio. Inotersen was being investigated in phase II programs for ATTR-CA, however, given the aforementioned side effects, toxicity profile and the development of longer acting ASOs, it is no longer being developed for use in ATTR-CA.
Patisiran (Onpattro, Alnylam), a siRNA which has been approved for ATTR-FAP in the US, targets the 3’ untranslated region of the TTR mRNA. siRNA are double stranded, hydrophilic molecules containing a sense and an antisense strand and prone to rapid renal excretion48. As such, patisiran is formulated as a lipid nanoparticle to target hepatocyte uptake. Once in the cytoplasm, the sense strand is removed by Ago2, an intracellular RNA endonuclease, leaving the pharmacologically active antisense strand-Ago2 complex to bind to the target mRNA. This forms the RNA-induced silencing complex (RISC) and facilitates subsequent degradation of the target mRNA. In a phase II study of patients with FAP, serum TTR levels were reduced by over 80% after the second dose of patisiran, when given at a dose of 0.3mg/kg every three weeks49. The APOLLO study, a phase 3 study, randomized 225 patients with ATTR-FAP to patisiran vs placebo, excluding NYHA class III and IV patients50. In the cardiac subpopulation (n=126) defined by a left ventricular wall thickness ≥13 mm at baseline and no history of hypertension or aortic valve disease, those who were randomized to patisiran demonstrated a reduction in mean LV wall thickness (~1mm), global longitudinal strain by −1.4% and N terminal pro-BNP (NT-proBNP) levels reduced by ~55%. A post-hoc analysis showed a reduction of 46% in cardiac hospitalizations and all-cause mortality. The APOLLO-B trial (NCT 03997383) is a phase III study of patisiran in 300 patients with ATTR-CA, with a 1:1 randomization to placebo. Subjects must be pre-medicated with antihistamines (H1 and H2), glucocorticoids and acetaminophen given the pro-inflammatory nature of the lipid nanoparticle-siRNA complex, which predominately manifests as infusion reactions. The study duration of APOLLO-B involves a 12-month double bind period followed by an open-label extension where all patients will receive treatment with patisiran. The primary endpoint will be change from baseline at month 12 in the 6-minute walk test.
Second Generation Therapies:
Vutrisiran (Alnylam) is an siRNA which is conjugated to a N-acetyl galactosamine (GalNAc), specifically targeting hepatocytes. siRNAs that are conjugated to GalNAc enter cells via interaction with the GalNAc moiety on the asialoglycoprotein receptor (ASGPR) on the hepatocyte. ASGPR is present on the hepatocytes at a high concentration and thus facilitates rapid uptake. Vutrisiran has a greater potency and longer duration of action than other knockdown therapies currently in clinical trial and as such can be administered at a lower dose (25 mg every 3 months), with lower injection volume and longer dosing intervals. Furthermore, as a subcutaneous injection it has greater ease of administration than IV infusions and no premedications are required given the absence of pro-inflammatory lipid nanoparticles in the formulation. In a phase 1 study (NCT02797847), a single dose of 25mg of vutrisiran resulted in a mean maximum TTR reduction of 83% by week 6, which was sustained for 90 days51, enabling quarterly administration. HELIOS-A was a phase III study which enrolled 164 patients with hereditary amyloidosis polyneuropathy with or without ATTR-CA, excluding NYHA class III-IV heart failure (NCT03759379). Patients received either vutrisiran or the reference comparator, patisiran, during the treatment period in a 3:1 randomization, after which all patients will be switched to vutrisiran during the treatment extension period. Vutrisiran met the primary endpoint, change in modified neurologic impairment score (mNIS+7) from baseline at month 9 as compared to historical placebo data from the APOLLO study in addition to both secondary endpoints (quality of life assessed by the Norfolk Quality of Life Questionnaire-Diabetic Neuropathy and gait speed assessed by the 10-meter walk test). In addition, vutrisiran showed improvement compared to placebo in the exploratory cardiac endpoint of change from baseline in NT-proBNP. HELIOS-B is a phase III study of approximately 600 ATTR-CA patients, randomized 1:1 to receive 25mg of vutrisiran every 3 months or placebo (NCT04153149). There will be a cap of 30% on those concurrently taking commercial tafamidis who are enrolled in the trial. The trial will run for 30–36 months with the primary outcome being a composite of all-cause mortality and recurrent CV hospitalizations.
AKCEA-TTR-LRx (ION 682884, Akcea Therapeutics, Inc) is a ligand (GalNAc linked to the 5’ end) conjugated ASO (LICA) in which the ASO portion shares the same base sequence as inotersen. Thus, when the GalNAc is cleaved, ION-682884 has the same mechanism of action as inotersen, however the GalNAc conjugated drug has a much greater potency (approximately 51-fold). This allows for lower dose of drug to be administered to achieve a similar therapeutic effect. The ION-682884-CS1 (NCT03728634) study, showed a greater than 85% mean reduction in serum TTR levels with a monthly 45mg dose52. Furthermore, this dose (and interval of administration) has reportedly occurred without the problematic adverse events seen with inotersen, thought due to the 27-fold lower exposure to active drug seen with ION-682884. Cardio-TTRansform (NCT04136171) is a phase 3 clinical trial which will enroll approximately 700 patients with ATTR-CA, randomized 1:1 to receive 45mg of ION-682884 or placebo subcutaneously once every 4 weeks53. All patients in this study will be allowed to receive commercial tafamidis concurrently. The treatment period will be 120 weeks with frequent clinical monitoring. The primary endpoint will be a composite of CV mortality and frequency of CV clinical events comparing the 2 study arms.
CRISPR /Cas9 stands for clustered regularly interspaced short palindromic repeats and CRISPR-associated protein 9 that is a genome editing approach which is being leveraged to knock down the production of hepatic transthyretin. Formulations in phase 1 human trials include NTLA-2001 that is composed of human single-guide ribonucleic acid and a messenger ribonucleic acid sequence encoding Cas9 protein encapsulated in a lipid nanoparticle (LNP), that facilitates delivery to hepatocytes. The drug is a highly specific gene editing LNP that disrupts expression of human serum TTR expression. The first in human study is designed to investigate the safety and tolerability of NTLA-2001 at doses that are expected to meaningfully decrease the level of the circulating TTR protein. Animal data demonstrate that with a single administration, significant editing of the mouse transthyretin (TTR) gene in the liver was enabled with a >97% reduction in serum protein levels that persisted for at least 12 months54.
Stabilizing the transthyretin tetramer (Table 3)
Diflunisal is a non-steroidal anti-inflammatory agent which binds to the T4 binding sites on TTR, though with lower affinity than tafamidis. When compared to 3 other kinetic stabilizing agents, diflunisal was found to be the least potent. Dissociation of TTR was shown to be limited to 10% of normal with concentrations of 5.7 μM AG10 (800mg bid), 10.3 μM tolcapone (3 × 100mg over 12hours), 12.0 μM tafamidis (80mg daily) and 188 μM diflunisal (250mg bid)55. Diflunisal is associated with adverse effects such as renal dysfunction, gastrointestinal bleeding, hypertension and fluid retention, which can exacerbate heart failure in susceptible individuals. However, when used in the treatment of ATTR-CA, diflunisal is prescribed at a dose of 250mg orally twice daily, which is below the dose recommended for anti-inflammatory activity and appears to be well tolerated56. Furthermore, in subjects who receive this therapy, prescribers should ensure an eGFR > 45 ml/min, < 1 mg/kg of furosemide (or equivalent bioavailable loop diuretic) daily without a recent heart failure decompensation or history of gastrointestinal bleeding. Lohrmann et al assessed the effect of diflunisal therapy on cardiac structure and function at 1 year in 81 patients with ATTR-CA57. They described a significant increase in serum TTR levels (33 vs 19 mg/dL, p=0.01), and improvement in both left atrial volume index (−1.4 vs +4.6 ml/m2) and cardiac troponin I (−0.01 vs +0.03 ng/ml, p=0.01) in treated compared with untreated groups. In addition, the ATTRwt subgroup were found to have stable global longitudinal strain on echocardiogram (+0.1% vs +1.2%, p=0.03 for treated vs untreated, respectively). As such, diflunisal has been used in an off-label manner in those without significant renal or hematologic comorbidities, and also in allele carriers of mutations who are at high risk of developing disease, with frequent monitoring of renal function and heart failure symptoms.
Tafamidis (Vyndamax/Vyndaqel, Pfizer Inc, New York, NY, USA)
In those of Portuguese descent the most common TTR variant is Val30Met (p.Val50Met), which leads to early onset familial amyloid polyneuropathy (FAP), often in the 4th decade of life. Val30Met (p.Val50Met) has a high clinical penetrance, though some carriers develop mild or no manifestations of disease. Coehlo and colleagues studied these individuals who were allele carriers of the Val30Met (p.Val50Met) variant but did not develop evidence of disease. They found that these individuals were compound heterozygotes for Val30Met/Thr119Met58, which led to the hypothesis that a stabilizing variant (Thr119Met [p.Thr139Met]) can prevent dissociation of the TTR tetramer into monomers in the presence of a destabilizing variant and highly pathogenic Val30Met mutation. In seminal work, Kelly et al, showed that Thr119Met enhances stability of the TTR protein by slowing the rate of dissociation of the dimers39. It was based on this discovery that the TTR stabilizer, tafamidis was developed. Tafamidis is a benzoxazole derivative which lacks nonsteroidal anti-inflammatory activity and selectively binds to TTR in the blood, at the T4 binding site. Bulawa et al, showed that tafamidis stabilizes wild type TTR and inhibits amyloidogenesis in a dose-dependent manner, with similar effects on both Val30Met and Val122Ile TTR variants. In a randomized clinical trial of 128 patients with Val30Met familial amyloid polyneuropathy (Fx-005), tafamidis 20mg daily delayed neuropathic progression and preserved quality of life compared with placebo in a predefined secondary analysis, after 18 months of treatment59. It was approved by the European Medicines Agency in 2011 for patients with stage 1 symptomatic polyneuropathy. In the ATTR-ACT trial60, 441 patients with ATTR-CA, NYHA class I-III HF were randomized to receive tafamidis 80mg, 20mg or placebo daily for 30 months. Tafamidis was associated with a lower all-cause mortality vs placebo (29.5% vs 42.9%) and a 32% lower risk of cardiovascular hospitalizations in those with NYHA class I or II HF. However, subjects with NYHA class III symptoms had higher rates of cardiovascular related hospitalization with tafamidis therapy compared to placebo, highlighting the importance of early diagnosis and treatment. Furthermore, assessment of functional capacity and quality of life parameters showed a lower rate of decline in the distance covered on 6-minute walk test and a lower rate of decline in the Kansas City Cardiomyopathy Questionnaire-Overall Summary score. It was based on this study that tafamidis became the first US Food and Drug Administration (FDA) approved TTR stabilizer to treat ATTR-CA in the US, in May 2019. It is formulated as tafamidis meglumine (20mg capsules, dose 80mg daily) and tafamidis free salt (61mg capsule daily), the latter of which was formulated for patient convenience as a single dose capsule. These formulations are bioequivalent, though are not substitutable on a per mg basis61.
