Abstract
Background
: The safety of COVID vaccines should be continuously followed. This study reports adverse events of the Oxford/AstraZeneca COVID-19 vaccine.
Methods
: A prospective single-cohort study design was conducted to assess adverse events following immunization and associated factors of the first dose of Oxford/AstraZeneca's COVID-19 vaccine in Ayder Comprehensive specialized hospital. A structured questionnaire was administered consecutively to 423 participants. Follow-up data were collected 72 hours after vaccination via phone. Bivariate and multivariate logistic regression models were used to find associations between adverse events and independent variables. Statistical significance was declared at P<0.05.
Results
: Out of 423 health care workers approached, 395 responded. At least one adverse event (95% CI: 63.58, 72.77) was reported by 270 participants. Local and systemic symptoms occurred in 46.8% (95% CI: 41.94, 51.79) and 58.48% (95% CI: 53.53, 63.26)], respectively. Muscle ache, fatigue, headache and fever were the most common local symptoms. No reports of hospitalization, disability or death. Age (adjusted odds ratio [AOR]=0.97, P=0.048), female sex (AOR=1.84, P=0.028), and comorbidity (AOR=2.28, P=0.040) were independent predictors of adverse events.
Conclusion and recommendation
: Adverse events following immunization are commonly reported after the first dose of the Oxford/AstraZeneca COVID-19 vaccine; age, female sex and comorbidity are independent predictors.
Keywords: COVID vaccine, Adverse Events Following Immunization, Oxford/AstraZeneca, Ayder Hospital
Background
Since its emergence, SARS-CoV-2 has disrupted global health and health systems, economies and interactions (Paul & Divyanshi, 2021). Upto 22 June 2021, it has infected approximately 180 million people and claimed approximately 3.8 million lives worldwide (Worldometer, 2021). In response to this catastrophe, hundreds of candidate vaccines have been on trial, some of which have passed the acceptable efficacy and safety standards and been deployed for use (Kirby, 2020; Onyeaka et al., 2021). The University of Oxford's and AstraZeneca's recombinant chimpanzee adenovirus-vectored nCoV-19 (ChAdOx1 nCoV-19) vaccine is one of these vaccines (Onyeaka et al., 2021).
The ChAdOx1 nCoV-19 vaccine is given by intramuscular injection, using as a vector the modified chimpanzee adenovirus ChAdOx1 (Folegatti et al., 2020). Vaccine efficacy is 76.0% at preventing symptomatic COVID-19 at 22 days following the first dose and 81.3% after the second dose (Voysey, Clemens, et al., 2021; Voysey, Costa Clemens, et al., 2021). On the 30th of December 2020, the vaccine was first approved for temporary use in the United Kingdom vaccination program (Gallagher & Triggle, 2020) and later by the World Health Organization (WHO) for emergency use (WHO, 2021a).
Following its use, various safety-related studies started to emerge. They have revealed that the vaccine has a good safety profile with some short-lived and self-limiting side effects, including injection-site pain, headache, nausea, vomiting, diarrhea, swelling, redness at the injection site, enlarged lymph nodes, decreased appetite, dizziness, sleepiness, sweating and abdominal pain. Rarely, however, some serious adverse effects, such as blood clots and anaphylaxis, have been reported(DIMITROVA, 2021).
The vaccine is new, and its safety has not been established in various geographic, demographic and ethnic populations. Therefore, post-market adverse events should be studied and sufficient evidence generated. We, therefore, aimed to determine the occurrence of adverse events following the first dose of the ChAdOx1 nCoV-19 vaccine among health care workers at Ayder Comprehensive Specialized Hospital (ACSH).
Methods and material
Study area and time
The study was conducted at ACSH from April 19, 2021, to May 3, 2021. ACSH is a tertiary care hospital located in Mekelle, Tigray, Northern Ethiopia. It provides a comprehensive set of specialized and general health care services. In addition, it serves as a teaching and research hospital under Mekelle University. It has approximately 1912 health care professionals, practitioners and academics.
