Abstract
Participant recruitment for pediatric palliative intervention studies is a chronic challenge for researchers. Digital recruitment strategies, or digital technology-assisted recruitment methods used to remotely reach and enroll research subjects, can help address these recruitment challenges for pediatric palliative care clinical trials. This study (a) describes Facebook recruitment procedures targeting children with cancer and their parents for a pediatric palliative intervention randomized clinical trial, (b) reports recruitment results, and (c) discusses successful strategies to recruit pediatric populations via Facebook advertisements. Researchers used Facebook advertisements to recruit children with advanced cancer (aged 7 to 17 years) for a web-based legacy intervention. Between years 2015 and 2018, our research team enrolled 150 child–parent dyads (N= 300) to participate in the web-based legacy program. Results suggest that Facebook advertisements can be a successful tool to access and recruit pediatric populations with life-threatening conditions. Further research is needed to determine how innovative social-media recruitment strategies could be used in other populations of patients with serious illnesses and their caregivers to further advance the science in palliative care.
Keywords: digital recruitment, Facebook, pediatric palliative care, childhood cancer
Introduction
Recruitment barriers create significant challenges for pediatric palliative care researchers. Poor recruitment and retention rates can lead to a waste of research resources and poor-quality results that cannot be generalized to other patient groups [1–4]. This challenge may be caused in part by the limited number of potential subjects, families’ needs and preferences about study participation including a reluctance to have time away from their very ill child, lack of communication opportunities during the consent process, and professional gatekeeping [4–7]. Many pediatric palliative researchers use multisite studies to achieve adequate sample sizes [8] or limited numbers of participants due to small pools of eligible children with serious health conditions. Potential participants often have limited opportunities to clarify their understanding of the study during the consent process, which can lead to poor recruitment and retention rates [9]. Recruitment through clinical gatekeepers also poses additional challenges, even though it is the most common recruitment method for pediatric palliative clinical trials. As many clinical studies require clinicians’ involvement in recruiting potential subjects, the clinicians’ positive perceptions, dedication, and thorough understanding of the study can affect the potential participants’ decisions to join pediatric clinical trials [10,11]. Recruiting only participants who receive and accept the clinicians’ invitation can lead to selection bias [5]. Thus, finding a direct way to reach a broader pediatric population that may be interested in participating in such palliative clinical trials and would provide accurate study information is essential for a successful research process.
Digital recruitment, a digital technology-assisted recruitment method used to remotely reach and enroll research subjects [12], is one strategy that may help address recruitment challenges for pediatric palliative care clinical trials. Digital recruitment allows investigators to reach a large pool of eligible participants, introduce their studies, monitor the recruitment process, and complete enrollment remotely, using diverse digital tools [12]. In the past decade, various digital recruitment strategies have begun to emerge, while traditional recruitment methods have revealed their limitations with low participation rates and poor efficiency [2]. Digital tools such as media and online advertisements, internet sites and forums, email, mobile applications, and social media have particularly been used to guide potential participants to locate trials for which they may be eligible and help investigators to reach them [1]. Specifically, social media can easily expose potential participants to study information via computers and mobile devices [3].
Among many social media platforms, Facebook can serve as a useful recruitment tool for researchers by providing customized paid advertisements and exposure rate check metrics [3]. Facebook is one of the largest global social networks and has 2.5 billion monthly active users, thereby offering researchers a platform to reach a large number of potential participants [13]. By using Facebook’s micro-targeting algorithm, researchers can create various types of advertisements tailored to specific study objectives and targets [13]. Researchers can easily expose advertisements to potential subjects based on their personal characteristics, and due to the ubiquitous nature of Facebook, a target population can easily check these advertisements via their electronic devices [3]. Facebook recruitment has been successfully applied in studies for hard-to-reach populations, such as vaccine-hesitant parents [14], low-income women [15], immigrant health care providers [16], and kidney transplant recipients [17], and is less expensive and time consuming than other recruitment methods [18].
