Abstract
The COVID-19 pandemic is an ongoing global pandemic of coronavirus disease 2019 as an atypical type of viral pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Many potential pharmacotherapies are currently being investigated against this disease. This article points to and justifies, the importance of investigating the potential therapeutic value of pharmacological agents acting on Toll-like Receptor (TLR) 7 and/or TLR8 as double-edged swords combating COVID-19. Induction of TLR7 and/or TLR8 may be investigated as a strategy to stimulate immunity and may be added to anti-COVID19 vaccines to cope with their current viral escape challenge. TLR7 stimulation may not only help viral clearance through Th1 antiviral responses but may also provide beneficial broncho- and vaso-dilatory, as well as, anti-inflammatory effects. Pharmacological compounds acting as TLR7 and/or TLR8 agonists may be of value if used by frontline healthcare workers with comorbidities who demonstrate mild symptoms of the disease. On the other hand, TLR7 and/or TLR8 antagonists may be used in combination with immune-modulatory/anti-inflammatory drugs in severe cases of the disease, with potential synergistic effects that could also help in reducing the doses of such therapies, and consequently their adverse effects.
Keywords: Agonists, Antagonists, COVID-19, TLR7, TLR8, Vaccines
Highlights
-
•
There is potential value in development of TLR7/8 agonists/antagonists as double-edged swords combating COVID-19.
-
•
TLR7/8 agonists, as immune-stimulants in mild disease, may also be added to preventive vaccines acting against viral escape.
-
•
TLR7/8 antagonists may be combined with immune-suppressants in severe cases for potential synergism and minimized toxicity.
1. Introduction
Toll-like receptors (TLRs) are one class of receptors that play a key role in the recognition of viral particles, activation of the innate immune system, and consequently developing better drugs for COVID-19 (Khanmohammadi and Rezaei, 2021). In humans, TLRs have ten family members some of which are located in the cell membrane, and the others are situated in endosomes, such as TLR3, TLR7, TLR8, and TLR9 (Lester and Li, 2014). As pathogen recognition receptors (PRRs), their role in the pathogenesis of pneumonia should be stressed since alveolar macrophages express TLRs and are among the first cells to sense and respond to pathogens that enter the lungs (Quinton and Mizgerd, 2015; Steiner et al., 2017). TLRs were specifically reported to play important role in the induction of immune responses which lead to intracellular bacterium Mycoplasma pneumoniae eradication (Naghib et al., 2018). In addition, TLR signaling is known to be involved in the inflammatory response of the lungs and there has been an association between mortality in elderly patients with severe pneumonia and decreased TLR2 and TLR4 expression (Tang et al., 2014). Worth noting is that we previously pointed to and justified the importance of investigating the potential pharmacotherapies for COVID-19 “pneumonia”, rather than the broad category of COVID-19 disease (Khalifa and Ghoneim, 2020). TLRs may also have an important role in COVID pneumonia since viral particles could induce coagulation disorders through TLR3 activation (Biswas and Khan, 2020). RNA viruses in general, including SARS-CoV-2 causing COVID, invade the innate immune system leading to infection, through TLRs 3, 7, and 8 (Angelopoulou et al., 2020). TLR7 and TLR8 are of special importance since they are phylogenetically and structurally very similar and both can recognize GU-rich sequences in single-stranded RNAs from viruses (Forsbach et al., 2011). TLR7/8 are also involved in a plethora of intracellular signaling cascades which culminate in the gene expression of pro-inflammatory cytokines and chemokines (Parker et al., 2007). In addition, TLR7/8 is coupled to the adaptor protein MyD88 which activates downstream NF-κB driven genes (Kawai and Akira, 2007). It was also revealed that women were affected by COVID-19 less than men and it was suggested that this may be through a different expression of TLRs, especially TLR7 and TLR8 (Conti and Younes, 2020). This was further explained by the levels of activation of the immune cells that are higher in women than in men, and it is correlated with the trigger of TLR7 that is higher in women than in men and its biallelic expression leads to higher immune responses and increases the resistance to viral infections. In addition, the X chromosome influences the immune system by acting on TLR8, among other proteins, which can be over-expressed in women, and influence the response to viral infections. Moreover, a higher frequency of rare TLR7 variants was reported in younger (<60 years) males with life-threatening COVID-19 than in a control group with asymptomatic or oligosymptomatic infection (Fallerini et al., 2021). Such deleterious TLR7 variants were suggested to account for up to 4% of severe disease in male subjects and these young males with TLR7 loss-of-function variants and severe COVID-19 represented a subset of male patients contributing to disease susceptibility in up to 2% of severe COVID-19.
