Abstract
Background
Older adults (aged 65 years and above) constitute the fastest growing population cohort in the western world. There is increasing evidence that the burden of infections disproportionately affects older adults, and hence this vulnerable population is frequently exposed to antimicrobials. There is currently no systematic review summarising the evidence for organ injury risk among older adults following antimicrobial exposure. This systematic review and meta-analysis examined the relationship between antimicrobial exposure and organ injury in older adults.
Methodology
We searched for original research articles in PubMed, Embase.com, Web of Science core collection, Web of Science BIOSIS citation index, Scopus, Cochrane Central Register of Controlled Trials, ProQuest, and PsycINFO databases, using key words in titles and abstracts, and using MeSH terms. We searched for all available articles up to 31 May 2021. After removing duplicates, articles were screened for inclusion into or exclusion from the study by two reviewers. The Newcastle-Ottawa scale was used to assess the risk of bias for cohort and case-control studies. We explored the heterogeneity of the included studies using the Q test and I2 test and the publication bias using the funnel plot and Egger’s test. The meta-analyses were performed using the OpenMetaAnalyst software.
Results
The overall absolute risks of acute kidney injury among older adults prescribed aminoglycosides, glycopeptides, and macrolides were 15.1% (95% CI: 12.8–17.3), 19.1% (95% CI: 15.4–22.7), and 0.3% (95% CI: 0.3–0.3), respectively. Only 3 studies reported antimicrobial associated drug-induced liver injury. Studies reporting on the association of organ injury and antimicrobial exposure by age or duration of treatment were too few to meta-analyse. The funnel plot and Egger’s tests did not indicate evidence of publication bias.
Conclusion
Older adults have a significantly higher risk of sustaining acute kidney injury when compared to the general adult population. Older adults prescribed aminoglycosides have a similar risk of acute kidney injury to the general adult population.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12877-021-02512-3.
Keywords: Systematic review, meta-analysis, Older adults, Antimicrobial, Organ injury
Background
Older adults aged 65 years and above comprise the fastest and largest expanding population age group in the developed world [1]. They are prone to infectious diseases such as pneumonia, skin and soft tissue infections (SSTI), urinary tract infections (UTI) and septicaemia when compared to younger people [1]. It is estimated that the older adults comprise 48.7% of individuals admitted to hospital intensive care units for these infections [2], resulting in their increased length of hospital stay and exposure to antimicrobials. Giarratano et al. [3] highlighted several predisposing factors that make older adults more susceptible to antimicrobial adverse events. These include physiological changes, higher comorbidities, drug-drug interactions, drug delivery routes used, and length of time they are in contact or exposed to the antimicrobial agents. In one large prospective cohort study, antimicrobial related adverse events accounted for 19.3% of all drug-related adverse events seen at the emergency department [2]. Several antimicrobial-associated adverse events become apparent years after the drug has been approved. The adverse events reported in clinical trials differ considerably from post-marketing surveillance [4]. Since most clinical trials exclude older adults, the true nature and incidence of antimicrobial related adverse events in this population are unknown. In their review, Giarratano et al. [3] concluded that there is a general lack of epidemiological studies on antimicrobials used among the older adults, yet this is essential in informing healthcare providers to achieve optimal safety and effectiveness when providing antimicrobial pharmacotherapy to the older adults.
This paper describes a systematic review and meta-analysis carried out to investigate the risk of antimicrobial-associated kidney, liver, or tissue injury among older adults. The main outcome was quantifying the association of organ injury among older adults following exposure to antimicrobials.
Methods
This systematic review was conducted after the study protocol was registered with PROSPERO (CRD4202015262).
Search strategy and selection criteria
For this systematic review and meta-analysis, we searched for original research articles for observational studies describing kidney, liver or tissue injury associated with exposure to antimicrobials among older adults (65 years or above). We restricted our searches to the English language only for all available articles up to 31 May 2021.
Searches were conducted in PubMed, Embase.com, Web of Science core collection, Web of Science BIOSIS citation index, Scopus, Cochrane Central Register of Controlled Trials, ProQuest, and PsycINFO databases, using key words in titles and abstracts, and using MeSH terms. The full search criteria for all the databases are in additional file 1, document 1. After removing duplicates, TC carried out the title and abstract screening on all the articles for preliminary inclusion into the systematic review. PN and SR independently carried out title and abstract screening of 50% of the articles each for preliminary inclusion into the systematic review. Among the preliminary included articles, TC and PN carried out full article review of the first 50% of the articles, and TC and SR reviewed the other half. Where there were disagreements between reviewers in both the preliminary review and full article review, the opinion of the third reviewer was sought.
Inclusion and exclusion criteria
Studies were included in the systematic review and meta-analysis if they reported the absolute risk of antimicrobial-associated kidney, liver, or tissue injury among older adults. Studies were also included if they reported a subgroup analysis of participants who met the inclusion criteria, thus 65 years or older. Studies were excluded if they included adults below 65 years of age without relevant subgroup analysis for those 65 years or older; the outcome of interest was not kidney, liver, or tissue injury; the exposure was not an antimicrobial; and the study design was not an original observational study.
Risk of bias assessment
The Newcastle-Ottawa scale [5] was used to assess the risk of bias for each observational study (see additional file 4, document 1). Each study that scored 6 or more points on the Newcastle-Ottawa scale was considered to have a low risk of bias provided it scored at least 3 points on the selection domain, one or more on comparability, and at least 2 points on the outcome domain. All studies considered to be of low risk to bias were further considered for meta-analysis.
Data analysis
Data extraction was completed by TC and PN independently, using a pre-piloted excel form. Data for the following variables were extracted: author details, country of study, study design, study setting, data source, any condition treated with antimicrobial, inclusion and exclusion criteria, outcome measurement and ascertainment, controlled confounders, sample size, exposure antibiotic, and absolute risk or odds ratio of organ injury among the participants. The full data extraction table is available as additional file 2, document 1.
Heterogeneity among the studies included for meta-analysis was assessed using the Cochrane Q statistic [6] at 5% significance level and quantified using Higgins and Thompson’s I2 statistic [6]. An I2 value of more than 50% was considered to reflect substantial heterogeneity, and therefore, sensitivity analysis to investigate the possible source of heterogeneity was done. For the meta-analysis results, publication bias was assessed using a funnel plot [7] and Egger’s regression test [8]. Regardless of the heterogeneity, the random-effects model, using the DerSimonian and Laird method [9], was used to meta-analyse the studies. The meta-analyses were performed using the OpenMetaAnalyst software [10].
