Summary
Chronic hepatitis C (HCV) infection is a major global public health threat and in 2019 there were an estimated 58 million infected globally and 290,000 deaths. Elimination of viral hepatitis B/C as a public health threat by 2030 is defined as a 90% incidence reduction and a 65% mortality reduction. The Western Pacific region is one of the most affected regions with 10 million people living with HCV, one-fifth of the global burden. We review progress towards HCV elimination in the Western Pacific region since 2015. Key developments in the region, which comprises of 37 high-and-middle-income countries, include the following: 20 countries have national hepatitis action plans, 19 have conducted recent disease burden and investment cases, 10 have scaled-up hepatitis services at primary health care level, and in 11 countries, domestic financing including social health insurance support DAA costs. We highlight six countries' experience in navigating the path towards HCV elimination: Cambodia, China, Malaysia, Mongolia, Philippines, and Viet Nam. Future initiatives to accelerate elimination are expanding access to community-based testing using HCV point-of-care tests among at-risk and general populations; adopting decentralized and integrated HCV one-stop services at harm reduction sites, detention settings and primary care; expanding treatment to include children and adolescents; address stigma and discrimination; and ensuring sustainable financing through domestic resources to scale-up testing, treatment and prevention. The COVID-19 pandemic has a significant impact on hepatitis response across the region on community and facility-based testing, treatment initiation, monitoring and cancer screening, which is projected to delay elimination goals.
Keywords: hepatitis C, Western Pacific, regional progress, hepatitis elimination, hepatitis action plans
Introduction
Chronic hepatitis C infection (HCV) is a major global public health threat and cause of liver disease globally, with a disproportionately high burden in low-and middle-income countries (LMICs) (1-3). HCV infection is most commonly associated in LMICs with unsafe injection or inadequate infection control practices in health-care facilities, and in high-and middle-income countries (HMICs), most HCV transmission occurs among people who use unsterile equipment to inject drugs (2,3). In 2019, an estimated 58 million persons were chronically infected with HCV and there were 290,000 HCV-related deaths (3). On 28 May 2016 during the 69th World Health Assembly, 194 Member States made a historic commitment to eliminate viral hepatitis as a public health threat by 2030 with the launch of the first-ever Global Health Sector Strategy (GHSS) for Viral Hepatitis 2016-2021 (1). This strategy outlined a set of global impact targets - a reduction in hepatitis B/C-related mortality by 65% and in incidence of chronic hepatitis B (HBV) and HCV infections by 90% to achieve the goal of elimination of viral hepatitis through scale-up of five key synergistic preventative and testing/treatment programmatic interventions.
The global response and opportunities for HCV elimination have been transformed by advances in treatment in 2014 with the advent of curative, short-course direct acting antiviral (DAA) therapy, followed by dramatic 1000-fold reductions in cost of 12-weeks treatment to under USD$ 100; and simplification of the diagnostic pathway with widespread availability of rapid diagnostic testing for HCV antibody and now access to laboratory-based as well as point-of-care nucleic acid testing (NAT) for confirmation of HCV viraemia with associated recent cost reductions (4).
There has been further support for the scale-up of testing and treatment through a rapid sequence of guideline updates from professional societies such as American Association for the Study of Liver Diseases (AASLD), European Association for the Study of the Liver (EASL) and Asian Pacific Association for the Study of the Liver (APASL) (5-8) as well as from the World Health Organization (WHO) in 2014, 2016 and 2018 leading to recommendations for a "treat all" approach regardless of stage of disease using a few pan-genotypic regimens and adoption of a public health approach (9).
Although good progress has been made in several champion countries in scale-up of treatment access and of preventative approaches to reduce transmission, such as blood and injection safety, globally, we are far from achieving the 2030 service delivery coverage targets of 90% diagnosis and treatment of 80% of those infected. As of 2019, only around 20% of persons with HCV infection had been tested but now 62% of those diagnosed have been treated, as a result of the marked increase in the cumulative number treated, from 5 million in 2017 to almost 9.4 million in 2019 (3). To achieve the targets for global elimination by 2030, a substantial scale-up in testing and treatment is needed, with simplification of the care pathway.
