Abstract
Background
The effectiveness of the best combination between different antiviral and anti-inflammatory drugs stills an interest in the treatment of COVID19 infection.
Patients and methods
A prospective randomized cohort study comprised 108 adult patients with confirmed PCR COVID 19 infection with systemic hyper inflammation state, divided into two groups according to the treatment regimen, 56 in the tocilizumab- hydroxychloroquine (TCZ-HCQ) treatment, and 52 in the tocilizumab-remdesivir (TCZ-RMV) treatment. The first group received a combination of I.V. TCZ (400–800 mg every 24 h for only two doses) and HCQ (400 mg twice in the first day then 200 mg twice for 5 days) while the second group of patients received I.V. RMV of 200 mg on day 1 followed by 100 mg once daily infused over 60 min for 5 days with the same TCZ regimen used in the first group. All clinical parameters and laboratory investigations were assessed before and after treatment.
Results
The CRP was significantly decreased while PaO2/FiO2 (P/F) ratio post-treatment was significantly improved in both treatment groups. TCZ-HCQ group showed a significant decrease in the ferritin, LDH, and D. Dimer levels. The median days of hospitalization with interquartile range (IQR) were 10 (6–16) and 8 (5–12) for TCZ-HCQ and TCZ-RMV groups, respectively. The numbers of mechanically ventilated patients were 25 and 43 for TCZ-HCQ and TCZ-RMV groups, respectively. Therapeutic failure was about 26.8% in the TCZ-HCQ group and 30.8% in the TCZ-RMV group but there was no significant difference between both groups. Some complications were recognized only in TCZ-RMV following treatment including secondary bacterial infections (42.3%), myocarditis (15.4%), and finally pulmonary embolism (7.7%).
Conclusion
Efficacy of both TCZ-RMV and TCZ-HCQ combinations are observed in the treatment of severe COVID-19 patients; however the increased need for ICU or mechanical ventilation in the TCZ-RMV arm contributed to the appearance of cardiac and thrombotic events.
The study was registered at the Clinical Trials registry (ClinicalTrials.gov; NCT04779047).
Keywords: COVID-19, Cytokine storm, Tocilizumab, Hydroxychloroquine, Remdesivir, Antiviral, Anti-inflammatory
Introduction
Coronavirus disease 2019 (COVID-19) is considered a great issue all over the world nowadays. The elevated mortality rate, in severe acute respiratory syndrome coronavirus, is the core of the challenge [1]. The capacities of intensive care units (ICU) worldwide are challenged to encounter this outbreak. There is no therapy proven to be effective yet. Consequently, there is a great need for more research on treatments that can reduce the mortality rate and the number of critical cases [2].
Also, the rapid spread of COVID-19 infection created an urgent need for effective treatments against this type of viral pneumonia. Antivirals and immunomodulators are the main therapeutic agents that have been proposed for treatment. They are all subjected to many in-vitro, animal, and clinical trials. An improvement in preclinical results was demonstrated with these agents but till now no one of them was approved [3].
Hydroxychloroquine (HCQ) is one of the immunomodulatory and antimalarial drugs that demonstrated antiviral activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [4]. The antiviral effect of HCQ is attributed to its ability to elevate intracellular pH resulting in reduced phagolysosome fusion and impairing glycosylation of the viral receptor. On the other hand, the immune-modulating effect of HCQ is represented in inhibition of signaling of the toll-like receptor and reducing the production of IL-1 and IL-6 [5]. Also, previous studies suggested that HCQ may have an anti-thrombotic effect [6,7]. Additional Studies, regarding HCQ associated clinical outcomes, are required. If the HCQ treatment is proved to be effective, a great precedent could be achieved in the management of COVID-19 especially in areas that have limited resources for health care [7,8].
On other hand, it was found that hydroxychloroquine was not able to reduce the viral loads in the upper or lower respiratory tract in hospitalized COVID 19 patients, despite its antiviral activity in many in vitro systems [9].
Remdesivir (RMV), another antiviral drug, was used for the treatment of Ebola fever and Marburg virus disease. It was found to be effective against many single-stranded RNA viruses. It acts by therapeutically targeting RNA-dependent RNA polymerase, needed for RNA virus self-renewal [10]. Some randomized clinical trials suggested that RMV could help in improving the recovery for many hospitalized COVID-19 patients especially those who need supplemental oxygen therapy all over the world [11,12].
In many studies, the use of remdesivir in hospitalized COVID-19 patients has been demonstrated variability among health systems due to the differences in the severity of patients' cases and the protocols of treatment. In addition, it was found that it may not be associated with improvements in survival rates but was associated with longer days on hospital beds [[13], [14], [15]].
Tocilizumab (TCZ) is a monoclonal antibody that is used in the treatment of systemic juvenile idiopathic arthritis and rheumatoid arthritis. It acts as an antagonist for the IL-6 receptor [16]. The drug was tested in many clinical trials for severe SARS-CoV-2 cases and was found to improve many laboratory parameters including reduction of C-reactive protein (CRP), Interleukin (IL-6), lactate dehydrogenase (LDH), ferritin, and Total Leukocyte Count (TLC). Also, it reduces lung complications, duration of hospitalization, needs for ICU admission and mechanical ventilation, and mortality rate [[17], [18], [19]].
The exaggerated immune response and inflammation in severe COVID-19 patients motivate many clinical studies to use a combination of different drugs to be able to control viral infection [12]. The present study followed this recommendation in an attempt to find the best combination of medicines for severe cases of COVID-19 infection.
Patients and methods
Study design
Between October 1, 2020, and March 10, 2021, one hundred eight adult patients aged ≥18 years old with confirmed severe COVID-19 infection were included in this prospective cohort study. Study patients from the Hospital of Health Insurance, Beni-Suef, and Hospital of Teachers, Cairo, Egypt; were enrolled in an off-label treatment protocol approved by the Research Ethics Committee of Faculty of Pharmacy, Beni-Suef University (REC-H-PhBSU-21011) and was registered at the Clinical Trials registry (ClinicalTrials.gov; NCT04779047). The current study was completed along with the good clinical practices recommended by the Declaration of Helsinki and its amendment.
