ABSTRACT
Background
Higher flavonoid intakes are hypothesized to confer protection against type 2 diabetes mellitus.
Objectives
We aimed to 1) investigate associations between flavonoid intakes and diabetes, 2) examine the mediating impact of body fat, and 3) identify subpopulations that may receive the greatest benefit from higher flavonoid intakes in participants of the Danish Diet, Cancer, and Health Study followed up for 23 y.
Methods
Cross-sectional associations between baseline flavonoid intake, estimated using FFQs and the Phenol Explorer database, and body fat, estimated by bioelectrical impedance, were assessed using multivariable-adjusted linear regression models. Nonlinear associations between flavonoid intake and incident diabetes were examined using restricted cubic splines with multivariable-adjusted Cox proportional hazards models.
Results
Among 54,787 participants (median age: 56 y; IQR: 52–60 y; 47.3% men), 6700 individuals were diagnosed with diabetes. Participants in the highest total flavonoid intake quintile (median, 1202 mg/d) had a 1.52 kg lower body fat (95% CI: –1.74, –1.30 kg) and a 19% lower risk of diabetes (HR: 0.81; 95% CI: 0.75, 0.87) after multivariable adjustments and compared with participants in the lowest intake quintile (median: 174 mg/d). Body fat mediated 57% (95% CI: 42, 83%) of the association between flavonoid intake and incident diabetes. Of the flavonoid subclasses, moderate to high intakes of flavonols, flavanol monomers, flavanol oligo + polymers, and anthocyanins were significantly associated with a lower risk of diabetes. Although associations were not modified by sex, smoking status, BMI, or physical activity (Pinteraction > 0.05 for all), findings on an absolute scale suggest that those at a higher risk (those with obesity) may benefit the most from a higher flavonoid intake.
Conclusions
The findings reported in this study suggest that a diet abundant in flavonoid-rich foods may help ameliorate diabetes risk, in part through a reduction in body fat.
Keywords: flavonoids, diabetes, body fat, prospective cohort study, obesity
Introduction
Diabetes, a leading cause of disability worldwide (1), results in >2 million deaths every year due to the neurological and vascular complications associated with this condition (2). Strategies to lessen the global health and economic burden of diabetes should focus on diabetes prevention through the promotion of healthy behaviors and evidence-based diets at the population level (3). Higher intakes of fruits and vegetables tend to be associated with a lower incidence of type 2 diabetes mellitus (4), with particular fruits showing stronger associations than others, not attributable to their glycemic index/load (5). Flavonoids, a class of polyphenols found ubiquitously in fruits, vegetables, and other plant-derived foods and beverages, have been proposed as potential moderators of diabetes risk (6). In vitro and animal studies highlight potential mechanisms by which flavonoids and their metabolites can modify obesity and type 2 diabetes risk through inhibition of adipogenesis, insulin signaling/secretion, and the modulation of inflammatory pathways (7–9). Based on their chemical structure, flavonoids are categorized into subclasses, with certain subclasses being common to specific foods. Structural variance between subclasses results in differences in bioavailability and biological activity (10).
In a meta-analysis of 7 prospective cohort studies, persons with the highest intakes of flavonoids had an 11% lower risk of type 2 diabetes mellitus (RR: 0.89; 95% CI: 0.82–0.96) compared with persons with the lowest intakes (11). Of the flavonoid subclasses, significant inverse associations were observed for flavonols, flavanols, and anthocyanins. Whether these inverse associations differ in subgroups at a higher risk of diabetes remains unknown.
Mitigating weight gain during middle age has a critical public health impact, with the risk of developing diabetes being up to 70% greater in those who gain ≥4.5 kg between the ages of 40 and 60 y (12). The reported inverse association between flavonoid intake and both body fat (13) and weight gain during 24 y of follow-up (14) may account for, at least in part, the association between flavonoid intake and incident diabetes. Because body fat may be a better determinant of diabetes development than BMI (in kg/m2) and waist circumference (15), estimation of the mediating effect of the association between flavonoids and body fat on diabetes risk is warranted in a large observational cohort.
Therefore, the primary aim of this study was to investigate the association of total flavonoid and flavonoid subclass intakes with body fat and incident diabetes in the Danish Diet, Cancer, and Health cohort. Secondary aims were to investigate the mediating effect of body fat and to identify subpopulations that may benefit the most from higher flavonoid intakes.
Methods
Study population
Participants of the Danish Diet, Cancer, and Health study, all between the ages of 50 and 64 y, were recruited from Copenhagen and Aarhus, Denmark, between 1993 and 1997. All Danish residents are assigned a unique number, allowing cross-linking of participants to the following nationwide registries: the Civil Registration System; the Integrated Database for Labor Market Research Database; the Danish National Prescription Registry, which holds information on all filled prescriptions from Danish pharmacies since 1994, with each drug classified by the anatomical therapeutic chemical (ATC) code (16); and The Danish National Patient Register, which contains information on all hospital admissions in Denmark since 1978. This includes 1 primary diagnosis and ≥1 secondary diagnoses defined by the International Classification of Diseases (ICD): the 8th revision (ICD-8) until 1993 and the 10th revision (ICD-10) from 1994 (17).
Of the 57,053 participants initially recruited into the study, 56,468 completed an FFQ and had no diagnosis of cancer prior to study enrolment. Participants were excluded if they had improbable energy intakes (<500 kcal/d or >5000 kcal/d; n = 198), missing data or extreme values for any covariates (n = 243), or prevalent diabetes at baseline (n = 1240); prevalent diabetes was defined as self-reported diabetes, ICD-10 diagnosis of diabetes (E10, E11), or use of insulin and other glucose-lowering medications (ATC; A10A, A10B) at or prior to baseline. This left 54,787 participants remaining for analysis in the current study (Supplemental Figure 1).
This study was approved by the Danish Data Protection Agency (Ref. 2012–58–0004 I-Suite nr: 6357, VD-2018–117).
Exposures
Exposures were baseline intakes of total flavonoids, flavonoid subclasses, individual flavonoid compounds with mean intakes >5 mg/d, and flavonoid-rich foods. Calculations of flavonoid intake are described in detail elsewhere (18). Briefly, participants completed a validated, 192-item FFQ at study entry (19); estimates of the flavonoid content of each food and beverage item in the FFQ were obtained from the Phenol-Explorer database (20). Because the average intakes of isoflavones, dihydrochalcones, dihydroflavonols, and chalcones were very low in this cohort (<5 mg/d), these flavonoid subclasses were not assessed discretely. Total flavonoid intake was calculated by summing the estimated intakes for each of the 219 flavonoid aglycones.
Study outcomes
Body fat
Body fat (kg) at baseline was estimated using bioelectrical impedance (BIA 101-F device; Akern/RJL) as described previously (21). The method used has been validated in a Danish population, aged 35–65 y, against measurements of total body potassium and total body water (22) and has been shown to have good agreement with body composition determined by DXA in middle-aged participants from the Leiden Longevity Study (23).
Incident diabetes
The outcome of the study consisted of either a primary or a secondary diagnosis of diabetes (ICD-10; E10, E11) for both inpatient and outpatient visits during follow-up. To capture events in people treated in a primary care setting, we included those who filled a prescription for insulin or noninsulin medication for diabetes treatment (ATC; A10A, A10B). This definition is based on that developed by the Danish Health Data Authority for identifying patients with diabetes and has a positive predictive value of 96.9% (95% CI: 89.5, 99.2%) (24).
Covariates
Participants completed questionnaires upon study enrolment, providing information on sex, age, education, smoking habits, alcohol consumption, daily activity, use of hormone replacement therapy, and diet. Anthropometry was measured at the study centers at baseline. Average annual income over 5 y was used to represent socioeconomic status and was defined as household income after taxation and interest, using the value of the Danish currency in 2015. The presence of hypertension was defined by a combination of self-reported hypertension and the use of ≥2 antihypertensive medications at baseline (24).