The 2018 WHO guidelines on care and treatment of persons with chronic hepatitis C infection endorsed eight key good practice approaches to simplify service delivery of viral hepatitis prevention, care and treatment, and improve access to effective hepatitis services, and implement the "Treat All" approach (Table 1) (9). However, until recently, most of the evidence to inform the simplified approaches of decentralization, integration and task-shifting were based on HIV literature and experience where adoption of these approaches had such a catalytic impact on antiretroviral treatment scale-up (10). There are even greater opportunities for simplification with HCV infection, as short course curative treatment requires minimal expertise and monitoring. A recent comprehensive WHO-led systematic review of 142 studies now provides a strong evidence-base supporting these approaches in HCV care (11). Full decentralization of HCV testing and treatment at the same site compared to no or only partial decentralization was associated with increased linkage and treatment uptake, especially among persons who inject drugs. Task-shifting to primary care providers was associated with high rates of HCV cure compared to specialist-delivered care in all subpopulations. The feasibility and effectiveness of other emerging models of care to achieve elimination has been demonstrated in different settings, including a comprehensive "educate, test and treat" in a high burden rural general population settings in Egypt (and now also in Pakistan) that achieved high testing and treatment coverage and 90% reduction in HCV incidence (12,13); a same day test-and-treat model (14); and micro-elimination initiatives in most affected populations such as people who inject drugs, people living with HIV, persons on haemodialysis, and in prisons (15).
Table 1. Eight good practice principles for simplified service delivery of viral hepatitis prevention, care and treatment.
| No. | Eight practice principles |
|---|---|
| 1 | Comprehensive national planning for the elimination of HCV infection based on local epidemiological context, existing health-care infrastructure, current coverage of testing, treatment and prevention, and available financial or human resources. |
| 2 | Simple and standardized algorithms across the continuum of care from testing, linkage to care and treatment. |
| 3 | Strategies to strengthen linkage from testing to care, treatment and prevention. |
| 4 | Integration of hepatitis testing, care and treatment with other services (e.g. HIV services) to increase the efficiency and reach of hepatitis services. |
| 5 |
Decentralized testing and treatment services at primary health facilities or harm reduction sites to promote access to care. This is facilitated by two approaches: i) task-sharing, supported by training and mentoring of health-care workers and peer workers; ii) a differentiated care strategy to assess level-of-care needs, with specialist referral as appropriate for those with complex problems. |
| 6 | Community engagement and peer support to promote access to services and linkage to the continuum of care, which includes addressing stigma and discrimination. |
| 7 | Strategies for more efficient procurement and supply management of quality-assured, affordable medicines and diagnostics. |
| 8 | Data systems to monitor the quality of individual care and coverage at key steps along the continuum or cascade of care at the population level. |
The WHO Global Hepatitis Programme also undertook in collaboration with key partners a global project in 2019 to collate good practices and lessons learned from different aspects of national viral hepatitis responses. Key success factors identified in planning the response included strong political will and leadership, effective community mobilization and engagement, and development of comprehensive and costed national plans. Optimal forecasting, national registration and strategic procurement approaches for both drugs and diagnostics through forecasting, as well as exploiting opportunities for diagnostic integration, and simplified integrated service delivery at harm reduction sites and in primary care, supported by a well-trained workforce accelerates service delivery.
Implementing the Regional Action Plan for Viral Hepatitis 2016-2020 towards HCV elimination
The Western Pacific Region comprises 37 high-and middle-income countries and is highly diverse in its hepatitis epidemiology across the large countries and small island states. WHO estimates that in 2019, 126 million people were living with chronic HBV infection (defined as hepatitis B surface antigen (HBsAg) positive) (116 million) and chronic HCV (HCV viraemic prevalence) (10 million) in the region, accounting for 40% of the global HBV burden (296 million) and 17% of the world's 58 million HCV burden (3). Half of the global burden of death due to hepatitis B and C are in this region, mostly due to cirrhosis or liver cancer from chronic hepatitis infection. The incidence of liver cancer is especially high, and five out of 10 countries with the highest incidence of new cases of liver cancer globally include Mongolia, Lao People's Democratic Republic, Cambodia, Viet Nam and China (16). Table 2 summarizes regional progress in 2019.
