Abstract
Psychiatric sequelae substantially contribute to the post-acute burden of disease associated with COVID-19, persisting months after clearance of the virus. Brain imaging shows white matter (WM) hypodensities/hyperintensities, and the involvement of grey matter (GM) in prefrontal, anterior cingulate (ACC) and insular cortex after COVID, but little is known about brain correlates of persistent psychopathology.
With a multimodal approach, we studied whole brain voxel-based morphometry, diffusion-tensor imaging, and resting-state connectivity, to correlate MRI measures with depression and post-traumatic distress (PTSD) in 42 COVID-19 survivors without brain lesions, at 90.59 ± 54.66 days after COVID. Systemic immune-inflammation index (SII) measured in the emergency department, which reflects the immune response and systemic inflammation based on peripheral lymphocyte, neutrophil, and platelet counts, predicted worse self-rated depression and PTSD, widespread lower diffusivity along the main axis of WM tracts, and abnormal functional connectivity (FC) among resting state networks. Self-rated depression and PTSD inversely correlated with GM volumes in ACC and insula, axial diffusivity, and associated with FC.
We observed overlapping associations between severity of inflammation during acute COVID-19, brain structure and function, and severity of depression and post-traumatic distress in survivors, thus warranting interest for further study of brain correlates of the post-acute COVID-19 syndrome. Beyond COVID-19, these findings support the hypothesis that regional GM, WM microstructure, and FC could mediate the relationship between a medical illness and its psychopathological sequelae, and are in agreement with current perspectives on the brain structural and functional underpinnings of depressive psychopathology.
Keywords: COVID-19, SARS-COV-2, Magnetic resonance imaging, Depression, Anxiety, PTSD, Grey matter, White matter, Diffusion-tensor imaging, Resting state, Functional connectivity
1. Introduction
Consistent prospective clinical studies affirm that neuropsychiatric sequelae substantially contribute to the post-acute burden of disease associated with COVID-19 (Nalbandian et al., 2021; Mazza et al., 2020). Over 30% of COVID-19 survivors show clinical depression and post-traumatic distress that persist for months after virus clearance and discharge from the hospital, and leads to the need for psychiatric intervention and psychotropic medication (Taquet et al., 2020, 2021; Mazza et al., 2021). In the first months after recovery from the acute phase, psychopathology associates with neurocognitive impairment in multiple domains, both predicted by the systemic inflammation during the acute phase and by its pattern of change over time (Mazza et al., 2020, 2021; Benedetti et al., 2021; Yuan et al., 2020). Beyond COVID, influenza and viral pneumonia are recognized among common physical diseases preceding the onset of a major depressive episode (Kaplan et al., 2009; Okusaga et al., 2011), and persistent low-grade inflammation in the absence of active medical illness is increasingly recognized as a core biological underpinning of depressive psychopathology in ‘endogenous’ mood disorders, such as major depressive disorder (MDD) and bipolar disorder (BD), affecting both pathogenesis and therapy outcomes (Branchi et al., 2021; Benedetti et al., 2020; Miller and Raison, 2016).
The brain magnetic resonance imaging (MRI) correlates of depressive psychopathology have been extensively studied (Spellman and Liston, 2020). During a major depressive episode, patients show abnormalities in neural response to emotional stimuli (Matt et al., 1992; Chamberlain and Sahakian, 2006; Elliott et al., 2000, 2002; Leppänen, 2006), while recovery from depression associates with the normalization of brain cortico-limbic neural responses and functional connectivity in brain areas implicated in the control of emotions and in the cognitive generation of affect (Davidson et al., 2002, 2003; Benedetti et al., 2007, 2009; Vai et al., 2015, 2016). Abnormal neural responses are paralleled by reduced regional grey matter (GM) volumes at MRI voxel-based morphometry (VBM), affecting in particular the anterior cingulate cortex (ACC) (Matsuo et al., 2019; Vai et al., 2020; Wise et al., 2017; Bora et al., 2012), and by abnormal resting state functional connectivity suggesting circuit dysfunction mainly involving the default mode network (DMN), dorsolateral prefrontal cortex, orbitofrontal cortex, ACC, insula, amygdala, hippocampus, basal ganglia, thalamus, and cerebellum (Spellman and Liston, 2020; Scalabrini et al., 2020; Vai et al., 2019). Consistent findings in patients with post-traumatic stress disorder (PTSD) also affirm aberrant GM reduction (Li et al., 2014; Meng et al., 2016) and resting-state connectivity (Koch et al., 2016; Sheynin et al., 2021) in corticolimbic structures, largely overlapping with those implicated in mood disorders. Moreover, GM volume reduction and altered connectivity associate with neuropsychiatric symptoms in inflammatory medical conditions (Schrepf et al., 2018; Schweinhardt et al., 2008), suggesting that regional GM microstructure and function could mediate the relationship between a medical illness and its psychopathological sequelae.
A consistent literature also associates mood disorders and PTSD with white matter microstructure as studied with Diffusion Tensor Imaging (DTI), associating both conditions with a decrease in fractional anisotropy of water diffusion (FA) in both conditions, and a decrease in diffusivity along the main axis of the fibers (axial diffusivity, AD) with an increase in mean and radial diffusivity (MD, RD) in mood disorders (Siehl et al., 2018; Benedetti and Bollettini, 2014; Ju et al., 2020; Favre et al., 2019), spread in large brain regions and including cortico-limbic circuitries. These measures reflect the myelination, orientational coherence, and microtubular axonal structure of fibers, and associate with peripheral markers of inflammation in mood disorders, suggesting disruption of myelin sheaths and axonal pathology (Benedetti et al., 2016, 2020).
Notwithstanding the consistent literature about brain correlates of neurological symptoms in patients who suffered brain damage during the acute phase of COVID-19 illness, little is known about the brain correlates of depression and post-traumatic distress in COVID-19 survivors (Nalbandian et al., 2021). Beyond the abnormalities in the olfactory system, systematic reviews showed diffuse cerebral WM hypodensities/hyperintensities, and the involvement of prefrontal, ACC and insular cortex (Egbert et al., 2020; Najt et al., 2021): these regions have been consistently implicated in mood and anxiety disorders, thus raising the hypothesis that psychopathological symptoms after COVID-19 could correlate with their structure and function. A very recent study associated PTSD after COVID with reduced volumes of the left hippocampus and amygdala (Tu et al., 2021). With a multimodal brain imaging approach, here we studied whole brain VBM, DTI, and resting-state connectivity, to correlate MRI measures with psychopathological ratings in COVID-19 survivors.
2. Material and methods
2.1. Participants and data collection
We studied 50 participants surviving COVID-19, from January 7 to July 13, 2021, during an ongoing prospective cohort study on COVID-19 sequelae at IRCCS San Raffaele Hospital in Milan (De Lorenzo et al., 2020). Diagnosis of COVID-19 was made after clinical and radiological findings at the admission to the Emergency Department, as confirmed by positive real-time reverse-transcriptase polymerase chain reaction from a nasopharyngeal and/or throat swab. To keep a naturalistic study design, exclusion criteria were limited to exceeding age range 18–70, having needed Intensive Care Unit treatment during the acute phase; and the presence of coarse brain disorder prior to the onset of COVID-19, or recent brain lesions typical of COVID such as multifocal cortical infarcts or microhaemorrhagic lesions (Pezzini and Padovani, 2020). Written informed consent was obtained from all participants, and the institutional review board approved the study in accordance with the principles in the Declaration of Helsinki.
Depressive psychopathology was self-rated on the Zung Self-Rating Depression Scale (ZSDS) (Zung, 1965), which thanks to its behavioral and somatic orientation in assessing depressive symptomatology proved a valid instrument to differentiate depressed from non-depressed groups in the general population (Sepehry and Michalos, 2014), including COVID-19 survivors (Mazza et al., 2020, 2021; Benedetti et al., 2021; Yuan et al., 2020; Nie et al., 2020), and on the 13-item Beck Depression Inventory (BDI). Post-traumatic symptomatology was self-rated on the Impact of Event Scale- Revised (IES-R) (Creamer et al., 2003), which is reliable and valid to measure subjective distress in the dimensions of intrusion, avoidance, and hyperarousal after traumatic experiences (Kolokotroni and Michalos, 2014), and to detect acute and persistent distress in COVID-19 survivors (Mazza et al., 2020, 2021). MRI was obtained within one week after the clinical assessment.