Randomly, COVID 19 patients with cytokine storm were divided into two groups. Simple randomization was made by allocating patients using a table of random numbers. The first group of patients (n = 56) received IV TCZ 400 mg–800 mg every 24 h for only two doses and hydroxychloroquine 400 mg twice daily at day 1 then 200 mg twice daily for 5 days. While the second group of patients (n = 52), received IV remdesivir of 200 mg on day 1 followed by 100 mg per day infused over 60 min for 5 days with the same TCZ regimen used in the first group. The Egyptian Ministry of Health COVID-19 protocol was followed in the management of the studied severe COVID-19 subjects. All study patients gave informed written consent and knew that their involvement is voluntary and was informed that they can withdraw from the study at any time.
Patients and study population
Clinical evaluations
Different demographic and clinical data were gathered as age, sex, recent exposure history, medical signs and symptoms, different comorbidities such as coronary artery disease, arterial hypertension, diabetes mellitus, chronic obstructive pulmonary disease, and cancer. Blood laboratory test results at the time of admission, were reviewed and abstracted by senior medical physicians and charted in a computerized database for subsequent verification. The baseline of clinical status included body temperature, need for supplementary oxygen therapy, invasive or noninvasive ventilation, and also need for ICU admission. Chest computed tomographic (CT) scans were carried out for all studied subjects and the ratio of the lesion and its progression were recognized. Besides, serum inflammatory markers as LDH, CRP, and ferritin. Since clinical outcomes are poor predictors of avian influenza severity, as stated by American Thoracic Society guidelines for community-acquired pneumonia, evaluated cases were considered as severe or non-severe COVID-19 because of its worldwide acceptance. Comorbidities were categorized depending on the organ system, counting the respiratory, cardiovascular, and endocrine systems. Our study's endpoint was a composite measure that included the intensive-care unit, invasive ventilation, or death.
Inclusion criteria
Patients included in the study who admitted to intensive care unit with confirmed COVID-19 infection through polymerase chain reaction (PCR); after one week of home isolation or after 7 days of inpatient isolation with significant clinical manifestation of systematic hyper inflammation defined as rapid deterioration in oxygen saturation (SaO2) for less than 92% at ambient air or respiratory rate (RR) for more than 30, or PaO2/FiO2 ratio for less than 250; or in radiological findings of CT chest according to CO-RADS classification defined as worsening of lung involvement as an increase in the number and /or expansion of pulmonary areas of consolidation, need for increased FiO2 to maintain stable O2 saturation or worsening O2 saturation of >3% with steady FiO2, as well as elevation on inflammatory marker C-reactive protein (CRP, ≥100 mg/L) or ferritin (≥900 ng/mL) and lactate dehydrogenase (LDH, >220 U/L) [20,21].
Exclusion criteria
Pregnant or lactating women, known hypersensitivity to all drugs or any ingredients of the formulation, patients with other severe primary diseases, Serious co-morbidities, history of a psychiatric or neurological disorder, history of abuse (alcohol or drug), baseline elevation of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels >3-fold the upper limit of the normal range and other factors affecting the observation of curative effects, such as irregular medication and taking other herbal medicine reparations within 14 days before the study, were all excluded.
Criteria for hospital discharge
To be eligible, all of the following criteria were essential: a) a normal body temperature for at least three days; b) better respiratory indicators; c) chest radiology indicating significant improvement in respiratory acute exudative lesions, and d) two consecutive negative PCR tests.
Patients follow up and clinical outcomes
The clinical improvement or recovery of the patients was determined depending on many factors which included:
Primary outcomes
Include a) oxygenation improvement defined as hospitalization not requiring low supplemental oxygen, or high flow oxygen or noninvasive or invasive ventilation (FiO2 < 40%) b) duration of hospitalization, and c) mortality rate.
Secondary outcomes
Include a) Analysis of all inflammatory markers linked to COVID-19 infection such as ferritin, D-dimer, CRP, LDH on admission and after completion of the treatment course, b) Analysis of total leucocyte count (TLC) and absolute lymphocyte count % on admission and after treatment completion, c) Different complications as a myocardial infarction, secondary bacterial infection, and/or hypertension were followed during the management period and d) Monitoring level of liver enzymes.
Statistical analysis
Descriptive analysis was performed where quantitative variables were reported as median with interquartile range (IQR) and categorical variables were described as numbers (percentage %). A Mann–Whitney U test was applied to compare clinical parameters between two treatment groups; (TCZ) plus (HCQ) group and TCZ plus (RMV) group. A Chi-square test was performed to locate the difference in categorical data (baseline patient characteristics and study outcomes) between the two groups. Within the same treatment group, a Wilcoxon Signed Ranks test was used to compare the difference of the clinical parameters before (baseline values) and after drug administration (post-treatment values). A Pairwise Kendall rank correlation test was performed to estimate the correlation between study findings (clinical parameters and outcomes) and baseline patient characteristics. All tests were achieved using SPSS v 22.0 (SPSS, Chicago, USA) and significance was defined as p < 0.05.
Results
The baseline characteristics of patients were summarized in Table 1 . One hundred and eight patients were included in the study where 56 patients were treated with TCZ-HCQ and 52 patients were treated with the TCZ-RMV group. The median age of the TCZ-HCQ group and TCZ-RMV group was 53 (46–68) and 61 (52–70), respectively (p = 0.015). Most patients were male of 45(80.4%) in the TCZ-RMV group versus 32 (61.5%) in the TCZ-RMV group (p = 0.03). Baseline oxygen saturation was 85% (69–89) for the TCZ-HCQ group and 82% (75–88) for the TCZ-RMV group which showed an insignificant difference. Also, both groups had similar baseline respiratory rate and body temperature: 29 (23–31.8) versus 30 (25–33) and 39 °C (38–39) versus 38 °C (37.5–39) for the TCZ-HCQ group and TCZ-RMV group, respectively.
Table 1.