Table 2. Monitoring regional hepatitis progress in the Western Pacific Region, 2021.
| Targets | Interventions | Global 2020 targets (Regional targets in parentheses) | Global 2030 targets | Western Pacific Region at the end of 2019 |
|---|---|---|---|---|
| Impact | Incidence and prevalence among the general population | -30% | -90% | HBV prevalence in children younger than 5 years: 0.3% |
| (< 1% HBsAg in five-year-olds born after HBV immunization started) | 0.1% HBsAg in children aged 5 years or less | HBV prevalence: 5.9% | ||
| HBV incidence: 140,000 cases | ||||
| HCV prevalence: 0.5% | ||||
| HCV incidence: 230,000 cases | ||||
| Mortality | -10% | -65% | HBV deaths: 470,000 | |
| HCV deaths: 77,000 | ||||
| Service coverage | 3 dose hepatitis B vaccination | 9% | 90% | 94% |
| (95% by 2017) | ||||
| Birth dose hepatitis B vaccination | 50% | 90% | 84% | |
| (95% by 2017) | ||||
| Safe blood (screened donations) | 95% | 100% | 100% | |
| Safe injections | 100% | 100% | 98.8% safe injections | |
| Harm reduction | 200 injection sets/PWID | 300 injection sets/PWID | 57 injection sets/PWID (2015 base-line) | |
| Tested | 30% | 90% | HBV: 21.4 million (18%) | |
| HCV: 3.5 million (25%)± | ||||
| Treatment | HBV: 5 million | 80% eligible treated | HBV: 5.6 million (5%) | |
| HCV: 3 million | HCV: 1.5 million (10%)± |
±2015 baseline data was used as the denominator (number of people living with hepatitis C). PWID: people who inject drugs. Source: WHO, 2021.
On 14 October 2015, as part of Resolution WPR/ RC66.1, the Regional Action Plan for Viral Hepatitis in the Western Pacific 2016-2020, was launched to help countries in the Region develop their national responses to viral hepatitis (17). The framework consists of five priority areas (advocacy and awareness, evidence-based policy, data and surveillance, stopping transmission, and the treatment cascade) and follows a systems approach. This was followed in October 2017, with a further preventative initiative towards elimination - the Regional Framework for Triple Elimination of Mother-to-Child Transmission (EMTCT) of HIV, Hepatitis B and Syphilis in Asia and the Pacific 2018- 2030 (triple elimination, WPR/RC68.R2). This outlines an integrated and coordinated approach towards triple elimination, and includes the additional intervention of antiviral prophylaxis for HBV EMTCT to help achieve the global 2030 target of 0.1% HBsAg prevalence among children aged 5 years by 2030 (18). This integrated platform will also be important in the future for prevention of HCV mother-to-child transmission, pending the outcome of ongoing trials on the safety and efficacy of DAAs in pregnancy.
Since the launch of the Regional Action Plan for Viral Hepatitis in 2015, national action plans are now in place for 20 countries, and disease burden and investment cases have been developed in 19 countries. Drug prices have also been reduced substantially, and hepatitis medicines have been included in national health insurance systems in many countries. Hepatitis services are being scaled up at primary health care level in 10 countries and strategic information plans were developed by seven countries.
Developing a comprehensive national plan using a "whole systems" approach
A key feature of the WHO Western Pacific regional response and support to countries in their journey towards elimination of viral hepatitis as a public health threat is the adoption of a "whole systems" approach in developing a comprehensive national hepatitis response under the umbrella of Universal Health Coverage (UHC) (19). A systems approach recognizes that all parts of a health system are interrelated and interactions among the multiple parts and stakeholders are dynamic. National plans play a vital initial role in establishing a comprehensive viral hepatitis response. A specific costed national strategy or action plan allows Member States to identify priority areas and mobilize resources to mount an effective response. By the end of 2020, 20 countries had developed or drafted their national action plans for HBV and HCV (Figure 1) (20). Concurrently, these countries have also established national steering committees or working groups that support governance and coordination of the multiple stakeholders, programmes, partners and civil society organizations to deliver on the target indicators for hepatitis elimination. The majority of these groups include representation of patient and civil society organizations which contribute both to policy and guideline development, as well as help expand the scope, reach and acceptability of hepatitis services (21).