Inflammatory markers at hospital admission and discharge during acute COVID-19 were extracted from charts levels: C-reactive protein (CRP), and systemic immune-inflammation index (SII) (SII = platelets X neutrophils/lymphocytes), which previous prospective research associated with severity of psychopathology up to three months after hospital discharge (Mazza et al., 2021; Benedetti et al., 2021).
2.2. MRI data processing and statistical methods
All imaging was performed on a 3.0 T scanner (Ingenia CX, Philips, The Netherlands) using a 32-channel sensitivity encoding (SENSE) head coil. T1 and T2-weighted FLAIR images were visually inspected as part of the quality check procedure. WM hyperintensities were first observed upon visual inspection and later confirmed via the SPM12 toolbox LST (v. 3.0.0), using the Lesion growth algorithm LGA (Schmidt et al., 2012) with the default threshold 0.3. Brain atrophy was observed and quantified via the FSL 6 package Sienax v. 2.6 (Smith et al., 2002).
For VBM, structural T1-weighted MRI scans were acquired at baseline using a T1-weighted magnetization-prepared rapid gradient-echo (MP-RAGE) sequence (TR 2500 ms, TE 4.6 ms, yielding 220 transversal slices with a thickness of 0.8 mm), and processed with the CAT12 toolbox (http://dbm.neuro.uni-jena.de/cat), integrated in the SPM12 software package (Statistical Parametric Mapping, Institute of Neurology, London, UK). Voxel-based morphometry (VBM) statistics were carried out within the general linear model (GLM) framework, to associate markers of inflammation and severity of psychopathology with regional GM volumes. Age, sex, and total intracranial volume were added as nuisance covariates. We searched the whole brain for differences surviving a threshold, family-wise error (FWE) corrected for multiple comparisons, of pFWE<0.05 at the cluster level.
For DTI, we used SE Echo-planar imaging (EPI) and the following parameters: TR/TE = 5900/78 ms, FoV (mm) 240 (ap), 129 (fh), 232 (rl); acquisition matrix 2.14 × 2.73 × 2.30; 56 contiguous, 2.3 mm thick axial slices reconstructed with in-plane pixel size 1.88 × 1.88 × 2.30 mm; SENSE acceleration factor = 2; 1 b0 and 40 non-collinear directions of the diffusion gradients; b value = 1000 s/mm2. Fat saturation was performed to avoid chemical shift artifacts. Image analyses and tensor calculations were done using the “Oxford Center for Functional Magnetic Resonance Imaging of the Brain Software Library” (FSL 6.0; www.fmrib.ox.ac.uk/fsl/index.html) (Smith et al., 2004; Woolrich et al., 2009). Anisotropy of water diffusion was estimated through the application of diffusion-sensitizing gradients and calculation of elements of the diffusion tensor matrix, i.e. the three eigenvalues λ1, λ2 and λ3 (Le Bihan, 2003; Taylor et al., 2004; Basser et al., 1994), representing the tendency to diffuse along the principal direction of the fiber (axial diffusivity, AD, λ1); perpendicular to axonal walls and myelin sheaths (radial diffusivity, RD, the average of λ2 and λ3); independent of tissue directionality (average molecular motion: mean diffusivity, MD, average of λ1, λ2 and λ3). Fractional anisotropy (FA) was calculated as the square root of the sum of squares (SRSS) of the diffusivity differences, divided by the SRSS of the three diffusivities. Estimated FA, eigenvector, and eigenvalue maps (least-square fits) were skeletonized and transformed into a common space as used in Tract-Based Spatial Statistics (TBSS). All volumes were nonlinearly warped to the FMRIB58_FA template, and normalized to the Montreal Neurological Institute (MNI) space, to create a mean FA skeleton and warping individual FA values onto it. The resulting tract invariant skeletons were fed into voxelwise permutation-based cross-subject statistics. Multiple regressions on DTI measures were performed with voxelwise nonparametric permutation-based testing, with markers of inflammation and severity of psychopathology as factors, and with age, sex, and time elapsed from COVID diagnosis to the MRI scan as covariates. Threshold-free cluster enhancement (TFCE) (Smith and Nichols, 2009) was used to avoid defining arbitrary cluster-forming thresholds and smoothing levels. Data were tested against an empirical null distribution generated by 5000 permutations for each contrast, thus providing statistical maps fully corrected for multiple comparisons across space. Corrected p < 0.05 in a minimum cluster size of k = 100 was considered significant.
Scanning sessions for resting-state fMRI images comprised 200 sequential T2∗-weighted volumes (interleaved ascending transverse slices covering whole brain, tilted 30° downward with respect to bicommissural line to reduce susceptibility artifacts in orbitofrontal region), acquired using an EPI pulse sequence (TR = 2000 ms; TE = 30 ms; flip angle = 85°; field of view = 192 mm; number of slices = 38; slice thickness = 3.7 mm; matrix size = 64 x 62 reconstructed up to 96 x 96 pixels). Six dummy scans before fMRI acquisition allowed obtaining longitudinal magnetization equilibrium. Total time acquisition was 6 min and 56 s. We studied whole-brain functional connectivity (FC) patterns with multivariate pattern analysis (MVPA), which can detect neuroimaging-based biomarkers of mood disorders (Zeng et al., 2012) and the effects of biomarkers of brain damage (Thompson et al., 2016), as implemented in the CONN Toolbox (http://web.mit.edu.sanraffaele.idm.oclc.org/swg/software.htm), to create multivariate connectivity maps for each subject by deriving seeds based on the data, and computing functional connectivity patterns for each voxel via a principal component analysis (PCA) on the connectivity patterns between each single voxel to all other voxels. We performed separate analyses exploring effects of SII, CPR, ZSDS, BDI, IES-R. All models included age, sex, (and days after blood draw for inflammatory markers) as nuisance covariates. Analyses were thresholdet at peak level: p < 0.001, uncorrected; cluster level: p < 0.05 FWE-corrected). This estimates between-subject variance in connectivity patterns in relation to severity of psychopathology and inflammatory markers, performed across all voxels' connectivity patterns, to identify clusters of voxels that displayed a similar between-subject variance of their spatial connectivity. Post-hoc analysis using significant MVPA clusters as seeds was performed to test which areas of the brain change their connectivity in relation to the independent variables. Considering the sample size, 4 PCA components were kept for each voxel.
Finally, to test their effects on severity of psychopathology, and considering the a priori expected significant interaction of independent factors (biomarkers of systemic inflammation, age, sex) and the non-normal distribution of inflammatory biomarkers, independent variables were entered into a Generalized Linear Model (GLZM) analysis of homogeneity of variances with an identity link function (McCullagh and Nelder, 1989). Parameter estimates were obtained with iterative re-weighted least squares maximum likelihood procedures (Agresti, 1996; Dobson, 1990). The quality of the statistical model was checked using the entropy maximization principle of the Akaike information criterion (AIC) (Akaike, 1974).
3. Results
Clinical and demographic characteristics of the studied sample are resumed in Table 1. Eight patients were excluded from the study after MRI acquisitions: 2 because of multifocal cortical lesions, suggestive of micro-thromboses during acute COVID-19; 3 because of signs of cerebrovascular disease with a diffuse pattern of WM hyperintensities (WMH volumes of the excluded subjects: 8.7 ml, 10.13 ml, 10.34 ml; average WMH volume of the included sample: 0.7 ± 0.9 ml); 2 because of brain atrophy; 1 because of movement artifacts.
Table 1.