Baseline characteristics determined for the study population (no, %).
| Item | TCZ-HCQ (n = 56) | TCZ-RMV (n = 52) | P-value |
|---|---|---|---|
| Age, yr. | 53(46−68) | 61(52−70) | 0.015 |
| Gender, male (%) | 45(80.4%) | 32(61.5%) | 0.03 |
| Oxygen saturation% | 85(69−89) | 82(75−88) | 0.4 |
| Respiratory rate | 29(23−31.8) | 30(25−33) | 0.15 |
| Max temperature, celsius | 39(38−39) | 38(37.5−39) | 0.05 |
| Serum creatinine | 1.2(1.1−1.6) | 1.4(1.2−2) | 0.041 |
| Comorbidities | |||
| Hypertension, no. (%) | 37(66.1%) | 29(55.8%) | 0.27 |
| Diabetes, no. (%) | 26(46.4%) | 25(48.1%) | 0.86 |
| Ischemic heart disease, no. (%) | 16(28.6%) | 7(13.5%) | 0.06 |
| Heart failure, no. (%) | 3(5.4%) | 1(1.9%) | 0.35 |
| Atrial fibrillation, no. (%) | 5(8.9%) | 1(1.9%) | 0.11 |
| Chronic kidney disease, no. (%) | 2(3.6%) | 3(5.8%) | 0.59 |
| Asthma, no. (%) | 10(17.9%) | 4(7.7%) | 0.12 |
| Chronic obstructive pulmonary disease, no. (%) | 2(3.6%) | 1(1.9%) | 0.6 |
| 2 or more comorbidities, no. (%) | 30(53.6%) | 26(50%) | 0.43 |
| Respiratory support | |||
| Supplemental oxygen at entry, no. (%) | 49(87.5%) | 9(17.3%) | <0.001 |
| Mechanical ventilation need, no. (%) | 25(44.6%) | 43(82.7%) | <0.001 |
| ICU admission, no. (%) | 44(78.6%) | 50(96.2%) | 0.007 |
Data are expressed as median IQR.
The main comorbidities identified in TCZ-HCQ and TCZ-RMV groups in percentage were hypertension (66.1% versus 55.8%), diabetes (46.4% versus 48.1%), ischemic heart disease (28.6% versus 13.5%), and asthma (17.9% versus 7.7%), respectively. Patients admitted to ICU were 44 (78.6%) and 50 (96.2%) in TCZ-HCQ and TCZ-RMV groups, respectively (p = 0.007) where 44% of patients in TCZ-HCQ required mechanical ventilation versus 82% in another group (p < 0.001).
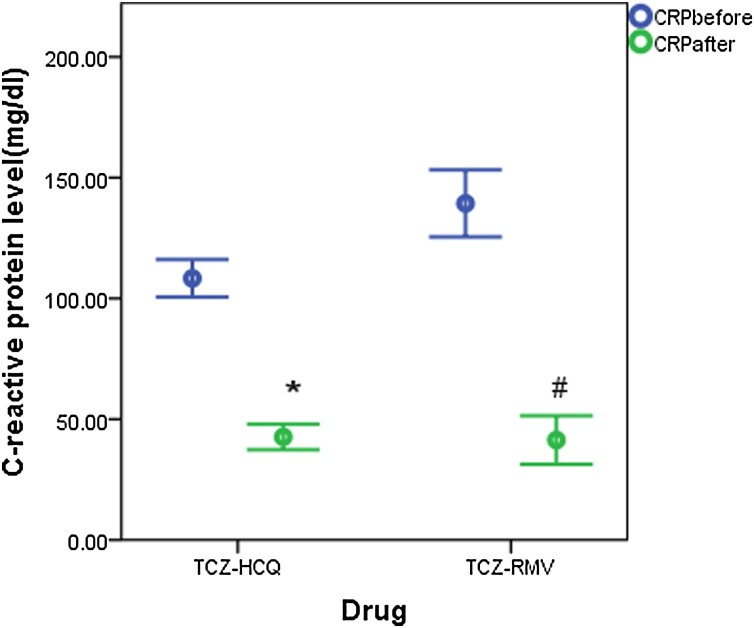
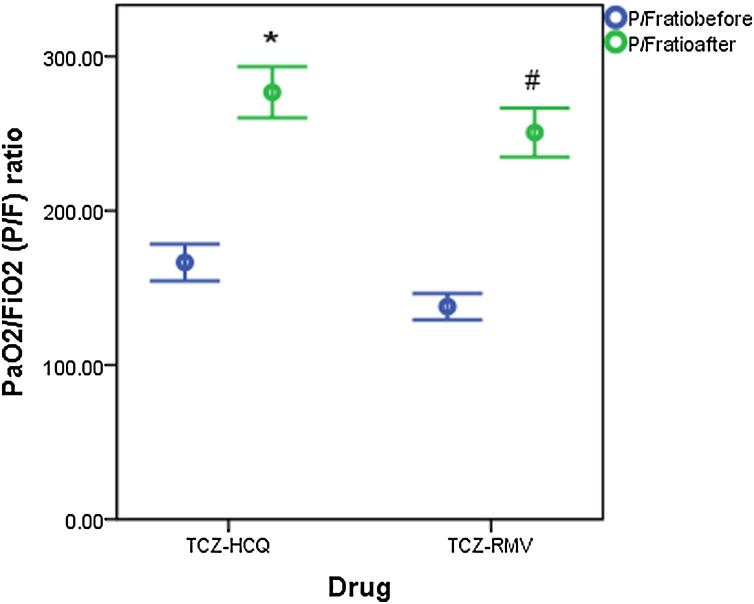
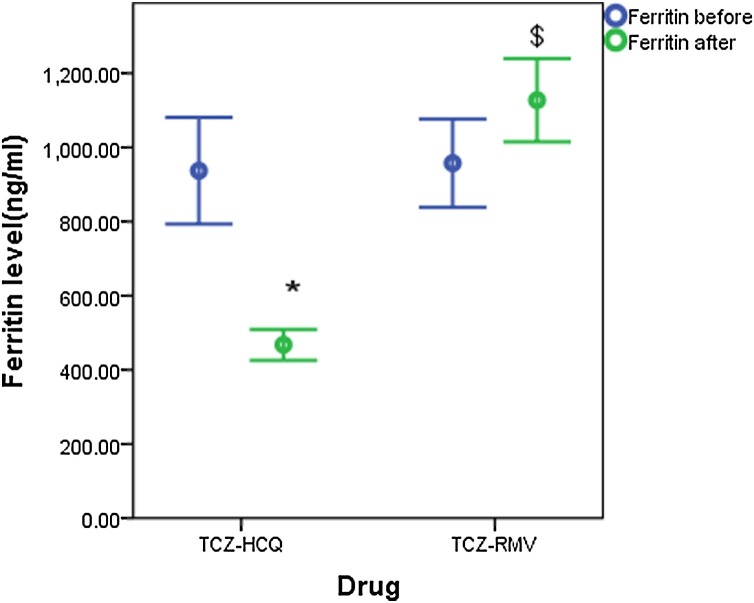
Both groups showed insignificant differences in measurements of baseline clinical parameters except for LDH which was higher in the TCZ-HCQ group (516 IU/L (351.8−710) for TCZ-HCQ group versus 397 IU/L (270−518) for TCZ-RMV group, p = 0.002). Baseline CRP was significantly decreased when compared with endpoint measurements in both groups (97 mg/dl (67.8–139.2) and 26 mg/dl (13.7–79.3), p < 0.001 for TCZ-HCQ group versus 125 mg/dl (43.7–210.8) and 20.1 mg/dl (6.4–40.7), p < 0.001, respectively) (Fig. 1 ). On the other hand, the decline in LDH level and D. Dimer level was only significant in the TCZ-HCQ group where baseline and endpoint values were 516 IU/L (351.8–710) and 397 IU/L (270–518), (p = 0.001) for LDH, 0.48 μg/mL (0.34–0.88) and 0.23 μg/mL (0.13–0.68) for D.Dimer level, p = 0.001, respectively. P/F ratio post-treatment was significantly improved in both groups as shown in Fig. 2 . Only the TCZ-RMV group showed an increase in serum ferritin level after receiving therapy but the difference wasn't significant between baseline and endpoint values (673 ng/mL (381–1408) and 1044 ng/mL (506–1563.5), respectively). On other hand, the TCZ-HCQ group showed a significant decrease in ferritin level post-treatment (540.5 ng/mL (377.5–1082) and 337 ng/mL (280.3–626.8), p < 0.001, respectively) as shown in Fig. 3 .