Statistical analysis
Two types of analyses were performed, a cross-sectional analysis and a time-to-event analysis. First, a cross-sectional analysis was performed to investigate the association between total flavonoid intake and body fat at baseline; a linear regression model was fit with total flavonoid intake as a continuous predictor and body fat as the response. Second, Cox proportional hazards models were used to investigate the relation between flavonoid intake and diabetes. Participants were followed from the date of enrolment until the date of diabetes diagnosis, death, emigration, or end of follow-up (August 2017), whichever came first. Quintiles were derived separately for each exposure variable. Restricted cubic splines were used to investigate nonlinear relations between flavonoid intake (continuous) and diabetes, with HRs and 95% CIs derived from Cox proportional hazards models. HR estimates were reported for the median intake in each quintile, using the median intake in quintile 1 as the reference point. We tested for nonlinearity using a chi-square test comparing nested models. The HRs derived from the cubic splines were graphed over a fine grid of x-axis values; for visual simplicity, x-axis values were restricted to intakes within 3 SDs of the mean for each exposure. Four models of adjustment were used in all analyses: model 1a adjusted for age, sex; model 2b adjusted for age, sex, smoking status (current, former, or never), physical activity [metabolic equivalent (MET); total daily MET], pure alcohol intake (g/d), social economic status (income), hormone replacement therapy use (current, former, or never), and education (≤7 y, 8–10 y, or ≥11 y); model 2 adjusted for all covariates in model 1b plus body fat (kg), hypertension status (yes/no), and cholesterol status (yes/no); and model 3 adjusted for all covariates in model 2 plus energy intake and intakes (g/d) of red meat, processed meat, PUFAs, MUFAs, SFAs, fiber, soft drinks, coffee, and added sugar. Covariates were chosen a priori to the best of our knowledge of potential confounders of flavonoid intake and diabetes. Cox proportional hazards assumptions were tested using log–log plots of the survival function compared with time and assessed for parallel appearance. All deaths were censored rather than treated as a competing risk. To identify subpopulations that may benefit the most from higher flavonoid intakes, first, interaction on the multiplicative scale was assessed by likelihood ratio tests of Cox proportional hazards models with and without the interaction terms. Second, analyses were stratified by risk factors for diabetes, namely smoking status (never/former smoker compared with current smoker), BMI (>30 compared with <30), physical activity (greater than compared with less than the median MET score), and sex. Because there is potential for residual confounding when stratifying by smoking status, BMI, and MET score, the corresponding continuous variables (smoking pack-years, BMI, and MET score) were included in the model. In addition, due to the miniscule loss to follow-up in this cohort (<0.3%), standard logistic regression models were used to obtain the predicted 20-y absolute risk estimates of diabetes for each subgroup. For these analyses, a binary outcome indicating a diagnosis of diabetes during 20 y of follow-up was used. Unless indicated by the stratification variable, these estimates are for the “average” cohort participant at baseline—that is, a nonsmoking participant, aged 56 y, who has completed 8–10 y of education, with a BMI of 25.5, a total daily MET score of 56, a mean household income of 394,701–570,930 Danish krone/y, an alcohol intake of 13 g/d, and who is not taking hormone replacement therapy. For flavonoid exposures for which clear inverse associations with both body fat and incident diabetes were observed, the extent to which the association between flavonoid intake and diabetes was mediated by baseline body fat was quantified through natural direct and indirect effects (25), which estimate how large an association we would observe if flavonoid intake had no impact on body fat (the natural direct effect) and if flavonoid intake only had an impact upon diabetes through its impact on body fat (the natural indirect effect), respectively. Estimation was done using a Cox proportional hazards model in the Medflex package for R (26). CIs were obtained by bootstrapping (1000 iterations). Body fat was included as a continuous variable in the mediation analysis, whereas total flavonoid intake was included as a categorical variable (quintiles). In supplementary analyses, Pearson's correlations were performed between flavanone intake and the primary dietary sources of flavanones and the association between body fat (per 1.52 kg), and incident diabetes was investigated using a Cox proportional hazards model. All analyses were undertaken using Stata/IC 14.2 (StataCorp) and R statistics (R Foundation for Statistical Computing).
Results
This population of 54,787 Danish residents, with a median age of 56 y (IQR: 52–60 y) at entry, had a median follow-up of 20.8 y (IQR: 17.3–21.6 y). During 1,010,191 person-years of follow-up, 6700 individuals were diagnosed with diabetes.
Baseline characteristics
The median intake of flavonoids was 495 mg/d (IQR: 287–213 mg/d). Compared with participants with the lowest flavonoid intakes, those with the highest intakes were more likely to be women, be more physically active, have a higher degree of education, have a higher income, and less likely to have ever smoked or to be hypercholesterolemic. Furthermore, they tended to have a healthier underlying dietary pattern, consuming more fish, dietary fiber, fruits, vegetables, and tea and consuming less red meat and processed meat (Table 1).
TABLE 1.
Baseline characteristics of study population1
| Total flavonoid intake quintiles | ||||||
|---|---|---|---|---|---|---|
| Total population (n = 54,787) | Quintile 1 (n = 10,958) | Quintile 2 (n = 10,957) | Quintile 3 (n = 10,957) | Quintile 4 (n = 10,957) | Quintile 5 (n = 10,958) | |