Clinical and demographic characteristics of the sample; severity of depressive symptoms and post-traumatic distress; and medical comorbidities and cardiovascular risk factors.
| Variables | Statistics |
|---|---|
| Age | 54.86 7.89 |
| Sex (M/F) | 29/13 |
| Hospitalized (managed at home) | 37/5 |
| Duration of hospitalization | 17.96 ± 16.67 |
| CRP at hospitalization / discharge | 80.03 ± 76.86 / 7.50 ± 10.39 |
| SII at hospitalization / discharge | 1912.63 ± 1461.88 / 809.59 ± 570.32 |
| Time from COVID-19 symptoms onset to MRI scan | 90.59 ± 54.66 |
| Total Intracranial Volume (ml) | 1386.83 ± 158.21 |
| Normalized Brain Volume (ml) | 1385.02 ± 52.45 |
| White Matter Hyperintensities Volume (ml) | 0.70 ± 0.90 |
| White Matter Hyperintensities Number | 5.88 ± 3.97 |
| New antidepressant treatment (n - %) | 6–14.3% |
| Chronic antidepressant treatment (n - %) | 1–2.4% |
| ZSDS Index ≥50 (n - %) | 13–30.9% |
| ZSDS Index score | 44.99 ± 12.52 |
| BDI score ≥9 (n - %) | 8–19% |
| BDI score | 4.95 ± 5 |
| IES-R score ≥33 (n - %) | 15–35.7% |
| IES-R total score | 27.57 ± 22.91 |
| IES-R hyperarousal subscale mean score | 1.23 ± 1.14 |
| IES-R Avoidance subscale mean score | 1.44 ± 1.49 |
| IES-R Intrusion subscale mean score | 1.26 ± 1.16 |
| Overweight (BMI>25) / Obese subjects (BMI>30) | 26 (61.9%) / 7 (16.7%) |
| Current or former Smokers | 18 (42.9%) |
| Hypertension | 15 (35.7%) |
| Coronary artery disease | 4 (9.5%) |
| Dyslipidemia | 4 (9.5%) |
| Diabetes Mellitus, type 2 | 3 (7.1%) |
| Gout | 1 (2.4%) |
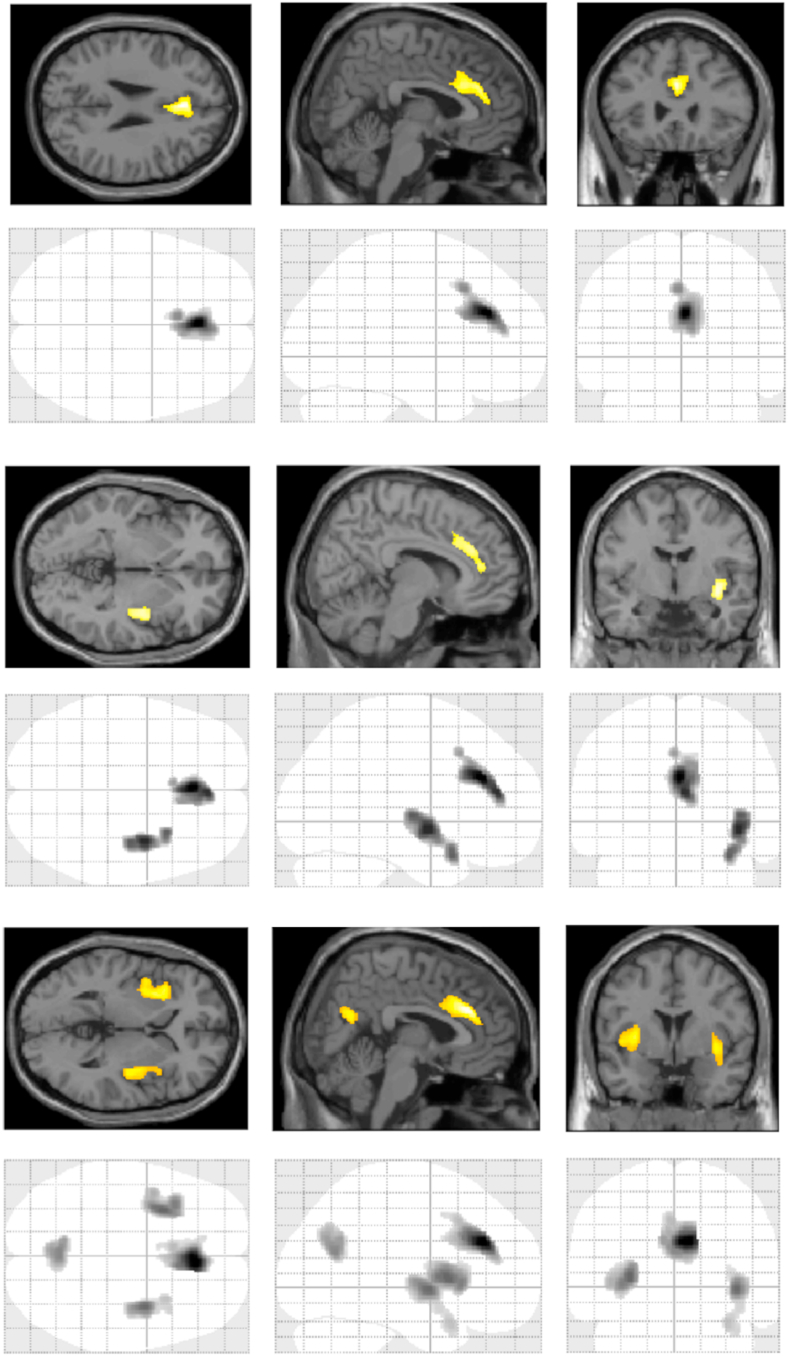
Severity of current psychopathology associated with regional GM volumes at VBM (Fig. 1). Both ZSDS, BDI, and IES-R scores negatively associated with GM in large clusters in bilateral ACC, encompassing Brodmann areas (BA) 24 and 32 (Table 2). BDI and IES-R scores also associated with GM in bilateral insular cortex. IES-R also associated with precuneus, the effects being driven by the hyperarousal and intrusion symptom dimensions, which correlated with GM in the same areas, while avoidance showed no effect.
Fig. 1.
Brain areas where severity of psychopathology associated with regional grey matter volumes. Top: Zung Self-rating Depression Scale (ZSDS); Middle: Beck Depression Inventory (BDI); Bottom: Impact of Event Scale (IES-R).
Table 2.
Results of VBM analysis. In all the listed brain areas, severity of psychopathology, as rated on ZSDS, BDI, and IES-R total and subscale scores, negatively associated with GM volumes. L = left; R = right; L/R = bilateral.
| Region | Brodmann Area (BA) | Peak MNI Coordinates | Cluster size (voxels) | t | Z | pFWE |
|---|---|---|---|---|---|---|
| ZSDS index scores | ||||||
| L/R ACC | 32, 24 | +0 +32 +26 | 1546 | 5.66 | 4.76 | 0.004 |
| BDI scores | ||||||
| L/R ACC | 32, 24 | +2 +30 +27 | 1239 | 4.72 | 4.14 | 0.011 |
| R Insula | 13 | +38 -3 -6 | 1105 | 4.50 | 3.98 | 0.017 |
| L Insula | 13 | -30 +12 +3 | 785 | 4.42 | 3.92 | 0.053 |
| IES-R total score | ||||||
| L/R ACC | 32, 24 | +6 +32 +27 | 1628 | 6.23 | 5.10 | 0.003 |
| L Insula | 13 | -30 +9 +4 | 1637 | 5.11 | 4.40 | 0.003 |
| R Insula | 13 | +38 -3 -3 | 1216 | 5.03 | 4.35 | 0.012 |
| Precuneus | 31 | 0 -63 +21 | 892 | 4.68 | 4.11 | 0.035 |
| IES-R - hyperarousal subscale score | ||||||
| L/R ACC | 32, 24 | +8 +32 +27 | 1458 | 6.66 | 5.34 | 0.005 |
| L Insula | 13 | -30 +10 +3 | 2102 | 5.49 | 4.65 | 0.001 |
| R Insula | 13 | +38 -3 -3 | 2081 | 5.09 | 4.39 | 0.001 |
| Precuneus | 31 | -9 -60 +28 | 1061 | 5.31 | 4.53 | 0.020 |
| IES-R - intrusion subscale score | ||||||
| L/R ACC | 32, 24 | +2 +30 +27 | 1532 | 6.12 | 5.04 | 0.004 |
| L Insula | 13 | -30 +9 +4 | 1520 | 4.95 | 4.30 | 0.005 |
| R Insula | 13 | +38 -3 -3 | 1497 | 5.01 | 4.33 | 0.005 |
| Precuneus | 31 | +2 -63 +21 | 1009 | 4.88 | 4.24 | 0.024 |
Inflammatory markers in the ED predicted post-acute psychopathology. A GLZM analysis of variance on ZSDS scores showed that in the best fitting model (forward entry/backward removal, AIC criterion) SII at hospital admission positively (b = 0.411, Wald W2 = 9.02, p = 0.0027), and its decrease during treatment negatively (b = −0.469, Wald W2 = 7.85, p = 0.0051) predicted them; accordingly, a highly significant effect was detected for SII at hospital discharge (b = 0.337, Wald W2 = 13.07, p = 0.0003). Similar opposite effects were detected for IES-R scores: a positive effect of SII at hospital discharge (b = 0.479, Wald W2 = 7.967, p = 0.0048), and a negative effect of delta SII (b = −0.053, Wald W2 = 3.997, p = 0.0456). In all these models, female sex robustly associated with worse severity (ZSDS: W2 = 10.842, p = 0.0010; IES-R: W2 = 9.332, p = 0.0023). Effects in the same direction were observed for CRP at hospital admission on ZSDS (Wald W2 = 7.954, p = 0.0048), but not on IES-R.