Fig. 1.
C-reactive protein (CRP) level (mg/dl) before and after receiving therapy (data are expressed as mean ± SE. TCZ-HCQ; tocilizumab plus hydroxychloroquine treatment group, TCZ-RMV; tocilizumab plus remdesivir treatment group.
(*) significantly different when compared with baseline CRP level before receiving TCZ-HCQ at p < 0.001.
(#) significantly different when compared with baseline CRP level before receiving TCZ-RMV at p < 0.001.
Fig. 2.
PaO2/FiO2 (P/F) ratio before and after receiving therapy (data are expressed as mean ± SE. TCZ-HCQ; tocilizumab plus hydroxychloroquine treatment group, TCZ-RMV; tocilizumab plus remdesivir treatment group.
(*) significantly different when compared with baseline P/F ratio before receiving TCZ-HCQ at p < 0.001.
(#) significantly different when compared with baseline P/F ratio before receiving TCZ-RMV at p < 0.001.
Fig. 3.
Ferritin level (ng/mL) before and after receiving therapy (data are expressed as mean ± SE. TCZ-HCQ; tocilizumab plus hydroxychloroquine treatment group, TCZ-RMV; tocilizumab plus remdesivir treatment group.
(*) significantly different when compared with baseline ferritin level before receiving TCZ-HCQ at p < 0.001.
($) significantly different when compared with endpoint ferritin level after receiving TCZ-HCQ at p < 0.001.
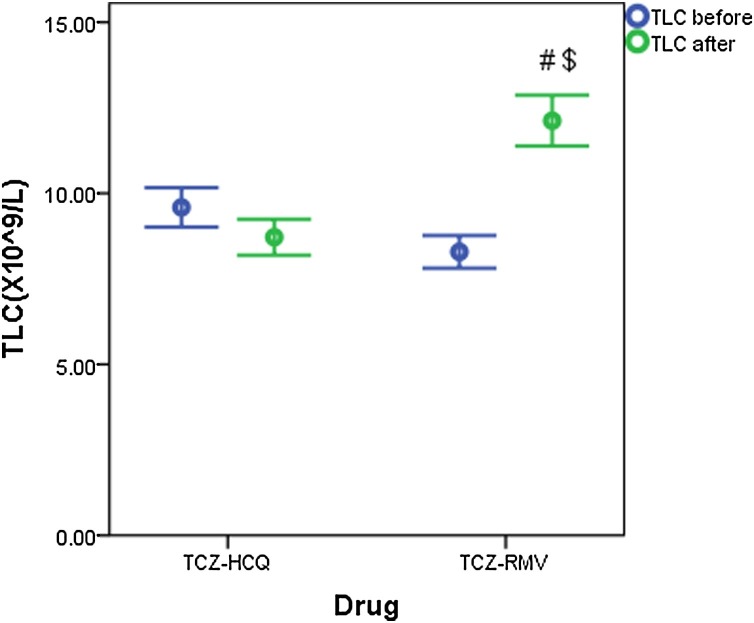
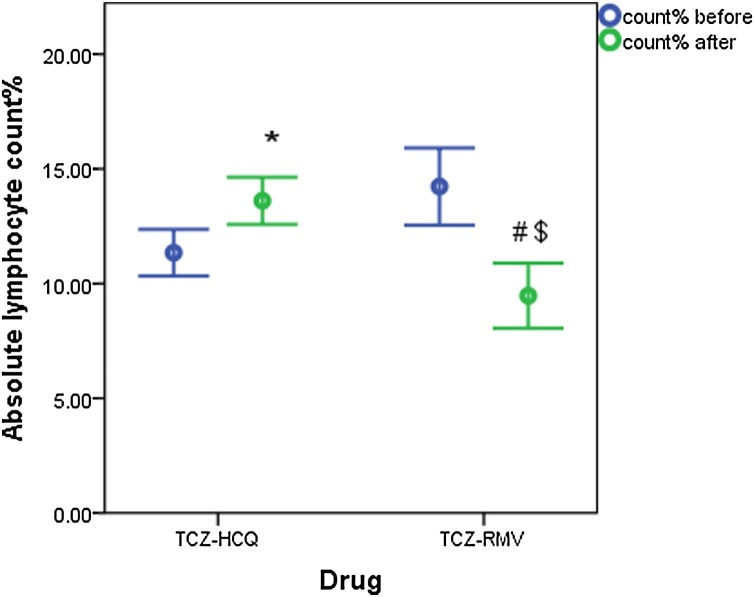
Fig. 4, Fig. 5 show the change in TLC and absolute lymphocyte count% in both treatment groups before and after receiving therapy. Liver enzymes (ALT and AST) are significantly increased in both treatment groups after therapy (Table 2 ).
Fig. 4.
Total leucocyte count (×109/L) before and after receiving therapy (data are expressed as mean ± SE. TCZ-HCQ; tocilizumab plus hydroxychloroquine treatment group, TCZ-RMV; tocilizumab plus remdesivir treatment group.