Results
Study identification
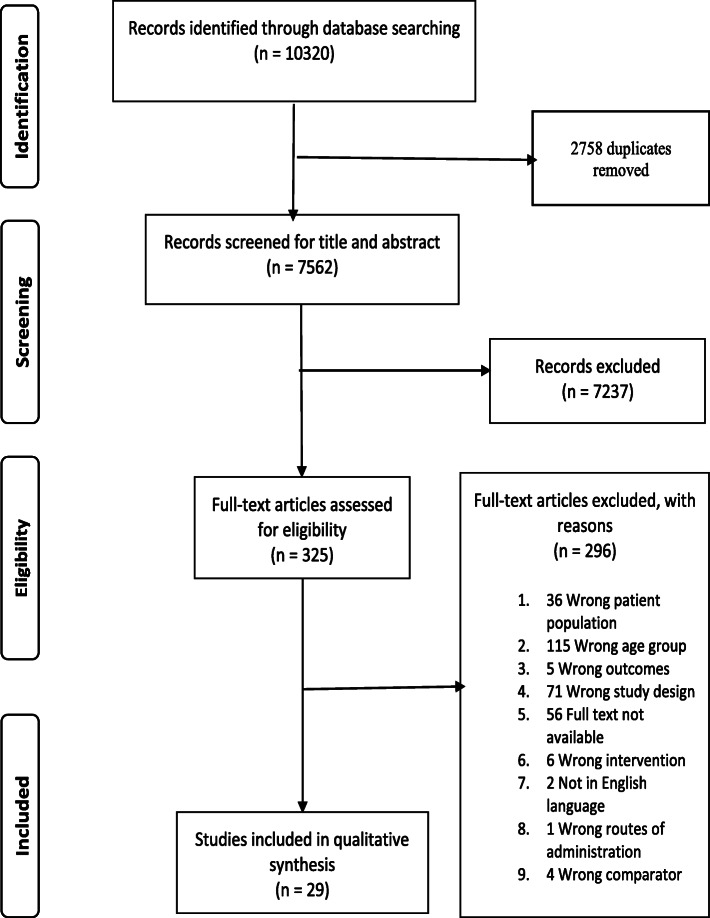
Following database searching, 10,320 studies were identified from the following databases: PubMed (1164), Embase.com (4880), Web of Science core collection (250), Web of Science BIOSIS citation index (301), Scopus (2901), Cochrane Central Register of Controlled Trials (817), ProQuest (6), and PsycINFO (1), of which 2758 were duplicates. Of the remaining 7562 studies, 7237 studies were excluded following title and abstract screening. A further 296 studies were excluded after the full-text screening, leaving 29 studies for inclusion into the the systematic review [11–39]. The full selection process is summarised in Fig. 1 below. Six of the included studies had data for two or more antimicrobials [16–18, 24, 27, 37], as shown in Table 1 below.
Fig. 1.
Flow chart of the literature search strategy to identify studies on antimicrobial exposure and organ injury risk
Table 1.
Main characteristics of the included studies on antimicrobials and organ injury
| Author, Year | Country | Method | Study Setting | Tool used to ascertain the outcome | Confounders | Sample size | Antibiotic class | Outcome | NOS Risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| Aggarwal, 2018 [31] | India | Prospective cohort | Hospital setting | Laboratory values | Age, gender, BMI, APACHE 2, DM, antibiotic dose | 26 | Polymyxin | AKI | Low |
| Ahmed, 2016 [38] | UK | Retrospective cohort | Hospital setting | AKIN | age, gender, surgeon grade, type of anaesthesia | 1500 | Aminoglycoside | AKI | Low |
| Ahmed, 2019 [37] | UK | Retrospective cohort | Primary care | ICD-10 codes | age, sex, coronary heart disease, renal disease, liver disease, respiratory disease, type 2 diabetes mellitus, heart failure | 28,800 | Cephalosporin | AKI | Low |
| Ahmed, 2019 [37] | UK | Retrospective cohort | Primary care | ICD-10 codes | age, sex, coronary heart disease, renal disease, liver disease, respiratory disease, type 2 diabetes mellitus, heart failure | 21,048 | Penicillin | AKI | Low |
| Ahmed, 2019 [37] | UK | Retrospective cohort | Primary care | ICD-10 codes | age, sex, coronary heart disease, renal disease, liver disease, respiratory disease, type 2 diabetes mellitus, heart failure | 14,868 | Quinolone | AKI | Low |
| Ahmed, 2018 [11] | UK | Retrospective cohort | Primary care | ICD-10 codes | age, diabetes, dementia, coronary heart disease, stroke, cancer, heart failure, polypharmacy | 29,936 | Trimethoprim | AKI | Low |
| Baciewicz, 2003 [12] | USA | Prospective cohort | Hospital setting | Laboratory values | age, sex, nephrotoxic agents, diabetes mellitus, coronary artery disease, hypertension and | 86 | Aminoglycoside | AKI | Low |
| Baghaei, 2010 [33] | Iran | Prospective cohort | Hospital setting | Laboratory values | Age, sex, concomitant hepatotoxic drugs | 295 | antituberculosis | DILI | Low |
| Baik, 2020 [36] | USA | Prospective cohort | Population based | ICD-9 codes | Concurrent antibiotics, gender, race, rural residency, income status | 685,018 | Fluoroquinolone | Tendon rupture | Low |
| Bright-Thomas, 2016 [13] | UK | Retrospective cohort | Primary care | Laboratory values | age, sex, ethnic origin | 200 | Antituberculosis drugs | DILI | Low |
| Carreno, 2013 [14] | USA | Retrospective cohort | Hospital setting | AKIN | age, history of AKI, vancomycin dose, concurrent receipt of nephrotoxins, concurrent receipt of vasopressors | 88 | Glycopeptide | AKI | Low |
| Craig, 2012 [15] | UK | Retrospective cohort | Hospital setting | Not stated | age, sex, operative procedure | 200 | Aminoglycoside | AKI | Low |
| Crellin, 2018 [16] | UK | Retrospective cohort | Multicentre Primary care | ICD-10 codes | sex, age, calendar period, chronic comorbidities, baseline renal function, history of renal or urological disease, and use of renin-angiotensin system blockers and potassium-sparing diuretics. | 55,961 | Cephalosporin | AKI | Low |
| Crellin, 2018 [16] | UK | Retrospective cohort | Multicentre Primary care | ICD-10 codes | sex, age, calendar period, chronic comorbidities, baseline renal function, history of renal or urological disease, and use of renin-angiotensin system blockers and potassium-sparing diuretics. | 56,736 | Nitrofurantoin | AKI | Low |
| Crellin, 2018 [16] | UK | Retrospective cohort | Multicentre Primary care | ICD-10 codes | sex, age, calendar period, chronic comorbidities, baseline renal function, history of renal or urological disease, and use of renin-angiotensin system blockers and potassium-sparing diuretics. | 33,130 | Quinolone | AKI | Low |
| Crellin, 2018 [16] | UK | Retrospective cohort | Multicentre Primary care | ICD-10 codes | sex, age, calendar period, chronic comorbidities, baseline renal function, history of renal or urological disease, and use of renin-angiotensin system blockers and potassium-sparing diuretics. | 153,201 | Trimethoprim | AKI | Low |
| Fraisse, 2014 [17] | France | Retrospective cohort | Hospital setting multicentre | NKF | age, sex, weight, concomitant use of other nephrotic drugs, types of infection | 109 | Aminoglycoside | AKI | Low |
| Fraisse, 2014 [17] | France | Retrospective cohort | Hospital setting multicentre | NKF | age, sex, weight, concomitant use of other nephrotic drugs, types of infection | 45 | Aminoglycoside | AKI | Low |
| Gandhi, 2013 [18] | Canada | Retrospective cohort | Population-based | ICD-10 codes | age, sex, chronic kidney disease, others | 96,226 | Macrolide | AKI | Low |
| Gandhi, 2013 [18] | Canada | Retrospective cohort | Population-based | ICD-10 codes | age, sex, chronic kidney disease, others | 94,083 | Macrolide | AKI | Low |
| Giri, 2016 [34] | India | Prospective cohort | Prospective cohort | Laboratory values | Overweight, age, sex, impaired renal status, pregnancy, immunocompromised | 26 | Aminoglycoside | AKI | High |
| Gyamlani, 2019 [19] | USA | Retrospective cohort | Hospital setting | CKD-EPI equation | age, sex, comorbidities, baseline eGFR, exposure to nephrotoxic medication | 22,057 | Glycopeptide | AKI | Low |
| Hall, 2014 [20] | USA | Retrospective cohort | Hospital setting | Laboratory values | age, sex, hospital stay, concomitant use of nephrotoxins | 92 | Glycopeptide | AKI | Low |