1.1. TLR7/8 agonists
Antiviral effects of TLR7 agonists have been reported against human papillomavirus (HPV), herpes simplex virus (HSV), human immunodeficiency viruses (HIV), hepatitis C virus (HCV), and hepatitis B virus (HBV) (Biffen et al., 2012; EnosiTuipulotu et al., 2018; Pockros et al., 2007a). It was also suggested that the available agonists against TLR7 may prevent the onset of severe COVID-19 in symptomatic patients and synergize with active anti-viral therapy (Onofrio et al., 2020). It was also hypothesized that remdesivir may synergize with TLR7 agonists and that the signs of efficacy of TLR7 agonists in combination with antiviral therapy may be captured by an early drop in viral load. In addition, we hypothesized earlier that the inhibition of TLR7 receptor function, by hydroxychloroquine may partially explain the ineffectiveness of this medication as an individual intervention in mild COVID since TLR7 function is critical for the activation of an appropriate innate immune response against ssRNA viruses that is utterly needed early in the course of the disease (Khalifa and Ghoneim, 2021). Imiquimod; as an immune-stimulator that activates TLR7, was also suggested to be used to enhance innate and adaptive immunity (Angelopoulou et al., 2020). As a potential pharmacotherapy for COVID-19, TLR7 stimulation may not only help viral clearance through Th1 antiviral responses (Medzhitov, 2001; Akira and Takeda, 2004) but may also provide bronchodilating and anti-inflammatory effects because these receptors are expressed in the bronchial epithelial cells and airway smooth muscle where the initiation of a Th1 immune response activation of TLR7 rapidly relaxes airway smooth muscle via production of NO (Lebold et al., 2016; Ekman et al., 2011). The produced NO may also selectively dilate pulmonary arteries which allow for more blood flow to open alveoli for gas exchange which is crucially needed in COVID-19 pneumonia patients. TLR8 has been also reported to recognize ssRNA viruses such as Influenza, HIV, HCV, Sendai, and Coxsackie B viruses, and the receptor binding to the viral RNA recruits MyD88 and leads to activation of the transcription factor NF-κB and hence an antiviral response (Heil et al., 2004; Zhang et al., 2016). Therefore, pharmacological compounds acting as TLR7 and/or TLR8 agonists such as imidazoquinoline, thiazoquinolone, or benzoazepine analogs may be of value if administered in combination with immune-modulatory/anti-inflammatory drugs. Induction of TLR7 and/or TLR8 stimulation may be investigated as a strategy to stimulate the innate immune system against ssRNA viruses, and consequently developing better drugs for COVID-19 (Khanmohammadi and Rezaei, 2021; Medzhitov, 2001; Akira and Takeda, 2004; Lebold et al., 2016; Ekman et al., 2011; Heil et al., 2004; Zhang et al., 2016).
TLR7/8 agonists can also be used to stimulate a strong immune response when used as vaccine adjuvants able to induce cell-mediated immunity (Tiboni et al., 2021). Bharat Biotech's vaccine; Covaxin®, developed in collaboration with the Indian Council of Medical Research (ICMR) and the National Institute of Virology (NIV), is similar to Sinovac® and Sinopharm® COVID-19 vaccines in that it is based on well-established vaccine technology and after inactivation, it is adjuvated with an imidazoquinoline (IMDG) class molecule (TLR7 and TLR8 agonist) chemisorbed on alum (Algel) (Algel-IMDG) (Tiboni et al., 2021). Moreover, it is to be mentioned that TLR7 agonists have previously demonstrated substantial utility as an infectious disease vaccine adjuvant, particularly for influenza (Hung et al., 2016; Weldon et al., 2012). Then, selectively targeting TLRs 7/8 would be beneficial in coping with the major challenge of current anti-COVID19 vaccines which is viral escape (vaccine efficacy; 50–96%). The underlying immunostimulatory mechanism of new adjuvants targeting TLRs is by stimulating the innate immunity via production of cytokines/chemokines and type I interferons, followed by immediate development of adaptive immune responses so that host protection against the virus is strengthened and lasts longer (Khanmohammadi and Rezaei, 2021; Tiboni et al., 2021). A conceptual diagram illustrating the immuno-stimulatory effects of TLR7/8 agonists against SARS-CoV-2-induced mild-to-moderate COVID-19 is depicted in Fig. 1.
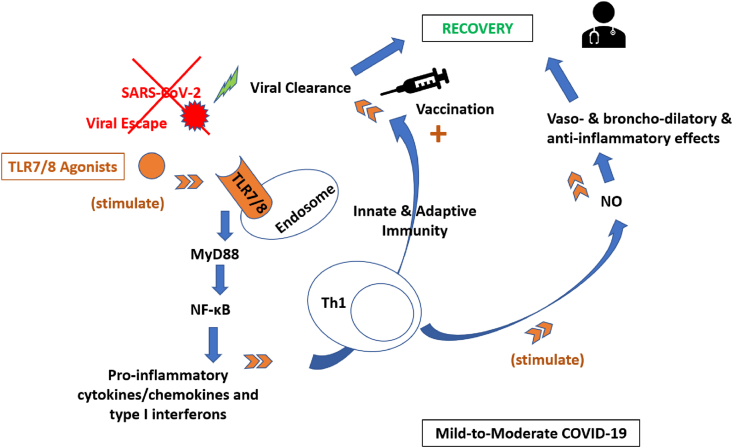
Fig. 1.