| Total flavonoid intake, mg/d | 495 [287–805] | 174 [128–213] | 321 [287–357] | 495 [442–549] | 727 [660–805] | 1 202 [1025–1 435] |
| Sex, % men | 25,903 (47.3) | 6302 (57.5) | 5592 (51.0) | 5168 (47.2) | 4851 (44.3) | 3990 (36.4) |
| Age, y | 56 [52–60] | 56 [52–60] | 56 [52–60] | 56 [52–60] | 56 [52–60] | 55 [52–60] |
| BMI, kg/m2 | 25.5 [23.3–28.2] | 26.1 [23.7–28.8] | 25.8 [23.6–28.2] | 25.6 [23.1–27.8] | 25.3 [23.1–27.8] | 24.9 [22.7–27.3] |
| Body fat, kg | 22.3 [17.8–27.7] | 22.8 [18.1–28.5] | 22.7 [18.2–28.2] | 22.4 [17.9–27.8] | 22.1 [17.8–27.4] | 21.5 [17.2–26.7] |
| MET score | 56.5 [37.0–84.8] | 51.0 [32.3–78.0] | 55.5 [36.3–84.0] | 57.5 [38.5–85.0] | 58.5 [38.8–87.0] | 60.0 [40.0–88.5] |
| Smoking status | ||||||
| Never | 19,281 (35.2) | 2669 (24.4) | 3662 (33.4) | 3928 (35.8) | 4370 (39.9) | 4652 (42.5) |
| Former | 15,746 (28.7) | 2630 (24.0) | 2952 (26.9) | 3172 (28.9) | 3508 (32.0) | 3484 (31.8) |
| Current | 19,760 (36.1) | 5659 (51.6) | 4343 (39.6) | 3857 (35.2) | 3079 (28.1) | 2822 (25.8) |
| Education, y | ||||||
| ≤7 | 17,939 (32.7) | 4995 (45.6) | 4156 (37.9) | 3495 (31.9) | 2945 (26.9) | 2348 (21.4) |
| 8–10 | 25,286 (46.2) | 4791 (43.7) | 5161 (47.1) | 5253 (47.9) | 5188 (47.3) | 4893 (44.7) |
| ≥11 | 11,537 (21.1) | 1166 (10.6) | 1637 (14.9) | 2206 (20.1) | 2818 (25.7) | 3710 (33.9) |
| Mean household income, DKK/y | ||||||
| ≤394,700 | 13,473 (24.6) | 3246 (29.6) | 2663 (24.3) | 2625 (24.0) | 2505 (22.9) | 2434 (22.2) |
| 394,701–570,930 | 13,659 (24.9) | 3184 (29.1) | 2929 (26.7) | 2656 (24.2) | 2533 (23.1) | 2357 (21.5) |
| 570,931–758,297 | 13,794 (25.2) | 2876 (26.2) | 2976 (27.2) | 2836 (25.9) | 2570 (23.5) | 2536 (23.1) |
| >758,297 | 13,861 (25.3) | 1652 (15.1) | 2389 (21.8) | 2840 (25.9) | 3349 (30.6) | 3631 (33.1) |
| Hypertensive | 8901 (16.2) | 1758 (16.0) | 1817 (16.6) | 1811 (16.5) | 1768 (16.1) | 1747 (15.9) |
| Hypercholesterolemic | 3964 (7.2) | 859 (7.8) | 794 (7.2) | 801 (7.3) | 814 (7.4) | 696 (6.4) |
| Medication use | ||||||
| Antihypertensive | 6480 (11.8) | 1275 (11.6) | 1341 (12.2) | 1315 (12.0) | 1280 (11.7) | 1269 (11.6) |
| Statin | 1026 (17.0) | 252 (18.2) | 208 (16.6) | 206 (17.3) | 197 (17.3) | 163 (15.2) |
| HRT (women only) | ||||||
| Never | 15,701 (54.4) | 2568 (55.2) | 2988 (55.7) | 3231 (55.8) | 3206 (52.5) | 3708 (53.2) |
| Current | 8707 (30.1) | 1278 (27.4) | 1550 (28.9) | 1677 (29.0) | 1983 (32.5) | 2219 (31.8) |
| Former | 4476 (15.5) | 810 (17.4) | 827 (15.4) | 881 (15.2) | 917 (15.0) | 1041 (14.9) |
| Dietary characteristics | ||||||
| Energy, kcal/d | 2270 [1877–2716] | 2059 [1679–2484] | 2213 [1844–2629] | 2327 [1941–2766] | 2375 [1989–2825] | 2372 [1976–2837] |
| Total fish intake, g/d | 38 [25–55] | 33 [22–48] | 37 [25–54] | 40 [27–57] | 41 [28–59] | 40 [27–57] |
| Red meat intake, g/d | 78 [56–107] | 80 [58–108] | 81 [59–109] | 80 [58–110] | 78 [57–107] | 72 [52–99] |
| Processed meat intake, g/d | 24 [14–40] | 28 [17–45] | 26 [15–42] | 25 [14–40] | 23 [14–38] | 20 [11–34] |
| Dietary fiber intake, g/d | 20 [16–25] | 16 [13–20] | 19 [16–23] | 21 [17–25] | 22 [18–27] | 23 [19–29] |
| SFA, g/d | 31 [24–39] | 29 [23–38] | 31 [24–39] | 32 [24–40] | 32 [25–41] | 32 [24–41] |
| PUFA, g/d | 13 [10–17] | 12 [9–16] | 13 [10–17] | 14 [10–18] | 14 [11–18] | 14 [10–18] |
| MUFA, g/d | 27 [21–35] | 26 [20–34] | 27 [21–35] | 28 [22–35] | 28 [22–35] | 27 [21–34] |
| Fruit intake, g/d | 171 [94–281] | 87 [44–141] | 161 [97–237] | 193 [114–300] | 224 [140–360] | 241 [141–390] |
| Vegetable intake, g/d | 161 [104–230] | 113 [71–168] | 149 [99–210] | 167 [113–234] | 184 [126–252] | 195 [135–271] |
| Alcohol intake, g/d | 13 [6–31] | 11 [4–24] | 13 [6–25] | 15 [6–34] | 14 [7–32] | 13 [6–32] |
| Tea intake, g/d | 7 [3–500] | 3 [0–16] | 16 [3–86] | 86 [7–200] | 500 [86–500] | 900 [500–1300] |
Values are medians [IQRs] or n (%), unless otherwise stated. CKD, chronic kidney disease; COPD, common obstructive pulmonary disease; DKK, Danish krone; HRT, hormone replacement therapy; MET, metabolic equivalent.
Association between flavonoid intake and baseline body fat
Total flavonoid intake was inversely associated with body fat in a linear dose–response manner (P for trend < 0.001) across the range of intakes in this cohort. After multivariable adjustments and compared with participants in quintile 1, participants in quintiles 2–5 had a 0.39 kg (95% CI: –0.60, –0.17 kg), 0.62 kg (95% CI: –0.84, –0.41 kg), 0.90 kg (95% CI: –1.11, –0.68 kg), and 1.52 kg (95% CI: –1.74, –1.30 kg) lower body fat, respectively (model 1b; Supplemental Table 1). In order to aid interpretation of this, in supplementary modeling, a 1.52-kg higher body fat translated to a 12% higher risk of diabetes (HR: 1.12; 95% CI: 1.11, 1.12). For the individual flavonoid subclasses, inverse associations with baseline body fat were observed for the flavonols, flavanol monomers, flavanol oligo + polymers, and anthocyanins (Supplemental Table 1).
Association between flavonoid intake and incident diabetes
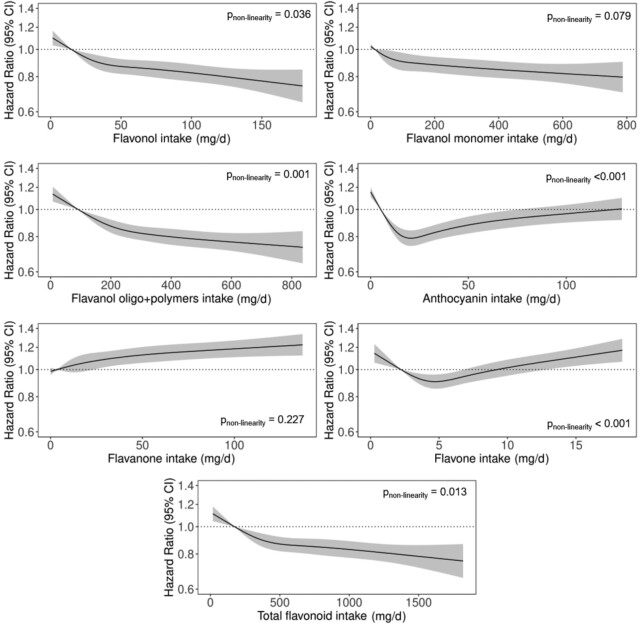
The association between total flavonoid intake and incident diabetes was nonlinear (P for nonlinearity = 0.013); the inverse association was steeper at lower intakes, and the gradient started to decrease at ∼400 mg/d (Figure 1). Compared with participants in quintile 1, participants in quintile 5 had a 19% lower risk of diabetes (HR: 0.81; 95% CI: 0.75, 0.87) after multivariable adjustments (model 1b; Table 2).
FIGURE 1.
HRs based on cubic splines to describe the association between flavonoid subclass intakes (mg/d) and incident diabetes among participants of the Danish Diet, Cancer, and Health cohort (n = 54,787). HRs (y axis on logarithmic scale) are based on Cox proportional hazards models adjusted for age, sex, smoking status, physical activity, alcohol intake, education, hormone replacement therapy, and socioeconomic status (income) and are comparing the specific level of flavonoid intake (horizontal axis) to the median intake for participants in the lowest intake quintile. P values for nonlinearity were obtained using a chi-square test to compare nested models.
TABLE 2.