AG10 (Eidos Therapeutics, Inc., San Francisco, CA, USA)
AG10 (Acoramidis) is a small molecule stabilizer with oral bioavailability which selectively binds to and stabilizes TTR. It mimics the super stabilizing properties of the Thr119Met variant62, which are thought to be due to the formation of hydrogen bonds between neighboring serine residues at position 117 of each monomer63. AG10 has been shown to be a potent and selective stabilizer of TTR, exceeding the efficacy of tafamidis in stabilizing WT and variant TTR in serum64. In the phase 2 AG10–201 study, there was near complete stabilization (>90%) of TTR at peak and trough serum levels, defined by percent occupancy of T4 binding site as measured by Fluorescent Probe Exclusion65. TTR stabilization was demonstrated by the fact that at 28 days after initiation of therapy, TTR levels rose on average by 51% in those taking 800mg twice daily of AG10 vs placebo. The Eidos AG10 study (ATTRIBUTE-CM) is a phase 3 trial, which has planned to enroll 510 subjects with ATTR-CA in a 2:1 ratio to AG10 800mg or placebo twice daily for 30 months (NCT03860935). The co-primary endpoints are change in distance walked on 6-minute walk test at 12 months (p<0.01) and all-cause mortality and frequency of cardiovascular related hospitalizations over a 30-month period (p<0.04). The 12-month data are expected towards the end of 2021.
Tolcapone
Tolcapone is a catechol-O-methyltransferase (COMT) inhibitor typically used for the treatment of Parkinson’s disease, though also observed to have TTR stabilizing properties. In vitro, it was found that tolcapone has a high affinity for the T4 binding site, displacing radiolabelled T4 from TTR with 4 times the efficiency of tafamidis and stabilizing the TTR dimer-dimer interface. Furthermore, tolcapone exhibits stronger TTR aggregation inhibitory activity for both wild-type and Val122Ile than tafamidis66 demonstrating dose-dependent kinetic stabilization of the TTR tetramer. When studied ex-vivo, it was shown to bind to and kinetically stabilize tetrameric TTR in human plasma from subjects with wild-type TTR and carriers of Val30Met-TTR. However, tolcapone has a short half-life and a FDA black box warning of potentially fatal, acute liver failure.
With the advent of effective therapies for cardiac amyloidosis and the potential role of combination therapy, providers may soon be in the enviable position of choosing among therapies. The cost effectiveness of emerging therapies has been questioned67, 68, especially for what may not be a rare disease (e.g. ATTRwt). Unfortunately, cost of such therapies could pose a significant obstacle to adoption and increase health disparities.
Anti-Plasma Cell Therapy (Table 4)
Table 4.
Therapies for Light Chain Cardiac Amyloidosis
| Drug | Mechanism of action | Dose | Side Effects | Concomitant Therapy | Efficacy Data | |
|---|---|---|---|---|---|---|
| Systemic Response | Organ response* | |||||
| Melphalan76, 122 (Alkeran, Evomela) | Alternation of guanine nucleotide, resulting in inter- and intra-strand DNA crosslinks, interfering with DNA replication and transcription | 100 – 200 mg/m2 oral | Fluid
retention Dizziness Gastrointestinal Myelosuppression Fever |
Dexamethasone (ineligible for ASCT) | HR:
|
18.7% cardiac response with dexamethasone (reduction in IVS of ≥ 2mm with resolution of HF)122 |
| Cyclophosphamide (Cytoxan) | Activity via the active metabolite, phosphoramide mustard, at the guanine N-7 position leading to cell apoptosis | 100mg/d oral OR 300 mg/m2 oral weekly |
Alopecia Hemorrhagic cystitis Gastrointestinal Myelosuppression Infection |
Proteasome inhibitors CD38 monoclonal antibodies |
See below under bortezomib | See below under bortezomib |
| Bendamustine79 (Belrapzo, Bendeka, Treanda) | Induces DNA interstrand crosslinks leading to
cytotoxicity. Inhibits several mitotic checkpoints, promotes inefficient DNA repair |
100 mg/m2 IV | Myelosuppression Fatigue Nausea / vomiting Fever |
Dexamethasone |
BDex:
|
BDex:
|
| Lenalidomide83, 84 (Revlimid) |
|
~15 mg/d oral | Fluid
retention Myelosuppression Elevated LFTs Thromboembolism Infection Increases NT-proBNP Skin rash |
Dexamethasone Melphalan Cyclophosphamide |
HR :
|
Overall organ response:
|
| Pomalidomide86, 123 (Pomalyst) | 2–4 mg/d oral | Fluid
retention Infection Thromboembolism Skin rash Increases NT-proBNP |
Dexamethasone |
PomDex:
|
Overall organ Response 15%123 Cardiac response 15%123 |
|
| Daratumumab100, 102 (Darzalex) | Human immunoglobulin G1 kappa (IgG1κ) monoclonal antibody against CD38 antigen which leads to cell death via multiple mechanisms including complement mediated and antibody dependent cytotoxicity and apoptosis | 1.8g SC | Infusion related reactions | Dexamethasone Bortezomib Cyclophosphamide (CyBorD) |
HR
|
Cardiac response 42% with Dara-CyBorD |
| Corticosteroids | Bind to cytosolic glucocorticoid receptors and translocate to the nucleus where they modulate gene expression resulting in anti-inflammatory and immunosuppressive activity | 20–40mg dexamethasone oral | Fluid
retention Thrombosis Gastrointestinal hemorrhage Infection Psychosis |
Used as part of a multidrug regimen | Poor hematologic response using high dose dexamethasone as a single agent | Unknown |
| Bortezomib90 (Velcade) | Proteasome inhibitor - reversible boronic acid inhibitor of the chymotrypsin-like activity to the proteasome | Variable dose and intervals, IV | Peripheral neuropathy | Dexamethasone Cyclophosphamide Melphalan |
CyBorD90:
|
CyBorD90:
|
Cardiac response relies heavily on cardiac stage at diagnosis, thus direct comparison between studies is not possible.
Response varies with dexamethasone dose
Cardiac staging is based on Mayo 2004 criteria91
B, bortezomib; D, dexamethasone; CR, complete response; CYP, cyclophosphamide; HR, hematologic response rate; IFN-γ, interferon-gamma; JNK, c-jun terminal kinase; M, melphalan; NT-proBNP, N terminal pro brain natriuretic peptide; PR, partial response; VGPR, very good partial response;
Therapies for AL amyloidosis have evolved considerably over the past decade. Autologous stem cell transplant (ASCT) has proven to have the best long-term outcomes, however only a fraction of patients with cardiac involvement are eligible and with a host of new, novel highly active therapies, the treatment landscape is rapidly evolving. The Mayo group reported that ~25% of patients are eligible for ASCT69 and only 3.4% (23 of 668 patients) of patients with overt heart failure and AL amyloidosis treated at their center over a 20-year period underwent heart transplant70. Cardiac damage in AL amyloidosis occurs as a result of light chain toxicity, and so the goal is rapid and complete normalization of the involved light chain. Indeed, the advent of effective anti-plasma cell therapies with limited toxicity has led to an evolution in the definition of a deep hematologic response from a complete response to modified, stringent and absolute involved free light chain response, the latter defined as a difference in involved and uninvolved light chain of < 10 mg/L71 and an involved free light chain of < 20 mg/L72. In those ineligible for ASCT, medical therapy is pursued with the goal of attaining complete remission (CR), defined as normalization of serum kappa and lambda free light chains and free light chain (FLC) ratio. Those who do not achieve CR may be classified as having very good partial response (VGPR), defined as a difference in FLC < 40mg/dl, partial response (PR, decrease in difference in FLC > 50%) or no response73. Cardiac involvement of AL amyloidosis predicts a poor prognosis, which is outlined in the staging system created by the Mayo group. Patients are categorized as stage I to IV based on TnT ≥ 0.025 ng/ml (high sensitivity TnT ≥ 40ng/L), NT-proBNP ≥ 1800pg/ml and difference in FLC ≥ 18mg/dL, with stage I defined as all below threshold to stage IV being all three elevated37. Typically, patients with a serum NT-proBNP ≥ 8,500 pg/ml or TnT ≥ 0.05 ng/ml (high sensitivity TnT ≥ 75 ng/mL) are considered ineligible for ASCT. Although patients may become transplant eligible over time, the benefit of ASCT compared to targeted anti-plasma cell therapy is unknown. Cardiac response is defined as a decrease in NT-proBNP by > 30% and 300 ng/L (if baseline NT-proBNP > 650ng/L)73 and is associated with a survival benefit in the setting of anti-plasma cell therapy.
Alkylating Agents:
Melphalan, cyclophosphamide and bendamustine belong to a group of chemotherapeutic agents called the nitrogen mustard alkylating agents. Melphalan exerts its effects via alteration of the DNA nucleotide guanine, and results in the formation of inter- and intra-strand DNA crosslinks74 which interfere with DNA replication and transcription. The cytotoxic effects of melphalan are related to cellular concentration and duration of cellular exposure, with some studies linking higher doses of melphalan to excess toxicity in older patients and those with renal impairment. As such, a lower dose of melphalan (~140 mg/m2) is often used in those over 65 years, and in patients with renal insufficiency, reduced cardiac function or lower performance status without a detrimental effect on long-term outcomes75. Oral melphalan is typically combined with dexamethasone in the treatment of patients who are ineligible for ASCT or as pre-ASCT conditioning treatment. Palladini et al evaluated 259 patients with AL amyloidosis treated with oral melphalan and dexamethasone, over 50% of whom had advanced cardiac disease76. In those with severe cardiac involvement, the dose of dexamethasone was reduced by 50% given the propensity for fluid retention and exacerbation of heart failure. Hematologic response rate in the full dose group was 76% vs 51% in the attenuated steroid dose group with a complete response occurring in 31% and 12% respectively. Median survival was also much lower in the attenuated dose group, 20 months as compared to 7.4 years in the full dose group. Patients with severe cardiac involvement are at high risk of early death after the initiation of treatment, have a short overall median survival of < 18 months and poorer response to therapy77. Cyclophosphamide acts in a similar manner to melphalan, though exerts its effect via the active metabolite, phosphoramide mustard, at the guanine N-7 position leading to cell apoptosis78. It is typically used in combination with other therapies such as proteasome inhibitors and CD38 monoclonal antibodies and is associated with side effects including hair loss and hemorrhagic cystitis. Bendamustine was investigated in a phase 2 trial of 31 patients with relapsed AL amyloidosis in combination with dexamethasone, in which 57% achieved a partial response or better (NCT01222260)79. The overall organ response was 29% with a median overall survival of 18.2 months. Bendamustine is considered a third line or salvage therapy.