TLR7/8 agonists as immuno-stimulants against SARS-CoV-2-induced mild-to-moderate COVID-19.
TLR7/8, situated in endosomes, play a key role in the recognition of SARS-CoV-2 causing COVID-19. Through activation of the innate immune system, TLR7/8 are involved in a plethora of intracellular signaling cascades which culminate in the gene expression of pro-inflammatory cytokines and chemokines. In addition, TLR7/8 are coupled to the adaptor protein MyD88 which activates downstream NF-κB driven genes. TLR7/8 agonists have been reported active against several ssRNA viruses including SARS-CoV-2, through enhancing both innate and adaptive immunity. They may not only help early viral clearance through Th1 antiviral responses but may also provide broncho-dilating and anti-inflammatory effects via production of NO. Moreover, TLR7/8 agonists can be used as vaccine adjuvants able to induce humoral and cellular immunity coping with the major challenge of current anti-COVID19 vaccines which is viral escape. The underlying adjuvant immunostimulatory mechanism is by stimulating the innate immunity via production of cytokines/chemokines and type I interferons, followed by immediate development of adaptive immune responses so that host protection against the virus is strengthened and lasts longer.
COVID-19, coronavirus disease 2019; MYD88, myeloid differentiation primary response 88; NF-kB, nuclear factor k-light-chain-enhancer; NO, nitric oxide; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ssRNA, single strand RNA; Th1, T-helper 1 cells; TLR7/8, toll-like receptors 7 and/or 8.
The fate of TLR7/8 agonists’ use as therapeutic targets will depend heavily on whether their use is associated with sustained systemic harms and intolerable sickness-related side effects. Recent studies demonstrated that TLR7 stimulation is capable of inducing acute sickness responses, but more studies are necessary to investigate the mechanisms and determine whether negative responses occur after acute or chronic use and whether such negative responses are self-limited or sustained (Michaelis et al., 2019). Some clinical trials with TLR7 agonists observed manifestations associated with acute sickness responses, including fever and flu-like symptoms (Sauder et al., 2003). Several small-molecule TLR7/8 agonists have also been clinically approved or are under ongoing investigation such as imiquimod (Rodell et al., 2019) with some side effects (headache, fever) that could hamper their clinical efficacy upon repeated systemic dosing (Pockros et al., 2007b). AZD8848 is also a novel TLR7 agonist that has been designed as an ante-drug to restrict the effects of the drug to the site of administration and to minimize any potential side effects associated with systemic cytokine production while being used for the treatment of asthma and allergic rhinitis (Leaker et al., 2019). It is a metabolically labile ester that is topically active but is rapidly hydrolysed by butyrylcholinesterase to a much less active metabolite upon entry into the circulation (Khan et al., 2005). One study investigated the efficacy, safety, and tolerability of intranasal AZD8848 60 μg administered once-weekly for 8 weeks in patients with physician-diagnosed mild allergic asthma who were subsequently challenged with an inhaled allergen. The drug was administered intranasally to minimize any risk of local inflammatory effects in the lungs and to further reduce the risk of the systemic activity. The results of this study revealed that the incidence of adverse events was similar in the two groups and that AZD8848 was generally well tolerated (Leaker et al., 2019). Murine models were also used to investigate acute and chronic effects of a therapeutically relevant dose of the imidazoquinoline TLR7/8 agonist R848 that is similar to the FDA-approved imiquimod but has a considerably higher potency. The results of this study revealed that the drug sickness responses are not protracted and do not result in cell death since they started following the initial dosing of TLR7/8 agonist and then receptor tolerance occurred thereafter and the drug did not continue to provoke behavioral or molecular evidence of inflammation. The lack of detected adverse outcomes in these studies further supports the notion that TLR7 agonists could be safely used in both acute and chronic applications (Michaelis et al., 2019).