HRs of incident diabetes by quintiles of flavonoid intake1
| Flavonoid intake quintiles | |||||
|---|---|---|---|---|---|
| Quintile 1 (n = 10,958) | Quintile 2 (n = 10,957) | Quintile 3 (n = 10,957) | Quintile 4 (n = 10,957) | Quintile 5 (n = 10,958) | |
| Total flavonoids | |||||
| No. events | 1734 | 1380 | 1337 | 1205 | 1044 |
| Intake,2 mg/d | 174 (6–251) | 321 (251–395) | 495 (395–602) | 727 (602–909) | 1202 (909–3552) |
| HR (95% CI) | |||||
| Model 1a | Ref | 0.83 (0.80, 0.87) | 0.74 (0.69, 0.78) | 0.69 (0.65, 0.73) | 0.63 (0.59, 0.68) |
| Model 1b | Ref | 0.92 (0.88, 0.96) | 0.87 (0.82, 0.92) | 0.85 (0.80, 0.90) | 0.81 (0.75, 0.87) |
| Model 2 | Ref | 0.92 (0.88, 0.96) | 0.89 (0.84, 0.94) | 0.89 (0.84, 0.95) | 0.88 (0.82, 0.94) |
| Model 3 | Ref | 0.92 (0.88, 0.96) | 0.88 (0.83, 0.94) | 0.88 (0.82, 0.94) | 0.84 (0.78, 0.92) |
| Flavonols | |||||
| No. events | 1684 | 1510 | 1263 | 1212 | 1031 |
| Intake,2 mg/d | 15 (0–20) | 26 (20–32) | 38 (32–50) | 66 (50–83) | 116 (83–251) |
| HR (95% CI) | |||||
| Model 1a | Ref | 0.84 (0.81, 0.88) | 0.75 (0.70, 0.79) | 0.68 (0.64, 0.72) | 0.62 (0.58, 0.66) |
| Model 1b | Ref | 0.93 (0.89, 0.97) | 0.89 (0.83, 0.94) | 0.86 (0.80, 0.91) | 0.81 (0.75, 0.87) |
| Model 2 | Ref | 0.91 (0.88, 0.95) | 0.87 (0.82, 0.93) | 0.89 (0.84, 0.95) | 0.87 (0.81, 0.94) |
| Model 3 | Ref | 0.92 (0.88, 0.97) | 0.88 (0.82, 0.94) | 0.88 (0.82, 0.95) | 0.84 (0.77, 0.91) |
| Flavanol monomers | |||||
| No. events | 1676 | 1419 | 1369 | 1191 | 1045 |
| Intake,2 mg/d | 14 (0–21) | 30 (21–45) | 66 (45–115) | 261 (115–281) | 473 (281–916) |
| HR (95% CI) | |||||
| Model 1a | Ref | 0.91 (0.89, 0.94) | 0.79 (0.74, 0.84) | 0.70 (0.65, 0.74) | 0.66 (0.62, 0.71) |
| Model 1b | Ref | 0.97 (0.94, 1.00) | 0.92 (0.86, 0.99) | 0.87 (0.81, 0.93) | 0.84 (0.78, 0.90) |
| Model 2 | Ref | 0.96 (0.93, 0.99) | 0.91 (0.85, 0.97) | 0.94 (0.88, 1.01) | 0.92 (0.86, 0.99) |
| Model 3 | Ref | 1.00 (0.97, 1.03) | 0.99 (0.92, 1.06) | 0.95 (0.89, 1.02) | 0.93 (0.87, 1.00) |
| Flavanol oligo + polymers | |||||
| No. events | 1750 | 1391 | 1328 | 1189 | 1042 |
| Intake,2 mg/d | 92 (0–136) | 179 (136–217) | 255 (217–303) | 359 (303–434) | 537 (434–2254) |
| HR (95% CI) | |||||
| Model 1a | Ref | 0.80 (0.77, 0.84) | 0.71 (0.68, 0.75) | 0.66 (0.62, 0.70) | 0.62 (0.58, 0.66) |
| Model 1b | Ref | 0.89 (0.85, 0.94) | 0.84 (0.79, 0.89) | 0.81 (0.76, 0.86) | 0.77 (0.72, 0.83) |
| Model 2 | Ref | 0.91 (0.87, 0.95) | 0.86 (0.82, 0.91) | 0.84 (0.79, 0.89) | 0.82 (0.77, 0.88) |
| Model 3 | Ref | 0.93 (0.88, 0.98) | 0.90 (0.85, 0.95) | 0.89 (0.83, 0.95) | 0.90 (0.83, 0.97) |
| Anthocyanins | |||||
| No. events | 1632 | 1298 | 1171 | 1220 | 1379 |
| Intake,2 mg/d | 5 (0–10) | 13 (10–17) | 20 (17–24) | 36 (24–53) | 70 (53–397) |
| HR (95% CI) | |||||
| Model 1a | Ref | 0.76 (0.72, 0.79) | 0.67 (0.63, 0.72) | 0.70 (0.66, 0.74) | 0.78 (0.73, 0.83) |
| Model 1b | Ref | 0.85 (0.81, 0.89) | 0.80 (0.75, 0.85) | 0.84 (0.79, 0.89) | 0.92 (0.85, 0.99) |
| Model 2 | Ref | 0.88 (0.84, 0.92) | 0.84 (0.79, 0.90) | 0.88 (0.83, 0.94) | 0.94 (0.87, 1.01) |
| Model 3 | Ref | 0.87 (0.83, 0.91) | 0.83 (0.78, 0.89) | 0.88 (0.83, 0.94) | 0.97 (0.90, 1.04) |
| Flavanones | |||||
| No. events | 1372 | 1239 | 1356 | 1348 | 1385 |
| Intake,2 mg/d | 4 (0–6) | 9 (6–13) | 18 (13–26) | 32 (26–49) | 70 (49–564) |
| HR (95% CI) | |||||
| Model 1a | Ref | 0.96 (0.92, 1.01) | 0.94 (0.88, 1.01) | 0.96 (0.90, 1.02) | 1.02 (0.95, 1.09) |
| Model 1b | Ref | 1.03 (0.98, 1.07) | 1.06 (0.99, 1.13) | 1.10 (1.03, 1.17) | 1.15 (1.08, 1.23) |
| Model 2 | Ref | 1.00 (0.96, 1.05) | 1.02 (0.95, 1.09) | 1.05 (0.99, 1.12) | 1.11 (1.04, 1.19) |
| Model 3 | Ref | 1.04 (0.99, 1.08) | 1.07 (1.00, 1.15) | 1.10 (1.03, 1.18) | 1.13 (1.05, 1.22) |
| Flavones | |||||
| No. events | 1446 | 1278 | 1299 | 1271 | 1406 |
| Intake,2 mg/d | 2 (0–3) | 4 (3–5) | 5 (5–6) | 7 (6–9) | 11 (9–51) |
| HR (95% CI) | |||||
| Model 1a | Ref | 0.85 (0.81, 0.89) | 0.81 (0.76, 0.86) | 0.84 (0.79, 0.89) | 0.92 (0.86, 0.98) |
| Model 1b | Ref | 0.92 (0.88, 0.97) | 0.91 (0.86, 0.97) | 0.96 (0.90, 1.02) | 1.04 (0.98, 1.12) |
| Model 2 | Ref | 0.93 (0.88, 0.97) | 0.91 (0.86, 0.97) | 0.95 (0.89, 1.01) | 1.01 (0.95, 1.08) |
| Model 3 | Ref | 0.95 (0.90, 1.00) | 0.96 (0.90, 1.02) | 1.02 (0.95, 1.09) | 1.12 (1.04, 1.21) |
HRs (95% CI) for incident diabetes during 23 y of follow-up, obtained from restricted cubic splines based on Cox proportional hazards models. Model 1a adjusted for age and sex; model 1b adjusted for age, sex, smoking status, physical activity, alcohol intake, education, hormone replacement therapy, and socioeconomic status (income); model 2 adjusted for all covariates in model 1b plus body fat, hypertension status, and cholesterol status; and model 3 adjusted for all covariates in model 2 plus energy intake and intakes (g/d) of red meat, processed meat, PUFAs, MUFAs, SFAs, fiber, soft drinks, coffee, and added sugar.