Steroids:
Glucocorticoids (prednisone and dexamethasone) bind to cytosolic glucocorticoid receptors and translocate to the nucleus where they modulate gene expression resulting in anti-inflammatory and immunosuppressive activity80. Dexamethasone treatment induces upregulation of pro-apoptotic genes, down-regulation of anti-apoptotic genes and activation of intrinsic apoptotic pathways80. It has a potency 6-fold that of prednisone, however, with greater efficacy comes greater toxicity, particularly in patients with advanced cardiac involvement. In a study by Dhopaker et al, 28% of patients experienced volume overload related to treatment with 40mg daily of dexamethasone (480mg per cycle)81. Treatment related mortality occurred in 6 patients, 4 of whom had advanced cardiac amyloidosis and experienced sudden death thought due to a cardiac event. Other severe adverse effects include thrombosis, gastrointestinal hemorrhage, infections and psychosis especially with high dose therapy and as such a low-dose strategy is often employed in older patients and those with heart failure76. In the recent era, due to their synergistic effect with immunomodulatory agents and proteasome inhibitors, glucocorticoids are used as part of a multidrug regimen to improve response rates and limit toxicity (see below).
Immunomodulatory agents
Originally marketed as a tranquilizer and antiemetic, thalidomide and its derivatives (lenalidomide and pomalidomide) have antiproliferative, anti-angiogenic and immunomodulatory effects. Their anti-angiogenic and antiproliferative properties are mediated via inhibition of interleukin-6 (IL-6) expression, a growth factor for the proliferation of myeloma cells. They activate apoptotic pathways through direct activation of caspase-8 mediated cell death. Within the mitochondria, they activate c-jun terminal kinase (JNK) which through a series of events result in the release of pro-apoptotic proteins into the cytosol82. In addition, by activating T cells, they increase expression of IL-2 and interferon-gamma (IFN-γ) genes, augmenting natural killer (NK) cell-dependent cytotoxicity82. Lenalidomide has a greater potency than thalidomide, being 50 to 2,000 times more potent at stimulating T-cell proliferation and 50 to 100 times more potent in augmenting IL-2 and IFN-γ production82. Dexamethasone activates caspase-9, a pro-apoptotic molecule and is associated with the release of second mitochondrial-derived activator of caspase (Smac) from the mitochondria into the cytosol, a regulator of the activity of molecules that affect apoptosis82. As such, it augments the anti-proliferative effects of lenalidomide. However, notable side-effects include neutropenia, thrombocytopenia, elevations in liver function tests and thromboembolism and lenalidomide tends to raise NT-proBNP in AL amyloidosis patients83. With the addition of melphalan to lenalidomide and dexamethasone, overall hematologic response rates of ~ 58% are observed84, with a higher dose of lenalidomide associated with a higher rate of CR, though limited by tolerability at doses >15mg84. The addition of cyclophosphamide in place of melphalan shows similar overall hematologic response rates (~60%), though again has a high burden of toxicity and poor outcomes in those with advanced cardiac disease85. Overall hematologic response rates with pomalidomide/dexamethasone are similar to those achieved with lenalidomide (40–60%)85, 86. Unfortunately, in those with advanced cardiac amyloidosis at the time of treatment initiation, outcomes of these treatment combinations remain poor.
Proteasome Inhibitors:
The ubiquitin-proteasome system (UPS) functions as a pathway for intracellular regulation of protein degradation, thus playing a role in maintaining protein homeostasis87. The UPS involves a series of enzymes which tag the protein with a polyubiquitin chain. The 26S proteasome comprises a 20S core flanked by two 19S caps. The 20S core contains subunits which have proteolytic activity, including caspase-like, trypsin-like and chymotrypsin-like activity. The proteasome recognizes and binds the tagged protein and subsequently hydrolyzes it into short polypeptides in the 20S core. Proteasome inhibitors bind to the proteasome binding pocket, thus rendering it inactive87 which results in a multitude of downstream events, including accumulation of ubiquitin tagged proteins, inhibition of NF-κB signaling, downregulation of growth factor receptors, suppression of adhesion molecule expression and inhibition of angiogenesis, all of which lead to apoptosis88. Bortezomib (Velcade) is a first-generation reversible boronic acid inhibitor of the chymotrypsin-like activity to the proteasome. Bortezomib, in conjunction with cyclophosphamide and dexamethasone (CyBorD), has been shown to induce a rapid reduction in light chains in patients with AL amyloidosis and appears to be relatively well tolerated in those with cardiac involvement, however, can cause significant peripheral neuropathic side effects. Furthermore, those with translocation t(11;14) have inferior hematologic response to bortezomib89. A study of 230 patients from the National Amyloidosis Center (London, England) and the Amyloidosis Research and Treatment Center (Pavia, Italy) of newly diagnosed patients with AL amyloidosis reported an overall hematologic response rate of 60% with a cardiac response of 17%90. Advanced cardiac stage IIIb patients (Mayo 2004 criteria91) (NT-proBNP > 8500ng/L) had a lower overall response of 42% and poorer survival (median survival 7 months), than those with stage II or IIIa disease (overall hematologic response 64% and 69%, respectively), though still showed a survival benefit in those who achieved a hematologic response compared to those who did not (median survival 26 months vs 6 months, respectively)90. In a retrospective study of bortezomib/melphalan/dexamethasone (BMDex) vs melphalan/dexamethasone (MDex), the former induced higher hematologic response (69 vs 51%) and CR (42 vs 19%) the difference in response between groups being most notable in those who could not tolerate full-dose dexamethasone92. The addition of bortezomib in patients with NYHA class III or IV HF and/or NT-proBNP > 8500ng/L did not improve survival. Second generation proteasome inhibitors include carfilzomib, ixazomib, marizomib and oprozomib. Carfilzomib (Kyprolis) is an irreversible tetrapeptide epoxyketone which has greater inhibitory activity than bortezomib and has shown activity in patients resistant to bortezomib93. In a phase 1 dose-escalation study of carfilzomib in patients with previously treated systemic AL amyloidosis however, cardiac events were common42. Three of 12 patients had a cardiac event: 1 with cardiac arrest due to ventricular tachycardia, 1 developed a restrictive cardiomyopathy (amyloid negative on biopsy) and 1 had a decline in LV function, all possibly related to carfilzomib therapy and highlighting the need for close cardiac monitoring if this therapy is utilized. However, in those for whom bortezomib is contraindicated due to peripheral neuropathy, carfilzomib may be effective in appropriately selected candidates without severe cardiac involvement94. Ixazomib (Ninlaro) is a reversible agent which is hydrolyzed to its active form in aqueous solution and has comparable inhibitory activity to bortezomib93. It binds to the proteasome beta-5 site to inhibit the chymotrypsin-like activity. In 27 patients with relapsed/refractory AL amyloidosis ixazomib showed encouraging hematologic (52%) and organ (56%) response rates with a 45% cardiac response rate (5 of 11 patients)95. It is being investigated in a phase 1/2 study to assess safety and hematologic response rate in combination with cyclophosphamide and dexamethasone (NCT03236792). Patients with NYHA class III or IV HF or NT-proBNP > 8500ng/L are excluded. TOURMALINE-AL1 is a phase 3 trial investigating the use of ixazomib with dexamethasone vs physician’s choice (NCT01659658), results of which showed no significant difference in hematologic overall response rate96. Nevertheless, CR was more frequent with ixazomib (26 vs 18%), and treatment with ixazomib was associated with a significantly longer progression-free survival. Furthermore, the patients who received ixazomib had a higher rate of cardiac and renal responses.
Daratumumab:
CD38 is a glycoprotein expressed on a variety of cell types, including normal myeloid and lymphoid cells, but is also highly overexpressed on neoplastic monoclonal plasma cells97. CD38 is involved in cell signaling and regulation of cytoplasmic calcium flux playing a role in activation, survival and growth of lymphoid and myeloid cells. Daratumumab (Darzalex) is a human immunoglobulin G1 kappa (IgG1κ) monoclonal antibody against CD38 antigen which leads to cell death via multiple mechanisms including complement mediated and antibody dependent cytotoxicity and apoptosis98. It has been shown to be highly effective in and is approved for the treatment of multiple myeloma99, which led to investigation of its use in AL amyloidosis. It has been shown to be effective in the treatment of patients with relapse or progression of AL amyloidosis, resulting in a 76% hematologic response rate and 36% CR rate with a median time to response of 1 month100. The speed with which daratumumab can achieve normalization of light chain levels was highlighted in a report by Hossein et al101. Two patients with advanced Mayo cardiac stage (stage III and IV) were treated with daratumumab monotherapy and achieved a normal light chain level within one cycle of therapy. ANDROMEDA investigated the safety and efficacy of daratumumab plus CyBorD compared with CyBorD alone in patients with new diagnosed AL amyloidosis (NCT03201965). ANDROMEDA enrolled 388 patients, randomized to CyBorD alone or with daratumumab (1:1). More than one-half of patients assigned to daratumumab achieved a complete hematological response (53%) compared with only 18% of patients assigned to CyBorD (odds ratio [OR] = 5.1; p<0.0001). The 6-month cardiac response rate was 42% for the daratumumab arm compared with 22% for CyBorD alone (p=0.0029)102. In comparison, CR rates in patients receiving CyBorD90, melphalan with dexamethasone76 or HDM with ASCT103 have been reported as 23%, 19% and up to 48%, respectively. Isatuximab acts by inducing internalization of CD38104. Daratumumab was approved by the FDA in combination with CyBorD for newly diagnosed light chain amyloidosis. A phase 2 study of isatuximab in patients with relapsed or refractory AL amyloidosis with organ involvement is underway (NCT03499808), however those with NYHA class IV symptoms or EF < 35% are excluded from participation. Elotuzumab is an IgG1κ monoclonal antibody against signaling lymphocytic activation molecule F7 (SLAMF7) which is used in the treatment of multiple myeloma combined with lenalidomide and dexamethasone. The mechanism of action in multiple myeloma cells appears to be antibody-dependent, cell-mediated cytotoxicity through recruitment and activation of NK cells105. Elotuzumab mediated cell death requires the presence of NK cells since binding to SLAMF7 marks the myeloma cells for recognition by NK cells and also direct binding to SLAMF7 on NK cells themselves causes direct activation and enhanced cytotoxicity106. Elotuzumab in conjunction with lenalidomide and dexamethasone was successful in inducing hematologic and renal response in a patient with relapsed/refractory multiple myeloma with AL amyloidosis despite two stem cell transplants and numerous combinations of chemotherapeutic agents107. It is currently being studied in a phase 2 trial in conjunction with lenalidomide and dexamethasone with or without cyclophosphamide in patients with relapsed AL amyloidosis (NCT03252600).