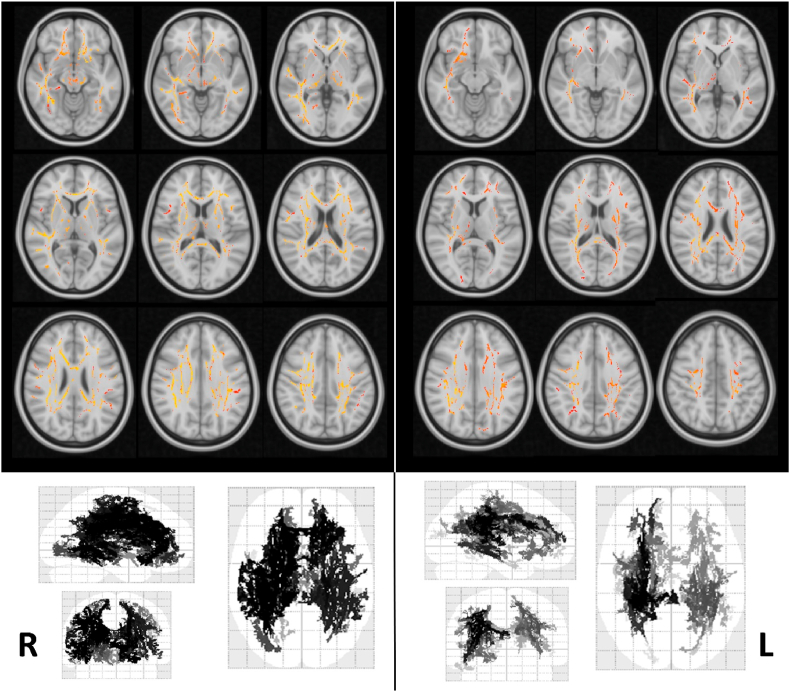
Inflammatory markers showed no significant effect on VBM, but predicted DTI measures of WM microstructure (Fig. 2). SII showed significant negative brain widespread associations with measures of water diffusion (AD, MD), with maximal effects in proportionally reducing AD in large portions of the WM skeleton. CRP also negatively associated with AD in spread brain WM tracts, with effects overlapping to those of SII (see detailed effects in Supplementary Table S1).
Fig. 2.
Widespread effects of biomarkers of inflammation at the admission in the emergency department (Left: SII; Right: CRP) on axial diffusivity in the white matter skeleton (TBSS analysis). Voxels surviving the statistical threshold of corrected p < 0.05 at TFCE are overlaid in radiologic projection on the 152 MNI T1 template and in glass brains.
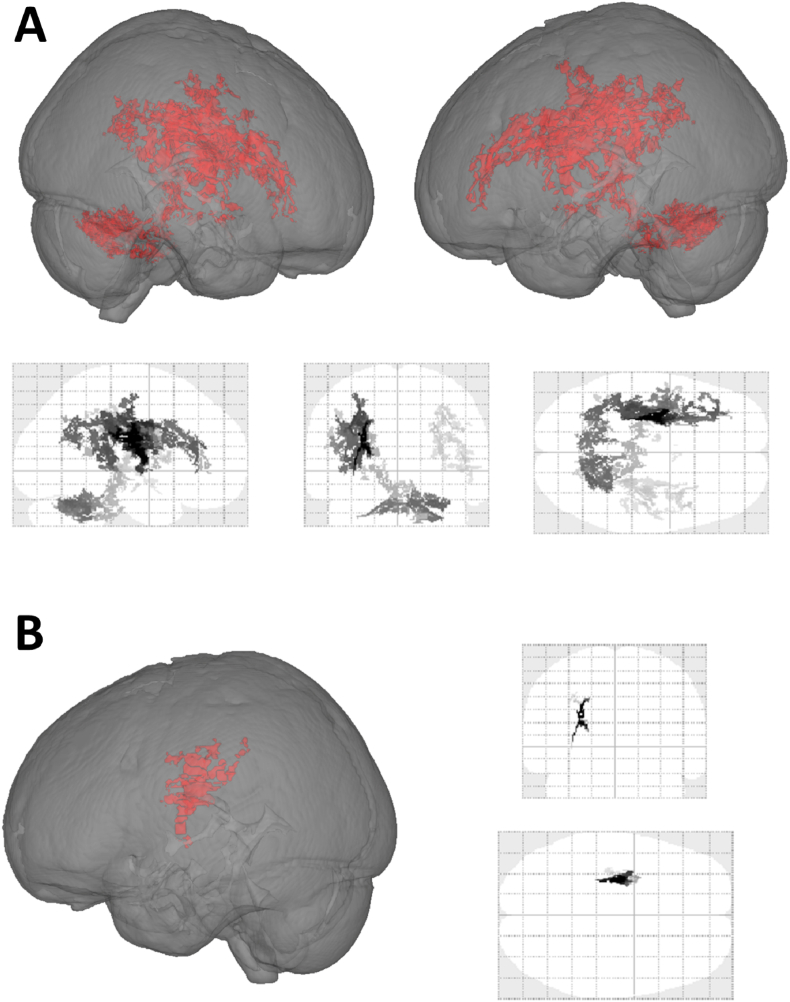
Severity of psychopathology also associated with WM microstructure in the same direction of inflammatory biomarkers. IES-R scores negatively associated with AD in several WM tracts in both hemispheres, with signal peaks in superior and posterior corona radiata and superior longitudinal fasciculus, but also involving Inferior longitudinal fasciculus, external capsule, anterior thalamic radiation. BDI negatively associated with AD in a small cluster including left superior corona radiata, superior longitudinal fasciculus, and posterior corona radiata (Fig. 3) (see detailed effects in Supplementary Table S2).
Fig. 3.
Negative association between severity of psychopathology (A, top: IES-R; B, bottom: BDI) with axial diffusivity in the white matter skeleton (TBSS analysis). Voxels surviving the statistical threshold of corrected p < 0.05 at TFCE are shown in a 3D rendering and in glass brains.