Median; range in parentheses (all such values).
For flavonoid subclasses, comparing high with low intakes (quintile 5 compared with quintile 1), the risk of diabetes was 19% lower for flavonols (HR: 0.81; 95% CI: 0.75, 0.87), 16% lower for flavanol monomers (HR: 0.86; 95% CI: 0.78, 0.90), and 23% lower for flavanol oligo + polymers (HR: 0.77; 95% CI: 0.72, 0.83) after multivariable adjustments (model 1b; Table 2). Conversely, the risk of diabetes appeared to be positively associated with flavanone intakes, and the association with anthocyanin and flavone intakes was U-shaped (Figure 1). After adjusting for potential dietary confounders, flavanol oligo + polymers were significantly inversely associated with diabetes, flavanones were positively associated with diabetes, and the association between anthocyanin and flavone intake and diabetes was U-shaped (model 3; Table 2).
Mediation analysis
The mediation analysis showed that after multivariable adjustments (model 1b), baseline body fat explained 57% (95% CI: 42, 83%) of the total association between total flavonoid intake and incident diabetes (Supplemental Table 2). For individual flavonoid subclasses, for which clear inverse associations between both baseline body fat and incident diabetes were observed (i.e., flavonols, flavanol monomers, and flavanol oligo + polymers), baseline body fat explained 52% (95% CI: 35, 81%), 66% (95% CI: 45, 89%), and 35% (95% CI: 26, 48%), respectively (Supplemental Table 2).
Associations between major flavonoid compound intakes and incident diabetes
Inverse associations between all individual flavonoid compounds with mean intakes >5 mg/d [except for the flavanol monomers, for which we present only the results for epicatechin due to the very high correlation (>0.89) between flavonol monomers] and incident diabetes are presented in Supplemental Table 3 and Supplemental Figure 2. Kaempferol, quercetin, epicatechin, procyanidin dimer, procyanidin trimer, and malvidin intakes were significantly inversely associated with incident diabetes, although for the latter 2 associations plateaued at moderate intakes. Conversely, hesperidin intakes were positively associated with diabetes, and associations between diabetes and apigenin, delphinidin, and cyanidin intakes were somewhat U-shaped (Supplemental Figure 2).
Associations between total flavonoid intake and incident diabetes stratified by risk factors for diabetes
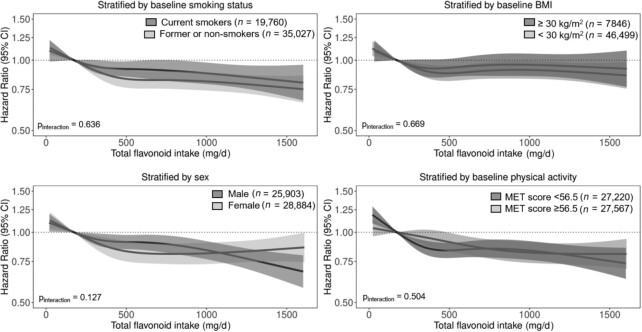
The association between total flavonoid intake and incident diabetes was present in all subgroups investigated (Pinteraction > 0.05 for all; Figure 2). However, because the prevalence of diabetes was higher in those with a BMI ≥30, the difference (total flavonoid intake quintile 5 – total flavonoid intake quintile 1) in the 20-y estimated risk of diabetes was greater in this subgroup (Table 3).
FIGURE 2.
Multivariable-adjusted association between total flavonoid intake and incident diabetes stratified by baseline smoking status, BMI, sex, and physical activity. HRs (y axis on logarithmic scale) are based on Cox proportional hazard models and are comparing the specific level of flavonoid intake (horizontal axis) to the median intake for participants in the lowest intake quintile (174 mg/d). All analyses were standardized for age, sex, smoking status, physical activity, alcohol intake, education, hormone replacement therapy, and socioeconomic status (income). MET, metabolic equivalent.
TABLE 3.
20-y predicted risk of diabetes1
| Total flavonoid intake | |||
|---|---|---|---|
| Quintile 1 risk (95% CI) | Quintile 5 risk (95% CI) | Risk difference (%) | |
| Men | |||
| Nonsmoker | 10.55 (9.64, 11.53) | 9.24 (8.36, 10.20) | 1.31 |
| Former smoker | 11.84 (10.87, 12.89) | 10.39 (9.44, 11.42) | 1.45 |
| Current smoker | 15.03 (13.93, 16.20) | 13.25 (12.07, 14.52) | 1.78 |
| BMI, kg/m2 | |||
| <30 | 11.17 (10.23, 12.19) | 9.38 (8.51, 10.34) | 1.79 |
| ≥30 | 35.24 (32.91, 37.63) | 30.94 (28.56, 33.43) | 4.30 |
| MET score | |||
| <56.5 | 10.72 (9.79, 11.72) | 9.39 (8.48, 10.37) | 1.33 |
| ≥56.5 | 10.30 (9.40, 11.27) | 9.02 (8.16, 9.96) | 1.28 |
| Women | |||
| Nonsmoker | 7.36 (6.70, 8.08) | 6.42 (5.82, 7.07) | 0.94 |
| Former smoker | 8.30 (7.53, 9.14) | 7.24 (6.55, 8.00) | 1.06 |
| Current smoker | 10.65 (9.77, 11.60) | 9.33 (8.47, 10.26) | 1.32 |
| BMI, kg/m2 | |||
| <30 | 7.53 (6.86, 8.25) | 6.28 (5.71, 6.91) | 1.25 |
| ≥30 | 26.04 (24.10, 28.08) | 22.47 (20.66, 24.40) | 3.57 |
| MET score | |||
| <56.5 | 7.48 (6.80, 8.23) | 6.52 (5.91, 7.20) | 0.96 |
| ≥56.5 | 7.18 (6.53, 7.90) | 6.26 (5.68, 6.90) | 0.92 |
1The 20-y predicted risk (percentage) of diabetes calculated from logistic regression models. Unless indicated by the stratification variable, these estimates are for a nonsmoking participant, aged 56 y, who has completed 8–10 y of education, with a BMI of 25.5, a total daily MET score of 56, with a mean household income of 394,701–570,930 DKK/y, an alcohol intake of 13 g/d, and who is not taking hormone replacement therapy. DKK, Danish krone; MET, metabolic equivalent.
Investigation into the positive association between flavanone intake and diabetes
In this cohort, flavanone intake was most strongly correlated with intakes of oranges (r = 0.35), orange juice (r = 0.89), grapefruit (r = 0.12), and soft drinks (r = 0.38; an assumption was made that lemonade consists of ∼6% lemon juice). Intakes of oranges, orange juice, and grapefruit were not significantly associated with incident diabetes, whereas intakes of soft drinks were significantly positively associated with incident diabetes (Supplemental Figure 3). There was no association between flavanone intake and incident diabetes in participants who consumed soft drinks <1 per month (n = 25 773; Supplemental Figure 4).