1.2. TLR7/8 antagonists
From another perspective, TLRs were suggested to play key roles in the pathogenesis of the cytokine release syndrome and they were hypothesized to be the main PRRs participating in the induction of cytokine storm in the COVID-19-infected patients (Safaei and Karimi-Googheri, 2020; Karimi-Googheri and Arababadi, 2014). Stimulation of viral-sensing toll-like receptors in vitro and administration of synthetic viral RNA in vivo induced features of hyper-inflammation, including cytokine elevation, immune cell airway infiltration, and MC-protease production (Gebremeskel et al., 2021). A phase IIa trial on the psoriasis patients revealed that using IMO-3100, an antagonist of TLR7, significantly modulated immune responses and down-regulated cytokine production in these patients (Kimball et al., 2013). Also, Merck as a leading science and technology company, announced that the U.S. Food and Drug Administration (FDA) has cleared its investigational new drug application (IND) for M5049, that blocks the activation of TLR7 and TLR8, for the treatment of patients with Covid-19 pneumonia (Merck, 2021). The company initiated a Phase II randomized, controlled clinical study evaluating the safety and efficacy of M5049 in this patient population at sites in the United States and Brazil based on the scientific hypothesis that activation of TLR7/8 leads to immune cell activation and inflammation, which when not properly controlled can cause severe immunopathology and that the intervention with the investigational drug may reduce life-threatening complications of Covid-19, including severe respiratory symptoms. Another study demonstrated that severe cases of COVID-19 differed from healthy controls and mild COVID-19 patients in exhibiting increased TLR7 activity (Root-Bernstein, 2021). Therefore, TLR7 and/or TLR8 antagonists could also be of value as add on therapies in severe cases of the disease since their corresponding TLR7 agonists were reported to induce high levels of Type-I interferons and the highest IFN-gamma levels were reported to be displayed by TLR8 and TLR7/8 agonists, which also induced the highest levels of pro-inflammatory cytokines IL-12, TNF-alpha, and IL-1 beta (Ghosh et al., 2006). In addition, TLR7 activation leads to the production of IL-1, IL-6, monocyte chemoattractant protein-1, MIP-1A, TNF-α, and type 1 IFN (Khanmohammadi and Rezaei, 2021; Yazdanpanah et al., 2020) and in cultured neurons, it was also reported to induce IL-6 and TNF-α expression through Myd88 (Liu et al., 2013). The combined administration of TLR7 and/or TLR8 antagonists with other biologics and/or pharmacotherapies classically used to target the cytokine storm, may have a synergistic effect that could help in reducing the dose of such therapies and consequently their adverse effects. It may also help in reducing the length of stay in the ICU for severe COVID patients which will have positive pharmacoeconomic value. A conceptual diagram illustrating the immuno-suppressant effects of TLR7/8 antagonists against SARS-CoV-2-induced severe COVID-19 is depicted in Fig. 2.
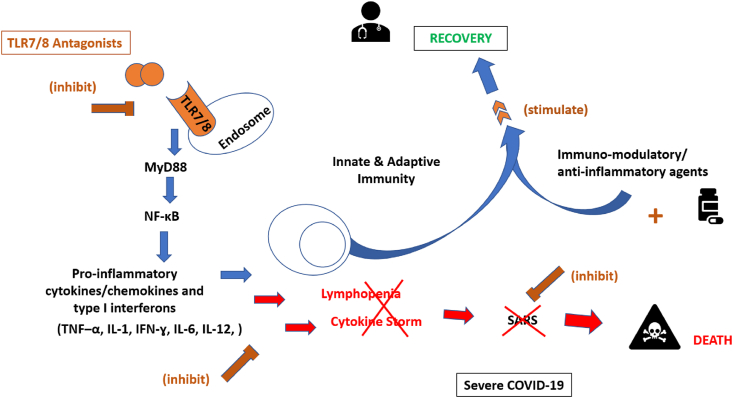
Fig. 2.
TLR7/8 antagonists as immuno-suppressants against SARS-CoV-2-induced severe COVID-19.
TLR7/8 antagonists were suggested to play key roles in suppressing the pathogenesis of the cytokine release syndrome in severe COVID-19 patients. TLR7/8 antagonists modulate immune responses and down-regulate cytokine production and may reduce life-threatening complications of COVID-19, including severe lymphopenia and SARS. They act against excessive levels of pro-inflammatory cytokines/chemokines and type I interferons including TNF–α, IL-1, IFN-ɣ, IL-6, & IL-12. The combined administration of TLR7/8 antagonists with immunomodulating, and anti-inflammatory pharmacotherapies classically used to target the cytokine storm, may have a synergistic effect that could help in reducing the dose of such therapies and consequently their adverse effects in more advanced late stages of the disease till returning with host immunity to recovery.
COVID-19, coronavirus disease 2019; IFN-ɣ, inteferon gamma; TNF–α, tumour necrosis factor alpha; IL, interleukin; MYD88, myeloid differentiation primary response 88; NF-kB, nuclear factor k-light-chain-enhancer; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TLR7/8, toll-like receptors 7 and/or 8.