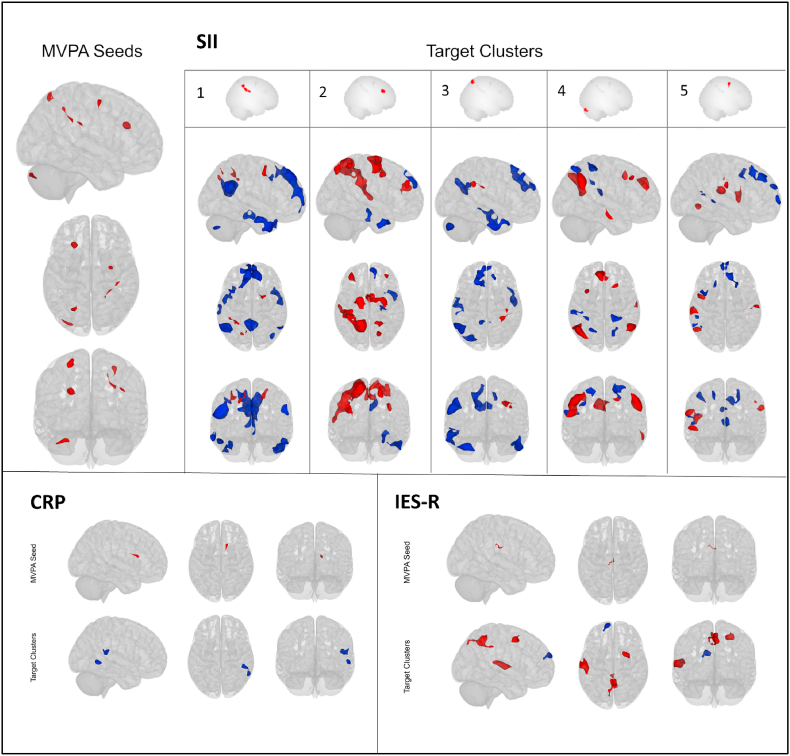
Inflammatory markers also influenced resting state FC (Table 3, Fig. 4). MVPA identified 5 seeds where SII significantly associated with rs-FC, located in left dorsolateral prefrontal cortex (salience network), inferior parietal sulcus (dorsal attentional network), cerebellum, right parietal lobe, and precentral gyrus (dorsal attentional network). Severity of inflammation negatively associated with rs-FC between these seeds and target clusters in the default mode network, language and frontoparietal networks; and positively associated with FC in dorsal attentional, salience, and sensorimotor network; with an opposite connectivity pattern for the seed in the cerebellum. CRP was negatively associated with rs-FC between right caudate and two clusters in right temporal cortex in the language network (see detailed effects in Supplementary Tables S3 and S4).
Table 3.
Resting-State seed regions significantly influenced by severity of psychopathology or inflammatory biomarkers according to the MVPA analyses, and related FC network according to the FSL Harvard-Oxford and AAL atlas.
| Variable of Interest | MNI coordinates (x,y,z) | Anatomical Label | Resting-State Network | Cluster size (voxels) | pFWE |
|---|---|---|---|---|---|
| IES-R | -8 -22 +32 | Cingulate Gyrus, anterior and posterior division | – | 77 | 0.016 |
| PCR | +14 +18 +16 | Caudate nucleus, Right | – | 77 | 0.015 |
| SII | +38 -28 +30 | Frontal and Parietal lobe, Right | – | 143 | <0.001 |
| -22 +24 +28 | Middle and superior Frontal Gyrus Left | Salience network | 135 | <0.001 | |
| -26 -68 +64 | Lateral Occipital Cortex, superior division Left | Dorsal Attention network | 106 | 0.002 | |
| -28 -88 -32 | Cerebellum Crus I/II, Left | Posterior Cerebellar Networks | 103 | 0.002 | |
| +30 -4 +56 | Precentral Gyrus Right | Dorsal Attention network | 80 | 0.012 |
Fig. 4.
Effects of inflammatory biomarkers (SII, CRP) and severity of post-traumatic distress (IES-R score) in reducing (blue) or enhancing (red) resting-state functional connectivity from MVPA seeds to target clusters. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Moreover, MVPA identified a seed in the dorsal cingulate cortex where severity of post-traumatic distress (IES-R score) negatively associated with connectivity to medial prefrontal cortex, and positively influenced connectivity to bilateral posterior cingulate cortex/precuneus in DMN, superior temporal cortex in sensorimotor network, and right middle frontal gyrus (see detailed effects in Supplementary Table S5).
4. Discussion
This is the first study to detect robust associations between anxiety and depression in the post-acute COVID-19 syndrome, inflammatory markers during acute COVID, brain regional GM volumes, DTI measures of WM microstructure, and resting-state functional connectivity. The main findings are that severity of depressive psychopathology associates with decreasing GM volumes in the ACC, while post-traumatic symptoms associate with decreasing GM volumes both, in the ACC and in bilateral insular cortex; and both associate with WM microstructure. Inflammation during acute COVID-19 predicted both, WM microstructure and current psychopathology several weeks after clearance of the virus. Moreover, resting-state FC associated both, with inflammation and psychopathology, confirming the brain functional implication of the structural effect.
These findings support the hypothesis that regional GM and WM microstructure and functional and structural connectivity could mediate the relationship between a medical illness and its psychopathological sequelae, and are in agreement with current perspectives on the brain structural and functional underpinnings of depressive psychopathology.
At the core of cortico-limbic structures involved in the control of emotions, in the cognitive generation of affect, and in the attribution of salience, the ACC and the insular cortex have been widely implicated in mood and post-traumatic stress disorders. In healthy subjects the ACC associates with the detection of unfavourable outcomes, response errors, response conflict, and decision uncertainty (Ridderinkhof et al., 2004). Smaller ACC volumes robustly associate with major depressive disorder (Matsuo et al., 2019; Vai et al., 2020; Wise et al., 2017; Bora et al., 2012), but are also proportional to the severity of mild depressive symptoms in the general population, as self-rated on the BDI (Webb et al., 2014), or observed (Hayakawa et al., 2014). Smaller ACC and insula GM volumes consistently associate with PTSD (O'Doherty et al., 2015; Bromis et al., 2018), are negatively impacted by the cumulative exposure to stress (Clausen et al., 2017; Butler et al., 2017), and can predict the occurrence of PTSD after mild brain trauma (Stein et al., 2021), with insular volumes decreasing over time together with PTSD worsening (Zantvoord et al., 2021). Our data now suggest that GM volumes in these structures also associate with post-acute COVID psychopathology, leading to the development of both, depressive and post-traumatic symptoms.
Previous prospective studies by our group showed that markers of inflammation during hospitalization predicted anxiety and depression up to three months after virus clearance (Mazza et al., 2021; Benedetti et al., 2021). Here we confirmed the same effects, with higher SII and CRP at hospitalization predicting the severity of both, persistent depressive symptoms and post-traumatic distress.
These same markers predicted widespread lower AD, with overlapping effects in several WM tracts. Effects were spread in large portions of the WM skeleton, as observed in patients with mood disorders (Siehl et al., 2018; Benedetti and Bollettini, 2014; Ju et al., 2020; Favre et al., 2019), in particular for the effects of inflammatory biomarkers on WM microstructure (Benedetti et al., 2016). Axial diffusivity represents the water diffusivity parallel to the axonal fibers, reflecting the greater freedom of water to diffuse along the principal fiber axis rather than to travel across the surrounding myelin sheaths. Both myelin and axonal microstructure, including microtubules and neurofilaments (Kinoshita et al., 1999), contribute to this diffusion anisotropy, with studies on neurodevelopment associating AD with fiber diameter or organization (Takahashi et al., 2000), and animal models associating a reduction of AD with axonal injury (Song et al., 2003; Sun et al., 2006; Boretius et al., 2012). We previously showed that neurons, and their axons, suffer during COVID-19, as revealed by higher blood levels of neurofilament light chain (NfL), a cytoskeletal intermediate filament protein of central and peripheral neurons which is released during disease progression in a variety of neurological diseases, and which can mark fatal brain damage during COVID-19 (De Lorenzo et al., 2021). It is then tempting to surmise that our observation of inverse relationship between AD post-COVID, and the severity of previous acute inflammation in the ED, could be the consequence of a slowly recovering axonal damage due to acute COVID-19. Further research and modelling, also using MRI techniques capable of discerning intra-from extra-neurite water diffusion (Pieri et al., 2021), are needed to clarify this issue.
These measures of WM microstructure also associated with severity of psychopathology. Both depressive symptoms and post-traumatic distress, as self-rated on BDI and IES-R, again associated with reduced AD. This observation supports the hypothesis that reduction in axonal integrity or directionality of WM tracts could influence post-COVID psychopathology, by mediating at the level of the brain the effects of inflammation on behavior. In patients with mood disorders the spread disruption of WM microstructure can change in parallel to the severity of depression and ameliorate after successful antidepressant treatment (Melloni et al., 2020). Further research will clarify if recovery from post-COVID symptomatology will be associated with changes in AD.