Study design and population
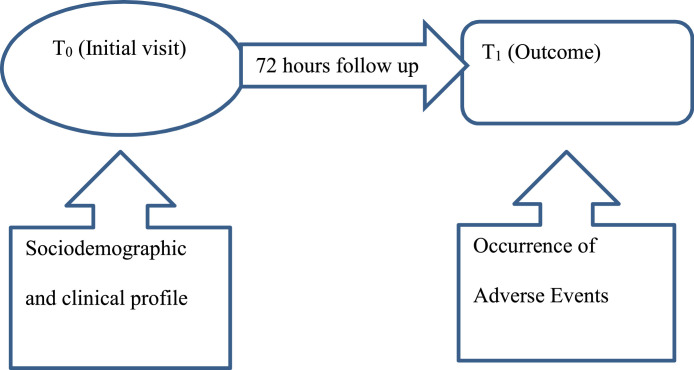
We employed a prospective single cohort study design among health care workers (practitioners, student-practitioners and academics) working in ACSH given the first dose of the ChAdOx1 nCoV-19 vaccine. All participants received the first dose of COVISHIELD, Oxford-AstraZeneca's COVID-19 vaccine, produced by the Serum Institute of India. The study was designed with two time periods. During their initial visit, sociodemographic data and information on comorbidities were collected from participants. Details about new symptoms occurring in the 72-hour post-vaccine period were then collected via telephone-based interviews (Figure 1).
Fig. 1.
Design of the study
Sample size and sampling procedure
The sample size was calculated based on the formula for a single population proportion, where Zα/2 = 1.96, margin of error = 0.05, p = proportion of health care workers with adverse events following immunization (AEFI) = 0.50 and q= 1-p:
Sample size (n)= = 384, adding a 10% non-response rate, the minimum sample size was found to be 423.
We used a consecutive sampling technique to collect data from the vaccination site as health care workers came for vaccination. Then we collected data consecutively until a sufficient sample was reached.
Variables and definitions
| Adverse events |
|
| Comorbidity |
|
| Local symptoms |
|
| Systemic symptoms |
|
Data collection tools and procedures
The data collection tool was prepared through expert consultation and literature review. Data were collected in two steps. Initially (T0), we approached the health care workers using face-to-face interviews to collect sociodemographic and comorbidity data when they received their vaccine at the service area. Their telephone number was recorded, and they were informed about a call after 72 hours. They were told to note and record any adverse effects following the vaccine. After 72 hours (T1), data about adverse effects and related timing were collected via interview over the phone. This study adopted 72 hours because most adverse events occur within this period, as evidenced by recent studies (Menni et al., 2021; Shrestha et al., 2021)(12,13)
The data collectors were briefed on the importance of the study and were from the vaccination area. They were constantly supervised to ensure the quality of data. Data were entered into EpiData, which minimizes data entry errors.
Data analysis
The collected data were coded and entered into EpiData version 4.6, then exported into and analyzed using Stata version 15.0. Categorical variables were described using frequencies with percentages. Continuous variables were described using an appropriate combination of measures of central tendency and dispersion. Proportions of occurrence of commonly reported adverse events were presented using a bar graph with error bars.
A binary logistic regression model was fitted to investigate associations between adverse events and the independent variables. We exported independent variables P<0.25 in the bivariate analysis to multivariable logistic regression models. Statistical significance was declared at P<0.05. Multicollinearity assumption and goodness of fit of the final model were checked using the Variance Inflation Factor (VIF) and Hosmer-Lemeshow test, respectively. No collinearity issue was found with maximum and average VIF values of 1.78 and 1.33, respectively. The final multivariable model was a good fit (Hosmer-Lemeshow test: Ȥ2=11.48, df=8, p=0.176).
Results
Sociodemographic and clinical characteristics of the participants
During the study period, 423 study participants who received the COVID-19 vaccine were recruited consecutively. Of these, 395 (93.38%) participants responded to the follow-up questionnaire over the phone. The median age of the subjects was 32 (interquartile range [IQR]=15). The majority (69.37%) of the study participants were males. Regarding religion, 355 (89.87%) were Orthodox Christians. More than half (53.67%) of the participants were married.