Venetoclax
Translocation t(11;14) is the most common cytogenic aberration in AL amyloidosis occurring in up to 60% of patients and confers a poor response to bortezomib89. Patients with t(11:14) overexpress B-cell lymphoma 2 (BCL-2), a protein involved in programmed cell death regulation108. BCL-2 mediates suppression of the proapoptotic pathway molecules BAX and BAK108. Venetoclax, an orally bioavailable agent, is an inhibitor of BCL-2 and thus promotes cell death. It has been shown to be effective in patients with multiple myeloma with translocation t(11;14)109. A phase 1 study (NCT03000660) investigating venetoclax and dexamethasone in relapsed/refractory AL amyloidosis was stopped given the FDA concerns that emerged from the BELLINI clinical trial (NCT02755597, Study M14–031) evaluating venetoclax with bortezomib in patients with multiple myeloma in which there was an increased risk of death for patients receiving venetoclax as compared to the control group. However, after subgroup analysis, the BELLINI study has reopened for patients with t(11;14). Similarly, it is expected that the study of venetoclax in AL amyloidosis will reopen for those with t(11;14).
Amyloid Degradation/Extraction:
Doxycycline (Table 3) is a tetracycline antibiotic which binds to the bacterial ribosome and inhibits protein synthesis. However, it is the ability to inhibit matrix metalloproteinase (MMP) which is thought to be the basis of its anti-amyloidogenic activity110. Levels of MMP are elevated in AL accompanied with marked diastolic dysfunction when compared to little or no elevation in TTR amyloidosis110. This led to a phase 2 study of the safety and efficacy of doxycycline in combination with CyBorD for the treatment of AL amyloidosis. D’Souza and colleagues reported a low 1-year mortality of 20% and a high stem cell transplant utilization rate of 60%, in this single arm study111.
The use of doxycycline in combination with ursodeoxycholic acid (ursodiol) for the treatment of ATTR CA was studied in 53 patients112. Ursodiol is a bile acid sequestrant which has antiamyloid fibril effects as has its taurine conjugate, tauroursodeoxycholic acid (TUDCA). Both have synergistic activity with doxycycline to reduce amyloid fibril burden. Karlstedt et al reported an 11% adverse event rate, mainly due to dermatologic and gastrointestinal side effects, and equivocal outcomes112 with no obvious benefit observed. Overall, it remains unclear as to the place doxycycline and ursodiol have in the treatment of either AL- or ATTR-CA.
NI006 is a recombinant human monoclonal IgG1 antibody that exclusively targets with high affinity the forms of TTR that are disease-associated with amyloid conformation but not physiological forms of transthyretin. NI006 targets both wild-type TTR as well as TTR variants and induces the clearance of pathological TTR in preclinical models. Currently, NI006 is in Phase 1 clinical development in ATTR cardiomyopathy patients (NCT04360434).
CAEL-101 (11–1F4) is an IgG1 monoclonal antibody that binds to kappa and lambda light chain amyloid fibrils, accumulating in amyloid laden organs113 leading to elimination of the amyloid protein. In the 1 year follow up of the phase 1a/1b study, 67% (12 of 18) of renal and/or cardiac-evaluable patients demonstrated organ response43. Currently, CAEL-101 is being evaluated in patients with Mayo stage IIIa (NCT04512235) and IIIb cardiac AL amyloidosis (NCT04504825).
Serum amyloid P (SAP) is a glycoprotein which binds avidly to all types of amyloid fibrils. As such, targeting this protein may provide a pathway to extraction of amyloid deposits from affected organs. Bodin et al showed that administration of anti-human-SAP antibodies to mice with amyloid deposits containing human SAP activated macrophage mediated phagocytosis of the SAP containing amyloid deposits114. In a phase 1 trial, a small-molecule drug, (R)-1-[6-[(R)-2-carboxy-pyrrolidin-1-yl]-6-oxo-hexanoyl]pyrrolidine-2-carboxylic acid (CPHPC), was first administered to 15 patients with systemic amyloidosis to reduce circulating SAP, followed by a fully humanized monoclonal IgG1 anti-SAP antibody41. Patients with cardiac involvement were excluded from the study. At 42 days following treatment, there was a decrease in amyloid organ burden as indicated by decreased hepatic stiffness and reduction in hepatic amyloid burden on SAP scintigraphy. A phase 2 study failed to show any improvement in cardiac amyloid burden after treatment with anti-SAP antibody (dezamizumab).
NEOD-001 (Prothena) is a humanized form of murine monoclonal antibody 2A4, which binds to an epitope on the misfolded light chain protein though does not bind in the protein’s native conformation. Therefore, it was posited that NEOD-001 could clear AL amyloid deposits in affected organs. Exploratory end points of a phase 1/2 study showed that 8 of 14 patients (57%) with cardiac involvement responded to therapy while the remaining 6 patients showed stable disease115. A phase 2b study (NCT02632786) in previously treated patients with persistent cardiac dysfunction did not meet its primary or secondary endpoints and the phase 3 study in treatment naïve patients was discontinued due to futility.
PRX004 (Prothena) is a monoclonal antibody which selectively binds to non-native misfolded TTR but not native TTR. PRX004 is being studied in patients with hereditary ATTR amyloidosis in a phase 1 study (NCT03336580).
Emerging therapeutics:
Anti-seeding (Table 3) refers to inhibition of the aggregation of native transthyretin onto preformed amyloid fibrils. This may be particularly beneficial in the setting of single organ liver transplantation for ATTRv, after which ongoing cardiac deposition can occur. Preformed amyloid fibrils in the heart can act as a template for further seeding of native transthyretin. TabFH2 is a peptide inhibitor which binds to the amyloid driving F- and H-stands of fragmented fibrils, thereby impeding self-recognition and seeding45.
The CD38-targeting antibody-drug conjugate (ADC) STI-6129/CD38–077, comprises a fully human anti-CD38 antibody conjugated to a microtubule inhibitor (duostatin 5.2)116. This ADC binds avidly to CD38 positive tumor cells, after which it is internalized to exert its cytotoxic effect. STI-6129 is being studied in patient with relapsed or refractory systemic AL amyloidosis in a phase 1 study (NCT04316442).
Advanced Therapies
Heart transplantation may be considered in select patients with end-stage cardiomyopathy secondary to ATTR-CA or AL-CA who have responded to light-chain depleting therapy. Heart transplant candidates should be carefully selected with particular attention being paid to extracardiac involvement such as neuropathic, gastrointestinal or hepatic manifestations of disease, which could affect post-transplant outcomes117. Patients with ATTRwt- or ATTRv-CA with the Val122Ile mutation generally require only single organ heart transplant, however, other variants, such as Thr60Ala may need consideration for dual heart/liver transplant. Recent single center studies have shown that outcomes of appropriately selected patients with either AL- or ATTR-CA are similar to that of non-amyloid cardiomyopathy patients118, 119. Furthermore, the new heart transplantation allocation policy120 provides a pathway for listing these patients with a restrictive cardiomyopathy, who often do not meet the hemodynamic criteria otherwise required for higher priority status. This has led to a decrease in waitlist time/delisting due to clinical deterioration and an increase in the number of patients being transplanted with amyloid cardiomyopathy121 without any change in short-term outcomes. Going forward, it is unclear where heart transplantation will fit in the treatment paradigm, as earlier detection of amyloid cardiomyopathies and novel medical therapies continue to change the landscape of this disease.
Summary/Conclusions
Basic science investigations in the last few decades have led to the elucidation of mechanisms underlying amyloidogenesis and have resulted in the development of effective therapies in multiple classes of compounds. The cardiologist caring for affected patients is in the enviable position of choosing from these therapies that can meaningfully improve the lives of affected patients especially when administered before significant cardiac dysfunction has ensured (Figure 4). The therapeutic landscape in this arena is rapidly evolving and additional breakthroughs are anticipated in the coming years.
Disclosures:
Dr. Maurer is funded by the National Institutes of Health (HL139671-01, AG R21AG058348, and AG K24AG036778 to M.S.M). Dr. Maurer’s institution received funding for clinical trials for Pfizer, Prothena, Eidos, and Alnylam. Dr. Maurer has received consulting income from Pfizer, EIdos, Prothena, Akcea, and Alnylam. Dr Griffin has no disclosures.