Effects of inflammation on DTI measures of WM microstructure were paralleled by effects on functional connectivity. Inflammation increased rs-FC within the dorsal attentional and salience networks, and between these networks and the sensorimotor network, which have all been implicated in depression and in PTSD. Interestingly, increased connectivity within Salience and Sensorimotor Networks has been previously associated to persistent physical pain and medical illness (Hegarty et al., 2020; Kutch et al., 2017), possibly reflecting an increased reliance of body ‘threat assessment’(83) and underpinning effects of persistent medical and psychopathological symptoms after COVID.
Higher SII also associated with a reduction of rs-FC between (i) DMN, language and frontoparietal networks, and (ii) the salience and dorsal attentional networks. Salience and dorsal attentional network exert an inhibitory influence on the DMN, inducing anticorrelations (Zhou et al., 2018), whereas frontoparietal system has been shown to mediate their interactions (Vincent et al., 2008; Spreng et al., 2010). Our results suggest that peripheral inflammatory status may be associated to an inhibition of DMN activity. Reduced rs-FC in this network was consistently associated to psychopathological conditions such as depression and PTSD (Koch et al., 2016; Yan et al., 2019). In line with these findings, in our sample post-traumatic symptomatology was associated with higher rs-FC between PCC/precuneus in the DMN and the mid portion of cingulate cortex, including dorsal ACC, a crucial brain region of the salience network (Seeley et al., 2007). Abnormal rs-FC in these regions was proposed as core neural underpinning of with PTSD (Koch et al., 2016), where increased FC in PCC/precuneus, rated soon after the traumatic experience, also predicted the onset of PTSD and its symptom severity several weeks after trauma (Lanius et al., 2010; Wang et al., 2012).
Finally, we detected that high level of CPR was associated to a reduced rs-FC between caudate and temporal cortex. Previous findings detected an inverse association between this inflammatory index and striatum rs-FC among patients with major depressive disorder (Felger et al., 2016), this effect may parallel the reduction of striatal dopamine release induced by inflammatory cytokines, as demonstrated in a monkey model of cytokine-induced depression (Felger et al., 2013).
Strengths of the present study include a focused research question, state-of-the-art imaging methods, a whole-brain study approach, and straightforward effects, but our results must be viewed in light of some methodological limitations. The relative small sample size and the monocentric recruitment in a single ethnic group, could affect the possibility of population stratifications limiting the generalizability of the findings. The widely heterogeneous drug treatments administered during the course of the illness could have influenced MRI measures. We obtained an excellent power to study the association of single psychopathological measures with MRI, but not to investigate their interaction with clinical and pharmacological variables. Finally, a word of caution is needed because of the correlational nature of neuroimaging studies, hampering interpretation of the neurobiological basis of GM and WM MRI measures (Furlan et al., 2019).
In conclusion, these limitations do not bias the main finding of overlapping associations of severity of inflammation during acute COVID-19, brain structure and function, and severity of depression and post-traumatic distress in survivors, thus warranting interest for further study of brain correlates of the post-acute COVID-19 syndrome. Beyond COVID-19, our results support current perspectives on the association between inflammation and depression, and on the usefulness of multimodal brain imaging to investigate its underpinnings.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2021.100387.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Agresti A. Wiley; New York: 1996. An Introduction to Categorical Data Analysis. [Google Scholar]
- Akaike H. A new look at the statistical model identification. IEEE Trans. Automat. Control. 1974;19(6):716–723. [Google Scholar]
- Basser P.J., Mattiello J., LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 1994;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F., Bollettini I. Recent findings on the role of white matter pathology in bipolar disorder. Harv. Rev. Psychiatr. 2014;22(6):338–341. doi: 10.1097/HRP.0000000000000007. [DOI] [PubMed] [Google Scholar]
- Benedetti F., Bernasconi A., Blasi V., Cadioli M., Colombo C., Falini A., et al. Neural and genetic correlates of antidepressant response to sleep deprivation - a functional magnetic resonance imaging study of moral valence decision, in bipolar depression. Arch. Gen. Psychiatr. 2007;64(2):179–187. doi: 10.1001/archpsyc.64.2.179. [DOI] [PubMed] [Google Scholar]
- Benedetti F., Radaelli D., Bernasconi A., Dallaspezia S., Colombo C., Smeraldi E. Changes in medial prefrontal cortex neural responses parallel successful antidepressant combination of venlafaxine and light therapy. Arch. Ital. Biol. 2009;147(3):83–94. [PubMed] [Google Scholar]
- Benedetti F., Poletti S., Hoogenboezem T.A., Mazza E., Ambree O., de Wit H., et al. Inflammatory cytokines influence measures of white matter integrity in Bipolar Disorder. J. Affect. Disord. 2016;202:1–9. doi: 10.1016/j.jad.2016.05.047. [DOI] [PubMed] [Google Scholar]
- Benedetti F., Aggio V., Pratesi M.L., Greco G., Furlan R. Neuroinflammation in bipolar depression. Front. Psychiatr. 2020;11:71. doi: 10.3389/fpsyt.2020.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F., Mazza M., Cavalli G., Ciceri F., Dagna L., Rovere-Querini P. Can cytokine blocking prevent depression in COVID-19 survivors? J. Neuroimmune Pharmacol. 2021;16(1):1–3. doi: 10.1007/s11481-020-09966-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E., Fornito A., Pantelis C., Yücel M. Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. J. Affect. Disord. 2012;138(1–2):9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- Boretius S., Escher A., Dallenga T., Wrzos C., Tammer R., Bruck W., et al. Assessment of lesion pathology in a new animal model of MS by multiparametric MRI and DTI. Neuroimage. 2012;59(3):2678–2688. doi: 10.1016/j.neuroimage.2011.08.051. [DOI] [PubMed] [Google Scholar]
- Branchi I., Poggini S., Capuron L., Benedetti F., Poletti S., Tamouza R., et al. Brain-immune crosstalk in the treatment of major depressive disorder. Eur. Neuropsychopharmacol. 2021;45:89–107. doi: 10.1016/j.euroneuro.2020.11.016. [DOI] [PubMed] [Google Scholar]
- Bromis K., Calem M., Reinders A.A., Williams S.C., Kempton M.J. Meta-analysis of 89 structural MRI studies in posttraumatic stress disorder and comparison with major depressive disorder. Am. J. Psychiatr. 2018;175(10):989–998. doi: 10.1176/appi.ajp.2018.17111199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler O., Adolf J., Gleich T., Willmund G., Zimmermann P., Lindenberger U., et al. Military deployment correlates with smaller prefrontal gray matter volume and psychological symptoms in a subclinical population. Transl. Psychiatry. 2017;7(2):e1031–e. doi: 10.1038/tp.2016.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain S.R., Sahakian B.J. The neuropsychology of mood disorders. Curr. Psychiatr. Rep. 2006;8(6):458–463. doi: 10.1007/s11920-006-0051-x. [DOI] [PubMed] [Google Scholar]
- Clausen A.N., Billinger S.A., Sisante J.-F.V., Suzuki H., Aupperle R.L. Preliminary evidence for the impact of combat experiences on gray matter volume of the posterior insula. Front. Psychol. 2017;8:2151. doi: 10.3389/fpsyg.2017.02151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creamer M., Bell R., Failla S. Psychometric properties of the impact of event scale - revised. Behav. Res. Ther. 2003;41(12):1489–1496. doi: 10.1016/j.brat.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Davidson R.J., Pizzagalli D., Nitschke J.B., Putnam K. Depression: perspectives from affective neuroscience. Annu. Rev. Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Davidson R.J., Irwin W., Anderle M.J., Kalin N.H. The neural substrates of affective processing in depressed patients treated with venlafaxine. Am. J. Psychiatr. 2003;160(1):64–75. doi: 10.1176/appi.ajp.160.1.64. [DOI] [PubMed] [Google Scholar]