Finally, TLR7/8 agonist/antagonist may play key roles in developing better drugs that may also be added to preventive vaccines, and combating early, as well as, late COVID-19 pathogenesis (Khanmohammadi and Rezaei, 2021; Chugh et al., 2021). The stage of the disease is important in targeting the condition with either the agonist or the antagonist of TLRs. Agonists of TLRs such as the innate immunity stimulator, imiquimod, could be an option to control COVID-19 since it binds to TLR7/8 and induces the production of pro-inflammatory cytokines, such as TNF-α, IL-1, IL-2, IL-6, IL-8, IL-12, as well as IFN-α. However, it should be mentioned that activation of TLR7/8 can induce a strong pro-inflammatory response in COVID patients, leading to acute lung injury and it may have a dual role in the progression of the disease (Moreno-Eutimio et al., 2020; Veras et al., 2020). It should also be noticed that imiquimod in the late stage of the infection could lead to cytokine storm and side effects of consistent inflammation. Therefore, imiquimod is appropriate in the early stages of COVID-19 infection (Angelopoulou et al., 2020). The early stages of the disease or mild COVID should meet the case definition for COVID-19 without evidence of viral pneumonia or hypoxia and the late stage of the disease or severe COVID should have clinical signs of severe pneumonia (fever, cough, dyspnoea, fast breathing) plus one of the following: respiratory rate >30 breaths/min; severe respiratory distress; or SpO2 < 90% on room air (World Health Organization, 2020).
Whole-genome sequencing of SARS-CoV, MERS-CoV, and SARS-CoV-2 revealed that TLR7 could be more involved in the pathogenesis of SARS-CoV-2 in comparison to SARS-CoV and MERS-CoV as it has more ssRNA motifs that could bind to TLR7 (van der Made et al., 2020). Nevertheless, clinical studies should determine the proper dose of TLR7 agonists to be used in the early stage of the disease if used as monotherapy or if combined with antiviral medications, since excessive activation of TLR7 is considered to be involved in the pathogenesis of several autoimmune diseases (Souyris et al., 2018). TLR7-specific antagonists such as Cpd-6 and Cpd-7 (Tojo et al., 2020), the TLR7/9 dual antagonists such as DV-1179 and IMO-3100, or the TLR7/8/9 antagonists such as IMO-8400 that are recently developed and were demonstrated to be very effective in preventing inflammation (Gao et al., 2017) may be used in the late stages of COVID since inhibition of TLR signaling pathways has been predicted to be an effective therapeutic strategy to suppress unwanted, disease-associated inflammatory responses. The outcome if TLR7/8 agonists are used in severe disease and if TLR7/8 antagonists are used in mild disease has not been investigated yet and may be difficult to be predicted given the multi-faceted nature of the pathophysiology of COVID-19 and the inter-individual variations in failure of host defence mechanisms along with other host-specific factors, that could determine whether an individual progresses to severe disease or not. Nevertheless, various treatment options are of value in different stages of the disease; ranging from antivirals and immune stimulants, that could be beneficial when mild symptoms are present, to immunomodulating, anti-inflammatory, antioxidant, and antithrombotic therapy that could be beneficial in more advanced stages of the disease (van Eijk et al., 2021).
2. Conclusion
Pharmacological compounds acting on TLR7 and/or TLR8 could be of therapeutic value in COVID-19. TLR7 and/or TLR8 agonists may be used by frontline healthcare workers with comorbidities who demonstrate mild disease that could progress to severe manifestations, and as beneficial vaccine adjuvants as well. On the other hand, TLR7 and/or TLR8 antagonists may be used in combination with other immunomodulatory/anti-inflammatory drugs in severe cases of the disease. These drug combinations may have potential synergistic effects and minimized toxicities; allowing for better pharmacological outcomes using reduced drug doses.
Consent to participate and to publish
The two contributing authors gave their consent to participate in and publish this manuscript.
Funding
The current research did not receive any grants from funding agencies in the public or commercial sectors.
Credit author statement