Few palliative care studies have used Facebook recruitment, especially for children with serious conditions and their family members. While this population has unique characteristics and complex information needs, most palliative studies require planned discussion about sensitive issues for children and their families with a delicate and well-tailored recruitment approach. Despite their impact on research, recruitment strategies have been under-reported in many pediatric palliative studies [19,20]. In particular, gaps exist for studies reporting results of Facebook recruitment methods in pediatric palliative care randomized controlled trials (RCTs). Thus, the aims of this study were to (a) describe a Facebook recruitment procedure targeting children with cancer and their parents for a RCT examining a legacy intervention, (b) report recruitment results, and (c) discuss successful strategies to recruit pediatric populations via Facebook advertisements.
Design and Methods
Facebook advertisements were used to recruit children (aged 7 to 17 years) with advanced cancer to receive a web-based legacy intervention. Legacy-making refers to actions or behaviors aimed at remembering the life of an individual to positively influence the ill person and their family [21–23]. The web-based intervention guided children with advanced cancer and their parents to create a digital story for the family to keep and be shared with family and friends, if desired [21]. In the following section, we describe the details of the Facebook recruitment method based on the four recruitment plan components—planning, initiating, conducting, and reporting [3]. Other results related to the web-based legacy intervention have been reported elsewhere [24–28].
Planning
We have previously used Facebook recruitment methods to enroll children with cancer and their parents [8,29]. These preliminary studies guided our methods for Facebook advertising, such as using a survey link in the Facebook advertisement, interest terms, advertisement wording for target audiences, and a flexible advertising approach [8].
Initiating
The principal investigator (PI) and study team created a study Facebook page and customized advertisements. Using the Facebook paid function, the study team designed a pool of advertisements, each including a brief study description, photograph, and survey/web link. As our study targeted children with cancer, we carefully developed the study descriptions and pictures with language sensitive to children’s treatment goals. Thus, we did not use the term ‘legacy’ or ‘legacy-making’ in study documents with children or parents, as this could have unintentionally implied that death was imminent. Instead, the project was described as a ‘digital-storytelling project’ for children with cancer. This same terminology was used and well-received by participants and staff in our pilot study [22]. We selected royalty-free photographs that would engage the target population. The study was approved by the university institutional review board (IRB; #140622). An IRB-approved study description was created and posted on the study Facebook page. Due to the nature of Facebook recruitment methods, the IRB waived the need for written consent.
The research team members determined advertisement goals and interest terms to reach the target audience. Among several Facebook advertisement cost models (e.g., cost-per-thousand-impressions, cost-per-like), we selected a pay-per-check option as our goal was for large numbers of interested individuals to click the link and complete the screening survey. As the average cost per click depends on characteristics of advertisements and the target audience, we carefully chose advertisement components, target groups, and designated a maximum budget. Legally, individuals must be at least 13 years of age to have a Facebook account. Thus, we aimed to reach parents via Facebook advertisements and then access the children with advanced cancer via their parents. Eligibility criteria included parents who were (a) aged 18 years or older, (b) living in the United States, (c) of any sex, and (d) interested in pediatric oncology (e.g., expressed interest in or liked other Facebook pages related to childhood cancer). Once we created the advertisements and chose the target audience details we wished to reach, including demographics, interests, and behaviors, the Facebook system provided estimated daily results (e.g., people reached, link clicks) per designated budget.
Conducting
The advertisements were exhibited on Facebook for three years (2015–2018). The PI regularly monitored the posted study advertisements and their performances and revised low-performing advertisements by changing the pictures or wording. Some advertisements included compensation information. In addition, each parent was eligible for a monthly $25 Amazon gift card draw if they completed the screening survey and provided their contact information. Two different types of advertising campaigns were conducted. In one type, once users clicked the link in the advertisements, they were taken to a REDCap screening survey that contained brief study descriptions and screening questions. If the parents were identified as eligible participants, the survey asked parents to provide their names and contact information at the end of this survey. Alternatively, users who clicked the link were guided to a research webpage and had to enter their contact information if they were interested. Either way, once they submitted their contact information, the coordinator contacted them via phone or email within one week to provide detailed study information and confirm their eligibility. As will be described later, the latter type of campaign was excluded in the early phase of the study because of its ineffectiveness.