Of the total participants approached, 43 (10.89%) had at least one comorbidity. Diabetic mellitus was the lead comorbidity (34.88%) reported, followed by hypertension (23.26%) and asthma (23.26%). Fourteen (3.54%) study participants had a known history of allergy to medications. Few (1.77%) patients were positive for COVID-19 previously (Table 1).
Table 1.
Sociodemographic and clinical characteristics among the participants, ACSH, 2021 (n=395)
| Variables | All casesn=395 |
Reported perceived side-effect |
||
|---|---|---|---|---|
| Yesn=270 | Non=125 | |||
| Age in years [median (IQR)] | 32(25-40) | 30(24-40) | 36(28-45) | |
| Gender (male), n (%) | 274(69.37) | 173(64.07) | 101(80.80) | |
| Religion, n (%) | ||||
| Orthodox | 355(89.87) | 244(90.37) | 111(88.80) | |
| Muslim | 22(5.57) | 15(5.56) | 7(5.60) | |
| Protestant | 11(2.79) | 7(2.59) | 4(3.20) | |
| Others A | 7(1.77) | 4(1.48) | 3(2.40) | |
| Marital status, n (%) | ||||
| Married | 212(53.67) | 129(47.78) | 83(66.40) | |
| Single | 179(45.32) | 137(50.74) | 42(33.60) | |
| Divorced/separated | 4(1.01) | 4(1.48) | 0(0.00) | |
| Comorbidity (yes), n (%) | 43(10.89) | 32(11.85) | 11(8.80) | |
| If comorbid, type of comorbidity (n = 43), n (%) | ||||
| Diabetes mellitus | 15(34.88) | 12(37.50) | 3(27.27) | |
| Hypertension | 10(23.26) | 6(18.75) | 4(36.36) | |
| Asthma | 10(23.26) | 7(21.88) | 3(27.27) | |
| Cardiac disease | 2(4.65) | 2(6.25) | 0(0.00) | |
| Other B | 6(13.95) | 5(15.62) | 1(9.10) | |
| Medication allergy history (yes), n (%) | 14(3.54) | 6(2.22) | 8(6.40) | |
| Positive Covid-19 test (yes), n (%) | 7(1.77) | 4(1.48) | 3(2.40) | |
A= (Did not tell their religion), B = (Arthritis and Gastric disease)
Adverse events following immunization
Among the 395 vaccinated individuals, 270 (68.35%) reported at least one AEFI with a 95% CI (63.58, 72.77). Local symptoms occurred in 185 (46.83%; 95% CI 41.94, 51.79). Pain at the injection site was the most frequently reported local symptom, reported by 155 (39.24%; 95% CI 34.52, 44.17) participants. Sixty (15.19%; 95% CI 11.96, 19.01) of the participants experienced at least one symptom related to the injected arm, weakness of the arm, 51 (12.91%), being the most common symptom.
Most notably, more than half (58.48%; 95% CI 53.53, 63.26) of the subjects indicated having one or more systemic adverse events. The most commonly reported systemic symptoms were muscle ache (43.4%), fatigue (42.53%), headache (36.20%) and fever (29.87%). Few cases (4.30%) experienced gastrointestinal symptoms. Other symptoms reported by respondents included: rash 2 (0.50%), nasal bleeding 1 (0.25%) and dyspnea 1 (0.25%). Among 134 who responded, 46 (34.34%) reported taking medication by themselves (Table 2).
Table 2.