AK and AG conceived, designed and performed research. AK and AG wrote and approved the manuscript, and all data were generated in-house and no paper mill was used.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Akira S., Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Angelopoulou A., Alexandris N., Konstantinou E., Mesiakaris K., Zanidis C., Farsalinos K., Poulas K. Imiquimod - a toll like receptor 7 agonist - is an ideal option for management of COVID 19. Environ. Res. 2020;188:109858. doi: 10.1016/j.envres.2020.109858. Sep. Epub 2020 Jun 23. PMID: 32846644; PMCID: PMC7309930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffen M., Matsui H., Edwards S., et al. Biological characterization of a novel class of toll-like receptor 7 agonists designed to have reduced systemic activity. Br. J. Pharmacol. 2012;166:573–586. doi: 10.1111/j.1476-5381.2011.01790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas I., Khan G.A. Coagulation disorders in COVID-19: role of toll-like receptors. J. Inflamm. Res. 2020;13:823–828. doi: 10.2147/JIR.S271768. Oct 29. PMID: 33149655; PMCID: PMC7605922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugh H., Awasthi A., Agarwal Y., Gaur R.K., Dhawan G., Chandra R. A comprehensive review on potential therapeutics interventions for COVID-19. Eur. J. Pharmacol. 2021;890:173741. doi: 10.1016/j.ejphar.2020.173741. https://doi:%2010.1016/j.ejphar.2020.173741 Jan 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti P., Younes A. Coronavirus COV-19/SARS-CoV2 affects women less than men: clinical response to viral infection. J. Biol. Regul. Homeost. Agents. 2020 doi: 10.23812/Editorial-Conti-3. (in press) [DOI] [PubMed] [Google Scholar]
- Ekman A.K., Adner M., Cardell L.O. Toll-like receptor 7 activation reduces the contractile response of airway smooth muscle. Eur. J. Pharmacol. 2011;652:145–151. doi: 10.1016/j.ejphar.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Enosi Tuipulotu D., Netzler N.E., Lun J.H., Mackenzie J.M., White P.A. TLR7 agonists display potent antiviral effects against norovirus infection via innate stimulation. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.02417-17. e02417-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallerini C., Daga S., Mantovani S., Benetti E., Picchiotti N., Francisci D., Paciosi F., Schiaroli E., Baldassarri M., Fava F., Palmieri M., Ludovisi S., Castelli F., Quiros-Roldan E., Vaghi M., Rusconi S., Siano M., Bandini M., Spiga O., Capitani K., Furini S., Mari F., GEN-COVID Multicenter Study. Renieri A., Mondelli M.U., Frullanti E. Association of Toll-like receptor 7 variants with life-threatening COVID-19 disease in males: findings from a nested case-control study. Elife. 2021;10 doi: 10.7554/eLife.67569. Mar 2. PMID: 33650967; PMCID: PMC7987337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsbach A., et al. Dual or triple activation of TLR7, TLR8, and/or TLR9 by single-stranded oligoribonucleotides. Nucleic Acid Therapeut. 2011;21:423–436. doi: 10.1089/nat.2011.0323. https://doi:%2010.1089/nat.2011.0323 [DOI] [PubMed] [Google Scholar]
- Gao W., Xiong Y., Li Q., Yang H. Inhibition of toll-like receptor signaling as a promising therapy for inflammatory diseases: a journey from molecular to nano therapeutics. Front. Physiol. 2017;8:508. doi: 10.3389/fphys.2017.00508. Published 2017 Jul 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremeskel S., Schanin J., Coyle K.M., Butuci M., Luu T., Brock E.C., Xu A., Wong A., Leung J., Korver W., Morin R.D., Schleimer R.P., Bochner B.S., Youngblood B.A. Mast cell and eosinophil activation are associated with COVID-19 and TLR-mediated viral inflammation: implications for an anti-siglec-8 antibody. Front. Immunol. 2021;12:650331. doi: 10.3389/fimmu.2021.650331. Mar 10. PMID: 33777047; PMCID: PMC7988091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh T.K., Mickelson D.J., Fink J., Solberg J.C., Inglefield J.R., Hook D., Gupta S.K., Gibson S., Alkan S.S. Toll-like receptor (TLR) 2-9 agonists-induced cytokines and chemokines: I. Comparison with T cell receptor-induced responses. Cell. Immunol. 2006;243(1):48–57. doi: 10.1016/j.cellimm.2006.12.002. Sep. Epub 2007 Jan 23. PMID: 17250816. [DOI] [PubMed] [Google Scholar]
- Heil F., Hemmi H., Hochrein H., et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- Hung I.F., Zhang A.J., To K.K., Chan J.F., Li P., Wong T.L., Zhang R., Chan T.C., Chan B.C., Wai H.H., Chan L.W., Fong H.P., Hui R.K., Kong K.L., Leung A.C., Ngan A.H., Tsang L.W., Yeung A.P., Yiu G.C., Yung W., Lau J.Y., Chen H., Chan K.H., Yuen K.Y. Topical imiquimod before intradermal trivalent influenza vaccine for protection against heterologous non-vaccine and antigenically drifted viruses: a single-centre, double-blind, randomised, controlled phase 2b/3 trial. Lancet Infect. Dis. 2016;16:209–221. doi: 10.1016/S1473-3099(15)00354-0. [DOI] [PubMed] [Google Scholar]
- Karimi-Googheri M., Arababadi M.K. TLR3 plays significant roles against hepatitis B virus. Mol. Biol. Rep. 2014;41:3279–3286. doi: 10.1007/s11033-014-3190-x. [DOI] [PubMed] [Google Scholar]