In the last six months of enrollment, we used additional recruitment strategies to successfully achieve our target sample size. Guided by a paid digital advertising agency, we developed and launched a recruitment video campaign for Facebook and evaluated target audiences. Ten monthly postings about childhood cancer were posted on our study Facebook page to increase the study’s credibility and create emotional connections with potential participants. To check the comments or questions left by participants and to answer them promptly, the PI/coordinator monitored the study Facebook page and the performance of the advertisements daily.
Reporting
To measure the impact of our advertisements and participants’ responses, we used the metrics built into Facebook, such as the number of impressions (i.e., the number of times the study’s advertisement appeared on users’ screens) and the number of link clicks (i.e., the number of times that users clicked the advertisement). These data were automatically documented and provided to us by Facebook.
Results
Participants responses and characteristics
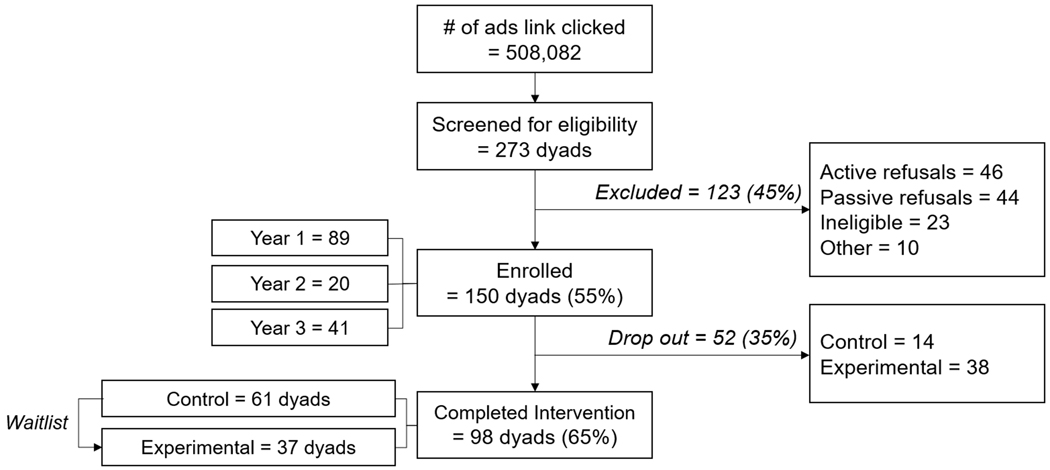
Our Facebook campaign resulted in 508,082 total link clicks to our screening survey. Table 1 summarizes the recruitment responses for each year. Among the 273 parents of children with cancer who were screened for eligibility, 150 child–parent dyads (55%) enrolled in the study to participate in the web-based legacy program (Figure 1).
Table 1.
Facebook Recruitment Metrics
| Year 1 | Year 2 | Year 3* | |
|---|---|---|---|
| Total impressions1 | 27,090,424 | 7,749,048 | 7,134,631 |
| Total reached2 | 4,831,838 | 2,456,647 | 1,619,796 |
| Unique clicks3 | 302,005 | 177,101 | 28,976 |
| Cost per result 4 | $0.30 | $0.17 | $0.69 |
| Number of recruited subjects | 89 | 20 | 41 |
Year 3 ad campaign managed by external company
Number of times ads on screen
Number of people who saw ads at least once
Unique link clicks
Average cost per link click
Figure 1.