(#) significantly different when compared with baseline TLC before receiving TCZ-RMV at p < 0.001.
($) significantly different when compared with endpoint TLC after receiving TCZ-HCQ at p < 0.001.
Fig. 5.
Absolute lymphocyte count before and after receiving therapy (data are expressed as mean ± SE. TCZ-HCQ; tocilizumab plus hydroxychloroquine treatment group, TCZ-RMV; tocilizumab plus remdesivir treatment group.
(*) significantly different when compared with baseline lymphocyte count before receiving TCZ-HCQ at p = 0.013.
(#) significantly different when compared with baseline lymphocyte count before receiving TCZ-RMV at p = 0.025.
($) significantly different when compared with endpoint lymphocyte count after receiving TCZ-HCQ at p = 0.002.
Table 2.
General lab findings before (baseline) and after (endpoint) receiving therapy.
| Clinical parameter | Normal range | Baseline TCZ-HCQ | Baseline TCZ-RMV | P-Value | Endpoint TCZ-HCQ | Endpoint TCZ-RMV | P-value |
|---|---|---|---|---|---|---|---|
| C-reactive protein level, mg/dl | 0−8 | 97 (67.8−139.2) | 125 (43.7−210.8) | 0.12 | 26 (13.7−79.3) | 20.1 (6.4−40.7) | 0.07 |
| Ferritin level, ng/mL | Male | 540.5 (377.5−1082) | 673 (381−1408) | 0.49 | 337 (280.3−626.8) | 1044 (506−1563.5) | <0.001 |
| 12−300 | |||||||
| Female | |||||||
| 12−150 | |||||||
| D-Dimer level, μg/mL | 0−0.5 | 0.48 (0.34−0.88) | 0.58 (0.28−1.2) | 0.41 | 0.23 (0.13−0.68) | 0.52 (0.32−2.5) | <0.001 |
| PaO2/FiO2 (P/F) ratio | >400 | 120.5 (99.6−218.8) | 113.5 (91.3−176.8) | 0.08 | 295.5 (182.3−386) | 280 (115−325.3) | 0.25 |
| Lactate dehydrogenase level (LDH), IU/L | 120−250 | 516 (351.8−710) | 397 (270−518) | 0.002 | 312 (251.8−465.5) | 370 (279−637) | 0.049 |
| TLC(×109/L) | 1.1−3.2 | 10.1 (5.2−13.2) | 7.6 (5.5−10.4) | 0.15 | 7.7 (6.1−10.7) | 10.7 (8.2−15.3) | <0.001 |
| Absolute lymphocyte count% | 18−45% | 9 (6−15) | 13 (9−17) | 0.07 | 11.5(9−20) | 8 (6−9) | 0.002 |
| ALT(IU/L) | 7−40 | 38 (24−57) | 37 (24−52) | 0.85 | 75.5 (48.5−201.8) | 68 (45.8−201.8) | 082 |
| AST(IU/L) | 13−35 | 42 (26−63) | 39.8 (27−60) | 0.93 | 55.5 (32.3−88.3) | 53 (31.3−87.3) | 0.77 |
Data are expressed as median (IQR).
A weak correlation was found between the P/F ratio and mechanical ventilation need at entry (r = 0.321, p = 0.004) and between the P/F ratio and heart failure comorbidity (r = 0.322, p = 0.004) in the TCZ-HCQ group. While LDH level following TZC-HCQ therapy was weakly correlated with baseline O2 saturation (r = −0.343, p < 0.001) and heart failure (r = −0.319, p = 0.004).
Shifting to study outcomes (Table 3 ), the length of hospital stay (LOS) was approximately similar in both groups where the median days of hospitalization (IQR) were 10 (6–16) and 8 (5–12) for TCZ-HCQ and TCZ-RMV groups, respectively. Therapeutic failure was identified with 15 patients (26.8%) in the TCZ-HCQ group and 16 patients (30.8%) in the TCZ-RMV group but the difference wasn't significant between both groups. Of those patients who experienced therapeutic failure, 12 patients (21.4%) and 15 patients (28.8%) regrettably died in the TCZ-HCQ group and TCZ-RMV, respectively. Only weak correlations were identified in both treatment groups between study outcomes and baseline patient characteristics. The length of hospital stay (LOS) was weakly correlated with diabetes comorbidity (r = −0.32, p = 0.005) and with ICU admission (r = −0.335, p = 0.004) in the TCZ-HCQ group. Additionally, therapeutic failure showed weak correlations with baseline O2 saturation (r = 0.375, p = 0.002), hypertension comorbidity (r = −0.418, p = 0.002) and heart failure (r = 0.393, p = 0.004) in TCZ-HCQ group. On the other hand, therapeutic failure in the TCZ-RMV group was weakly correlated with baseline O2 saturation (r = 0.328, p = 0.005), initial supplemental oxygen therapy (r=-0.305, p = 0.029), and mechanical ventilation need at entry (r = 0.305, p = 0.029). Furthermore, Mortality in the TCZ-HCQ group was weakly correlated with mechanical ventilation need at entry (r = 0.406, p = 0.003) and heart failure comorbidity (r = 0.456, p = 0.001). Similarly, a weak correlation was identified in the TCZ-RMV group between death and baseline O2 saturation (r = 0.366, p = 0.002). Some complications have emerged only in TCZ-RMV following treatment: secondary bacterial infections occurred in 22 patients (42.3%), myocarditis identified in 8 patients (15.4%) and finally, pulmonary embolism occurred in 4 patients (7.7%).
Table 3.
Clinical observational indices.
| TCZ-HCQ | TCZ-RMV | P-value | |
|---|---|---|---|
| Length of hospitalization (days) | 10 (6−16) | 8 (5−12) | 0.06 |
| Death (n, %) | 12 (21.4%) | 15 (28.8%) | 0.4 |
| Patient discharge after improvememt (n, %) | 44 (78.6%) | 37 (71.2%) | 0.4 |
Discussion
In this prospective, cohort study of cases with severe COVID-19, patients were randomly allocated into two treatment groups; either receiving TCZ-HCQ or TCZ-RMV. Both of them showed significant clinical improvement in CRP level and P/F ratio after completion of study doses with nearly similar LOS and mortality. It could be attributed to the significant therapeutic effects of TCZ which prove its superiority when combined with the standard management of moderate to severe COVID-19 cases as repeatedly reported in recent studies [18,22]. As a monoclonal antibody, it targets the IL-6 pathways to calm the inflammatory storm and decrease mortality [23,24]. Moreover, both HCQ and RMV were proven to prevent the in-vitro SARS-CoV-2 growth owing to non-specific, antiviral activity [25].