| Huang, 2018 [21] | China | Retrospective cohort | Hospital setting | AKIN | age, sex, type of infection, comorbidities, APACH II score | 50 | Glycopeptide | AKI | Low |
| Karino, 2014 [22] | Japan | Prospective cohort | Hospital setting | Categorical scale of mild, moderate, or serious (state recommended). | sex, age, underlying disease | 22 | Penicillin | AKI | Low |
| Li, 2015 [24] | Canada | Retrospective cohort | Population-based | ICD-10 codes | age, sex, baseline evidence of CKD, CVD, cancer, diabetes | 52,518 | Macrolide | AKI | Low |
| Li, 2015 [24] | Canada | Retrospective cohort | Population-based | ICD-10 codes | age, sex, baseline evidence of CKD, CVD, cancer, diabetes | 51,523 | Macrolide | AKI | Low |
| Liu, 2015 [23] | China | Retrospective cohort | Hospital setting | AKIN | age, sex, weight, comorbidities, concomitant use of ACEI, ARBs, NSAIDs, aminoglycosides, immunosuppressants | 124 | Glycopeptide | AKI | Low |
| Mizokami, 2013 [25] | Japan | Retrospective cohort | Hospital setting | Laboratory values | age, gender, infection severity, body weight, comorbidity index | 94 | Glycopeptide | AKI | Low |
| Morimoto, 2017 [32] | Japan | Prospective cohort | Hospital setting | KDIGO | Age, gender, concomitant antibiotics, BMI, BUN, severity of pneumonia | 57 | Penicillin | AKI | Moderate |
| Noh, 2019 [26] | South Korea | Retrospective cohort | Hospital setting | Laboratory values | age, sex, comorbidities (e.g., hypertension, etc.) | 77 | Antituberculosis drugs | DILI | Low |
| Ong, 2016 [27] | Singapore | Retrospective cohort | Hospital-based | KDIGO | age, sex, critical illness, comorbidities (e.g. diabetes, etc.), nephrotoxic medication | 194 | Aminoglycoside | AKI | Low |
| Ong, 2016 [27] | Singapore | Retrospective cohort | Hospital-based | KDIGO | age, sex, critical illness, comorbidities (e.g. diabetes, etc.), nephrotoxic medication | 84 | Aminoglycoside | AKI | Low |
| Pan, 2018 [39] | China | Retrospective cross-sectional | Hospital-based | KDIGO | age, gender, baseline serum creatinine, vasopressors, beta-blockers, furosemide, carbapenems, ICU admittance, etc. | 647 | Glycopeptide | AKI | Low |
| Pan, 2017 [28] | China | Retrospective cohort | Hospital-based | KDIGO | sex, age, baseline serum creatinine, the reason for vancomycin therapy, ICU admittance, etc. | 279 | Glycopeptide | AKI | Low |
| Paterson. 1998 [29] | Australia | Prospective cohort | Hospital-based | Laboratory values | age, duration of therapy, allopurinol use, | 88 | Aminoglycoside | AKI | Low |
| Raveh, 2002 [30] | Israel | Prospective cohort | Hospital-based | Laboratory values | age, gender, BMI, infectious diagnosis, diabetes mellitus, etc. | 209 | Aminoglycoside | AKI | Low |
| Sia, 2018 [35] | Australia | Retrospective cohort | Hospital based | KDIGO | Age, gender, treatment indicator, ICU admission | 242 | Aminoglycoside | AKI | Low |
KEY: AKI Acute kidney injury, DILI Drug induced liver injury, KDIGO Kidney Disease: Improving Global Outcome, AKIN Acute Kidney Injury Network, ICD-10 International Classification of Diseases version 10, CKD-EPI Chronic kidney Disease – Epidemiology collaborator, NKF National Kidney Foundation, CVD Cardiovarscular disease, ACEI Angiotensin converting enzyme Inhibitor, ARB Angiotensin II receptor blocker, NSAID Nonsteroidal anti-inflammatory drug, ICU Intensive care unit, BMI Body-Mass index
NOS risk of bias grades: low, moderate, high
Characteristics of included studies
Out of the 29 studies included for qualitative analysis, eight were prospective cohort studies [12, 22, 29–31, 33, 34, 36] and the rest were all retrospective cohort studies. Most of the studies were hospital-based except four that were based on primary care data [11, 13, 16, 37] and three that were based on population data [18, 24, 36]. Six of the studies were carried out in the United Kingdom [11, 13, 15, 16, 37, 38], five from the United States of America [12, 14, 19, 20, 36], four from China [21, 23, 28, 39], three from Japan [22, 25, 32], two each from Australia [29, 35], Canada [18, 24] and India [31, 34], and one each from France [17], Iran [33], Israel [30], Singapore [27], and South Korea [26]. Out of the twenty-nine studies included for qualitative analysis, one reported on tendon rupture [36], three reported on drug-induced liver injury (DILI) [13, 26, 33], and the rest reported on acute kidney injury (AKI). After considering the classes of antimicrobials used, nine, eight and four studies reported on AKI due to aminoglycosides, glycopeptides, and macrolides, respectively. Cephalosporins, penicillin, quinolone, and trimethoprim had a couple of studies, each reporting on AKI, while nitrofurantoin had a single study reporting on AKI. Three studies were on antituberculosis antimicrobials, and they reported on DILI. One study on quinolones reported on tendon rupture. Table 1 below summarises the main characteristics of all the included studies.
Risk of bias
The Newcastle-Ottawa Scale (NOS) [5] with slight modifications was used to assess the risk of bias for each of the included observational studies. This tool is well validated and commonly used for assessing the risk of bias for observational studies included in a systematic review [40]. In this systematic review, the modified tool is shown in additional file 4_doc1_appendices. Table 2 above summarises the risk of bias for each included observational study. All but two of the included cohort studies had a low risk of bias. The study by Morimoto et al. [32] had moderate risk of bias due to low score on selection criteria, while the study by Giri et al. [34] had high risk of bias due to low score on comparability.
Table 2.
Quality assessment of included studies based on the modified Newcastle-Ottawa scoring system (summarised from additional file 3, document 1)
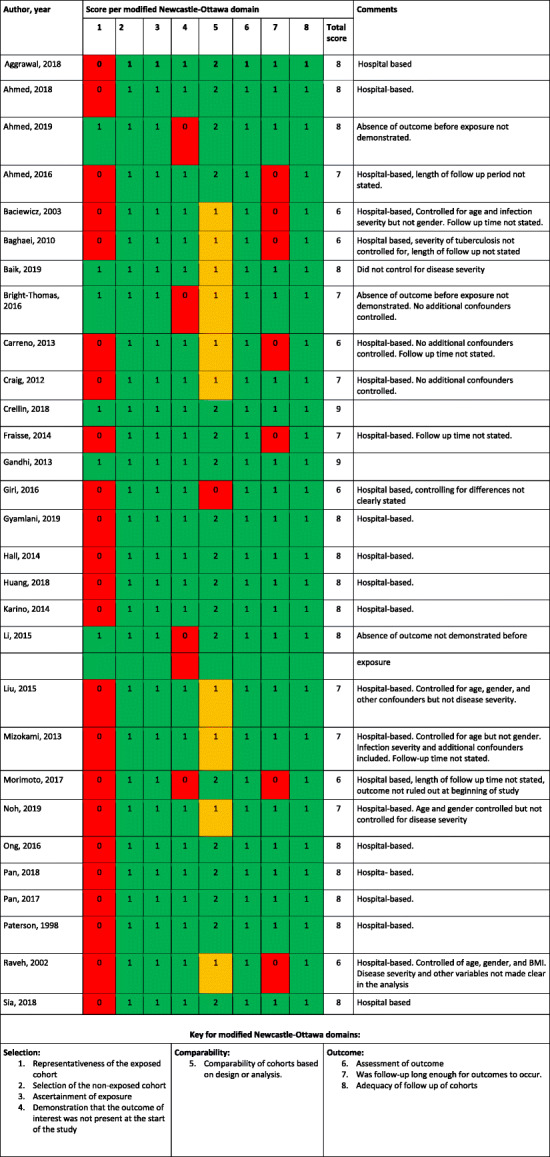
Key for modified Newcastle-Ottawa domains:
Selection:
1. Representativeness of the exposed cohort
2. Selection of the non-exposed cohort
3. Ascertainment of exposure
4. Demonstration that the outcome of interest was not present at the start of the study
Comparability:
5. Comparability of cohorts based on design or analysis.
Outcome:
6. Assessment of outcome
7. Was follow-up long enough for outcomes to occur.
8. Adequacy of follow up of cohorts
Red = Failed to satisify domain (Reduces quality)
Amber = Moderately satisfied domain (moderately affects quality)
Green = Satisfied domain (Increases quality)
Outcomes
Out of the 29 studies included in this systematic review, two studies were not considered for meta-analysis due to their moderate or high risk of bias [32, 34]. A further two studies were not included in the meta-analysis of absolute risk because the researchers reported odds ratios only [16, 37]. However, the remaining twenty-five studies expressed the absolute risk of organ injury among exposed older adults. Eight studies [12, 15, 17, 27, 29, 30, 35, 38] reported absolute risk of AKI due to aminoglycoside exposure, with two antimicrobials reported in each of the studies by Fraisse et al. [17] and Ong et al. [27]. Eight studies reported absolute risk of AKI due to glycopeptide exposure [14, 19–21, 23, 25, 28, 39], and two studies reported the absolute risk of AKI due to macrolide exposure [18, 24] on two antimicrobials each. Two studies each on penicillin [11, 22], quinolones [16, 37], cephalosporins [16, 37], and trimethoprim [11, 16] also reported an absolute risk of AKI due to exposure to the respective antimicrobial. Only three studies [13, 26, 33] reported an absolute risk of DILI due to antituberculosis antimicrobials. Meta-analyses were done on absolute risks for AKI among older adults who received aminoglycoside, glycopeptide, or macrolide antimicrobials, and the respective attributable risk percentages were determined.
Amino glycopeptides
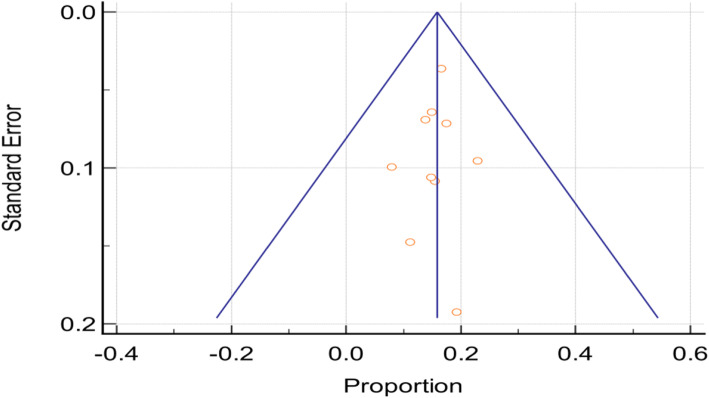
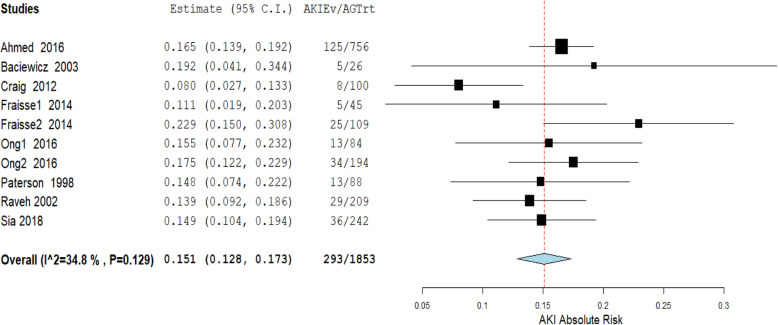
A total of 1853 participants were exposed to aminoglycosides, and 293 of them were reported to have developed antimicrobial associated AKI across the eight studies. Figure 2 below, is the funnel plot of the included studies showing a very low degree of publication bias. The random-effects model was used to meta analyse the studies to establish the overall risk of AKI among older adults using aminoglycoside antimicrobials. Figure 3 below, is the forest plot of the meta-analysis and the studies show a high degree of homogeneity (I2 = 34.8%, p = 0.129). The overall absolute risk of AKI among older adults exposed to aminoglycosides was 15.1% (95% CI: 12.8–17.3%). This was significantly higher (p < 0.0001) than the average risk of AKI among adults of 18 years and above, following aminoglycoside antimicrobial exposure (10.5%; 95% CI: 10.1–10.8%) [41]. Therefore, the attributable risk per cent of AKI among older adults exposed to aminoglycosides was 30.5% (95% CI: 6.6–54.4%).
Fig. 2.
Funnel plot for studies included in the meta-analysis of the proportion of acute kidney injury among older adults prescribed aminoglycosides
Fig. 3.
Meta-analysis of the proportion of acute kidney injury among older adults prescribed aminoglycosides. AKIEv = acute kidney injury cases. AGTrt = number exposed to aminoglycosides
Glycopeptides
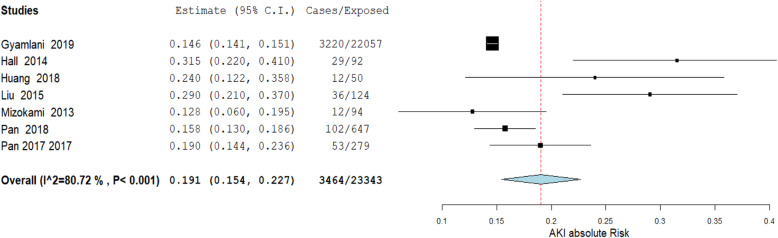
A total of 23,431 participants were included in the eight studies that reported AKI due to glycopeptide exposure among older adults. Figure 4 (additional file 5, document 1) is a funnel plot showing the distribution of the studies when the standard error was plotted against the proportion of cases, and Fig. 5 (additional file 5, document 1) shows the forest plot before removing outliers, with an absolute risk of 21.4% (95% CI: 17.1–25.7%). After performing the leave-one-out meta-analysis, the overall absolute risk reduced significantly (p = 0.017) after excluding the study by Carreno et al. [14]. As shown in Fig. 6 (additional file 5, document 1), excluding other studies did not significantly impact the overall absolute risk of AKI. Figure 7 (additional file 5, document 1) shows the funnel plot following the outlier’s exclusion, and Fig. 4 shows the forest plot for a random-effects model for the studies after removing the outlier. In this systematic review, the overall absolute risk of AKI following the use of glycopeptides is, therefore, 19.1% (95% CI: 15.4–22.7%). There is no significant difference (p = 0.117) with the established risk of AKI among adults (18 years and above) on glycopeptide antimicrobial treatment (absolute risk = 18.7%; 95% CI: 15.6–21.7%) [42].
Fig. 4.
Meta-analysis of the proportion of acute kidney injury among older adults prescribed glycopeptides after removing the outlier
Macrolides
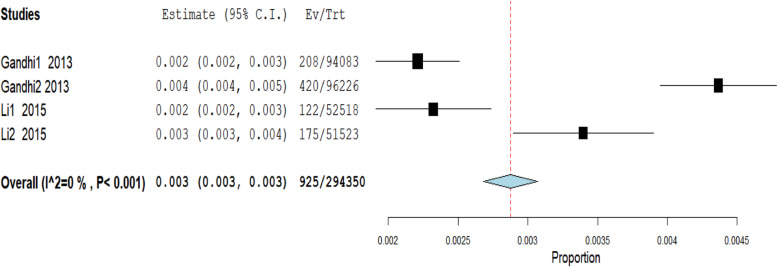
Only two studies reported on the risk of macrolide-associated AKI, but among them, a total of 294,350 participants were involved [18, 24]. Each of these two studies reported on two antimicrobials. The fixed-effects model was used to analyse these studies. Figure 5 is the forest plot summarising the combined outcome of these four studies. The overall risk of AKI among older adults exposed to macrolides was 0.3% (95% CI: 0.3–0.3%).