Recruitment Results
Advertisement Campaign types
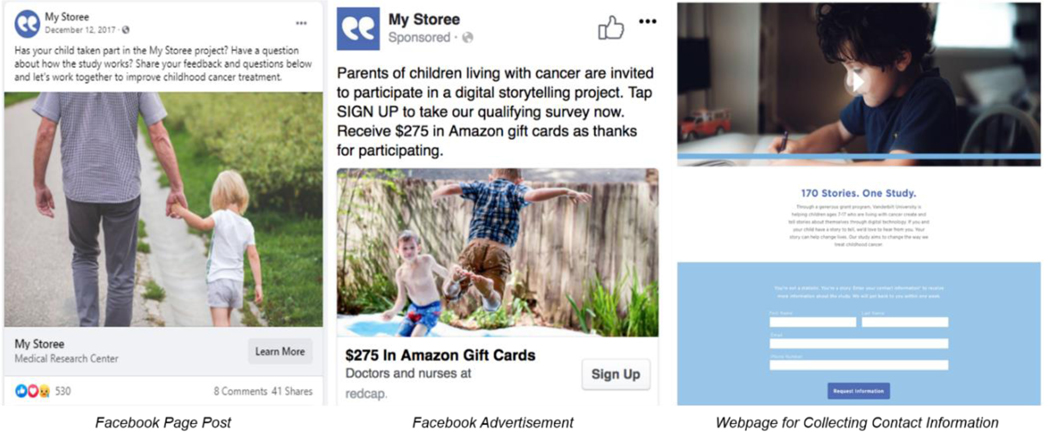
Our original advertisement campaign consisted of images, textual study information, and a link to a REDCap survey or a study webpage. The advertisements were distributed throughout the first two and a half years of enrollment. We recruited 89 participants in the first year of enrollment, and 20 participants in the second year. Due to the declining recruitment rate, we hired a professional agency to modify, manage and host the Facebook advertisement campaign during the third year of enrollment by adding a recruitment video, and regularly posting to our Facebook study page to be more engaged and connected with potential participants (Figure 2).
Figure 2.
Recruitment Materials
This modification led us to recruit 41 more participants during the final six months of enrollment in Year 3. The recruitment rate increased from 2 to 6 subjects per month and our study Facebook page had 3,895 followers by the end of the campaign. Link click advertisement campaigns yielded the majority (n=24,269 unique clicks; 84%) of clicks to the REDCap screening survey. In this case, participants were asked to complete a brief background information questionnaire and then enter their contact information. On the other hand, driving potential participants to a splash page (n=4,098 unique clicks; 14%) or to a website (n=83 unique clicks; <1%) to enter their contact information first were costly and produced limited enrollments. Due to their ineffectiveness, the latter types of advertising campaigns were promptly withdrawn from the study.
Costs
We spent a total of $120,000 purchasing Facebook advertisements in Years 1 and 2 plus additional money paid to the external company that managed the campaign in Year 3 of recruitment. Average cost per click ranged from $0.30 in Year 1, $0.17 in Year 2, and $0.69 in Year 3 (Table 1). Our Facebook advertising expenses were substantially higher than previous studies (e.g., $1130 [29]; $537 [8]) due to the large sample size and inclusion criteria targeting a very specific and hard-to-reach population. However, costs remained substantially lower when compared to expenses related to use of multiple data-collections sites and personnel salaries that would have been necessary to recruit the same sample size using traditional in-person enrollment methods. Thus, target population characteristics and sample size are driving factors in costs associated with Facebook advertising recruitment methods.
Communication
During the advertising period, users communicated with the research team by commenting on the advertisements or posts on our Facebook study page. The most frequent comments were emotional responses aimed at the target population, such as ‘God bless the children.’ Some users raised questions related to their child’s eligibility to participate in the study. For example, one user asked why their four-year-old child could not participate, perceiving that this age group would benefit from the intervention used in our study. We clarified that the intervention was developmentally appropriate for our targeted age group and responded that we hoped to expand to additional age groups in future studies.
Discussion
Facebook recruitment strategies could be successfully applied to web-based pediatric palliative intervention RCTs targeting children with advanced cancer and their parents. Traditionally, participant recruitment is one of the biggest challenges in pediatric palliative research [20]. Social media recruitment has proved to be an excellent countermeasure to overcome this challenge with lower costs, shorter recruitment times, and a greater reach to hard-to-access populations [18,30]. These advantages have also been demonstrated in our study by enabling effective geographically diverse participant recruitment and a sufficient sample size. Also, we reported each step of our recruitment process with enrollment results to provide general guidelines for implementing Facebook recruitment strategies for future pediatric palliative studies.