Non-standard Abbreviations and Acronyms
- AL
immunoglobulin light chain
- ASCT
autologous stem cell transplant
- ASGPR
asialoglycoprotein receptor (ASGPR)
- ASO
antisense oligonucleotide
- ATTRv
variant transthyretin amyloidosis
- ATTRwt
wild-type transthyretin amyloidosis
- BCL-2
B-cell lymphoma 2 receptor
- BMDex
bortezomib, melphalan, dexamethasone
- BNP
brain natriuretic peptide
- CA
cardiac amyloidosis
- COMT
catechol-O-methyltransferase
- CPHPC
(R)-1-[6-[(R)-2-carboxy-pyrrolidin-1-yl]-6-oxo-hexanoyl]pyrrolidine-2-carboxylic acid
- CR
complete response
- CRISPR/Cas9
clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9
- CyBorD
cyclophosphamide, bortezomib, dexamethasone
- FAP
familial amyloid polyneuropathy
- GalNAc
N-acetyl galactosamine
- HDM
high dose melphalan
- IAA
isolated atrial amyloidosis
- IFN-γ
interferon-gamma
- JNK
c-jun terminal kinase
- LICA
ligand conjugated antisense oligonucleotide
- LNP
lipid nanoparticle
- MAPK
mitogen-activated protein kinase
- MMP
matrix metalloproteinase
- NT-proBNP
N terminal pro-BNP
- PR
partial response
- SAP
serum amyloid P-component
- Smac
second mitochondrial-derived activator of caspase
- siRNA
small interfering ribonucleic acid
- SLAMF7
signaling lymphocytic activation molecule F7
- SSA
Serum amyloid associated protein
- Tn
troponin
- TUDCA
tauroursodeoxycholic acid
- UPS
ubiquitin-proteasome system
- VGPR
very good partial response
REFERENCES
- 1.Ruberg FL, Grogan M, Hanna M, Kelly JW and Maurer MS. Transthyretin Amyloid Cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:2872–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maurer MS and Mann DL. The Tafamidis Drug Development Program: A Translational Triumph. JACC Basic Transl Sci. 2018;3:871–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maurer MS, Elliott P, Comenzo R, Semigran M and Rapezzi C. Addressing Common Questions Encountered in the Diagnosis and Management of Cardiac Amyloidosis. Circulation. 2017;135:1357–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manral P and Reixach N. Amyloidogenic and non-amyloidogenic transthyretin variants interact differently with human cardiomyocytes: insights into early events of non-fibrillar tissue damage. Bioscience reports. 2015;35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao L, Buxbaum JN and Reixach N. Age-related oxidative modifications of transthyretin modulate its amyloidogenicity. Biochemistry. 2013;52:1913–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reixach N, Deechongkit S, Jiang X, Kelly JW and Buxbaum JN. Tissue damage in the amyloidoses: Transthyretin monomers and nonnative oligomers are the major cytotoxic species in tissue culture. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imperlini E, Gnecchi M, Rognoni P, Sabidò E, Ciuffreda MC, Palladini G, Espadas G, Mancuso FM, Bozzola M, Malpasso G, Valentini V, Palladini G, Orrù S, Ferraro G, Milani P, Perlini S, Salvatore F, Merlini G and Lavatelli F. Proteotoxicity in cardiac amyloidosis: amyloidogenic light chains affect the levels of intracellular proteins in human heart cells. Scientific reports. 2017;7:15661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobson CM. Protein folding and misfolding. Nature. 2003;426:884–90. [DOI] [PubMed] [Google Scholar]
- 9.Hipp MS, Kasturi P and Hartl FU. The proteostasis network and its decline in ageing. Nature reviews Molecular cell biology. 2019;20:421–435. [DOI] [PubMed] [Google Scholar]
- 10.Wickner S, Maurizi MR and Gottesman S. Posttranslational quality control: folding, refolding, and degrading proteins. Science (New York, NY). 1999;286:1888–93. [DOI] [PubMed] [Google Scholar]
- 11.Merlini G and Bellotti V. Molecular mechanisms of amyloidosis. The New England journal of medicine. 2003;349:583–96. [DOI] [PubMed] [Google Scholar]
- 12.Eisenberg D and Jucker M. The amyloid state of proteins in human diseases. Cell. 2012;148:1188–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khurana R, Gillespie JR, Talapatra A, Minert LJ, Ionescu-Zanetti C, Millett I and Fink AL. Partially Folded Intermediates as Critical Precursors of Light Chain Amyloid Fibrils and Amorphous Aggregates. Biochemistry. 2001;40:3525–3535. [DOI] [PubMed] [Google Scholar]
- 14.Buxbaum JN and Reixach N. Transthyretin: the servant of many masters. Cellular and molecular life sciences : CMLS. 2009;66:3095–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sekijima Y, Wiseman RL, Matteson J, Hammarström P, Miller SR, Sawkar AR, Balch WE and Kelly JW. The biological and chemical basis for tissue-selective amyloid disease. Cell. 2005;121:73–85. [DOI] [PubMed] [Google Scholar]
- 16.Misra P, Blancas-Mejia LM and Ramirez-Alvarado M. Mechanistic Insights into the Early Events in the Aggregation of Immunoglobulin Light Chains. Biochemistry. 2019;58:3155–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellotti V, Mangione P and Merlini G. Review: immunoglobulin light chain amyloidosis--the archetype of structural and pathogenic variability. Journal of structural biology. 2000;130:280–9. [DOI] [PubMed] [Google Scholar]
- 18.Perfetti V, Palladini G, Casarini S, Navazza V, Rognoni P, Obici L, Invernizzi R, Perlini S, Klersy C and Merlini G. The repertoire of λ light chains causing predominant amyloid heart involvement and identification of a preferentially involved germline gene, IGLV1–44. Blood. 2012;119:144–50. [DOI] [PubMed] [Google Scholar]
- 19.Merlini G AL amyloidosis: from molecular mechanisms to targeted therapies. Hematology American Society of Hematology Education Program. 2017;2017:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Obici L and Merlini G. Amyloidosis in autoinflammatory syndromes. Autoimmun Rev. 2012;12:14–7. [DOI] [PubMed] [Google Scholar]
- 21.Morris AD, Smith RN and Stone JR. The pathology and changing epidemiology of dialysis-related cardiac beta-2 microglobulin amyloidosis. Cardiovasc Pathol. 2019;42:30–35. [DOI] [PubMed] [Google Scholar]
- 22.Podduturi V, Armstrong DR, Hitchcock MA, Roberts WC and Guileyardo JM. Isolated atrial amyloidosis and the importance of molecular classification. Proc (Bayl Univ Med Cent). 2013;26:387–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiti F and Dobson CM. Protein misfolding, functional amyloid, and human disease. Annual review of biochemistry. 2006;75:333–66. [DOI] [PubMed] [Google Scholar]
- 24.Pepys MB, Rademacher TW, Amatayakul-Chantler S, Williams P, Noble GE, Hutchinson WL, Hawkins PN, Nelson SR, Gallimore JR, Herbert J and et al. Human serum amyloid P component is an invariant constituent of amyloid deposits and has a uniquely homogeneous glycostructure. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:5602–5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tennent GA, Lovat LB and Pepys MB. Serum amyloid P component prevents proteolysis of the amyloid fibrils of Alzheimer disease and systemic amyloidosis. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:4299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arosio P, Knowles TP and Linse S. On the lag phase in amyloid fibril formation. Physical chemistry chemical physics : PCCP. 2015;17:7606–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saelices L, Johnson LM, Liang WY, Sawaya MR, Cascio D, Ruchala P, Whitelegge J, Jiang L, Riek R and Eisenberg DS. Uncovering the Mechanism of Aggregation of Human Transthyretin. The Journal of biological chemistry. 2015;290:28932–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riek R and Eisenberg DS. The activities of amyloids from a structural perspective. Nature. 2016;539:227–235. [DOI] [PubMed] [Google Scholar]
- 29.Stevens FJ and Kisilevsky R. Immunoglobulin light chains, glycosaminoglycans, and amyloid. Cellular and molecular life sciences : CMLS. 2000;57:441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ihse E, Ybo A, Suhr O, Lindqvist P, Backman C and Westermark P. Amyloid fibril composition is related to the phenotype of hereditary transthyretin V30M amyloidosis. The Journal of pathology. 2008;216:253–61. [DOI] [PubMed] [Google Scholar]
- 31.Bergström J, Gustavsson A, Hellman U, Sletten K, Murphy CL, Weiss DT, Solomon A, Olofsson BO and Westermark P. Amyloid deposits in transthyretin-derived amyloidosis: cleaved transthyretin is associated with distinct amyloid morphology. The Journal of pathology. 2005;206:224–32. [DOI] [PubMed] [Google Scholar]
- 32.Larsen BT, Mereuta OM, Dasari S, Fayyaz AU, Theis JD, Vrana JA, Grogan M, Dogan A, Dispenzieri A, Edwards WD, Kurtin PJ and Maleszewski JJ. Correlation of histomorphological pattern of cardiac amyloid deposition with amyloid type: a histological and proteomic analysis of 108 cases. Histopathology. 2016;68:648–56. [DOI] [PubMed] [Google Scholar]
- 33.Bhuiyan T, Helmke S, Patel AR, Ruberg FL, Packman J, Cheung K, Grogan D and Maurer MS. Pressure-volume relationships in patients with transthyretin (ATTR) cardiac amyloidosis secondary to V122I mutations and wild-type transthyretin: Transthyretin Cardiac Amyloid Study (TRACS). Circ Heart Fail. 2011;4:121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quarta CC, Solomon SD, Uraizee I, Kruger J, Longhi S, Ferlito M, Gagliardi C, Milandri A, Rapezzi C and Falk RH. Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation. 2014;129:1840–9. [DOI] [PubMed] [Google Scholar]