On the other hand, the decline in LDH level, D-Dimer and ferritin level was only significant in the TCZ-HCQ group. Even worse, the TCZ-RMV group showed a non-significant increase in serum ferritin level after receiving therapy. The design of this study may contribute to the poor clinical outcomes of the TCZ-RMV group due to significant differences in baseline clinical parameters between the two groups including; higher age, serum creatinine, mechanical ventilation need, ICU admission, and lower oxygen saturation% in the TCZ-RMV group. Also, there was an unequal distribution of gender in both two groups (including males more than females). The COVID-19 infection more commonly affects older males with comorbidities and can cause fatal respiratory disorders including acute respiratory distress syndrome (ARDS) [[26], [27], [28]]. On the other hand, more favorable outcomes were expected to be achieved with females if compared to males [29]. Moreover, the higher need for oxygen therapy and mechanical ventilation results in increasing admission in the ICU and affects clinical outcomes despite receiving therapy [27,30].
Although the number of COVID-19 patients studied for HCQ in trials was found to be significantly larger than the number of COVID-19 patients studied for RMV; RMV has been reported as an active agent against SARS-CoV-2 in vitro testing. Also, an adequate clinical safety profile for RMV was reported in about 500 healthy volunteers and patients infected with acute Ebola virus [31,32]. Also, a previous study has found that RMV can decrease the risk of progression and shorten the time needed for recovery in COVID-19 patients who are early diagnosed with increased risk of hyper inflammation and the need for oxygen supplementation [33]. In our study's findings, there was no significant difference between TCZ-HCQ and TCZ-RMV in both therapeutic failure and mortality rates which is indicating that both HCQ and RMV could be considered equally safe when used for treating severe COVID-19 patients. The mortality rate in both groups was comparable to many previous studies [18,27], higher than some reported rates of 4.3–11% [26,30], and lower than others reporting mortality of 60.5% [34], which is attributed to different patient characteristics [18]. On the other hand, other previous studies did not agree with the clinical efficacy of TCZ, RMV, and HCQ in the treatment of COVID-19 patients [[35], [36], [37]].
Liver enzymes (ALT and AST) significantly increased in both treatment groups after therapy. No superiority of RMV in liver injury was found, which disagrees with previous findings [38,39]. This could be explained on the basis that antivirals were proven to cause hepatocellular injury [39,40]. Also, there are limited and contradictory data regarding the use of TCZ in COVID-19 treatment in clinical practice. A previous study reported Liver injury and hepatic interactions after TCZ administration in COVID-19 cases, another study observed clinical improvement of respiratory symptoms without any adverse events with its use, while a previous case series reported a resolution of liver function elevation, resulted from previous treatment with different antivirals or as an inflammatory response; after 3 weeks of administration of TCZ [[41], [42], [43]]. Also, it was repeatedly reported that SARS-CoV-2 infection contributes to aminotransferase elevation which necessitates close monitoring of liver functions in COVID-19 patients especially those with prior liver disease [39,[44], [45], [46]]. In addition, previous studies found an exponential increase in aminotransferases with increased disease severity of COVID-19 cases or increased need for intensive care unit admission [47,48]. Consequently, the definite cause of liver function elevation in all studied patients is difficult to be determined, but it is advisable to make a follow-up for aminotransferases before, during, and after treatment in COVID-19 patients to avoid liver injury.
However, some complications emerged only after treatment with TCZ-RMV including secondary bacterial infections, myocarditis, and pulmonary embolism. Although a previous study, in the United States (US), reported superinfections upon treatment with TCZ in mechanically ventilated patients with COVID 19 [49]; our study demonstrated superinfection only in the TCZ-RMV group. This difference can be attributed to the increased number of mechanically ventilated patients in the TCZ-RMV group. A previous study agreed with this finding, as it identified secondary infections in 13.5%–44% of mechanically ventilated ICU patients with COVID-19 [50].
Several studies indicated that cardiac events and myocarditis were common in hospitalized patients due to COVID-19 infection and this was attributed to the elevation in cardiac enzymes as troponin and lactate dehydrogenase (LDH) [51,52]. On the other hand, elevation in the level of troponin was observed in hospitalized COVID-19 patients taking remdesivir. Besides, these adverse events were more common for patients who were mechanically ventilated compared to those who were not [31]. Wang et al. showed that the use of remdesivir therapy was related to hyponatremia and hypokalemia which was the leading cause of discontinuing remdesivir in some patients [53]. In addition, as in our study, Rafaniello et al. showed that remdesivir was associated with a two-fold increased risk in cardiac adverse reactions compared to both hydroxychloroquine and azithromycin [54]. Again, the elevated number of mechanically ventilated patients in the TCZ-RMV group, compared to the TCZ-HCQ group, might contribute to the detection of myocarditis either due to mechanical ventilation or the use of RMV. Taking into consideration the level of LDH as a cardiac enzyme, although the baseline of LDH was elevated in the TCZ-HCQ group than the other group, the decline in LDH level was only significant in the TCZ-HCQ. This can be attributed to the increased need for mechanical ventilation in the TCZ-RMV group. Further studies are required to examine the impact of both HCQ or RMV regarding the appearance of myocarditis taking into consideration the need for mechanical ventilation of patients to clarify their safety.
Although all patients included in the study had cytokine storm, pulmonary embolism was recognized only in the TCZ-RMV group. According to a previous study, despite the reduction in the inflammatory markers by using TCZ and following a proactive anticoagulative way in treatment, in SARS-CoV-2 patients with acute respiratory distress syndrome, thrombotic events were also observed. This can raise concerns regarding the role played by COVID-19 in the coagulative pathways [55]. This fact has been reflected in our study in the arm that included a higher number of mechanically ventilated patients with a higher need for ICU admission. Consequently, it can be assumed that an increased need for ICU or mechanical ventilation may be followed by an increased risk of thrombotic events.