Fig. 5.
Meta-analysis of the proportion of acute kidney injury among older adults prescribed macrolides
Drug-induced liver injury (DILI)
Only three studies, with 572 participants, reported antimicrobial-associated DILI [13, 26, 33]. In their study, Noh et al. [26] followed up 77 outpatients who were diagnosed and being treated for latent tuberculosis infection (LTBI), of which 14.3% (=11) had raised aminotransferases (the laboratory marker used to determine DILI) due to antituberculosis antimicrobials. All the participants were 65 years or above. Similarly, Bright-Thomas et al. [13] followed up 2070 adults (18 years and above) with active tuberculosis over 30 years (1981 to 2010). A subgroup of two hundred of these patients was 70 years old or more, hence included in this systematic review. Five per cent (n = 10) of these patients had raised aminotransferases, an indication of antimicrobial associated DILI. Baghaei et al. [33] carried out a prospective cohort study on risk of drug induced hepatitis among adult patients on anti-tuberculosis treatment. A subgroup of participants aged 65 years or above (n = 295) was considered for this systematic review, of which 16.6% (n = 49) had antimicrobial associated hepatitis. All three studies agreed that antimicrobial associated DILI increased with age.
Subgroup analysis
No studies included in this systematic review investigated the relative risk or odds ratio of organ injury associated with broad-spectrum antimicrobials compared to narrow-spectrum antimicrobials prescribed to older adults. However, three studies in one article [37] and another separate study [11] compared the odds of AKI among older adults when empirically prescribed different antimicrobials for urinary tract infection (UTI) with empirical prescription of nitrofurantoin. In another four studies published in one research paper [16], the odds of AKI among older adults prescribed different antimicrobials following UTI were compared with the odds following amoxicillin prescription. Another five studies each had data on long term compared to short term treatment and an age group comparison of those between 65 and 79 years versus those 80 years and above.
Organ injury following empirical antimicrobial prescription for UTI
Ahmed et al. [37] and Ahmed et al. [11] carried out several studies to determine the odds ratios of AKI when older adults have prescribed either cephalexin, co-amoxiclav, ciprofloxacin or trimethoprim when compared to nitrofurantoin for UTI. All exposure antimicrobials, except trimethoprim, had similar odds of causing UTI to nitrofurantoin among older adults. Trimethoprim was 1.9 times more likely to be associated with AKI when compared to nitrofurantoin (95% CI: 1.5–2.5). Crellin et al. [16] also reported the odds ratios of AKI among the older adults when cefalexin, ciprofloxacin, nitrofurantoin and trimethoprim were compared to amoxicillin. The odds of AKI associated with nitrofurantoin or cefalexin was not significantly different to that of amoxicillin among the older adults (OR = 0.89; 95% CI: 0.65–1.24, and OR = 1.01, 95% CI: 0.74–1.37, respectively). Ciprofloxacin was 1.48 (95% CI: 1.03–2.13) times, and trimethoprim was 1.72 (95% CI: 1.31–2.24) times more likely to be associated with AKI among older adults when compared to amoxicillin when prescribed for UTI.
Organ injury following treatment duration
Among the five studies that included duration of treatment as part of their analysis, three of them [27, 29, 30] compared up to 7 days of treatment with aminoglycosides versus more than 7 days, while the other two studies [19, 20] compared short and long term treatments with glycopeptides. In the study by Paterson et al. [29], there was a strong association of AKI among the patients who received an aminoglycoside for more than 7 days, with a risk ratio of 7.5 (95% CI: 2.7–20.5, p = 0.0001), when compared to those treated for seven or fewer days. Contrary, in both of their studies, Ong et al. [27] and Raveh et al. [30] found no association of risk of AKI among those who received an aminoglycoside for more than 7 days, when compared to 7 or fewer days of treatment, with relative risks of 0.99 (95% CI: 0.58–1.68) and 1.35 (95% CI: 0.68–2.68) respectively. Similarly, Gyamlani et al. [19] and Hall et al. [20] did not find any association between the risk of AKI among those who received long term glycopeptide treatment compared to short term treatment.
Organ injury according to age group
Three of the five studies determined the association of AKI due to glycopeptide treatment among those at least 80 years when compared to those between 65 and 79 years [14, 20, 28]. According to Pan et al. [28], there was a significant association of AKI among those at least 80 years old and on glycopeptide therapy when compared to those between 65 and 79 years of age, with a relative risk of 2.3 (95% CI: 1.5–3.8, p = 0.0004). However, the studies by Hall et al. [20] and Carreno et al. [14] did not show any significant association of older age with AKI due to glycopeptide therapy. Similarly, both Bright-Thomas et al. [13] and Noh et al. [26] did not find any significant association of older age with DILI due to antituberculosis antimicrobials, with respective relative risks of 1.52 (95% CI: 0.44–5.19) and 1.18 (95% CI: 0.18–7.75).
Heterogeneity and publication bias
The included aminoglycoside studies were tested for heterogeneity and the Q-test was 11.59 (p = 0.2376), and I2 was 22.32% (95% CI: 0.00–62.07%). Figure 2 (above) is the funnel plot of the included studies showing a central and uniform distribution of both small and large studies around the mean, indicating a low degree of publication bias. The Egger’s regression test intercept was − 0.3564 (95% CI: − 2.341 to 1.628).
The funnel plot for studies on glycopeptide exposure (Fig. 4, additional file 5, document 1) was skewed to the right, with one study possibly an outlier. Figure 5 (additional file 5, document 1) is the forest plot before sensitivity analysis, with an absolute risk of 21.4% (95% CI: 17.1–25.7%). After performing the leave-one-out meta-analysis, the overall absolute risk reduced significantly (p = 0.017) after excluding the study by Carreno et al. (16). As shown in Fig. 6 (additional file 5, document 1), excluding other studies did not significantly impact the overall absolute risk of AKI. The Q – test was 39.08 (p < 0.0001) and the I2 test was 84.65% (95% CI: 70.15–92.10%). The funnel plot (Fig. 7, additional file 5, document 1) was skewed, and Egger’s regression test intercept was 2.2664 (p = 0.0340).
Discussion
This is the first systematic review with meta-analysis to investigate kidney, liver, and tissue injury associated with antimicrobial exposure among older adults 65 years and above. This systematic review’s primary outcome of interest was to determine the overall risk of organ injury among older adults who received antimicrobial therapy. Most of the studies included in our systematic review expressed the risk of kidney injury following exposure to aminoglycoside or glycopeptide antimicrobials.
Antimicrobial exposure and risk of organ injury
In our systematic review, older adults had a 15.1% (95% CI: 12.8–17.3%) risk of developing AKI following exposure to aminoglycosides. Several reviews and original research studies have found a wide variation of AKI risk among patients prescribed these antimicrobials. Selby et al. [43] found a 24.4% risk of AKI among hospitalised patients associated with increased mortality. Oliveira et al. [44] found the prevalence of AKI among intensive care unit patients prescribed aminoglycosides to be as high as 58% and associated with increased mortality. On the lower end, Paquette et al. [45] found the risk of AKI from aminoglycoside exposure to be 12% after excluding children, seriously ill patients, and patients treated for less than 5 days. In a large study of 8270 participants, Fuhrman et al. [46] found the incidence of AKI associated with aminoglycosides to be only 4 %. Oliveira et al. [44] argued that this huge variation in prevalence is due to varying study population characteristics. However, the majority of published literature estimates the prevalence to be 10 to 20%. In their systematic review and meta-analysis, Hayward et al. [41] found a 10.5% incidence of AKI among adults of 18 years to 95 years of age, from a total of 24,107 non-intensive care patients. Using this systematic review as our reference, we concluded that older adults are at significantly higher risk of AKI when prescribed aminoglycosides (p < 0.0001), with attributable risk from 6.6 to 54.4%.