We used multiple strategies to maximize the benefits of digital recruitment methods. Advertisement language and photographs were carefully evaluated and selected through multiple team meetings and preliminary tests due to the sensitive nature of palliative clinical trials for our target population. Engagement strategies included adding a video and regular posts to the study Facebook page to increase our presence and emotional connection with potential participants. Users could actively learn about the study and easily communicate with the research team through our study Facebook advertisements and research page. Potential subjects could be fully informed about the study and carefully consider the benefits and risks of participating. Moreover, we were able to successfully recruit children with advanced cancer by targeting their parents since many adults have Facebook accounts. We frequently checked the advertisements’ performance and made modifications when necessary for low performing ads. Having potential participants first answer a brief background information questionnaire and then provide their contact information for future contact was more effective for recruitment, than first collecting their names and phone numbers/email addresses. Asking non-sensitive screening and demographic questions first and then collecting more personal information may reduce any psychological barriers and build a sense of trust between the researchers and potential participants. As a result of this effort, 55% of users who were screened for eligibility were enrolled in the study (n=150 dyads), and 98 dyads (65%) were included in the analysis sample for primary study outcomes [21]. This was an encouraging result compared with the retention rate of other intervention studies that involved patient-support person dyads, which have ranged from 36 to 100% [31,32]. We will continue to investigate effective strategies to improve recruitment and retention rates through Facebook recruitment methods.
Despite the promising findings, demographic factors of the target population may impact success of Facebook recruitment [33]. A review of 30 medical studies using social media recruitment strategies provided evidence that only 12 out of 30 studies showed better recruitment outcomes than those that used traditional recruitment methods [34]. Our Facebook campaign recruited parents who were mostly female, white, non-Hispanic, living in the middle-west region, and college-level educated [21]. This finding is consistent with the study by Whitaker et al. [18] who found that Facebook recruitment methods attracted more responses from young, white, females. For optimal results, researchers should clearly identify the characteristics of their target populations and determine whether Facebook recruitment is the best strategy to successfully complete study aims. In some research situations, such as seeking an ethically diverse sample, supplemental strategies could be implemented given the recognition of access to internet surveys may be overrepresented by white middle class females. Researchers can pilot various advertisements for a short period of time and select the most effective advertisements tailored to the specific needs of their target audiences.
While Facebook recruitment has been identified as an effective research strategy, ethical considerations, such as breach of privacy and confidentiality, recruitment bias, and data security, have been raised [3,34,35]. The Facebook system can reveal users’ private information that has not been disclosed to other users by displaying comments and tags on the advertisements or by leaving an unconscious online trail [35,36]. To promote an ethical recruitment process and prevent any unintentional harm to potential participants, researchers should be fully informed of the latest Facebook policies, design the best suited recruitment modality, and discuss wordings in the advertisements with the pertinent IRBs at the early stages of their research [35]. In addition, researchers should clearly understand how social media data is presented, carefully review social media research policies and guidelines such as Internet Research Ethics 3.0 [37] and hold frequent discussions with the IRB to provide maximum privacy protection for their participants [35,36]. Future studies may need to devise ethical guidelines for other digital recruitment methods (e.g., MyChart), specifically tailored to pediatric palliative clinical trials that use digital recruitment tools. Despite the benefits, Facebook recruitment has several limitations. As this method can be applied only to individuals with access to technology and internet, there is a possibility of selection bias [36]. As Facebook recruitment relies on self-report, this method has a potential risk of inaccurate demographics or medical information [17]. Even though selection bias or misrepresentation can also occur using traditional recruitment methods [17,18], researchers should be mindful of the potential impact on internal validity when interpreting the study results and using strategies to prevent these problems. Finally, Facebook advertisements can have a wide range of cost-effectiveness depending on the circumstances and target population. The average cost per click can vary based on multiple factors (e.g., target sex, location, interest). Researchers should therefore carefully budget and consider including a consultant who has experience using Facebook advertisements to assist in determining if and how Facebook could be used to aid in recruitment strategies for their studies. Finally, researchers should keep up with the ever-changing Facebook algorithms and the latest technology to use this method most effectively and accurately. With all of this in mind, researchers need to fully understand these limitations when conducting research, collecting data, and interpreting their findings.