Adverse events following immunization of COVID-19 among the participants, ACSH, 2021 (n=395)
| AEFI | Frequency | Percentage (95% CI) | |
| Any AEFI | 270 | 68.35 (63.58, 72.77) | |
| Local symptoms | |||
| Any | 185 | 46.83 (41.94, 51.79) | |
| Pain | 155 | 39.24 (34.52, 44.17) | |
| Swelling | 13 | 3.29 (1.92, 5.59) | |
| Induration | 22 | 5.57 (3.69, 8.33) | |
| Stiffness of the arm | 11 | 2.78 (1.54, 4.97) | |
| Weakness of the arm | 51 | 12.91 (9.94, 16.61) | |
| Numbness of the arm | 18 | 4.56 (2.88, 7.13) | |
| Systemic symptoms | |||
| Any | 231 | 58.48 (53.53, 63.26) | |
| Headache | 143 | 36.20 (31.59, 41.08) | |
| Fever | 118 | 29.87 (25.54, 34.59) | |
| Myalgia | 170 | 43.04 (38.22, 47.99) | |
| Fatigue | 168 | 42.53 (37.72, 47.48) | |
| Joint pain | 3 | 0.76 (0.24, 2.34) | |
| Back pain | 3 | 0.76 (0.24, 2.34) | |
| Nausea | 14 | 3.54 (2.10, 5.91) | |
| Vomiting | 1 | 0.25 (0.035, 1.19) | |
| Abdominal pain | 3 | 0.76 (0.24, 2.34) | |
| Nasal bleeding | 1 | 0.25 (0.035, 1.19) | |
| Dyspnea | 1 | 0.25 (0.035, 1.19) | |
| Rash | 2 | 0.50 (0.061, 1.82) | |
| What did you do after AEFI? (n=134) | |||
| Took self-medication | 46 | 34.33 (26.7, 42.86) | |
| Spontaneously cured | 88 | 65.67 (57.13, 73.30) | |
AEFI = Adverse Events Following Immunization
Timing of the occurrence of commonly reported adverse events
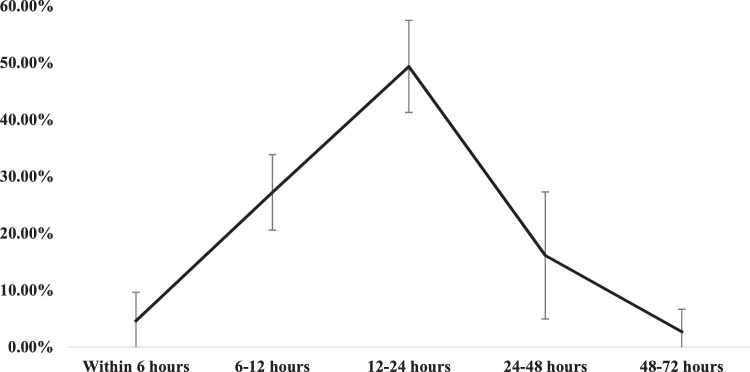
Among the 10 most commonly reported adverse events, the vast majority (81.19%) occurred within the first 24 hours, of which nearly half (49.37%) happened between 12 to 24 hours post-vaccination. As shown in Figure 2, the proportion of adverse effect occurrence decreased substantially from the first (81.19%) to the second (16.11%) and third day (2.70%).
Fig. 2.
Timing of the occurrence of commonly reported adverse events, ACSH, 2021 (n=395)
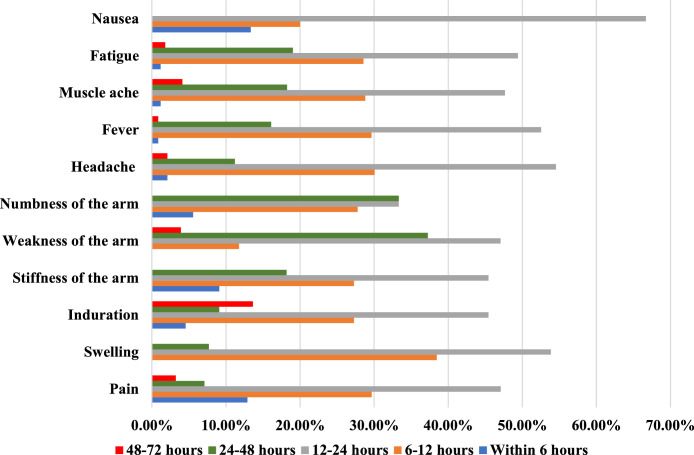
Specific AEFIs most frequently occurred from 12 to 24 hours, followed by 6 to 12 hours after vaccination. A considerable proportion of nausea, stiffness of the arm into which the vaccine was injected, and injection site pain occurred in the first 6 hours. Nausea did not occur after 24 hours, while weakness of the arm and swelling on the injection site happened after 6 hours (Figure 3)
Fig. 3.