- 35.Brenner DA, Jain M, Pimentel DR, Wang B, Connors LH, Skinner M, Apstein CS and Liao R. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circulation research. 2004;94:1008–10. [DOI] [PubMed] [Google Scholar]
- 36.Shi J, Guan J, Jiang B, Brenner DA, Del Monte F, Ward JE, Connors LH, Sawyer DB, Semigran MJ, Macgillivray TE, Seldin DC, Falk R and Liao R. Amyloidogenic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38alpha MAPK pathway. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C, Laumann K, Zeldenrust SR, Leung N, Dingli D, Greipp PR, Lust JA, Russell SJ, Kyle RA, Rajkumar SV and Gertz MA. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30:989–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palladini G, Lavatelli F, Russo P, Perlini S, Perfetti V, Bosoni T, Obici L, Bradwell AR, D’Eril GM, Fogari R, Moratti R and Merlini G. Circulating amyloidogenic free light chains and serum N-terminal natriuretic peptide type B decrease simultaneously in association with improvement of survival in AL. Blood. 2006;107:3854–8. [DOI] [PubMed] [Google Scholar]
- 39.Hammarström P, Wiseman R, Powers E and Kelly J. Prevention of Transthyretin Amyloid Disease by Changing Protein Misfolding Energetics. Science. 2003;299:713–716. [DOI] [PubMed] [Google Scholar]
- 40.Morgan GJ, Yan NL, Mortenson DE, Rennella E, Blundon JM, Gwin RM, Lin CY, Stanfield RL, Brown SJ, Rosen H, Spicer TP, Fernandez-Vega V, Merlini G, Kay LE, Wilson IA and Kelly JW. Stabilization of amyloidogenic immunoglobulin light chains by small molecules. Proc Natl Acad Sci U S A. 2019;116:8360–8369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richards DB, Cookson LM, Berges AC, Barton SV, Lane T, Ritter JM, Fontana M, Moon JC, Pinzani M, Gillmore JD, Hawkins PN and Pepys MB. Therapeutic Clearance of Amyloid by Antibodies to Serum Amyloid P Component. N Engl J Med. 2015;373:1106–14. [DOI] [PubMed] [Google Scholar]
- 42.Cohen AD, Scott EC, Liedtke M, Kaufman JL, Landau H, Vesole DH, Gomes CL, Gasparetto C, Lentzsch S, Rosenzweig M, Sanchorawala V, Smith DD, Comenzo RL and Durie BGM. A Phase I Dose-Escalation Study of Carfilzomib in Patients with Previously-Treated Systemic Light-Chain (AL) Amyloidosis. Blood. 2014;124:4741–4741. [Google Scholar]
- 43.Edwards CV, Bhutani D, Mapara M, Radhakrishnan J, Shames S, Maurer MS, Leng S, Wall JS, Solomon A, Eisenberger A and Lentzsch S. One year follow up analysis of the phase 1a/b study of chimeric fibril-reactive monoclonal antibody 11–1F4 in patients with AL amyloidosis. Amyloid. 2019;26:115–116. [DOI] [PubMed] [Google Scholar]
- 44.Saelices L, Chung K, Lee JH, Cohn W, Whitelegge JP, Benson MD and Eisenberg DS. Amyloid seeding of transthyretin by ex vivo cardiac fibrils and its inhibition. Proc Natl Acad Sci U S A. 2018;115:E6741–E6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saelices L, Nguyen BA, Chung K, Wang Y, Ortega A, Lee JH, Coelho T, Bijzet J, Benson MD and Eisenberg DS. A pair of peptides inhibits seeding of the hormone transporter transthyretin into amyloid fibrils. J Biol Chem. 2019;294:6130–6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crooke ST, Wang S, Vickers TA, Shen W and Liang XH. Cellular uptake and trafficking of antisense oligonucleotides. Nat Biotechnol. 2017;35:230–237. [DOI] [PubMed] [Google Scholar]
- 47.Benson MD, Waddington-Cruz M, Berk JL, Polydefkis M, Dyck PJ, Wang AK, Plante-Bordeneuve V, Barroso FA, Merlini G, Obici L, Scheinberg M, Brannagan TH 3rd, Litchy WJ, Whelan C, Drachman BM, Adams D, Heitner SB, Conceicao I, Schmidt HH, Vita G, Campistol JM, Gamez J, Gorevic PD, Gane E, Shah AM, Solomon SD, Monia BP, Hughes SG, Kwoh TJ, McEvoy BW, Jung SW, Baker BF, Ackermann EJ, Gertz MA and Coelho T. Inotersen Treatment for Patients with Hereditary Transthyretin Amyloidosis. N Engl J Med. 2018;379:22–31. [DOI] [PubMed] [Google Scholar]
- 48.Crooke ST, Witztum JL, Bennett CF and Baker BF. RNA-Targeted Therapeutics. Cell Metab. 2018;27:714–739. [DOI] [PubMed] [Google Scholar]
- 49.Suhr OB, Coelho T, Buades J, Pouget J, Conceicao I, Berk J, Schmidt H, Waddington-Cruz M, Campistol JM, Bettencourt BR, Vaishnaw A, Gollob J and Adams D. Efficacy and safety of patisiran for familial amyloidotic polyneuropathy: a phase II multi-dose study. Orphanet J Rare Dis. 2015;10:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adams D, Gonzalez-Duarte A, O’Riordan WD, Yang CC, Ueda M, Kristen AV, Tournev I, Schmidt HH, Coelho T, Berk JL, Lin KP, Vita G, Attarian S, Plante-Bordeneuve V, Mezei MM, Campistol JM, Buades J, Brannagan TH 3rd, Kim BJ, Oh J, Parman Y, Sekijima Y, Hawkins PN, Solomon SD, Polydefkis M, Dyck PJ, Gandhi PJ, Goyal S, Chen J, Strahs AL, Nochur SV, Sweetser MT, Garg PP, Vaishnaw AK, Gollob JA and Suhr OB. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. The New England journal of medicine. 2018;379:11–21. [DOI] [PubMed] [Google Scholar]
- 51.Adams D and Verena K. Phase 1 study of ALN-TTRsc02, a subcutaneously administered investigational RNAi therapeutic for the treatment of transthyretin-mediated amyloidosis. Revue Neurologique. 2019;175:S129. [Google Scholar]
- 52.Viney NJ, Guo S, Tai LJ, Baker BF, Aghajan M, Jung SW, Yu RZ, Booten S, Murray H, Machemer T, Burel S, Murray S, Buchele G, Tsimikas S, Schneider E, Geary RS, Benson MD and Monia BP. Ligand conjugated antisense oligonucleotide for the treatment of transthyretin amyloidosis: preclinical and phase 1 data. ESC Heart Fail. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Falk RH, Gertz MA, Benson MD, Buchele G, Brambatti M, Tsimikas S, Viney NJ, Tai L-J, Monteiro C, Yang Q, O’Dea LSL, Karwatowska-Prokopczuk E, Schneider E, Geary RS and Monia BP. Rationale and Design of a Phase 3 Study to Evaluate the Efficacy and Safety of ION-682884 in Patients with Transthyretin-Mediated Amyloid Cardiomyopathy (ATTR-CM). Blood. 2019;134:5764–5764. [Google Scholar]
- 54.Finn JD, Smith AR, Patel MC, Shaw L, Youniss MR, van Heteren J, Dirstine T, Ciullo C, Lescarbeau R, Seitzer J, Shah RR, Shah A, Ling D, Growe J, Pink M, Rohde E, Wood KM, Salomon WE, Harrington WF, Dombrowski C, Strapps WR, Chang Y and Morrissey DV. A Single Administration of CRISPR/Cas9 Lipid Nanoparticles Achieves Robust and Persistent In Vivo Genome Editing. Cell Reports. 2018;22:2227–2235. [DOI] [PubMed] [Google Scholar]
- 55.Nelson LT, Paxman RJ, Xu J, Webb B, Powers ET and Kelly JW. Blinded potency comparison of transthyretin kinetic stabilisers by subunit exchange in human plasma. Amyloid. 2020:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castano A, Helmke S, Alvarez J, Delisle S and Maurer MS. Diflunisal for ATTR cardiac amyloidosis. Congest Heart Fail. 2012;18:315–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lohrmann G, Pipilas A, Mussinelli R, Gopal DM, Berk JL, Connors LH, Vellanki N, Hellawell J, Siddiqi OK, Fox J, Maurer MS and Ruberg FL. Stabilization of Cardiac Function With Diflunisal in Transthyretin (ATTR) Cardiac Amyloidosis. J Card Fail. 2020;26:753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coehlo T, Chorao R, Sousa A, Alves I, Torres M and Saraiva M. Compound heterozygotes of transthyretin Met30 and transthyretin Met119 are protected from the devastating effects of familial amyloid polyneuropathy. Neuromuscul Disord. 1996;6:27.8845715 [Google Scholar]
- 59.Coelho T, Maia L, Martins da Silva A, Cruz M, Plante-Bordeneuve V, Lozeron P, Suhr O, Campistol J, Conceic ã o I, Schmidt H, Trigo P, Kelly J, Labaudinière R, Chan J, Packman J, Wilson A and Grogan D. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology. 2012;79:785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, Drachman BM, Shah SJ, Hanna M, Judge DP, Barsdorf AI, Huber P, Patterson TA, Riley S, Schumacher J, Stewart M, Sultan MB, Rapezzi C and Investigators A-AS. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N Engl J Med. 2018;379:1007–1016. [DOI] [PubMed] [Google Scholar]
- 61.Lockwood PA, Le VH, O’Gorman MT, Patterson TA, Sultan MB, Tankisheva E, Wang Q and Riley S. The Bioequivalence of Tafamidis 61-mg Free Acid Capsules and Tafamidis Meglumine 4 × 20-mg Capsules in Healthy Volunteers. Clin Pharmacol Drug Dev. 2020;9:849–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miller M, Pal A, Albusairi W, Joo H, Pappas B, Haque Tuhin MT, Liang D, Jampala R, Liu F, Khan J, Faaij M, Park M, Chan W, Graef I, Zamboni R, Kumar N, Fox J, Sinha U and Alhamadsheh M. Enthalpy-Driven Stabilization of Transthyretin by AG10 Mimics a Naturally Occurring Genetic Variant That Protects from Transthyretin Amyloidosis. J Med Chem. 2018;61:7862–7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim JH, Oroz J and Zweckstetter M. Structure of Monomeric Transthyretin Carrying the Clinically Important T119M Mutation. Angew Chem Int Ed Engl. 2016;55:16168–16171. [DOI] [PubMed] [Google Scholar]
- 64.Penchala SC, Connelly S, Wang Y, Park MS, Zhao L, Baranczak A, Rappley I, Vogel H, Liedtke M, Witteles RM, Powers ET, Reixach N, Chan WK, Wilson IA, Kelly JW, Graef IA and Alhamadsheh MM. AG10 inhibits amyloidogenesis and cellular toxicity of the familial amyloid cardiomyopathy-associated V122I transthyretin. Proc Natl Acad Sci U S A. 2013;110:9992–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Judge DP, Heitner SB, Falk RH, Maurer MS, Shah SJ, Witteles RM, Grogan M, Selby VN, Jacoby D, Hanna M, Nativi-Nicolau J, Patel J, Rao S, Sinha U, Turtle CW and Fox JC. Transthyretin Stabilization by AG10 in Symptomatic Transthyretin Amyloid Cardiomyopathy. J Am Coll Cardiol. 2019;74:285–295. [DOI] [PubMed] [Google Scholar]