Future studies can consider multiple types of digital recruitment strategies in other potential social media platforms (e.g., Instagram, Snapchat) to reach more target audiences and deliver more detailed study information. Since the ages of individuals using various social media platforms are different [38], further research is needed to examine the effectiveness of various social medial platforms for palliative care research recruitment efforts. Various customized advertising formats, such as slideshows, augmented reality, and playable advertisements can provide more thorough information about a study and help inform potential participants’ decision to participate. Finally, more research is needed to develop specific strategies to target more diverse participants (e.g., male, Hispanic, non-Caucasian). Facebook recruitment may reduce the potential burden on participants and increase the understanding of study activities that include sensitive topics such as palliative care clinical trials. Ultimately, innovative social media recruitment can be an effective tool to recruit vulnerable populations to further advance the science to improve care to patients with serious conditions and their family members.
Acknowledgements
We thank the children and their parents who generously participated in this study. We also thank Drs. Cynthia A. Gerhardt and Barbara Given (study consultants) for providing guidance on the recruitment approach. We acknowledge Paramore Digital for assisting in Facebook recruitment.
Funding details.
This work was supported by the National Institute of Nursing Research (grant RO1 NR015353; ClinicalTrials.gov number NCT04059393; principal investigator: T.F.A.) and NIH/National Center for Advancing Translational Sciences (grant UL1 TR000445). Additionally, this project was conducted in collaboration with the Palliative Care Research Cooperative Group funded by the National Institute of Nursing Research (grant U24 NR014637).
Biography
Eunji Cho, PhD, RN is a postdoctoral fellow at the Vanderbilt University School of Nursing. She received a doctoral degree in nursing from Duke University. Her research interest is exploring the concept of human flourishing in children, adolescents, and young adults with life-threatening conditions and developing psychosocial interventions for this population.
Mary Jo Gilmer, PhD, MBA, RN-BC, FAAN is a professor of nursing at Vanderbilt University School of Nursing and a professor of medicine (pediatrics) at the Monroe Carell Jr. Children’s Hospital at Vanderbilt. Her research interests focus on ways to reduce suffering in children with cancer through interventions such as animal-assisted interactions and promotion of parent-child communication.
Debra L. Friedman, MD, MS is the E. Bronson Ingram chair in pediatric oncology and associate professor of pediatrics; leader, Vanderbilt-Ingram Cancer Center Cancer Health Outcomes; and control program director, Division of Pediatric Hematology-Oncology Vanderbilt University Medical Center and Vanderbilt-Ingram Cancer Center, Nashville, Tennessee.
Verna Hendricks-Ferguson, PhD, RN, FPCN, FAAN is the Irene Riddle Endowed Chair at Saint Louis University’s Trudy Busch Valentine School of Nursing, St. Louis, Missouri. Her program of research includes: (a) evaluation of interventions focused on offering earlier discussions by pediatric oncology providers to parents of children and adolescents with a poor prognosis cancer about the benefits of palliative and end-of-life care support and (b) evaluation of psychosocial focused palliative care interventions (i.e., music, art, and mindfulness) for pediatric and adult patients.
Pamela S. Hinds, PhD, RN, FAAN is The William and Joanne Conway chair in nursing research and executive director, Department of Nursing Science, Professional Practice, and Quality Outcomes; and research integrity officer and professor, Department of Pediatrics, Children’s National Health System, and The George Washington University, Washington, DC. Her research interests include patient-reported outcomes in pediatric palliative care and the roles of clinicians in supporting families of seriously ill children.