Discussion
In this prospective cohort of 54,787 Danish men and women, a flavonoid-rich diet was inversely associated with incident diabetes; after adjusting for potential lifestyle and dietary confounders, this association plateaued at moderate flavonoid intakes (∼400 to 600 mg/d). This association did not appear to be modified by smoking status, BMI, physical activity level, or sex; however, findings on an absolute scale suggest that those at a higher risk of diabetes (those with obesity) may benefit the most from a higher flavonoid consumption. Of the flavonoid subclasses, moderate to high intakes of flavonols, flavanol monomers, flavanol oligo + polymers, and anthocyanins were associated with a lower risk of diabetes. Cross-sectionally, total flavonoid intake was inversely associated with body fat, explaining approximately half of the association between total flavonoid intake and incident diabetes.
Limitations inherent to observational studies apply to the current study in that we are not able to infer causality or rule out residual confounding. Furthermore, we acknowledge common FFQ limitations, particularly that not all dietary sources of flavonoids, especially berries other than strawberries, were included in the questionnaire. Although flavonoid intake may have changed during the 23 y of follow-up, the resulting measurement error would likely result in a bias of observed associations toward the null. We acknowledge that although bioelectrical impedance has good agreement with measures of body fat, it is not the gold standard measure for estimating body fat, particularly in obese/morbidly obese and underweight individuals (27), and we only had a single measure at baseline and as such could not investigate changes in body fat or rule out residual confounding. Furthermore, the cross-sectional and mediation analyses should be interpreted with caution because temporality of the flavonoid–body fat association is not known. Unfortunately, we were unable to distinguish between type 1 and type 2 diabetes or capture diabetes treated nonpharmacologically in a primary care setting; due to the age of the cohort (between 50 and 64 y at baseline), we assume that the majority of incident cases were type 2 diabetes. Despite these limitations, the current study has many strengths, including a large sample size with 23 y of follow-up, allowing for the accumulation of a large number of incident cases of diabetes and thus affording sufficient power to examine associations in subgroups of interest; a negligible loss to follow-up, permitting the calculation of absolute risk differences; and the availability of key participant characteristics, enabling appropriate model adjustment to reduce residual confounding.
Evidence from both prospective cohort studies and randomized controlled trials points to dietary patterns high in fruits and vegetables, wholegrains, nuts, and legumes, low in both red and processed meats, sugar-sweetened beverages, and refined grains, and with a moderate alcohol intake, for the prevention and management of type 2 diabetes (28). The association between flavonoid-rich dietary patterns and incident type 2 diabetes has been investigated in several large prospective cohort studies, a meta-analysis of which reports a curvilinear relation between total flavonoid intake and incident type 2 diabetes, with a significantly lower risk at intakes ≥550 mg/d (11). In the current study, the gradient of the inverse association between total flavonoid intake and incident diabetes decreased beyond moderate intakes (quintile 3: 395– 602 mg/d), indicating the existence of a threshold, beyond which higher intakes may afford little added benefit. Of the individual flavonoid subclasses, a lower risk of diabetes has been observed for anthocyanins (29, 30) [RR of 0.89 (95% CI: 0.82, 0.95) in meta-analysis (11)], flavanols (29, 31) [RR of 0.86 (95% CI: 0.78, 0.95) in meta-analysis (11)], flavonols (31, 32) [RR of 0.86 (95% CI: 0.80, 0.94) in meta-analysis (11)], flavanones (29, 33) [RR of 1.02 (95% CI: 0.94, 1.10) in meta-analysis (11)], and flavones (29) [RR of 0.96 (95% CI: 0.85, 1.08) in meta-analysis (11)]. Although the reported relative risks from the meta-analysis are similar to those described in the current study for flavonols, flavanols, and anthocyanins, we observed that higher intakes of flavanones were associated with a higher risk of incident diabetes. From our results, we conclude that this positive association was driven by the assumption that lemonade contains lemon juice (34) and is therefore a contributor to flavanone intake. Intakes of other major sources of flavanones, namely oranges, orange juice, and grapefruit, were not significantly associated with incident diabetes. Important to note is that a major source of anthocyanins in this cohort was fruit squash/cordial, which has a high sugar content. The co-occurrence of dietary components that may increase type 2 diabetes risk within flavonoid-rich foods and beverages, such as sugar in the previous instances, may explain the U-shaped associations observed for the anthocyanin and flavone subclasses and compounds within those subclasses. Interestingly, although malvidin belongs to the anthocyanin subclass, we observed an inverse association between malvidin intake and diabetes. In this cohort, it is likely that the primary dietary source of malvidin was red wine. A moderate intake of red wine is associated with a lower risk of type 2 diabetes in numerous cohort studies, and red wine has been shown to improve insulin sensitivity, an effect not attributable to its alcohol content, in randomized controlled trials (35). In the current study, apples and tea were likely major contributors to total flavonoid intake (31). In a meta-analysis of 16 cohort studies, higher tea intakes were associated with a lower risk of type 2 diabetes (36). The current study exemplifies the need to interpret associations between dietary sources of flavonoids and health outcomes, in observational studies, in the context of the whole food matrix. It may be that the differing associations for flavonoid subclass intakes and diabetes between studies are due to differing dietary patterns and key sources of flavonoids in the different populations.
Risk factors for type 2 diabetes include smoking (37) and obesity (38). Although associations between total flavonoid intakes and diabetes were present in all subpopulations investigated, the difference (flavonoid intake quintile 5 – quintile 1) in the 20-y estimated absolute risk of diabetes was greatest for those with a BMI ≥30 (men: 4.30%; women: 3.57%), likely due to the higher prevalence of diabetes in this “at-risk” subgroup. Thus, if the association between higher flavonoid intakes and lower diabetes risk is truly causal, ensuring adequate intakes of flavonoid-rich foods in obese individuals will translate to a greater reduction in cases of type 2 diabetes at a population level.
Unlike smoking, obesity may be influenced by flavonoid intake (9) and, therefore, may be an intermediate on the causal pathway between flavonoid intake and diabetes. In randomized controlled trials, short-term ingestion of green tea extract (39), powdered black tea (40), and cocoa or dark chocolate (≥30 g/d) (41), all rich in flavonoids, has been shown to reduce body weight and improve body fat distribution. In a study of 2734 healthy female twins from the TwinsUK registry, higher habitual intakes of anthocyanins, flavanols, flavonols, and proanthocyanidins were associated with lower fat mass, independent of genetic and environmental factors (13). Furthermore, in a pooled analysis of 3 large prospective cohorts, a higher habitual intake of flavonoids (with the exception of flavones and flavanones) was associated with less weight gain during follow-up, with the strongest inverse associations observed for intakes of anthocyanins and flavanol polymers (14). In the current study, persons with the highest total flavonoid intakes had a 1.52-kg lower body fat at baseline; body fat explained 57% of the total association between total flavonoid intake and incident diabetes. In this cohort, a 1.52-kg lower body fat translated to a 12% lower risk of diabetes. These findings suggest that a protective effect of flavonoids against diabetes may be mediated, in part, by the effect of flavonoids on the metabolism of fat in the body.
There are numerous purported mechanisms by which flavonoids and their metabolites reduce obesity and type 2 diabetes incidence, including enhancing insulin secretion and reducing insulin resistance, potentially by decreasing apoptosis and promoting proliferation of pancreatic β-cells, and reducing inflammation and oxidative stress in muscle (8, 9). A meta-analysis of randomized controlled trials concluded that green tea consumption significantly reduces fasting glucose and hemoglobin A1c concentrations (42), whereas flavonoid-rich chocolate or cocoa consumption significantly reduces insulin resistance and fasting insulin (43). However, the exact mechanisms remain to be elucidated (44); findings from preclinical studies are often not replicated in human studies (45) likely due to limited studies on flavonoid metabolites to date. Evidence for the antidiabetic effects of a diet rich in flavonoids is growing. Despite this, there has been minimal translation of this research into either policy or practice. This is likely due, at least in part, to an incomplete understanding of the mechanisms and pathways linking flavonoid intake to health benefits (46). Complete characterization is challenging because bioavailability and bioactivity differ between flavonoid compounds, and both phase 2 and gut-derived flavonoid metabolites likely also exert biologically relevant effects (47).