- 66.Sant’Anna R, Gallego P, Robinson LZ, Pereira-Henriques A, Ferreira N, Pinheiro F, Esperante S, Pallares I, Huertas O, Almeida MR, Reixach N, Insa R, Velazquez-Campoy A, Reverter D, Reig N and Ventura S. Repositioning tolcapone as a potent inhibitor of transthyretin amyloidogenesis and associated cellular toxicity. Nat Commun. 2016;7:10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gurwitz JH and Maurer MS. Tafamidis-A Pricey Therapy for a Not-So-Rare Condition. JAMA Cardiol. 2020;5:247–248. [DOI] [PubMed] [Google Scholar]
- 68.Kazi DS, Bellows BK, Baron SJ, Shen C, Cohen DJ, Spertus JA, Yeh RW, Arnold SV, Sperry BW, Maurer MS and Shah SJ. Cost-Effectiveness of Tafamidis Therapy for Transthyretin Amyloid Cardiomyopathy. Circulation. 2020;141:1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gertz MA. Immunoglobulin light chain amyloidosis: 2018 Update on diagnosis, prognosis, and treatment. Am J Hematol. 2018;93:1169–1180. [DOI] [PubMed] [Google Scholar]
- 70.Grogan M, Gertz M, McCurdy A, Roeker L, Kyle R, Kushwaha S, Daly R, Dearani J, Rodeheffer R, Frantz R, Lacy M, Hayman S, McGregor C, Edwards B and Dispenzieri A. Long term outcomes of cardiac transplant for immunoglobulin light chain amyloidosis: The Mayo Clinic experience. World J Transplant. 2016;6:380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Manwani R, Cohen O, Sharpley F, Mahmood S, Sachchithanantham S, Foard D, Lachmann HJ, Quarta C, Fontana M, Gillmore JD, Whelan C, Hawkins PN and Wechalekar AD. A prospective observational study of 915 patients with systemic AL amyloidosis treated with upfront bortezomib. Blood. 2019;134:2271–2280. [DOI] [PubMed] [Google Scholar]
- 72.Muchtar E, Dispenzieri A, Leung N, Lacy MQ, Buadi FK, Dingli D, Hayman SR, Kapoor P, Hwa YL, Fonder A, Hobbs M, Gonsalves W, Kourelis TV, Warsame R, Russell SJ, Lust JA, Lin Y, Go RS, Zeldenrust SR, Kyle RA, Rajkumar SV, Kumar SK and Gertz MA. Optimizing deep response assessment for AL amyloidosis using involved free light chain level at end of therapy: failure of the serum free light chain ratio. Leukemia. 2019;33:527–531. [DOI] [PubMed] [Google Scholar]
- 73.Palladini G, Dispenzieri A, Gertz MA, Kumar S, Wechalekar A, Hawkins PN, Schonland S, Hegenbart U, Comenzo R, Kastritis E, Dimopoulos MA, Jaccard A, Klersy C and Merlini G. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 2012;30:4541–9. [DOI] [PubMed] [Google Scholar]
- 74.Falco P, Bringhen S, Avonto I, Gay F, Morabito F, Boccadoro M and Palumbo A. Melphalan and its role in the management of patients with multiple myeloma. Expert Rev Anticancer Ther. 2007;7:945–57. [DOI] [PubMed] [Google Scholar]
- 75.Katragadda L, McCullough LM, Dai Y, Hsu J, Byrne M, Hiemenz J, May S, Cogle CR, Norkin M, Brown RA, Wingard JR, Chang M and Moreb JS. Effect of melphalan 140 mg/m(2) vs 200 mg/m(2) on toxicities and outcomes in multiple myeloma patients undergoing single autologous stem cell transplantation-a single center experience. Clin Transplant. 2016;30:894–900. [DOI] [PubMed] [Google Scholar]
- 76.Palladini G, Milani P, Foli A, Obici L, Lavatelli F, Nuvolone M, Caccialanza R, Perlini S and Merlini G. Oral melphalan and dexamethasone grants extended survival with minimal toxicity in AL amyloidosis: long-term results of a risk-adapted approach. Haematologica. 2014;99:743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dietrich S, Schonland SO, Benner A, Bochtler T, Kristen AV, Beimler J, Hund E, Zorn M, Goldschmidt H, Ho AD and Hegenbart U. Treatment with intravenous melphalan and dexamethasone is not able to overcome the poor prognosis of patients with newly diagnosed systemic light chain amyloidosis and severe cardiac involvement. Blood. 2010;116:522–8. [DOI] [PubMed] [Google Scholar]
- 78.Hall AG and Tilby MJ. Mechanisms of action of, and modes of resistance to, alkylating agents used in the treatment of haematological malignancies. Blood Rev. 1992;6:163–73. [DOI] [PubMed] [Google Scholar]
- 79.Lentzsch S, Lagos GG, Comenzo RL, Zonder JA, Osman K, Pan S, Bhutani D, Pregja S, Sanchorawala V and Landau H. Bendamustine With Dexamethasone in Relapsed/Refractory Systemic Light-Chain Amyloidosis: Results of a Phase II Study. J Clin Oncol. 2020;38:1455–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Burwick N and Sharma S. Glucocorticoids in multiple myeloma: past, present, and future. Ann Hematol. 2019;98:19–28. [DOI] [PubMed] [Google Scholar]
- 81.Dhodapkar MV, Hussein MA, Rasmussen E, Solomon A, Larson RA, Crowley JJ, Barlogie B and United States Intergroup Trial Southwest Oncology G. Clinical efficacy of high-dose dexamethasone with maintenance dexamethasone/alpha interferon in patients with primary systemic amyloidosis: results of United States Intergroup Trial Southwest Oncology Group (SWOG) S9628. Blood. 2004;104:3520–6. [DOI] [PubMed] [Google Scholar]
- 82.Anderson KC. Lenalidomide and thalidomide: mechanisms of action--similarities and differences. Semin Hematol. 2005;42:S3–8. [DOI] [PubMed] [Google Scholar]
- 83.Kastritis E, Terpos E, Roussou M, Gavriatopoulou M, Pamboukas C, Boletis I, Marinaki S, Apostolou T, Nikitas N, Gkortzolidis G, Michalis E, Delimpasi S and Dimopoulos MA. A phase 1/2 study of lenalidomide with low-dose oral cyclophosphamide and low-dose dexamethasone (RdC) in AL amyloidosis. Blood. 2012;119:5384–90. [DOI] [PubMed] [Google Scholar]
- 84.Moreau P, Jaccard A, Benboubker L, Royer B, Leleu X, Bridoux F, Salles G, Leblond V, Roussel M, Alakl M, Hermine O, Planche L, Harousseau JL and Fermand JP. Lenalidomide in combination with melphalan and dexamethasone in patients with newly diagnosed AL amyloidosis: a multicenter phase 1/2 dose-escalation study. Blood. 2010;116:4777–82. [DOI] [PubMed] [Google Scholar]
- 85.Kumar SK, Hayman SR, Buadi FK, Roy V, Lacy MQ, Gertz MA, Allred J, Laumann KM, Bergsagel LP, Dingli D, Mikhael JR, Reeder CB, Stewart AK, Zeldenrust SR, Greipp PR, Lust JA, Fonseca R, Russell SJ, Rajkumar SV and Dispenzieri A. Lenalidomide, cyclophosphamide, and dexamethasone (CRd) for light-chain amyloidosis: long-term results from a phase 2 trial. Blood. 2012;119:4860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Palladini G, Milani P, Foli A, Basset M, Russo F, Perlini S and Merlini G. A phase 2 trial of pomalidomide and dexamethasone rescue treatment in patients with AL amyloidosis. Blood. 2017;129:2120–2123. [DOI] [PubMed] [Google Scholar]
- 87.Potts BC, Albitar MX, Anderson KC, Baritaki S, Berkers C, Bonavida B, Chandra J, Chauhan D, Cusack JC Jr., Fenical W, Ghobrial IM, Groll M, Jensen PR, Lam KS, Lloyd GK, McBride W, McConkey DJ, Miller CP, Neuteboom ST, Oki Y, Ovaa H, Pajonk F, Richardson PG, Roccaro AM, Sloss CM, Spear MA, Valashi E, Younes A and Palladino MA. Marizomib, a proteasome inhibitor for all seasons: preclinical profile and a framework for clinical trials. Curr Cancer Drug Targets. 2011;11:254–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Narayanan S, Cai CY, Assaraf YG, Guo HQ, Cui Q, Wei L, Huang JJ, Ashby CR Jr. and Chen ZS. Targeting the ubiquitin-proteasome pathway to overcome anti-cancer drug resistance. Drug Resist Updat. 2020;48:100663. [DOI] [PubMed] [Google Scholar]
- 89.Bochtler T, Hegenbart U, Kunz C, Granzow M, Benner A, Seckinger A, Kimmich C, Goldschmidt H, Ho AD, Hose D, Jauch A and Schonland SO. Translocation t(11;14) is associated with adverse outcome in patients with newly diagnosed AL amyloidosis when treated with bortezomib-based regimens. J Clin Oncol. 2015;33:1371–8. [DOI] [PubMed] [Google Scholar]
- 90.Palladini G, Sachchithanantham S, Milani P, Gillmore J, Foli A, Lachmann H, Basset M, Hawkins P, Merlini G and Wechalekar AD. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood. 2015;126:612–5. [DOI] [PubMed] [Google Scholar]
- 91.Dispenzieri A, Gertz MA, Kyle RA, Lacy MQ, Burritt MF, Therneau TM, Greipp PR, Witzig TE, Lust JA, Rajkumar SV, Fonseca R, Zeldenrust SR, McGregor CG and Jaffe AS. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol. 2004;22:3751–7. [DOI] [PubMed] [Google Scholar]
- 92.Palladini G, Milani P, Foli A, Vidus Rosin M, Basset M, Lavatelli F, Nuvolone M, Obici L, Perlini S and Merlini G. Melphalan and dexamethasone with or without bortezomib in newly diagnosed AL amyloidosis: a matched case-control study on 174 patients. Leukemia. 2014;28:2311–6. [DOI] [PubMed] [Google Scholar]
- 93.Driscoll J and Girnius S. Proteasome Inhibitors to Treat AL Amyloidosis. In: Fernandez-Escamilla A-M, ed. Exploring new findings on amyloidosis: IntechOpen; 2016. [Google Scholar]
- 94.Manwani R, Mahmood S, Sachchithanantham S, Lachmann HJ, Gillmore JD, Yong K, Rabin N, Popat R, Kyriakou C, Worthington S, Sharpley F, Smith M, Shah R, Cheesman S, Hawkins PN and Wechalekar AD. Carfilzomib is an effective upfront treatment in AL amyloidosis patients with peripheral and autonomic neuropathy. Br J Haematol. 2019;187:638–641. [DOI] [PubMed] [Google Scholar]