A previous study has been found that the baseline characteristics of patients, such as hypertension and older age, could affect treatment outcomes especially increased mortality [56]. On the other hand, in the present study population, only weak correlations were identified in both treatment groups between study outcomes and baseline patient characteristics in agreement with previous findings, which demonstrated that coexisting conditions, days of symptoms, sex, and enrollment region before initiation of treatment had no significant association with clinical improvement [31]. One explanation of that is the fact that SARS-CoV-2 appears to have tropism for diverse tissues including primarily the respiratory tract but also the heart, brain, liver, and kidney [48].
Conclusion
TCZ-HCQ and TCZ-RMV combinations have reasonable clinical efficacy in the treatment of severe COVID-19 patients with cytokine storm with a low percent of clinical failure. The need for mechanical ventilation and ICU should be taken into consideration in the follow-up of patients with cytokine storm, as it may constitute a risk for COVID-19 complications as myocarditis, superinfections, elevated liver enzymes, and pulmonary embolism.
CRediT authorship contribution statement
Rania M Sarhan: Conceptualization, Methodology, Writing – original draft preparation, Investigation, Writing – review & editing. Hadeer S. Harb: Data curation, Software, Writing – original draft preparation. Ahmed E. Abou Warda: Data curation, Visualization, Supervision. Mounir M. Salem-Bekhit: Reviewing and Funding. Faiyaz Shakeel: Reviewing and Funding. Sami Ali Alzahrani: Reviewing and Funding; Yasmin M. Madney: Data curation, Software, Writing – original draft preparation. Marian S. Boshra: Conceptualization, Methodology, Writing – original draft preparation, Investigation, Writing – review & editing.
Competing interests
None.
Acknowledgments
“The authors extend their appreciation to the Vice Deanship of Research Chair at King Saud University, Saudi Arabia for funding the work through Kayyali Chair for Pharmaceutical Industry, grant number MS-2021”.
References
- 1.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klopfenstein T., Zayet S., Lohse A., Balblanc J.-C., Badie J., Royer P.-Y., et al. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Médecine Mal Infect. 2020;50(5):397–400. doi: 10.1016/j.medmal.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziaie S., Koucheck M., Miri M., Salarian S., Shojaei S., Haghighi M., et al. Review of therapeutic agents for the treatment of COVID-19. J Cell Mol Anesth. 2020;5(1):32–36. [Google Scholar]
- 4.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases. Lancet Infect Dis. 2003;3(11):722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung H., Bobba R., Su J., Shariati-Sarabi Z., Gladman D.D., Urowitz M., et al. The protective effect of antimalarial drugs on thrombovascular events in systemic lupus erythematosus. Arthritis Rheum. 2010;62(3):863–868. doi: 10.1002/art.27289. [DOI] [PubMed] [Google Scholar]
- 7.Kashour Z., Riaz M., Garbati M.A., AlDosary O., Tlayjeh H., Gerberi D., et al. Efficacy of chloroquine or hydroxychloroquine in COVID-19 patients: a systematic review and meta-analysis. J Antimicrob Chemother. 2021;76(1):30–42. doi: 10.1093/jac/dkaa403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayerbe L., Risco-Risco C., Ayis S. The association of treatment with hydroxychloroquine and hospital mortality in COVID-19 patients. Intern Emerg Med. 2020;15(8):1501–1506. doi: 10.1007/s11739-020-02505-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta H.B., An H., Andersen K.M., Mansour O., Madhira V., Rashidi E.S., et al. Use of hydroxychloroquine, remdesivir, and dexamethasone among adults hospitalized with COVID-19 in the United States: a retrospective cohort study. Ann Intern Med. 2021 doi: 10.7326/M21-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown A.J., Won J.J., Graham R.L., Dinnon K.H., III, Sims A.C., Feng J.Y., et al. Broad-spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res. 2019;169 doi: 10.1016/j.antiviral.2019.104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCreary E.K., Angus D.C. Efficacy of Remdesivir in COVID-19. JAMA. 2020;324(11):1041–1042. doi: 10.1001/jama.2020.16337. [DOI] [PubMed] [Google Scholar]
- 12.Elsawah H.K., Elsokary M.A., Abdallah M.S., ElShafie A.H. Efficacy and safety of remdesivir in hospitalized Covid-19 patients: systematic review and meta-analysis including network meta-analysis. Rev Med Virol. 2021;31(4):e2187. doi: 10.1002/rmv.2187. [DOI] [PubMed] [Google Scholar]
- 13.Ohl M.E., Miller D.R., Lund B.C., Kobayashi T., Miell K.R., Beck B.F., et al. Association of remdesivir treatment with survival and length of hospital stay among US veterans hospitalized with COVID-19. JAMA Network Open. 2021;4(7) doi: 10.1001/jamanetworkopen.2021.14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vegivinti C.T.R., Pederson J.M., Saravu K., Gupta N., Barrett A., Davis A.R., et al. Remdesivir therapy in patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials. Ann Med Surg. 2021 doi: 10.1016/j.amsu.2020.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spinner C.D., Gottlieb R.L., Criner G.J., López J.R.A., Cattelan A.M., Viladomiu A.S., et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324(11):1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott L.J. Tocilizumab: a review in rheumatoid arthritis. Drugs. 2017;77(17):1865–1879. doi: 10.1007/s40265-017-0829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scavone C., Brusco S., Bertini M., Sportiello L., Rafaniello C., Zoccoli A., et al. Current pharmacological treatments for COVID-19: what’s next? Br J Pharmacol. 2020;177(21):4813–4824. doi: 10.1111/bph.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarhan R.M., Madney Y.M., Abou Warda A.E., Boshra M.S. Therapeutic efficacy, mechanical ventilation, length of hospital stay, and mortality rate in severe COVID‐19 patients treated with tocilizumab. Int J Clin Pract. 2021 doi: 10.1111/ijcp.14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu X., Han M., Li T., Sun W., Wang D., Fu B., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kafaja S., Clements P.J., Wilhalme H., Tseng C.-h, Furst D.E., Kim G.H., et al. Reliability and minimal clinically important differences of FVC. Results from the scleroderma lung studies (SLS-I and SLS-II) Am J Respir Crit Care Med. 2018;197(5):644–652. doi: 10.1164/rccm.201709-1845OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sciascia S., Aprà F., Baffa A., Baldovino S., Boaro D., Boero R., et al. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in severe patients with COVID-19. Clin Exp Rheumatol. 2020;38(3):529–532. [PubMed] [Google Scholar]