- Kawai T., Akira S. Signaling to NF-κB by toll-like receptors. Trends Mol. Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. https://doi:%2010.1016/j.molmed.2007.09.002 [DOI] [PubMed] [Google Scholar]
- Khalifa A.E., Ghoneim A.I. Ongoing COVID-19 clinical trials: a pharmacological perspective on pneumonia. Curr Tr Clin & Med Sci. 2020;2(2):1–3. https://irispublishers.com/ctcms/pdf/CTCMS.MS.ID.000533.pdf Dec. [Google Scholar]
- Khalifa A.E., Ghoneim A.I. Plausible pharmacological interpretation of hydroxychloroquine ineffectiveness for treatment of COVID-19 in hospitalized patients. Archives of Pharmacy and Pharmacology Research. 2021;2(5):1–5. https://irispublishers.com/appr/pdf/APPR.MS.ID.000549.pdf May. [Google Scholar]
- Khan M.O.F., Park K.K., Lee H.J. Antedrugs: an approach to safer drugs. Curr Med Chem. 2005;12:2227–2239. doi: 10.2174/0929867054864840. [DOI] [PubMed] [Google Scholar]
- Khanmohammadi S., Rezaei N. Role of Toll-like receptors in the pathogenesis of COVID-19. J Med Virol. 2021;93(5):2735–2739. doi: 10.1002/jmv.26826. May. Epub 2021 Feb 9. PMID: 33506952; PMCID: PMC8014260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball A., Krueger J., Sullivan T., et al. IMO-3100, an antagonist of Toll-like receptor (TLR) 7 and TLR9, demonstrates clinical activity in psoriasis patients with 4 weeks of treatment in a Phase 2a trial. J Invest Dermatol. 2013;133:S26. [Google Scholar]
- Leaker B.R., Singh D., Lindgren S., et al. Effects of the Toll-like receptor 7 (TLR7) agonist, AZD8848, on allergen-induced responses in patients with mild asthma: a double-blind, randomised, parallel-group study. Respir Res. 2019;20:288. doi: 10.1186/s12931-019-1252-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebold K.M., Jacoby D.B., Drake M.G. Toll-like receptor 7-targeted therapy in respiratory disease. Transfus. Med. Hemother. 2016;43:114–119. doi: 10.1159/000445324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester S.N., Li K. Toll-like receptors in antiviral innate immunity. J Mol Biol. 2014;426(6):1246-1264. doi: 10.1016/j.jmb.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.Y., Hong Y.F., Huang C.M., Chen C.Y., Huang T.N., Hsueh Y.P. TLR7 negatively regulates dendrite outgrowth through the Myd88-c-Fos-IL-6 pathway. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33(28):11479–11493. doi: 10.1523/JNEUROSCI.5566-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- Merck . Merck Initiates First Clinical Trial of TLR7 and 8 Inhibitor as a Potential Treatment for Severe Symptoms of CoViD-19 Infection. Merck Group; 2021. https://www.merckgroup.com/en/news/m5049-treatment-covid-19-pneumonia.html [Google Scholar]
- Michaelis K.A., Norgard M.A., Levasseur P.R., Olson B., Burfeind K.G., Buenafe A.C., Zhu X., Jeng S., McWeeney S.K., Marks D.L. Persistent Toll-like receptor 7 stimulation induces behavioral and molecular innate immune tolerance. Brain Behav Immun. 2019;82:338–353. doi: 10.1016/j.bbi.2019.09.004. Nov. Epub 2019 Sep 6. PMID: 31499172; PMCID: PMC6956569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Eutimio M.A., López-Macías C., Pastelin-Palacios R. Bioinformatic analysis and identification of single-stranded RNA sequences recognized by TLR7/8 in the SARS-CoV-2, SARS-CoV, and MERS-CoV genomes. Microb Infect. 2020;22(4–5):226–229. doi: 10.1016/j.micinf.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghib M., Hatam-Jahromi M., Niktab M., Ahmadi R., Kariminik A. Mycoplasma pneumoniae and toll-like receptors: a mutual avenue. Allergol Immunopathol (Madr). 2018;46(5):508–513. doi: 10.1016/j.aller.2017.09.021. Sep-Oct. Epub 2018 Jan 10. PMID: 29331619. [DOI] [PubMed] [Google Scholar]
- Onofrio L., Caraglia M., Facchini G., Margherita V., Placido S., Buonerba C. Toll-like receptors and COVID-19: a two-faced story with an exciting ending. Future Sci OA. 2020;6(8) doi: 10.2144/fsoa-2020-0091. Jul 30. FSO605. PMID: 32974046; PMCID: PMC7434222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker L.C., Prince L.R., Sabroe I. Translational mini-review series on Toll-like receptors. Networks regulated by Toll-like receptors mediate innate and adaptive immunity. Clin. Exp. Immunol. 2007;147:199–207. doi: 10.1111/j.1365-2249.2006.03203.x. https://doi:%2010.1111/j.1365-2249.2006.03203.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockros P.J., Guyader D., Patton H., et al. Oral resiquimod in chronic HCV infection: safety and efficacy in 2 placebo-controlled, double-blind phase IIa studies. J Hepatol. 2007;47:174–182. doi: 10.1016/j.jhep.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Pockros P.J., Guyader D., Patton H., Tong M.J., Wright T., McHutchison J.G., et al. Oral resiquimod in chronic HCV infection: safety and efficacy in 2 placebo-controlled, double-blind phase IIa studies. J Hepatol. 