- 95.Sanchorawala V, Palladini G, Kukreti V, Zonder JA, Cohen AD, Seldin DC, Dispenzieri A, Jaccard A, Schonland SO, Berg D, Yang H, Gupta N, Hui AM, Comenzo RL and Merlini G. A phase 1/2 study of the oral proteasome inhibitor ixazomib in relapsed or refractory AL amyloidosis. Blood. 2017;130:597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kastritis E, Dispenzieri A, Wechalekar AD, Schönland SO, Kim K, Sanchorawala V, Landau HJ, Kwok F, Suzuki K, Comenzo R, Palladini G, Berg D, Liu G, Kumar A, Faller DV and Merlini G. Ixazomib-dexamethasone (Ixa-Dex) vs physician’s choice (PC) in relapsed/refractory (RR) primary systemic AL amyloidosis (AL) patients (pts) by prior proteasome inhibitor (PI) exposure in the phase III TOURMALINE-AL1 trial. Journal of Clinical Oncology. 2020;38:8546–8546. [Google Scholar]
- 97.de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, Oomen LA, Peipp M, Valerius T, Slootstra JW, Mutis T, Bleeker WK, Anderson KC, Lokhorst HM, van de Winkel JG and Parren PW. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186:1840–8. [DOI] [PubMed] [Google Scholar]
- 98.van de Donk NW, Janmaat ML, Mutis T, Lammerts van Bueren JJ, Ahmadi T, Sasser AK, Lokhorst HM and Parren PW. Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol Rev. 2016;270:95–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, Minnema MC, Lassen U, Krejcik J, Palumbo A, van de Donk NW, Ahmadi T, Khan I, Uhlar CM, Wang J, Sasser AK, Losic N, Lisby S, Basse L, Brun N and Richardson PG. Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma. N Engl J Med. 2015;373:1207–19. [DOI] [PubMed] [Google Scholar]
- 100.Kaufman GP, Schrier SL, Lafayette RA, Arai S, Witteles RM and Liedtke M. Daratumumab yields rapid and deep hematologic responses in patients with heavily pretreated AL amyloidosis. Blood. 2017;130:900–902. [DOI] [PubMed] [Google Scholar]
- 101.Hossein Taghizadeh M, Reiter T, Duca F, Binder C, Kornauth M-T and Christoph HA. Daratumumab – a safe first-line treatment of cardiac AL amyloidosis in heavily compromised patients. The XVIth International Symposium on Amyloidosis March 26–29; Kumamoto, Japan2018. [Google Scholar]
- 102.Kastritis E, Palladini G, Minnema M, Wechalekar A, Jaccard A, Lee H, Sanchorawala V, Gibbs Simon, Mollee Peter, Venner Christopher P., Jin Lu, Stefan Schonland, Gatt Moshe E., Suzuki Kenshi, Kim Kihyun, Cibeira M. Teresa, Beksac Meral, Libby Edward, Valent Jason, Hungria Vania, Wong Sandy W, Rosenzweig Michael, Bumma Naresh, Chauveau Dominique, Ahmadi Tahamtan, Tran NamPhuong, Qin Xiang, Vasey Sandra Y., Tromp Brenda, Schecter Jordan M., Weiss Brendan M., Vermeulen Jessica, Merlini Giampaolo and RL Comenzo. Subcutaneous daratumumab + cyclophosphamide, bortezomib, and dexamethasone (CyBorD) in patients with newly diagnosed light chain amyloidosis. Paper presented at: EHA25 Virtual Congress 2020. [Google Scholar]
- 103.Landau H, Smith M, Landry C, Chou JF, Devlin SM, Hassoun H, Bello C, Giralt S and Comenzo RL. Long-term event-free and overall survival after risk-adapted melphalan and SCT for systemic light chain amyloidosis. Leukemia. 2017;31:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moreno L, Perez C, Zabaleta A, Manrique I, Alignani D, Ajona D, Blanco L, Lasa M, Maiso P, Rodriguez I, Garate S, Jelinek T, Segura V, Moreno C, Merino J, Rodriguez-Otero P, Panizo C, Prosper F, San-Miguel JF and Paiva B. The Mechanism of Action of the Anti-CD38 Monoclonal Antibody Isatuximab in Multiple Myeloma. Clin Cancer Res. 2019;25:3176–3187. [DOI] [PubMed] [Google Scholar]
- 105.Tai Y-T, Dillon M, Song W, Leiba M, Li X-F, Burger P, Lee AI, Podar K, Hideshima T, Rice AG, van Abbema A, Jesaitis L, Caras I, Law D, Weller E, Xie W, Richardson P, Munshi NC, Mathiot C, Avet-Loiseau H, Afar DEH and Anderson KC. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood. 2008;112:1329–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Collins SM, Bakan CE, Swartzel GD, Hofmeister CC, Efebera YA, Kwon H, Starling GC, Ciarlariello D, Bhaskar S, Briercheck EL, Hughes T, Yu J, Rice A and Benson DM Jr. Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: evidence for augmented NK cell function complementing ADCC. Cancer Immunol Immunother. 2013;62:1841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Iqbal SM, Stecklein K, Sarow J, Krabak M, Hillengass J and McCarthy P. Elotuzumab in Combination with Lenalidomide and Dexamethasone for Treatment-resistant Immunoglobulin Light Chain Amyloidosis With Multiple Myeloma. Clin Lymphoma Myeloma Leuk. 2019;19:e33–e36. [DOI] [PubMed] [Google Scholar]
- 108.Vaxman I, Sidiqi MH and Gertz M. Venetoclax for the treatment of multiple myeloma. Expert Rev Hematol. 2018;11:915–920. [DOI] [PubMed] [Google Scholar]
- 109.Touzeau C, Dousset C, Le Gouill S, Sampath D, Leverson JD, Souers AJ, Maiga S, Bene MC, Moreau P, Pellat-Deceunynck C and Amiot M. The Bcl-2 specific BH3 mimetic ABT-199: a promising targeted therapy for t(11;14) multiple myeloma. Leukemia. 2014;28:210–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Biolo A, Ramamurthy S, Connors LH, O’Hara CJ, Meier-Ewert HK, Soo Hoo PT, Sawyer DB, Seldin DC and Sam F. Matrix metalloproteinases and their tissue inhibitors in cardiac amyloidosis: relationship to structural, functional myocardial changes and to light chain amyloid deposition. Circ Heart Fail. 2008;1:249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.D’Souza A, Szabo A, Flynn KE, Dhakal B, Chhabra S, Pasquini MC, Weihrauch D and Hari PN. Adjuvant doxycycline to enhance anti-amyloid effects: Results from the dual phase 2 trial. EClinicalMedicine. 2020;23:100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Karlstedt E, Jimenez-Zepeda V, Howlett JG, White JA and Fine NM. Clinical Experience With the Use of Doxycycline and Ursodeoxycholic Acid for the Treatment of Transthyretin Cardiac Amyloidosis. J Card Fail. 2019;25:147–153. [DOI] [PubMed] [Google Scholar]
- 113.Wall JS, Kennel SJ, Stuckey AC, Long MJ, Townsend DW, Smith GT, Wells KJ, Fu Y, Stabin MG, Weiss DT and Solomon A. Radioimmunodetection of amyloid deposits in patients with AL amyloidosis. Blood. 2010;116:2241–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bodin K, Ellmerich S, Kahan MC, Tennent GA, Loesch A, Gilbertson JA, Hutchinson WL, Mangione PP, Gallimore JR, Millar DJ, Minogue S, Dhillon AP, Taylor GW, Bradwell AR, Petrie A, Gillmore JD, Bellotti V, Botto M, Hawkins PN and Pepys MB. Antibodies to human serum amyloid P component eliminate visceral amyloid deposits. Nature. 2010;468:93–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gertz MA, Landau H, Comenzo RL, Seldin D, Weiss B, Zonder J, Merlini G, Schonland S, Walling J, Kinney GG, Koller M, Schenk DB, Guthrie SD and Liedtke M. First-in-Human Phase I/II Study of NEOD001 in Patients With Light Chain Amyloidosis and Persistent Organ Dysfunction. J Clin Oncol. 2016;34:1097–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li L, Tong W, Lau M, Fells K, Zhu T, Sun Y, Kovacs E, Khasanov A, Yan Z, Deng D, Takeshita K, Kaufmann GF, Ji H, Li H and Zhang H. Preclinical Development of an Anti-CD38 Antibody-Drug Conjugate for Treatment of Hematological Malignancies. Blood. 2019;134:5621. [Google Scholar]
- 117.Barrett C, Alexander K, Zhao H, Haddad F, Cheng P, Liao R, Wheeler M, Liedtke M, Schrier S, Arai S, Weisshaar D and Witteles R. Outcomes in Patients With Cardiac Amyloidosis Undergoing Heart Transplantation. J Am Coll Cardiol HF. 2020;8:461–8. [DOI] [PubMed] [Google Scholar]
- 118.Kristen A, Kreusser M, Blum P, Schönland S, Frankenstein L, Dösch A, Knop B, Helmschrott M, Schmack B, Ruhparwar A, Hegenbart U, Katus H and Raake P. Improved outcomes after heart transplantation for cardiac amyloidosis in the modern era. J Heart Lung Transplant. 2018;37:611–618. [DOI] [PubMed] [Google Scholar]
- 119.Griffin JM, Chiu L, Axsom KM, Bijou R, Clerkin KJ, Colombo P, Cuomo MO, De Los Santos J, Fried JA, Goldsmith J, Habal M, Haythe J, Helmke S, Horn EM, Latif F, Lee SH, Lin EF, Naka Y, Raikhelkar J, Restaino S, Sayer GT, Takayama H, Takeda K, Teruya S, Topkara V, Tsai EJ, Uriel N, Yuzefpolskaya M, Farr MA and Maurer MS. Outcomes after heart transplantation for al compared to attr cardiac amyloidosis. Clin Transplant. 2020:e14028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thoracic Organ Transplant Committee. Review Board Guidance for Hypertrophic/Restrictive (HCM/RCM) Cardiomyopathy Exception Requests. October 2018. Available at: https://optn.transplant.hrsa.gov/media/2637/thoracic_guidance_review_board_hcm_rcm_201806.pdf. Accessed May 26th, 2020.
- 121.Griffin JM, DeFilippis EM, Rosenblum H, Topkara VK, Fried JA, Uriel N, Takeda K, Farr MA, Maurer MS and Clerkin KJ. Comparing Outcomes for Infiltrative and Restrictive Cardiomyopathies under the New Heart Transplant Allocation System. Clin Transplant. 2020:e14109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Palladini G, Perfetti V, Obici L, Caccialanza R, Semino A, Adami F, Cavallero G, Rustichelli R, Virga G and Merlini G. Association of melphalan and high-dose dexamethasone is effective and well tolerated in patients with AL (primary) amyloidosis who are ineligible for stem cell transplantation. Blood. 2004;103:2936–8. [DOI] [PubMed] [Google Scholar]
- 123.Dispenzieri A, Buadi F, Laumann K, LaPlant B, Hayman SR, Kumar SK, Dingli D, Zeldenrust SR, Mikhael JR, Hall R, Rajkumar SV, Reeder C, Fonseca R, Bergsagel PL, Stewart AK, Roy V, Witzig TE, Lust JA, Russell SJ, Gertz MA and Lacy MQ. Activity of pomalidomide in patients with immunoglobulin light-chain amyloidosis. Blood. 2012;119:5397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]