In our systematic review, glycopeptides were associated with a 19.1% (95% CI: 15.4–22.7%) risk of AKI among older adults. Gyamlani et al. [19] noted that AKI incidence due to glycopeptide exposure varies between 5 and 43%, depending on population and baseline risk factors. In their study, Gyamlani et al. found a 10.4% incidence of AKI among patients prescribed glycopeptides. In another study, Fuhrman et al. [46] found out that 17% of adults prescribed glycopeptides developed AKI during or post-exposure. In their systematic review and meta-analysis, Sinha Ray et al. [42] found the incidence of AKI among adults 18 years and above to be 18.7% (95% CI: 15.6–21.7%). Using this study as our baseline, there was no significant difference with older adults’ findings (p = 0.117). We, therefore, concluded that older adults of 65 years and above have a similar risk of developing AKI when prescribed glycopeptides as the general adult population of 18 years and above.
Only 0.3% of AKI was associated with macrolides to older adults in our systematic review. This is a rare outcome; therefore, there are not many studies done to explore this relationship. Similarly, we only found three studies that reported antimicrobial associated DILI among older adults. The number of studies was too small to perform a meaningful meta-analysis. However, the three studies had an average of 12.3% risk of antimicrobial associated DILI among older adults. Although fewer studies report antimicrobial-associated DILI among older adults, several studies have been reported on the general adult population. In a Swedish study, 27% of DILI was attributed to antimicrobials [47]. Similar studies were also done in Spain [48], India [49] and the United States of America [50] and found 32, 65, and 45% of DILI associated with antimicrobials, respectively. Several antimicrobials have been implicated, but most commonly were amoxicillin-clavulanic acid [48, 50], erythromycin [51] and nitrofurantoin [50].
Antimicrobial exposure and risk of organ injury by the duration
In our systematic review, the three studies that reported on the association of AKI with aminoglycoside treatment duration did not agree on their findings. Paterson et al. [29] found a relative risk ratio of 7.5 when patients were prescribed aminoglycosides for more than 7 days compared to those less than 7 days. However, Raveh et al. [30] reported an increased risk of AKI when aminoglycoside treatment was prolonged to 11 days and above. However, Selby et al. [43] did not find any association between AKI and treatment duration.
Antimicrobial exposure and risk of organ injury by age
We did not find any studies that assessed the association of aminoglycoside prescription with AKI by age group. However, some authors have found an increased risk of AKI with age from the general adult population [43]. Only one out of the three studies in our systematic review showed a significant association of age with AKI following glycopeptide prescription to older adults [28]. In their systematic review, van Hal et al. [52] found out that AKI risk following glycopeptide prescription increases with age if patients are exposed for more than 7 days.
Heterogeneity and publication bias
In our systematic review, we used the random-effects model to summarise the risk of AKI among older adults prescribed aminoglycosides and glycopeptides. Several authors, for example, Ioannidis [53] and Huedo-Medina et al. [54], decide on whether to use a fixed-effect or random-effects model based on heterogeneity results. However, we chose our model before heterogeneity testing, based on recommendations by Borenstein et al. [55] and further supported by Spinelli and Pandis [56]. Our studies were drawn from across the globe and were carried out under different conditions, so we assumed they were not identical. In this instance, heterogeneity testing helped us determine whether these studies have enough in common to be meta-analysed [56]. We also used the funnel plot [7] and Egger’s regression test [8] to assess our studies’ publication bias. We meta-analysed nine studies on aminoglycosides and seven studies on glycopeptides.
Strengths of our study
The study focused on older adults aged 65 and above, who are usually underrepresented in randomised controlled trials. Real-world data, which is more representative of the older adults prescribed antimicrobials, was used. The quality of each included study was thoroughly assessed on representativeness on study participants, ascertainment of exposure of interest, absence of outcome of interest at the beginning of the study, control of possible confounders including age, gender, and severity of the disease, outcome assessment, and adequacy of follow up for outcome to occur.
Most of the included studies shared some common strengths. All the studies considered a wide range of possible confounders and adjusted for them accordingly in their analyses. Well known and validated tools were used to ascertain the presence or absence of outcomes. For example, the international classification of diseases version 10 (ICD-10) was used by authors whose study settings were primary care or population-based [11, 16, 18, 24, 37]. Most hospital-based studies used tools that relied on laboratory markers for organ injury, such as a two-fold rise in serum creatinine or doubling of liver transaminases. Some of the tools used include the acute kidney injury network (AKIN) [14, 21, 38] and kidney disease, improving global outcome (KDIGO) [27, 28, 39]. Only three studies [13, 24, 37] did not describe how they determined the absence of outcome of interest at the beginning of their studies.
Limitations of our study
Despite their strengths, the included studies in this systematic review also came with their weaknesses. All the included studies, except five [13, 16, 18, 24, 37], were hospital-based. The general major drawback of using hospitalised patients is the generalisability of the findings [57]. Most of our data were obtained from subgroups embedded in larger studies. This led to our data lacking detailed information, for example, gender differences among the older adults, since we relied heavily on limited results from tables or otherwise.
Conclusion
In conclusion, the findings from this systematic review and meta-analysis suggest that there is a significantly increased risk of AKI among older adults when compared to the general adult population following exposure to aminoglycosides. Our results indicated that the risk of AKI in older adults is 15.1% (95% CI: 12.8–17.3). The risk of AKI following exposure to glycopeptides for older adults is similar to that previously found in the general adult population. We did not get enough studies to determine whether age or duration of treatment influences the risk of AKI among older adults.
Supplementary Information
Additional file 1. Risk of antimicrobial-associated organ injury among older adults: A systematic Review and Meta-Analysis.
Additional file 2. Risk factors for nephrotoxicity in elderly patients receiving once-daily aminoglycosides.
Additional file 3. Study appraisals – Risk of Bias assessment using Newcastle-Ottawa scale.
Additional file 4: Appendix 1. Modified Newcastle-Ottawa Scale: Cohort Studies tool.
Additional file 5. Funnel plots and leave-one-meta-analysis figures.
Acknowledgements
The authors would like to thank Marylou Francoise, the acting librarian for the Faculty of Science at University of Bath, for her immense help in training and supporting article search strategies from various databases.
Abbreviations
- ACEI
Angiotensin converting enzyme Inhibitor
- AKI
Acute kidney injury
- AKIN
Acute Kidney Injury Network
- ARB
Angiotensin II receptor blocker
- CKD-EPI
Chronic kidney Disease – Epidemiology collaborator
- DILI
Drug-induced liver injury
- DILI
Drug induced liver injury
- ICD-10
International Classification of Diseases version 10
- ICU
Intensive care unit; BMI- Body-Mass index
- KDIGO
Kidney Disease: Improving Global Outcome
- LTBI
Latent tuberculosis infection
- NKF
National Kidney Foundation
- CVD
Cardiovarscular disease
- NSAID
Nonsteroidal anti-inflammatory drug
- UTI
Urinary tract infection
Authors’ contributions
TC is the first author of the systematic review and meta-analysis paper. He led all the stages of the development and writing of this script. At the same time, PN and SR supervised and contributed to developing plans for searching, screening, extracting, and writing phases. All authors have read and approved the manuscript.
Funding
No funding was received for this systematic review and meta-analysis..