Conclusions
In this Danish prospective cohort study, we observed that higher flavonoid intakes were cross-sectionally associated with lower estimates of body fat and longitudinally associated with a lower risk of diabetes, particularly for the flavonol, flavanol monomer, and flavanol oligo + polymer subclasses. Our results suggest that promoting a diet abundant in healthy flavonoid-rich foods may ameliorate diabetes risk, in part through a reduction in body fat.
Supplementary Material
Acknowledgments
Ethics approval: In Denmark, register studies do not require approval from ethics committees. This study was approved by the Danish Data Protection Agency (no. 2012–58–0004 I-Suite nr: 6357, VD-2018–117). The authors’ responsibilities were as follows—NPB, FD, and JMH: designed the research (project conception, development of overall research plan, and study oversight); AT: conducted the original cohort study; AS and CK: calculated flavonoid intake from FFQ data; NPB, FD, and KM: analyzed the data; NPB: wrote the manuscript and had primary responsibility for final content; FD, KM, CPB, AC, JRL, CK, GG, AS, AT, and JMH: assisted with interpretation of the results and critically reviewed the manuscript; and all authors: read and approved the final manuscript.
Notes
NPB is funded by a National Health and Medical Research Council Early Career Fellowship (grant APP1159914), Australia. The salary of JMH is supported by a National Health and Medical Research Council of Australia Senior Research Fellowship, Australia (grant APP1116937). The salary of JRL is supported by a National Heart Foundation of Australia Future Leader Fellowship (ID 102817).
Author disclosures: The authors report no conflicts of interest.
Disclaimer: Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/World Health Organization.
Supplemental Figures 1–4 and Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: ATC, anatomical therapeutic chemical code; ICD, International Classification of Diseases; MET, metabolic equivalent.
Contributor Information
Nicola P Bondonno, Institute for Nutrition Research, School of Medical and Health Sciences, Edith Cowan University, Perth, Australia; Health and Medical Sciences, University of Western Australia, Royal Perth Hospital, Perth, Australia.
Frederik Dalgaard, Department of Cardiology, Herlev & Gentofte University Hospital, Copenhagen, Denmark.
Kevin Murray, School of Population and Global Health, University of Western Australia, Perth, Australia.
Raymond J Davey, School of Physiotherapy and Exercise Science, Curtin University, Perth, Australia.
Catherine P Bondonno, Institute for Nutrition Research, School of Medical and Health Sciences, Edith Cowan University, Perth, Australia; Health and Medical Sciences, University of Western Australia, Royal Perth Hospital, Perth, Australia.
Aedin Cassidy, Institute for Global Food Security, Queen's University Belfast, Northern Ireland.
Joshua R Lewis, Institute for Nutrition Research, School of Medical and Health Sciences, Edith Cowan University, Perth, Australia; Health and Medical Sciences, University of Western Australia, Royal Perth Hospital, Perth, Australia.
Cecilie Kyrø, The Danish Cancer Society Research Center, Copenhagen, Denmark.
Gunnar Gislason, Department of Cardiology, Herlev & Gentofte University Hospital, Copenhagen, Denmark; The National Institute of Public Health, University of Southern Denmark, Odense, Denmark; The Danish Heart Foundation, Copenhagen, Denmark.
Augustin Scalbert, International Agency for Research on Cancer, Lyon, France.
Anne Tjønneland, The Danish Cancer Society Research Center, Copenhagen, Denmark; Department of Public Health, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Jonathan M Hodgson, Institute for Nutrition Research, School of Medical and Health Sciences, Edith Cowan University, Perth, Australia; Health and Medical Sciences, University of Western Australia, Royal Perth Hospital, Perth, Australia.
Data Availability
The data, codebook, and analytic code will be made available upon request pending application and approval by the Diet, Cancer, and Health Steering Committee at the Danish Cancer Society.
References
- 1. Vos T, Barber RM, Bell B, Bertozzi-Villa A, Biryukov S, Bolliger I, Charlson F, Davis A, Degenhardt L, Dicker D. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet North Am Ed. 2015;386(9995):743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Danaei G, Lu Y, Singh GM, Carnahan E, Stevens GA, Cowan MJ, Farzadfar F, Lin JK, Finucane MM, Rao M. Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: a comparative risk assessment. Lancet Diabetes Endocrinol. 2014;2(8):634–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou B, Lu Y, Hajifathalian K, Bentham J, Di Cesare M, Danaei G, Bixby H, Cowan MJ, Ali MK, Taddei C. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schwingshackl L, Hoffmann G, Lampousi A-M, Knüppel S, Iqbal K, Schwedhelm C, Bechthold A, Schlesinger S, Boeing H. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. 2017;32(5):363–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Muraki I, Imamura F, Manson JE, Hu FB, Willett WC, van Dam RM, Sun Q. Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies. BMJ. 2013;347:f5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caro-Ordieres T, Marín-Royo G, Opazo-Ríos L, Jiménez-Castilla L, Moreno JA, Gómez-Guerrero C, Egido J. The coming age of flavonoids in the treatment of diabetic complications. J Clin Med. 2020;9(2):346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Williamson G, Kay CD, Crozier A. The bioavailability, transport, and bioactivity of dietary flavonoids: a review from a historical perspective. Compr Rev Food Sci Food Saf. 2018;17(5):1054–112. [DOI] [PubMed] [Google Scholar]
- 8. Kawser Hossain M, Abdal Dayem A, Han J, Yin Y, Kim K, Kumar Saha S, Yang G-M, Choi HY, Cho S-G. Molecular mechanisms of the anti-obesity and anti-diabetic properties of flavonoids. Int J Mol Sci. 2016;17(4):569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vinayagam R, Xu B. Antidiabetic properties of dietary flavonoids: a cellular mechanism review. Nutr Metab. 2015;12(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodriguez-Mateos A, Vauzour D, Krueger CG, Shanmuganayagam D, Reed J, Calani L, Mena P, Del Rio D, Crozier A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: an update. Arch Toxicol. 2014;88(10):1803–53. [DOI] [PubMed] [Google Scholar]
- 11. Xu H, Luo J, Huang J, Wen Q. Flavonoids intake and risk of type 2 diabetes mellitus: a meta-analysis of prospective cohort studies. Medicine (Baltimore). 2018;97(19):e0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holbrook T, Barrett-Connor E, Wingard D. The association of lifetime weight and weight control patterns with diabetes among men and women in an adult community. Int J Obes. 1989;13(5):723–9. [PubMed] [Google Scholar]
- 13. Jennings A, MacGregor A, Spector T, Cassidy A. Higher dietary flavonoid intakes are associated with lower objectively measured body composition in women: evidence from discordant monozygotic twins. Am J Clin Nutr. 2017;105(3):626–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bertoia ML, Rimm EB, Mukamal KJ, Hu FB, Willett WC, Cassidy A. Dietary flavonoid intake and weight maintenance: three prospective cohorts of 124,086 US men and women followed for up to 24 years. BMJ. 2016;352:i17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gómez-Ambrosi J, Silva C, Galofré JC, Escalada J, Santos S, Gil MJ, Valentí V, Rotellar F, Ramírez B, Salvador J. Body adiposity and type 2 diabetes: increased risk with a high body fat percentage even having a normal BMI. Obesity. 2011;19(7):1439–44. [DOI] [PubMed] [Google Scholar]