- De Lorenzo R., Conte C., Lanzani C., Benedetti F., Roveri L., Mazza M.G., et al. Residual clinical damage after COVID-19: a retrospective and prospective observational cohort study. PLoS One. 2020;15(10) doi: 10.1371/journal.pone.0239570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorenzo R., Loré N.I., Finardi A., Mandelli A., Cirillo D.M., Tresoldi C., et al. Blood neurofilament light chain and total tau levels at admission predict death in COVID-19 patients. J. Neurol. 2021:1–7. doi: 10.1007/s00415-021-10595-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson A.J. Chapman & Hall; New York: 1990. An Introduction to Generalized Linear Models. [Google Scholar]
- Egbert A.R., Cankurtaran S., Karpiak S. Brain, behavior, and immunity; 2020. Brain Abnormalities in COVID-19 Acute/subacute Phase: a Rapid Systematic Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R., Rubinsztein J.S., Sahakian B.J., Dolan R.J. Selective attention to emotional stimuli in a verbal go/no-go task: an fMRI study. Neuroreport. 2000;11(8):1739–1744. doi: 10.1097/00001756-200006050-00028. [DOI] [PubMed] [Google Scholar]
- Elliott R., Rubinsztein J.S., Sahakian B.J., Dolan R.J. The neural basis of mood-congruent processing biases in depression. Arch. Gen. Psychiatr. 2002;59(7):597–604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- Favre P., Pauling M., Stout J., Hozer F., Sarrazin S., Abe C., et al. Widespread white matter microstructural abnormalities in bipolar disorder: evidence from mega-and meta-analyses across 3033 individuals. Neuropsychopharmacology. 2019;44(13):2285–2293. doi: 10.1038/s41386-019-0485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger J.C., Mun J., Kimmel H.L., Nye J.A., Drake D.F., Hernandez C.R., et al. Chronic interferon-α decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacology. 2013;38(11):2179–2187. doi: 10.1038/npp.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger J.C., Li Z., Haroon E., Woolwine B.J., Jung M.Y., Hu X., et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatr. 2016;21(10):1358–1365. doi: 10.1038/mp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlan R., Melloni E., Finardi A., Vai B., Di Toro S., Aggio V., et al. Natural killer cells protect white matter integrity in bipolar disorder. Brain Behav. Immun. 2019;81:410–421. doi: 10.1016/j.bbi.2019.06.037. [DOI] [PubMed] [Google Scholar]
- Hayakawa Y.K., Sasaki H., Takao H., Hayashi N., Kunimatsu A., Ohtomo K., et al. Depressive symptoms and neuroanatomical structures in community-dwelling women: a combined voxel-based morphometry and diffusion tensor imaging study with tract-based spatial statistics. Neuroimage: Clinica. 2014;4:481–487. doi: 10.1016/j.nicl.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty A.K., Yani M.S., Albishi A., Michener L.A., Kutch J.J. Salience network functional connectivity is spatially heterogeneous across sensorimotor cortex in healthy humans. Neuroimage. 2020;221:117177. doi: 10.1016/j.neuroimage.2020.117177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y., Ou W., Su J., Averill C.L., Liu J., Wang M., et al. White matter microstructural alterations in posttraumatic stress disorder: an ROI and whole-brain based meta-analysis. J. Affect. Disord. 2020;266:655–670. doi: 10.1016/j.jad.2020.01.047. [DOI] [PubMed] [Google Scholar]
- Kaplan H.I., Sadock B.J. In: Comprehensive Textbook of Psychiatry. ninth ed. Sadock B.J., Sadock V.A., editors. Williams & Wilkins Co; 2009. [Google Scholar]
- Kinoshita Y., Ohnishi A., Kohshi K., Yokota A. Apparent diffusion coefficient on rat brain and nerves intoxicated with methylmercury. Environ. Res. 1999 May;80(4):348–354. doi: 10.1006/enrs.1998.3935. [DOI] [PubMed] [Google Scholar]
- Koch S.B., van Zuiden M., Nawijn L., Frijling J.L., Veltman D.J., Olff M. Aberrant resting-state brain activity in posttraumatic stress disorder: a meta-analysis and systematic review. Depress. Anxiety. 2016;33(7):592–605. doi: 10.1002/da.22478. [DOI] [PubMed] [Google Scholar]
- Kolokotroni F. In: Encyclopedia of Quality of Life and Well-Being Research. Michalos A.C., editor. Springer; 2014. Impact of event scale; pp. 3102–3105. [Google Scholar]
- Kutch J.J., Ichesco E., Hampson J.P., Labus J.S., Farmer M.A., Martucci K.T., et al. Brain signature and functional impact of centralized pain: a multidisciplinary approach to the study of chronic pelvic pain (MAPP) network study. Pain. 2017;158(10):1979. doi: 10.1097/j.pain.0000000000001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius R., Bluhm R., Coupland N., Hegadoren K., Rowe B., Theberge J., et al. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr. Scand. 2010;121(1):33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat. Rev. Neurosci. 2003;4(6):469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Leppänen J.M. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr. Opin. Psychiatr. 2006;19(1):34–39. doi: 10.1097/01.yco.0000191500.46411.00. [DOI] [PubMed] [Google Scholar]
- Li L., Wu M., Liao Y., Ouyang L., Du M., Lei D., et al. Grey matter reduction associated with posttraumatic stress disorder and traumatic stress. Neurosci. Biobehav. Rev. 2014;43:163–172. doi: 10.1016/j.neubiorev.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Harada K., Fujita Y., Okamoto Y., Ota M., Narita H., et al. Distinctive neuroanatomical substrates for depression in bipolar disorder versus major depressive disorder. Cerebr. Cortex. 2019;29(1):202–214. doi: 10.1093/cercor/bhx319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt G.E., Vázquez C., Campbell W.K. Mood-congruent recall of affectively toned stimuli: a meta-analytic review. Clin. Psychol. Rev. 1992;12(2):227–255. [Google Scholar]
- Mazza M.G., De Lorenzo R., Conte C., Poletti S., Vai B., Bollettini I., et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav. Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., Palladini M., De Lorenzo R., Magnaghi C., Poletti S., Furlan R., et al. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021;94:138–147. doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh P., Nelder J.A. second ed. Chapman & Hall; New York: 1989. Generalized Linear Models. [Google Scholar]
- Melloni E.M., Poletti S., Dallaspezia S., Bollettini I., Vai B., Barbini B., et al. Changes of white matter microstructure after successful treatment of bipolar depression. J. Affect. Disord. 2020;274:1049–1056. doi: 10.1016/j.jad.2020.05.146. [DOI] [PubMed] [Google Scholar]
- Meng L., Jiang J., Jin C., Liu J., Zhao Y., Wang W., et al. Trauma-specific grey matter alterations in PTSD. Sci. Rep. 2016;6(1):1–9. doi: 10.1038/srep33748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H., Raison C.L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016;16(1):22. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najt P., Richards H.L., Fortune D.G. Brain, Behavior, & Immunity-Health. 2021. Brain imaging in patients with COVID-19: a systematic review; p. 100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., et al. Post-acute COVID-19 syndrome. Nat. Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie X.-D., Wang Q., Wang M.-N., Zhao S., Liu L., Zhu Y.-L., et al. Anxiety and depression and its correlates in patients with coronavirus disease 2019 in Wuhan. Int. J. Psychiatr. Clin. Pract. 2020:1–6. doi: 10.1080/13651501.2020.1791345. [DOI] [PubMed] [Google Scholar]
- O'Doherty D.C., Chitty K.M., Saddiqui S., Bennett M.R., Lagopoulos J. A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatr. Res. Neuroimaging. 2015;232(1):1–33. doi: 10.1016/j.pscychresns.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Okusaga O., Yolken R.H., Langenberg P., Lapidus M., Arling T.A., Dickerson F.B., et al. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J. Affect. Disord. 2011;130(1–2):220–225. doi: 10.1016/j.jad.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzini A., Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nat. Rev. Neurol. 2020;16(11):636–644. doi: 10.1038/s41582-020-0398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieri V., Sanvito F., Riva M., Petrini A., Rancoita P.M., Cirillo S., et al. Along-tract statistics of neurite orientation dispersion and density imaging diffusion metrics to enhance MR tractography quantitative analysis in healthy controls and in patients with brain tumors. Hum. Brain Mapp. 2021;42(5):1268–1286. doi: 10.1002/hbm.25291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof K.R., Ullsperger M., Crone E.A., Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306(5695):443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Scalabrini A., Vai B., Poletti S., Damiani S., Mucci C., Colombo C., et al. All roads lead to the default-mode network—global source of DMN abnormalities in major depressive disorder. Neuropsychopharmacology. 2020;45(12):2058–2069. doi: 10.1038/s41386-020-0785-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt P., Gaser C., Arsic M., Buck D., Förschler A., Berthele A., et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in multiple sclerosis. Neuroimage. 2012;59(4):3774–3783. doi: 10.1016/j.neuroimage.2011.11.032. [DOI] [PubMed] [Google Scholar]