Availability of data and materials
All data generated or analysed during this study are included in this published article (and its supplementary information files).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tichawona Chinzowu, Email: tc888@bath.ac.uk.
Sandipan Roy, Email: sr2081@bath.ac.uk.
Prasad S. Nishtala, Email: pn403@bath.ac.uk
References
- 1.Augustine S, Bonomo RA. Taking stock of infections and antibiotic resistance in the elderly and long-term care facilities: a survey of existing and upcoming challenges. Eur J Microbiol Immunol (Bp) 2011;1(3):190–197. doi: 10.1556/EuJMI.1.2011.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esme M, Topeli A, Yavuz BB, Akova M. Infections in the Elderly Critically-Ill Patients. Front Med (Lausanne) 2019;6:118. doi: 10.3389/fmed.2019.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giarratano A, Green SE, Nicolau DP. Review of antimicrobial use and considerations in the elderly population. Clin Interv Aging. 2018;13:657–667. doi: 10.2147/CIA.S133640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin K, Bégaud B, Latry P, Miremont-Salamé G, Fourrier A, Moore N. Differences between clinical trials and postmarketing use. Br J Clin Pharmacol. 2004;57(1):86–92. doi: 10.1046/j.1365-2125.2003.01953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wells GA SB, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [cited 2020 01 October]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 6.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–1055. doi: 10.1016/S0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 8.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 10.Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end-users: R as a computational Back-end. J Stat Softw. 2012;49(5). 10.18637/jss.v049.i05.
- 11.Ahmed H, Farewell D, Francis NA, Paranjothy S, Butler CC. Risk of adverse outcomes following urinary tract infection in older people with renal impairment: retrospective cohort study using linked health record data. PLoS Med. 2018;15(9):e1002652. doi: 10.1371/journal.pmed.1002652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baciewicz AM, Sokos DR, Cowan RI. Aminoglycoside-associated nephrotoxicity in the elderly. Ann Pharmacother. 2003;37(2):182–186. doi: 10.1177/106002800303700203. [DOI] [PubMed] [Google Scholar]
- 13.Bright-Thomas RJ, Gondker AR, Morris J, Ormerod LP. Drug-related hepatitis in patients treated with standard anti-tuberculosis chemotherapy over a 30-year period. Int J Tuberc Lung Dis. 2016;20(12):1621–1624. doi: 10.5588/ijtld.16.0370. [DOI] [PubMed] [Google Scholar]
- 14.Carreno JJ, Jaworski A, Kenney RM, Davis SL. Comparative incidence of nephrotoxicity by age group among adult patients receiving Vancomycin. Infect Dis Ther. 2013;2(2):201–208. doi: 10.1007/s40121-013-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Craig P, Starks I, Bancroft G, Roberts P. Is prophylactic gentamicin associated with acute kidney injury in patients undergoing surgery for fractured neck of femur? Injury. 2012;43(12):2152–2155. doi: 10.1016/j.injury.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Crellin E, Mansfield KE, Leyrat C, Nitsch D, Douglas IJ, Root A, Williamson E, Smeeth L, Tomlinson LA. Trimethoprim use for urinary tract infection and risk of adverse outcomes in older patients: cohort study. BMJ. 2018;360:k341. doi: 10.1136/bmj.k341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraisse T, Gras Aygon C, Paccalin M, Vitrat V, De Wazieres B, Baudoux V, et al. Aminoglycosides use in patients over 75 years old. Age Ageing. 2014;43(5):676–681. doi: 10.1093/ageing/afu023. [DOI] [PubMed] [Google Scholar]
- 18.Gandhi S, Fleet JL, Bailey DG, McArthur E, Wald R, Rehman F, Garg AX. Calcium-channel blocker-clarithromycin drug interactions and acute kidney injury. JAMA. 2013;310(23):2544–2553. doi: 10.1001/jama.2013.282426. [DOI] [PubMed] [Google Scholar]
- 19.Gyamlani G, Potukuchi PK, Thomas F, Akbilgic O, Soohoo M, Streja E, Naseer A, Sumida K, Molnar MZ, Kalantar-Zadeh K, Kovesdy CP. Vancomycin-associated acute kidney injury in a large veteran population. Am J Nephrol. 2019;49(2):133–142. doi: 10.1159/000496484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall RG, Blaszczyk AT, Thompson KA, Brouse SD, Giuliano CA, Frei CR, et al. Impact of empiric weight-based vancomycin dosing on nephrotoxicity and mortality in geriatric patients with methicillin-resistant Staphylococcus aureus bacteraemia. J Clin Pharm Ther. 2014;39(6):653–657. doi: 10.1111/jcpt.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang M, Wu H, Zhou J, Xu M, Zhou S. Efficacy of Vancomycin on gram-positive bacterial infection in elderly critical patients and risk factors associated with nephrotoxicity. Arch Iran Med. 2018;21(8):349–355. [PubMed] [Google Scholar]
- 22.Karino F, Nishimura N, Ishihara N, Moriyama H, Miura K, Hamaguchi S, Sutani A, Kuraki T, Ikawa K, Morikawa N, Naora K, Isobe T. Nephrotoxicity induced by piperacillin–tazobactam in late elderly Japanese patients with nursing and healthcare associated pneumonia. Biol Pharm Bull. 2014;37(12):1971–1976. doi: 10.1248/bpb.b14-00362. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Yin Y, Liu XZ, Yao HJ, Li LX, Chen JH, Chen T, Lu XT, Bu SH, Zhang J. Retrospective analysis of vancomycin nephrotoxicity in elderly Chinese patients. Pharmacology. 2015;95(5–6):279–284. doi: 10.1159/000381783. [DOI] [PubMed] [Google Scholar]
- 24.Li DQ, Kim R, McArthur E, Fleet JL, Bailey DG, Juurlink D, Shariff SZ, Gomes T, Mamdani M, Gandhi S, Dixon S, Garg AX. Risk of adverse events among older adults following co-prescription of clarithromycin and statins not metabolized by cytochrome P450 3A4. CMAJ. 2015;187(3):174–180. doi: 10.1503/cmaj.140950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mizokami F, Shibasaki M, Yoshizue Y, Noro T, Mizuno T, Furuta K. Pharmacodynamics of vancomycin in elderly patients aged 75 years or older with methicillin-resistant Staphylococcus aureus hospital-acquired pneumonia. Clin Interv Aging. 2013;8:1015–1021. doi: 10.2147/CIA.S50238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noh CS, Kim HI, Choi H, Kim Y, Kim CH, Choi JH, Hyun IG, Baek MS. Completion rate of latent tuberculosis infection treatment in patients aged 65 years and older. Respir Med. 2019;157:52–58. doi: 10.1016/j.rmed.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Ong LZ, Tambyah PA, Lum LH, Low ZJ, Cheng I, Murali TM, Wan MQ, Chua HR. Aminoglycoside-associated acute kidney injury in elderly patients with and without shock. J Antimicrob Chemother. 2016;71(11):3250–3257. doi: 10.1093/jac/dkw296. [DOI] [PubMed] [Google Scholar]
- 28.Pan K, Ma L, Xiang Q, Li X, Li H, Zhou Y, Yang L, Cui Y. Vancomycin-associated acute kidney injury: a cross-sectional study from a single center in China. PLoS One. 2017;12(4):e0175688. doi: 10.1371/journal.pone.0175688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paterson DL, Robson JM, Wagener MM. Risk factors for toxicity in elderly patients given aminoglycosides once daily. J Gen Intern Med. 1998;13(11):735–739. doi: 10.1046/j.1525-1497.1998.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raveh D, Kopyt M, Hite Y, Rudensky B, Sonnenblick M, Yinnon AM. Risk factors for nephrotoxicity in elderly patients receiving once-daily aminoglycosides. QJM. 2002;95(5):291–297. doi: 10.1093/qjmed/95.5.291. [DOI] [PubMed] [Google Scholar]