Proportion of commonly reported specific side-effects, ACSH, 2021 (n=395)
Factors associated with adverse events following vaccination
All three explanatory variables (age, sex, and comorbidity) included in the final model were statistically significant predictors of AEFI. Accordingly, as age increases by 1 year, the odds of experiencing adverse events decrease by 3.0% (adjusted odds ratio (AOR)=0.97, P=0.003) after adjusting for sex and comorbidity. In addition, the likelihood of side effect experience was higher among women than men (AOR=1.84, P=0.028) after adjusting for age and comorbidity. Participants with comorbidity experienced more post-vaccine symptoms (AOR=2.28, P=0.040) than those without (Table 3) after adjusting for age and sex.
Table 3.
Factors associated with adverse events following immunization among health professionals in ACSH, May 2021.
| Variables | COR (95% CI) | AOR (95% CI) | P-value | |
|---|---|---|---|---|
| Age in years | 0.97 (0.95, 0.98) | 0.97 (0.95, 0.99) | 0.003 | |
| Sex | ||||
| Female | 2.36 (1.42, 3.93) | 1.84(1.07, 3.18) | 0.028 | |
| Male | 1 | 1 | ||
| Comorbidity | ||||
| Yes | 1.39 (0.68, 2.86) | 2.28 (1.04, 5.01) | 0.040 | |
| No | 1 | 1 | ||
Discussion
The study aimed to determine the magnitude of adverse events and associated factors following ChAdOx1 nCoV-19 vaccine immunization among health care workers. More than two-thirds of study participants experienced at least one adverse event. Most adverse events happened within 24 hours after vaccination, showing a sharp decline after this period. Younger age, being female and having comorbidity were independent predictors for AEFI incidence.
The magnitude of occurrence of at least one adverse event following the first dose of the ChAdOx1 nCoV-19 vaccine varies in different studies. Studies from India report occurrence of 57% and 69.7% (Kamal et al., 2021; Inbaraj et al., 2021) and in Togo 71.6% (Konu et al., 2021), which is consistent with the finding of this study (68.58%), however, the number is much higher (90%) in Korean studies (Jeon et al., 2021; Bae et al., 2021). One potential reason may be ethnic differences, as there is some evidence that ethnic differences predict vulnerability for adverse drug reactions in general (Baehr et al., 2015).
Reports of systemic reaction following the first dose of the ChAdOx1 nCoV-19 vaccine in a randomized controlled trial are as high as 86% (Ramasamy et al., 2020). However, the findings in this study (58.48%) are much lower. A large-scale community-based study in the United Kingdom reported an even much lower rate of 33.7% (Menni et al., 2021). The variation may be due to the difference in study design and the heterogeneity of the populations.
Most AEFIs revealed in this study are, to a lesser or greater rate, in line with symptoms from any vaccine, including different COVID-19 vaccines and, specifically, ChAdOx1 nCoV-19 (WHO, 2021b). They are short-lived, self-limiting symptoms and are mild or moderate in severity. In our study, of those who reported AEFIs, more than half said that they spontaneously recovered, and the rest took over-the-counter drugs. There was no report of serious adverse events, such as hospitalization, disability or death, which is similar to results from other studies (Menni et al., 2021; Ramasamy et al., 2020). Most AEFI happened within 24 hours following vaccination. There was a sharp decline in the incidence of adverse events between 24 and 72 hours following vaccination, corroborating findings elsewhere (Folegatti et al., 2020; Inbaraj et al., 2021).
Age, sex and comorbidity were associated with the incidence rate of AEFIs. Our findings show that younger study participants had more frequent symptoms, consistent with other findings (Menni et al., 2021; Kamal et al., 2021); this may be due to higher reactogenicity among young people than their older counterparts (Ramasamy et al., 2020). In our study, the probability of developing adverse effects following ChAdOx1 nCoV-19 vaccination is significantly higher among women (1.84 times) than men, which aligns with other reports (Gee, 2021; Blumenthal et al., 2021). In general, evidence shows that women encounter an increased risk of vaccine-related adverse events than men (Klein & Flanagan, 2016), which could be due to estrogen hormone, which triggers a robust immune response. Our study found that more pronounced AEFIs occurred in participants with comorbidity than those without. However, two other studies from India (Kamal et al., 2021; Inbaraj et al., 2021) found no significant association between these two attributes. The difference might be due to the difference in methods and sample size.