- 23.Fu B., Xu X., Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med. 2020;18(1):1–5. doi: 10.1186/s12967-020-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID‐19: a single-center experience. J Med Virol. 2020;92(7):814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Million M., Lagier J.-C., Gautret P., Colson P., Fournier P.-E., Amrane S., et al. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille, France. Travel Med Infect Dis. 2020;35 doi: 10.1016/j.tmaid.2020.101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallo Marin B., Aghagoli G., Lavine K., Yang L., Siff E.J., Chiang S.S., et al. Predictors of COVID‐19 severity: a literature review. Rev Med Virol. 2021;31(1):1–10. doi: 10.1002/rmv.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scully E.P., Haverfield J., Ursin R.L., Tannenbaum C., Klein S.L. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020:1–6. doi: 10.1038/s41577-020-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mulangu S., Dodd L.E., Davey Jr R.T., Tshiani Mbaya O., Proschan M., Mukai D., et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381(24):2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whittington M.D., Campbell J.D. Alternative pricing models for remdesivir and other potential treatments for COVID-19. Inst Clin Econ Rev. 2020:1–7. [Google Scholar]
- 34.Yang X., Yu Y., Xu J., Shu H., Liu H., Wu Y., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinot M., Jary A., Fafi-Kremer S., Leducq V., Delagreverie H., Garnier M., et al. Remdesivir failure with SARS-CoV-2 RNA-dependent RNA-polymerase mutation in a B-cell immunodeficient patient with protracted Covid-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen J., Liu D., Liu L., Liu P., Xu Q., Xia L., et al. A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19. Journal of Zhejiang University (Medical Science). 2020;49(2):215–219. doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tirupathi R., Bharathidasan K., Areti S., Kaur J., Salim S., Al-Tawfiq J.A. The shortcomings of tocilizumab in COVID-19. Infez Med. 2020;28(4):465–468. [PubMed] [Google Scholar]
- 38.Yapali S. What hepatologists need to know about COVID-19? Hepatology. 2020;2:41–43. doi: 10.14744/hf.2020.2020.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zampino R., Mele F., Florio L.L., Bertolino L., Andini R., Galdo M., et al. Liver injury in remdesivir-treated COVID-19 patients. Hepatol Int. 2020;14(5):881–883. doi: 10.1007/s12072-020-10077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cichoż-Lach H., Michalak A. Liver injury in the era of COVID-19. World J Gastroenterol. 2021;27(5):377. doi: 10.3748/wjg.v27.i5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gatti M., Fusaroli M., Caraceni P., Poluzzi E., De Ponti F., Raschi E. Serious adverse events with tocilizumab: Pharmacovigilance as an aid to prioritize monitoring in COVID-19. Br J Clin Pharmacol. 2021;87(3):1533–1540. doi: 10.1111/bcp.14459. [DOI] [PubMed] [Google Scholar]
- 42.Di Giambenedetto S., Ciccullo A., Borghetti A., Gambassi G., Landi F., Visconti E., et al. Off‐label use of tocilizumab in patients with SARS‐CoV‐2 infection. J Med Virol. 2020 doi: 10.1002/jmv.25897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Serviddio G., Villani R., Stallone G., Scioscia G., Foschino-Barbaro M.P., Lacedonia D. <? covid19?> Tocilizumab and liver injury in patients with COVID-19. Therap Adv Gastroenterol. 2020;13 doi: 10.1177/1756284820959183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao B., Ni C., Gao R., Wang Y., Yang L., Wei J., et al. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11(10):771–775. doi: 10.1007/s13238-020-00718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lozano-Sepulveda S.A., Galan-Huerta K., Martínez-Acuña N., Arellanos-Soto D., Rivas-Estilla A.M. SARS-CoV-2 another kind of liver aggressor, how does it do that? Ann Hepatol. 2020;19(6):592–596. doi: 10.1016/j.aohep.2020.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y., Liu S., Liu H., Li W., Lin F., Jiang L., et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73(4):807–816. doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang S., Wang R., Li L., Hong D., Ru R., Rao Y., et al. Liver injury in critically ill and non-critically ill COVID-19 patients: a multicenter, retrospective, observational study. Front Med. 2020;7:347. doi: 10.3389/fmed.2020.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guan W-j, Ni Z.-y, Hu Y., Liang W.-h, Ou C-q, He J.-x, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Somers E.C., Eschenauer G.A., Troost J.P., Golob J.L., Gandhi T.N., Wang L., et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis. 2021;73(2):e445–e454. doi: 10.1093/cid/ciaa954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clancy C.J., Nguyen M.H. Coronavirus disease 2019, superinfections, and antimicrobial development: what can we expect? Clin Infect Dis. 2020;71(10):2736–2743. doi: 10.1093/cid/ciaa524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manocha K.K., Kirzner J., Ying X., Yeo I., Peltzer B., Ang B., et al. Troponin and other biomarker levels and outcomes among patients hospitalized with COVID‐19: derivation and validation of the HA2T2 COVID‐19 mortality risk score. J Am Heart Assoc. 2021;10(6) doi: 10.1161/JAHA.120.018477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agdamag A.C.C., Edmiston J.B., Charpentier V., Chowdhury M., Fraser M., Maharaj V.R., et al. Update on COVID-19 myocarditis. Medicina. 2020;56(12):678. doi: 10.3390/medicina56120678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Idhrees A.M., Suthakaran P.K., Valooran G.J., Bashir M. Will remdesivir reshape cardiovascular practice in COVID 19 era? Int J Angiol. 2021;30(02):155–159. doi: 10.1055/s-0040-1721403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rafaniello C., Ferrajolo C., Sullo M.G., Gaio M., Zinzi A., Scavone C., et al. Cardiac events potentially associated to remdesivir: an analysis from the european spontaneous adverse event reporting system. Pharmaceuticals. 2021;14(7):611. doi: 10.3390/ph14070611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Atallah B., El Nekidy W., Mallah S.I., Cherfan A., AbdelWareth L., Scavone C., et al. Thrombotic events following tocilizumab therapy in critically ill COVID-19 patients: a Façade for prognostic markers. Thromb J. 2020;18(1):1–6. doi: 10.1186/s12959-020-00236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zangrillo A., Beretta L., Scandroglio A.M., Monti G., Fominskiy E., Colombo S., et al. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc. 2020;22(3):200–211. doi: 10.1016/S1441-2772(23)00387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]