- 31.Aggarwal R, Dewan A. Comparison of nephrotoxicity of Colistin with Polymyxin B administered in currently recommended doses: a prospective study. Ann Clin Microbiol Antimicrob. 2018;17(1):15. doi: 10.1186/s12941-018-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morimoto T, Nagashima H, Morimoto Y, Tokuyama S. Frequency of acute kidney injury caused by Tazobactam/Piperacillin in patients with pneumonia and chronic kidney disease: a retrospective observational study. Yakugaku Zasshi. 2017;137(9):1129–1136. doi: 10.1248/yakushi.17-00002. [DOI] [PubMed] [Google Scholar]
- 33.Baghaei P, Tabarsi P, Chitsaz E, Saleh M, Marjani M, Shemirani S, Pooramiri MV, Kazempour M, Farnia P, Fahimi F, Mansouri D, Masjedi M. Incidence, clinical and epidemiological risk factors, and outcome of drug-induced hepatitis due to antituberculous agents in new tuberculosis cases. Am J Ther. 2010;17(1):17–22. doi: 10.1097/MJT.0b013e31818f9eae. [DOI] [PubMed] [Google Scholar]
- 34.Giri VP, Giri OP, Bajracharya S, Khan FA, Sinha SP, Kanodia S, Bansal C. Risk of acute kidney injury with amikacin versus gentamycin both in combination with metronidazole for surgical prophylaxis. J Clin Diagn Res. 2016;10(1):FC09–FC12. doi: 10.7860/JCDR/2016/15621.7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sia CS, Ananda-Rajah MR, Adler NR, Yi-Wei B, Liew D, Tong EY, et al. Renal safety of short-term empiric gentamicin therapy in aged patients. Australas J Ageing. 2018;37(3):227–231. doi: 10.1111/ajag.12541. [DOI] [PubMed] [Google Scholar]
- 36.Baik S, Lau J, Huser V, McDonald CJ. Association between tendon ruptures and use of fluoroquinolone, and other oral antibiotics: A 10-year retrospective study of 1 million US senior Medicare beneficiaries. BMJ Open. 2020;10(12):e034844. doi: 10.1136/bmjopen-2019-034844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed H, Farewell D, Francis NA, Paranjothy S, Butler CC. Choice of Empirical Antibiotic Therapy and Adverse Outcomes in Older Adults With Suspected Urinary Tract Infection: Cohort Study. Open Forum Infect Dis. 2019;6(3):ofz039. doi: 10.1093/ofid/ofz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmed I, Khan MA, Allgar V, Mohsen A. The effectiveness and safety of two prophylactic antibiotic regimes in hip-fracture surgery. Eur J Orthop Surg Traumatol. 2016;26(5):483–492. doi: 10.1007/s00590-016-1794-7. [DOI] [PubMed] [Google Scholar]
- 39.Pan KM, Wu Y, Chen C, Chen ZZ, Xu JA, Cao L, Xu Q, Wu W, Dai PF, Li XY, Lv QZ. Vancomycin-induced acute kidney injury in elderly Chinese patients: a single-Centre cross-sectional study. Br J Clin Pharmacol. 2018;84(8):1706–1718. doi: 10.1111/bcp.13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moskalewicz A, Oremus M. No clear choice between Newcastle-Ottawa scale and appraisal tool for cross-sectional studies to assess methodological quality in cross-sectional studies of health-related quality of life and breast cancer. J Clin Epidemiol. 2020;120:94–103. doi: 10.1016/j.jclinepi.2019.12.013. [DOI] [PubMed] [Google Scholar]
- 41.Hayward RS, Harding J, Molloy R, Land L, Longcroft-Neal K, Moore D, Ross JDC. Adverse effects of a single dose of gentamicin in adults: a systematic review. Br J Clin Pharmacol. 2018;84(2):223–238. doi: 10.1111/bcp.13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinha Ray A, Haikal A, Hammoud KA, Yu AS. Vancomycin and the risk of AKI: a systematic review and Meta-analysis. Clin J Am Soc Nephrol. 2016;11(12):2132–2140. doi: 10.2215/CJN.05920616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selby NM, Shaw S, Woodier N, Fluck RJ, Kolhe NV. Gentamicin-associated acute kidney injury. Qjm. 2009;102(12):873–880. doi: 10.1093/qjmed/hcp143. [DOI] [PubMed] [Google Scholar]
- 44.Oliveira JF, Silva CA, Barbieri CD, Oliveira GM, Zanetta DM, Burdmann EA. Prevalence and risk factors for aminoglycoside nephrotoxicity in intensive care units. Antimicrob Agents Chemother. 2009;53(7):2887–2891. doi: 10.1128/AAC.01430-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paquette F, Bernier-Jean A, Brunette V, Ammann H, Lavergne V, Pichette V, Troyanov S, Bouchard J. Acute kidney injury and renal recovery with the use of aminoglycosides: a large retrospective study. Nephron. 2015;131(3):153–160. doi: 10.1159/000440867. [DOI] [PubMed] [Google Scholar]
- 46.Fuhrman DY, Kane-Gill S, Goldstein SL, Priyanka P, Kellum JA. Acute kidney injury epidemiology, risk factors, and outcomes in critically ill patients 16-25 years of age treated in an adult intensive care unit. Ann Intensive Care. 2018;8(1):26. doi: 10.1186/s13613-018-0373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Björnsson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology. 2005;42(2):481–489. doi: 10.1002/hep.20800. [DOI] [PubMed] [Google Scholar]
- 48.Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, García-Ruiz E, García-Muñoz B, González-Grande R, Pizarro A, Durán JA, Jiménez M, Rodrigo L, Romero-Gomez M, Navarro JM, Planas R, Costa J, Borras A, Soler A, Salmerón J, Martin-Vivaldi R, Spanish Group for the Study of Drug-Induced Liver Disease Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129(2):512–521. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 49.Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105(11):2396–2404. doi: 10.1038/ajg.2010.287. [DOI] [PubMed] [Google Scholar]
- 50.Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, et al. Features and Outcomes of 899 Patients With Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology. 2015;148(7):1340–52.e7. doi: 10.1053/j.gastro.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Björnsson ES, Hoofnagle JH. Categorization of drugs implicated in causing liver injury: critical assessment based on published case reports. Hepatology. 2016;63(2):590–603. doi: 10.1002/hep.28323. [DOI] [PubMed] [Google Scholar]
- 52.van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57(2):734–744. doi: 10.1128/AAC.01568-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ioannidis JP. Interpretation of tests of heterogeneity and bias in meta-analysis. J Eval Clin Pract. 2008;14(5):951–957. doi: 10.1111/j.1365-2753.2008.00986.x. [DOI] [PubMed] [Google Scholar]
- 54.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11(2):193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 55.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 56.Spineli LM, Pandis N. Meta-analysis: random-effects model. Am J Orthod Dentofac Orthop. 2020;157(2):280–282. doi: 10.1016/j.ajodo.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 57.Kukull WA, Ganguli M. Generalizability: the trees, the forest, and the low-hanging fruit. Neurology. 2012;78(23):1886–1891. doi: 10.1212/WNL.0b013e318258f812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Risk of antimicrobial-associated organ injury among older adults: A systematic Review and Meta-Analysis.
Additional file 2. Risk factors for nephrotoxicity in elderly patients receiving once-daily aminoglycosides.
Additional file 3. Study appraisals – Risk of Bias assessment using Newcastle-Ottawa scale.
Additional file 4: Appendix 1. Modified Newcastle-Ottawa Scale: Cohort Studies tool.
Additional file 5. Funnel plots and leave-one-meta-analysis figures.
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its supplementary information files).