Terrah Foster Akard, PhD, RN, CPNP, FAAN is an associate professor and director of graduate studies for the PhD in Nursing Science Program at the Vanderbilt University School of Nursing. Her research interests are developing psychosocial interventions to reduce suffering and improve quality of life for pediatric palliative care populations. Her research focuses on web-based recruitment methods and legacy interventions for children with serious illness and their family members.
Footnotes
Disclosure of interest
This work was supported by the National Institute of Nursing Research (grant RO1 NR015353; ClinicalTrials.gov number NCT04059393; principal investigator: T.F.A.) and NIH/National Center for Advancing Translational Sciences (grant UL1 TR000445). The authors report no further conflicts of interest.
Data availability statement
Raw data were generated at the Vanderbilt University School of Nursing. The data that support the results of this study are available from the corresponding author upon reasonable request.
References
- 1.Frampton GK, Shepherd J, Pickett K, et al. Digital tools for the recruitment and retention of participants in randomised controlled trials: A systematic map. Trials. 2020;21:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson EJ, Loux T, Arnold LD, et al. Obtaining contextually relevant geographic data using Facebook recruitment in public health studies. Health Place. 2019;55:37–42. [DOI] [PubMed] [Google Scholar]
- 3.Reagan L, Nowlin SY, Birdsall SB, et al. Integrative review of recruitment of research participants through Facebook. Nurs Res. 2019;68(6):423–32. [DOI] [PubMed] [Google Scholar]
- 4.Beasant L, Brigden A, Parslow RM, et al. Treatment preference and recruitment to pediatric RCTs: A systematic review. Contemp Clin Trials Commun. 2019;14:100335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crocker JC, Beecham E, Kelly P, et al. Inviting parents to take part in paediatric palliative care research: A mixed-methods examination of selection bias. Palliat Med. 2015;29(3):231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norman K. Survey reveals patient recruitment is the biggest challenge in pediatric trials. Appl Clin Trials. 2012;21(6). [Google Scholar]
- 7.Weaver MS, Mooney-Doyle K, Kelly KP, et al. The Benefits and Burdens of Pediatric Palliative Care and End-of-Life Research: A Systematic Review. J Palliat Med. 2019;22(8):915–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akard TF, Gerhardt CA, Hendricks-Ferguson V, et al. Facebook advertising to recruit pediatric populations. J Palliat Med. 2016;19(7):692–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woolfall K, Shilling V, Hickey H, et al. Parents’ agendas in paediatric clinical trial recruitment are different from researchers’ and often remain unvoiced: A qualitative study. PLoS One. 2013;8(7):e67352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaguelidou F, Amiel P, Blachier A, et al. Recruitment in pediatric clinical research was influenced by study characteristics and pediatricians’ perceptions: A multicenter survey. J Clin Epidemiol. 2013;66(10):1151–7. [DOI] [PubMed] [Google Scholar]
- 11.Schoot RA, van Ommen CH, Caron HN, et al. Accrual in supportive care trials in pediatric oncology, a challenge! Support Care Cancer. 2012;20(12):3149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baca-Motes K, Edwards AM, Waalen J, et al. Digital recruitment and enrollment in a remote nationwide trial of screening for undiagnosed atrial fibrillation: Lessons from the randomized, controlled mSToPS trial. Contemp Clin Trials Commun. 2019;14:100318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter-Harris L. Facebook targeted advertisement for research recruitment: A primer for nurse researchers. Appl Nurs Res. 2016;32:144–147. [DOI] [PubMed] [Google Scholar]
- 14.Tustin JL, Crowcroft NS, Gesink D, et al. Facebook recruitment of vaccine-hesitant Canadian parents: Cross-sectional study. JMIR Public Health Surveill. 2017;3(3):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lohse B. Facebook is an effective strategy to recruit low-income women to online nutrition education. J Nutr Educ Behav. 2013;45(1):69–76. [DOI] [PubMed] [Google Scholar]
- 16.McAleese S, Clyne B, Matthews A, et al. Gone for good? An online survey of emigrant health professionals using Facebook as a recruitment tool. Hum Resour Health. 2016;14:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung SY, Hacker ED, Rawl S, et al. Using Facebook in recruiting kidney transplant recipients for a REDCap study. West J Nurs Res. 2019;41(12):1790–812. [DOI] [PubMed] [Google Scholar]