Our findings may have positive policy and practice implications and provide data on local vaccine-related adverse effects for health care professionals and the public. However, our study has limitations, and we advise a caution in interpretation of the findings. The first limitation is that our participants were all health care workers, making it difficult to extrapolate the results to the broader population. The short follow-up time (only 72 hours after vaccination) could be considered as a second limitation since it leaves adverse events occurring after 72 hours untraced. Additional studies should be conducted that address the limitations of our study and we advise researchers to conduct research with more frequent data collection, including immediate follow-up after vaccination and trace adverse events happening after 72 hours. Furthermore, studies that include the wider population are highly recommended.
Conclusion
AEFI of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca COVID-19) vaccine was common among health care workers in ACSH, Tigray. Nearly half of study participants had at least one local symptom, and more than half had at least one systemic symptom. Younger age, being female and having comorbidity were independent predictors for AEFI incidence.
Funding
This project was funded by Mekelle University.
Ethical approval
Ethical clearance was received from Mekelle University College of Health Sciences institutional review board. We also received written informed consent from each study participant. In addition, secured handling of the collected data was ensured.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
The authors would like to thank the study participants.
References
- Bae S., Lee Y.W., Lim S.Y., Lee J.H., Lim J.S., Lee S., Park S., Kim S.K., Lim Y.J., Kim E.O., Jung J., Kwon H.S., Kim T.B., Kim S.H. Adverse Reactions Following the First Dose of ChAdOx1 nCoV-19 Vaccine and BNT162b2 Vaccine for Healthcare Workers in South Korea. Journal of Korean Medical Science. 2021;36(17):e115. doi: 10.3346/jkms.2021.36.e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehr A., Peña J.C., Hu D.J. Racial and Ethnic Disparities in Adverse Drug Events: A Systematic Review of the Literature. Journal of Racial and Ethnic Health Disparities. 2015;2(4):527–536. doi: 10.1007/s40615-015-0101-3. [DOI] [PubMed] [Google Scholar]
- Blumenthal K.G., Robinson L.B., Camargo C.A., Jr, Shenoy E.S., Banerji A., Landman A.B., Wickner P. Acute Allergic Reactions to mRNA COVID-19 Vaccines. JAMA. 2021;325(15):1562–1565. doi: 10.1001/jama.2021.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIMITROVA, 2021.Dimitrova E.K. Vaxzevria (previously COVID-19 Vaccine AstraZeneca) [Text] European Medicines Agency. 2021 https://www.ema.europa.eu/en/medicines/human/EPAR/vaxzevria-previously-covid-19-vaccine-astrazeneca [Google Scholar]
- Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., Bellamy D., Bibi S., Bittaye M., Clutterbuck E.A., Dold C., Faust S.N., Finn A., Flaxman A.L., Hallis B., Heath P., Jenkin D., Lazarus R., Makinson R.…Oxford COVID Vaccine Trial Group Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomized controlled trial. Lancet (London, England) 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J., Triggle N. Covid-19: Oxford-AstraZeneca vaccine approved for use in UK. BBC News. 2020 https://www.bbc.com/news/health-55280671 [Google Scholar]
- Gee J. First Month of COVID-19 Vaccine Safety Monitoring—United States, 14 December, 2020–13 January, 2021. MMWR. Morbidity and Mortality Weekly Report. 2021;70 doi: 10.15585/mmwr.mm7008e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbaraj L.R., George C.E., Franklyn N.N. Experience from a tertiary care hospital in South India. 2021. How safe is Covishield (ChAdOx1nCoV-19) vaccine? 2021.03.16.21253744. [DOI] [Google Scholar]