- 16. Wallach Kildemoes H, Toft Sørensen H, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7 Suppl):38–41. [DOI] [PubMed] [Google Scholar]
- 17. Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7 Suppl):30–3. [DOI] [PubMed] [Google Scholar]
- 18. Bondonno NP, Dalgaard F, Kyrø C, Murray K, Bondonno CP, Lewis JR, Croft KD, Gislason G, Scalbert A, Cassidy Aet al. Flavonoid intake is associated with lower mortality in the Danish Diet Cancer and Health Cohort. Nat Commun. 2019;10(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Overvad K, Tjønneland A, Haraldsdottir J, Ewertz M, Møller Jensen O. Development of a semiquantitative food frequency questionnaire to assess food, energy and nutrient intake in Denmark. Int J Epidemiol. 1991;20(4):900–5. [DOI] [PubMed] [Google Scholar]
- 20. Neveu V, Perez-Jiménez J, Vos F, Crespy V, Du Chaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart D. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database. 2010;2010:bap024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frost L, Benjamin EJ, Fenger-Grøn M, Pedersen A, Tjønneland A, Overvad K. Body fat, body fat distribution, lean body mass and atrial fibrillation and flutter: a Danish cohort study. Obesity. 2014;22(6):1546–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heitmann B. Prediction of body water and fat in adult Danes from measurement of electrical impedance: a validation study. Int J Obes. 1990;14(9):789–802. [PubMed] [Google Scholar]
- 23. Ling CH, de Craen AJ, Slagboom PE, Gunn DA, Stokkel MP, Westendorp RG, Maier AB. Accuracy of direct segmental multi-frequency bioimpedance analysis in the assessment of total body and segmental body composition in middle-aged adult population. Clin Nutr. 2011;30(5):610–15. [DOI] [PubMed] [Google Scholar]
- 24. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lange T, Rasmussen M, Thygesen LC. Assessing natural direct and indirect effects through multiple pathways. Am J Epidemiol. 2014;179(4):513–18. [DOI] [PubMed] [Google Scholar]
- 26. Steen J, Loeys T, Moerkerke B, Vansteelandt S. Medflex: an R package for flexible mediation analysis using natural effect models. J Stat Softw. 2017;76(11). doi: 10.18637/jss.v076.i11. [DOI] [Google Scholar]
- 27. Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, Heitmann BL, Kent-Smith L, Melchior J-C, Pirlich M. Bioelectrical impedance analysis—Part II: utilization in clinical practice. Clin Nutr. 2004;23(6):1430–53. [DOI] [PubMed] [Google Scholar]
- 28. Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet North Am Ed. 2014;383(9933):1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grosso G, Stepaniak U, Micek A, Kozela M, Stefler D, Bobak M, Pajak A. Dietary polyphenol intake and risk of type 2 diabetes in the Polish arm of the Health, Alcohol and Psychosocial Factors in Eastern Europe (HAPIEE) study. Br J Nutr. 2017;118(1):60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wedick NM, Pan A, Cassidy A, Rimm EB, Sampson L, Rosner B, Willett W, Hu FB, Sun Q, van Dam RM. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am J Clin Nutr. 2012;95(4):925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zamora-Ros R, Knaze V, Rothwell JA, Hémon B, Moskal A, Overvad K, Tjønneland A, Kyrø C, Fagherazzi G, Boutron-Ruault M-C. Dietary polyphenol intake in Europe: the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Nutr. 2016;55(4):1359–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jacques PF, Cassidy A, Rogers G, Peterson JJ, Meigs JB, Dwyer J. Higher dietary flavonol intake is associated with lower incidence of type 2 diabetes. J Nutr. 2013;143(9):1474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tresserra-Rimbau A, Guasch-Ferré M, Salas-Salvadó J, Toledo E, Corella D, Castaner O, Xiaohui G, Gomez-Gracia E, Lapetra J, Aros Fet al. Intake of total polyphenols and some classes of polyphenols is inversely associated with diabetes in elderly people at high cardiovascular disease risk. J Nutr. 2015;146(4):767–77. [DOI] [PubMed] [Google Scholar]
- 34. Knaze V, Rothwell JA, Zamora-Ros R, Moskal A, Kyrø C, Jakszyn P, Skeie G, Weiderpass E, Santucci de Magistris M, Agnoli C. A new food-composition database for 437 polyphenols in 19,899 raw and prepared foods used to estimate polyphenol intakes in adults from 10 European countries. Am J Clin Nutr. 2018;108(3):517–24. [DOI] [PubMed] [Google Scholar]
- 35. Martin MA, Goya L, Ramos S. Protective effects of tea, red wine and cocoa in diabetes: evidences from human studies. Food Chem Toxicol. 2017;109:302–14. [DOI] [PubMed] [Google Scholar]
- 36. Yang W-S, Wang W-Y, Fan W-Y, Deng Q, Wang X. Tea consumption and risk of type 2 diabetes: a dose–response meta-analysis of cohort studies. Br J Nutr. 2014;111(8):1329–39. [DOI] [PubMed] [Google Scholar]
- 37. Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298(22):2654–64. [DOI] [PubMed] [Google Scholar]
- 38. Boles A, Kandimalla R, Reddy PH. Dynamics of diabetes and obesity: epidemiological perspective. Biochim Biophys Acta Mol Basis Dis. 2017;1863(5):1026–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nagao T, Hase T, Tokimitsu I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity. 2007;15(6):1473–83. [DOI] [PubMed] [Google Scholar]
- 40. Bøhn SK, Croft KD, Burrows S, Puddey IB, Mulder TP, Fuchs D, Woodman RJ, Hodgson JM. Effects of black tea on body composition and metabolic outcomes related to cardiovascular disease risk: a randomized controlled trial. Food Funct. 2014;5(7):1613–20. [DOI] [PubMed] [Google Scholar]
- 41. Kord-Varkaneh H, Ghaedi E, Nazary-Vanani A, Mohammadi H, Shab-Bidar S. Does cocoa/dark chocolate supplementation have favorable effect on body weight, body mass index and waist circumference? A systematic review, meta-analysis and dose–response of randomized clinical trials. Crit Rev Food Sci Nutr. 2019;59(15):2349–62. [DOI] [PubMed] [Google Scholar]
- 42. Liu K, Zhou R, Wang B, Chen K, Shi L-Y, Zhu J-D, Mi M-T. Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. Am J Clin Nutr. 2013;98(2):340–8. [DOI] [PubMed] [Google Scholar]
- 43. Hooper L, Kay C, Abdelhamid A, Kroon PA, Cohn JS, Rimm EB, Cassidy A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr. 2012;95(3):740–51. [DOI] [PubMed] [Google Scholar]
- 44. Cao H, Ou J, Chen L, Zhang Y, Szkudelski T, Delmas D, Daglia M, Xiao J. Dietary polyphenols and type 2 diabetes: human study and clinical trial. Crit Rev Food Sci Nutr. 2019;59(20):3371–9. [DOI] [PubMed] [Google Scholar]
- 45. Martin MÁ, Goya L, Ramos S. Antidiabetic actions of cocoa flavanols. Mol Nutr Food Res. 2016;60(8):1756–69. [DOI] [PubMed] [Google Scholar]
- 46. Chen L, Teng H, Xie Z, Cao H, Cheang WS, Skalicka-Woniak K, Georgiev MI, Xiao J. Modifications of dietary flavonoids towards improved bioactivity: an update on structure–activity relationship. Crit Rev Food Sci Nutr. 2018;58(4):513–27. [DOI] [PubMed] [Google Scholar]
- 47. Teng H, Chen L. Polyphenols and bioavailability: an update. Crit Rev Food Sci Nutr. 2019;59(13):2040–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data, codebook, and analytic code will be made available upon request pending application and approval by the Diet, Cancer, and Health Steering Committee at the Danish Cancer Society.