- 18.Whitaker C, Stevelink S, Fear N. The use of Facebook in recruiting participants for health research purposes: A systematic review. J Med Internet Res. 2017;19(8):e290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beecham E, Hudson BF, Oostendorp L, et al. A call for increased paediatric palliative care research: Identifying barriers. Palliat Med. 2016;30(10):979–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hudson BF, Oostendorp LJ, Candy B, et al. The under reporting of recruitment strategies in research with children with life-threatening illnesses: A systematic review. Palliat Med. 2017;31(5):419–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akard TF, Wray S, Friedman DL, et al. Transforming a face-to-face legacy intervention to a web-based legacy intervention for children with advanced cancer. J Hosp Palliat Nurs. 2020;22(1):49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akard TF, Dietrich MS, Friedman DL, et al. Digital storytelling: An innovative legacy-making intervention for children with cancer. Pediatr Blood Cancer. 2015;62(4):658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foster TL. Personal reflections on legacy making. Palliat Support Care. 2010;8(1):99–100. [DOI] [PubMed] [Google Scholar]
- 24.Robson PC, Dietrich MS, Akard TF. Associations of age, gender, and family income with quality of life in children with advanced cancer. J Pediatr Oncol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akard TF, Dietrich MS, Friedman DL, et al. Effects of a web-based pediatric oncology legacy intervention on parental coping. Onco Nurs Forum. [DOI] [PubMed] [Google Scholar]
- 26.Akard TF, Gilmer MJ. Research Cooperative Groups in Pediatric Palliative Care Research. Palliative Medicine Reports. 2020;1(1):321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akard TF, Dietrich MS, Friedman DL, et al. Improved parent–child communication following a RCT evaluating a legacy intervention for children with advanced cancer. Prog Palliat Care. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akard TF, Dietrich MS, Friedman DL, et al. Randomized Clinical Trial of a Legacy Intervention for Quality of Life in Children with Advanced Cancer. J Palliat Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akard TF, Wray S, Gilmer MJ. Facebook advertisements recruit parents of children with cancer for an online survey of web-based research preferences. Cancer Nurs. 2015;38(2):155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McRobert CJ, Hill JC, Smale T, et al. A multi-modal recruitment strategy using social media and internet-mediated methods to recruit a multidisciplinary, international sample of clinicians to an online research study. PLoS One. 2018;13(7):e0200184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trivedi RB, Szarka JG, Beaver K, et al. Recruitment and retention rates in behavioral trials involving patients and a support person: A systematic review. Contemp Clin Trials. 2013;36(1):307–18. [DOI] [PubMed] [Google Scholar]
- 32.Badr H, Smith CB, Goldstein NE, et al. Dyadic psychosocial intervention for advanced lung cancer patients and their family caregivers: Results of a randomized pilot trial. Cancer. 2015;121(1):150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Topolovec-Vranic J, Natarajan K. The use of social media in recruitment for medical research studies: A scoping review. J Med Internet Res. 2016;18(11):e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dworkin J, Hessel H, Gliske K, et al. A comparison of three online recruitment strategies for engaging parents. Fam Relat. 2016;65(4):550–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamp K, Herbell K, Magginis WH, et al. Facebook recruitment and the protection of human subjects. West J Nurs Res. 2019;41(9):1270–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benedict C, Hahn AL, Diefenbach MA, et al. Recruitment via social media: Advantages and potential biases. Digit Health. 2019;5:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franzke AS, Bechmann A, Zimmer M, et al. Internet research: Ethical guidelines 3.0. 2020. [Retrieved 2021 from https://aoir.org/reports/ethics3.pdf]
- 38.Kozinets RV. Introducing: Netnography, qualitative social media research methods, and this book. In: Kozinets RV, editor. Netnography: The essential guide to qualitative social media research. Thousand Oaks, CA: Sage; 2020. p. 1–32. [Google Scholar]