- Jeon M., Kim J., Oh C.E., Lee J.Y. Adverse Events Following Immunization Associated with Coronavirus Disease 2019 Vaccination Reported in the Mobile Vaccine Adverse Events Reporting System. J Korean Med Sci. 2021:e114. doi: 10.3346/jkms.2021.36.e114. –e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal D., Thakur V., Nath N., Malhotra T., Gupta A., Batlish R. Adverse events following ChAdOx1 nCoV-19 Vaccine (COVISHIELD) amongst health care workers: A prospective observational study. Medical Journal Armed Forces India. 2021;77:S283–S288. doi: 10.1016/j.mjafi.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby T. Development of potential COVID-19 vaccines continues to accelerate. The Lancet Microbe. 2020;1(3):e109. doi: 10.1016/s2666-5247(20)30070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S.L., Flanagan K.L. Sex differences in immune responses. Nature Reviews Immunology. 2016;16(10):626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- Konu Y.R., Gbeasor-Komlanvi F.A., Yerima M., Sadio A., Tchankoni M.K., Zida-Compaore W.I.C., Nayo-Apetsianyi J., Afanvi K.A., Agoro S., Salou M., Landoh D.E., Nyansa A.B., Boko E., Mijiyawa M., Ekouevi D.K. 2021. Prevalence of severe adverse events in health professionals after receiving the first dose of the COVID-19 vaccination in Togo, March 2021. 2021.04.20.21254863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menni C., Klaser K., May A., Polidori L., Capdevila J., Louca P., Sudre C.H., Nguyen L.H., Drew D.A., Merino J., Hu C., Selvachandran S., Antonelli M., Murray B., Canas L.S., Molteni E., Graham M.S., Modat M., Joshi A.D.…Spector T.D. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. The Lancet Infectious Diseases. 2021;21(7):939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyeaka H., Al-Sharify Z.T., Ghadhban M.Y., Al-Najjar S.Z. A review on the advancements in the development of vaccines to combat coronavirus disease 2019. Clinical and Experimental Vaccine Research. 2021;10(1):6–12. doi: 10.7774/cevr.2021.10.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul B., Divyanshi W. 2021. 2020 Year in Review: The impact of COVID-19 in 12 charts.https://blogs.worldbank.org/voices/2020-year-review-impact-covid-19-12-charts [Google Scholar]
- Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., Voysey M., Aley P.K., Angus B., Babbage G., Belij-Rammerstorfer S., Berry L., Bibi S., Bittaye M., Cathie K., Chappell H., Charlton S., Cicconi P., Clutterbuck E.A.…Zizi D. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomized, controlled, phase 2/3 trial. The Lancet. 2020;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S., Devbhandari R.P., Shrestha A., Aryal S., Rajbhandari P., Shakya B., Pandey P., Shrestha R.K., Gupta M., Regmi A. Adverse events following the first dose of ChAdOx1 nCoV-19 (COVISHIELD) vaccine in the first phase of vaccine roll out in Nepal. Journal of Patan Academy of Health Sciences. 2021;8(1):9–17. https://www.jpahs.edu.np/index.php/jpahs/article/view/453 [Google Scholar]
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., Bibi S., Briner C., Cicconi P., Collins A.M., Colin-Jones R., Cutland C.L., Darton T.C., Dheda K., Duncan C.J.A.…Zuidewind P. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomized controlled trials in Brazil, South Africa, and the UK. The Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voysey M., Costa Clemens S.A., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., Bibi S., Briner C., Cicconi P., Clutterbuck E.A., Collins A.M., Cutland C.L., Darton T.C., Dheda K., Dold C.…Zuidewind P. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomized trials. The Lancet. 2021;397(10277):881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organisation; 2021. WHO lists two additional COVID-19 vaccines for emergency use and COVAX roll-out.https://www.who.int/news/item/15-02-2021-who-lists-two-additional-covid-19-vaccines-for-emergency-use-and-covax-roll-out 15 February. [Google Scholar]
- WHO . World Health Organisation; 2021. Background document on the AZD1222 vaccine against COVID-19 developed by Oxford University and AstraZeneca.https://www.who.int/publications-detail-redirect/background-document-on-the-azd1222-vaccine-against-covid-19-developed-by-oxford-university-and-astrazeneca March 1. [Google Scholar]
- Worldometer . 2021. COVID Live Update: 211,002,568 Cases and 4,419,834 Deaths from the Coronavirus—Worldometer.https://www.worldometers.info/coronavirus/ [Google Scholar]