- Schrepf A., Kaplan C.M., Ichesco E., Larkin T., Harte S.E., Harris R.E., et al. A multi-modal MRI study of the central response to inflammation in rheumatoid arthritis. Nat. Commun. 2018;9(1):1–11. doi: 10.1038/s41467-018-04648-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinhardt P., Kalk N., Wartolowska K., Chessell I., Wordsworth P., Tracey I. Investigation into the neural correlates of emotional augmentation of clinical pain. Neuroimage. 2008;40(2):759–766. doi: 10.1016/j.neuroimage.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepehry A.A. In: Encyclopedia of Quality of Life and Well-Being Research. Michalos A.C., editor. Springer; 2014. Self-rating depression scale (SDS) pp. 5790–5798. [Google Scholar]
- Sheynin S., Wolf L., Ben-Zion Z., Sheynin J., Reznik S., Keynan J.N., et al. Deep learning model of fMRI connectivity predicts PTSD symptom trajectories in recent trauma survivors. Neuroimage. 2021:118242. doi: 10.1016/j.neuroimage.2021.118242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siehl S., King J.A., Burgess N., Flor H., Nees F. Structural white matter changes in adults and children with posttraumatic stress disorder: a systematic review and meta-analysis. Neuroimage: Clinica. 2018;19:581–598. doi: 10.1016/j.nicl.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Zhang Y., Jenkinson M., Chen J., Matthews P.M., Federico A., et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17(1):479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H., et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Song S.K., Sun S.W., Ju W.K., Lin S.J., Cross A.H., Neufeld A.H. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20(3):1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Spellman T., Liston C. Toward circuit mechanisms of pathophysiology in depression. Am. J. Psychiatr. 2020;177(5):381–390. doi: 10.1176/appi.ajp.2020.20030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R.N., Stevens W.D., Chamberlain J.P., Gilmore A.W., Schacter D.L. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53(1):303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M.B., Yuh E., Jain S., Okonkwo D.O., Mac Donald C.L., Levin H., et al. Smaller regional brain volumes predict posttraumatic stress disorder at 3 months after mild traumatic brain injury. Biol. Psychiatr.: cognitive neuroscience and neuroimaging. 2021;6(3):352–359. doi: 10.1016/j.bpsc.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S.W., Liang H.F., Trinkaus K., Cross A.H., Armstrong R.C., Song S.K. Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn. Reson. Med. 2006;55(2):302–308. doi: 10.1002/mrm.20774. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Ono J., Harada K., Maeda M., Hackney D.B. Diffusional anisotropy in cranial nerves with maturation: quantitative evaluation with diffusion MR imaging in rats. Radiology. 2000;216(3):881–885. doi: 10.1148/radiology.216.3.r00se41881. [DOI] [PubMed] [Google Scholar]
- Taquet M., Luciano S., Geddes J.R., Harrison P.J. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. The Lancet Psychiatry. 2020;8(2):130–140. doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. The Lancet Psychiatry. 2021;8(5):416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W.D., Hsu E., Krishnan K.R., MacFall J.R. Diffusion tensor imaging: background, potential, and utility in psychiatric research. Biol. Psychiatr. 2004;55(3):201–207. doi: 10.1016/j.biopsych.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Thompson W.H., Thelin E.P., Lilja A., Bellander B.-M., Fransson P. Functional resting-state fMRI connectivity correlates with serum levels of the S100B protein in the acute phase of traumatic brain injury. Neuroimage: Clinica. 2016;12:1004–1012. doi: 10.1016/j.nicl.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y., Zhang Y., Li Y., Zhao Q., Bi Y., Lu X., et al. Post-traumatic stress symptoms in COVID-19 survivors: a self-report and brain imaging follow-up study. Mol. Psychiatr. 2021;20:1–6. doi: 10.1038/s41380-021-01223-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vai B., Poletti S., Radaelli D., Dallaspezia S., Bulgarelli C., Locatelli C., et al. Successful antidepressant chronotherapeutics enhance fronto-limbic neural responses and connectivity in bipolar depression. Psychiatr. Res. 2015;233(2):243–253. doi: 10.1016/j.pscychresns.2015.07.015. [DOI] [PubMed] [Google Scholar]
- Vai B., Bulgarelli C., Godlewska B.R., Cowen P.J., Benedetti F., Harmer C.J. Fronto-limbic effective connectivity as possible predictor of antidepressant response to SSRI administration. Eur. Neuropsychopharmacol. 2016;26(12):2000–2010. doi: 10.1016/j.euroneuro.2016.09.640. [DOI] [PubMed] [Google Scholar]
- Vai B., Bertocchi C., Benedetti F. Cortico-limbic connectivity as a possible biomarker for bipolar disorder: where are we now? Expert Rev. Neurother. 2019;19(2):159–172. doi: 10.1080/14737175.2019.1562338. [DOI] [PubMed] [Google Scholar]
- Vai B., Parenti L., Bollettini I., Cara C., Verga C., Melloni E., et al. Predicting differential diagnosis between bipolar and unipolar depression with multiple kernel learning on multimodal structural neuroimaging. Eur. Neuropsychopharmacol. 2020;34:28–38. doi: 10.1016/j.euroneuro.2020.03.008. [DOI] [PubMed] [Google Scholar]
- Vincent J.L., Kahn I., Snyder A.Z., Raichle M.E., Buckner R.L. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Sun Y-w, Wan J-q, Su S-s, Zhou Y., Xu J-r. A preliminary study of alterations in default network connectivity in post-traumatic stress disorder patients following recent trauma. Brain Res. 2012;1484:50–56. doi: 10.1016/j.brainres.2012.09.029. [DOI] [PubMed] [Google Scholar]
- Webb C.A., Weber M., Mundy E.A., Killgore W.D. Reduced gray matter volume in the anterior cingulate, orbitofrontal cortex and thalamus as a function of mild depressive symptoms: a voxel-based morphometric analysis. Psychol. Med. 2014;44(13):2833. doi: 10.1017/S0033291714000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise T., Radua J., Via E., Cardoner N., Abe O., Adams T., et al. Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Mol. Psychiatr. 2017;22(10):1455–1463. doi: 10.1038/mp.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M.W., Jbabdi S., Patenaude B., Chappell M., Makni S., Behrens T., et al. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45(1 Suppl. l):S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Yan C.-G., Chen X., Li L., Castellanos F.X., Bai T.-J., Bo Q.-J., et al. Reduced default mode network functional connectivity in patients with recurrent major depressive disorder. Proc. Natl. Acad. Sci. Unit. States Am. 2019;116(18):9078–9083. doi: 10.1073/pnas.1900390116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan B., Li W., Liu H., Cai X., Song S., Zhao J., et al. Correlation between immune response and self-reported depression during convalescence from COVID-19. Brain Behav. Immun. 2020;88:39–43. doi: 10.1016/j.bbi.2020.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zantvoord J.B., Zhutovsky P., Ensink J.B., den Kelder R.O., van Wingen G.A., Lindauer R.J. Trauma-focused psychotherapy response in youth with posttraumatic stress disorder is associated with changes in insula volume. J. Psychiatr. Res. 2021;132:207–214. doi: 10.1016/j.jpsychires.2020.10.037. [DOI] [PubMed] [Google Scholar]
- Zeng L.-L., Shen H., Liu L., Wang L., Li B., Fang P., et al. Identifying major depression using whole-brain functional connectivity: a multivariate pattern analysis. Brain. 2012;135(5):1498–1507. doi: 10.1093/brain/aws059. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Friston K.J., Zeidman P., Chen J., Li S., Razi A. The hierarchical organization of the default, dorsal attention and salience networks in adolescents and young adults. Cerebr. Cortex. 2018;28(2):726–737. doi: 10.1093/cercor/bhx307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zung W.W. A self-rating depression scale. Arch. Gen. Psychiatr. 1965;12(1):63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.