2007;47:174–182. doi: 10.1016/j.jhep.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Quinton L.J., Mizgerd J.P. Dynamics of lung defense in pneumonia: resistance, resilience, and remodeling. Annu Rev Physiol. 2015;77:407–430. doi: 10.1146/annurev-physiol-021014-071937. https://doi:10.1146/annurev-physiol-021014-071937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodell C.B., Ahmed M.S., Garris C.S., Pittet M.J., Weissleder R. Development of adamantane-conjugated TLR7/8 agonists for supramolecular delivery and cancer immunotherapy. Theranostics. 2019;9(26):8426–8436. doi: 10.7150/thno.35434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root-Bernstein R. Innate receptor activation patterns involving TLR and NLR synergisms in COVID-19, ALI/ARDS and sepsis cytokine storms: a review and model making novel predictions and therapeutic suggestions. International journal of molecular sciences. 2021;22(4):2108. doi: 10.3390/ijms22042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaei S., Karimi-Googheri M. 2020. Letter to the Editor: Toll-like Receptor Antagonists as a Potential Therapeutic Strategy against Cytokine Storm in COVID-19-Infected Patients. Viral Immunol. Oct 1. Epub ahead of print. PMID: 33012270. [DOI] [PubMed] [Google Scholar]
- Sauder Daniel N., Smith Michael H., Senta-McMillian Therese, Soria Inmaculada, Meng Tze-Chiang. Randomized, single-blind, placebo-controlled study of topical application of the immune response modulator resiquimod in healthy adults. Antimicrobial agents and chemotherapy. 2003;47(12):3846–3852. doi: 10.1128/AAC.47.12.3846-3852.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souyris M., Cenac C., Azar P., Daviaud D., Canivet A., Grunenwald S., Pienkowski C., Chaumeil J., Mejía J.E., Guéry J.C. TLR7 escapes X chromosome inactivation in immune cells. Sci Immunol. 2018;3(19) doi: 10.1126/sciimmunol.aap8855. Jan 26. PMID: 29374079. [DOI] [PubMed] [Google Scholar]
- Steiner D.J., Furuya Y., Jordan M.B., Metzger D.W. Protective role for macrophages in respiratory Francisella tularensis infection. Infect Immun. 2017;85 doi: 10.1128/IAI.00064-17. https://doi:10.1128/IAI.00064-1 e00064-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L., Li Q., Bai J., Zhang H., Lu Y., Ma S. Severe pneumonia mortality in elderly patients is associated with downregulation of Toll-like receptors 2 and 4 on monocytes. Am J Med Sci. 2014;347(1):34–41. doi: 10.1097/MAJ.0b013e3182798583. Jan. PMID: 23406892. [DOI] [PubMed] [Google Scholar]
- Tiboni M., Casettari L., Illum L. Nasal vaccination against SARS-CoV-2: synergistic or alternative to intramuscular vaccines? Int J Pharm. 2021;603:120686. doi: 10.1016/j.ijpharm.2021.120686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojo S., Zhang Z., Matsui H., et al. Structural analysis reveals TLR7 dynamics underlying antagonism. Nat Commun. 2020;11:5204. doi: 10.1038/s41467-020-19025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Made C.I., Simons A., Schuurs-Hoeijmakers J., et al. Presence of genetic variants among young men with severe COVID-19. JAMA. 2020;324(7):663–673. doi: 10.1001/jama.2020.13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eijk L.E., Binkhorst M., Bourgonje A.R., Offringa A.K., Mulder D.J., Bos E.M., Kolundzic N., Abdulle A.E., van der Voort P.H., Olde Rikkert M.G., van der Hoeven J.G., den Dunnen W.F., Hillebrands J.L., van Goor H. COVID-19: immunopathology, pathophysiological mechanisms, and treatment options. J Pathol. 2021;254(4):307–331. doi: 10.1002/path.5642. Jul. Epub 2021 Mar 25. PMID: 33586189; PMCID: PMC8013908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veras F.P., Pontelli M.C., Silva C.M., et al. SARS-CoV-2–triggered neutrophil extracellular traps mediate COVID-19 pathology. J Exp Med. 2020;217(12) doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldon W.C., Zarnitsyn V.G., Esser E.S., Taherbhai M.T., Koutsonanos D.G., Vassilieva E.V., Skountzou I., Prausnitz M.R., Compans R.W. Effect of adjuvants on responses to skin immunization by microneedles coated with influenza subunit vaccine. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0041501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization “ . 2020. Clinical Management of COVID-19” Interim Guidance. 27 May. [Google Scholar]
- Yazdanpanah F., Hamblin M.R., Rezaei N. The immune system and COVID-19: friend or foe? Life Sci. 2020;256:117900. doi: 10.1016/j.lfs.2020.117900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., El-Far M., Dupuy F.P., et al. HCV RNA activates APCs via TLR7/TLR8 while virus selectively stimulates macrophages without inducing antiviral responses. Sci Rep. 2016;6:29447. doi: 10.1038/srep29447. [DOI] [PMC free article] [PubMed] [Google Scholar]