Figure 1.
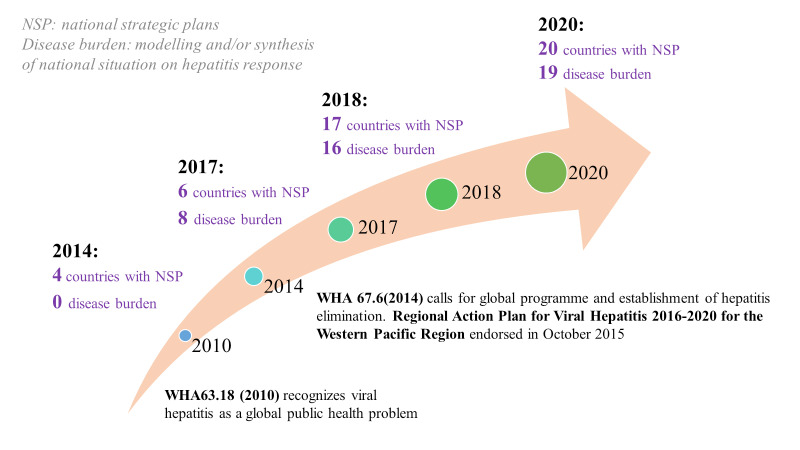
Scaling-up national action plans, Western Pacific Region, 2014-2020.
Integration of financing of hepatitis care with existing social health insurance programmes
By the end of 2020, most countries with existing social health insurance or government-financed universal coverage systems had incorporated financing of hepatitis care and, in particular, the costs of HCV DAA medicines (Table 3). This represents marked progress from the baseline of 2015 where only six countries funded the costs of direct-acting antiviral medicines - all of them high-income countries in the Western Pacific region, and a significant step to ensure sustainability of services needed to achieve elimination. Lessons learned from vertically funded programmes in transitioning to integrated financing and service delivery such as HIV, tuberculosis (TB) and malaria illustrate the complexities for decision makers in balancing the public health, political, economic and social needs (22).
Table 3. Coverage of hepatitis treatment through domestic resources (social health insurance and government financing) at the end of 2020 for selected countries in the Western Pacific Region.
| Country | HBV | HCV-DAA |
|---|---|---|
| Australia | Financed | Financed |
| Brunei Darussalam | Financed | Financed# |
| Cambodia | OOP | OOP |
| China | Financed | Financed |
| Hong Kong SAR | Financed | Financed |
| Japan | Financed | Financed |
| Lao PDR | OOP | OOP |
| Macao SAR | Financed | Financed |
| Malaysia | Financed | Financed |
| Mongolia | Financed | Financed |
| New Zealand | Financed | Financed |
| Papua New Guinea | OOP± | OOP |
| Philippines | * | OOP* |
| Republic of Korea | Financed | Financed |
| Singapore | Financed | Financed |
| Viet Nam | Financed | Financed |
OOP: out-of-pocket; PDR: People's Democratic Republic; SAR: Special Administrative Region (China). #Brunei Darussalam: transitioned to DAA and "Treat All" from mid-2020. ±Papua New Guinea: hepatitis B (HBV) testing and treatment pilot established in Oro province since 2019. *Philippines: pilots for HBV and hepatitis C (HCV) testing and treatment started with government financing in 2019, with HBV national expansion in April 2020. Source: WHO Western Pacific Region, 2021.
Country profiles on national viral hepatitis response
Implementation of these good practices to achieve elimination by 2030, is highly varied and adapted to the context of each country. The following section focusses on progress in six selected middle-income countries, accounting for more than 95% of the HCV burden in the region.
Cambodia
Cambodia has the fifth highest incidence of liver cancer globally and an estimated prevalence of 1.6% HCV viraemic infection. At the end of 2019, Cambodia launched its first-ever national strategic plan for viral hepatitis 2020-2024, just before the onset of the COVID-19 pandemic (WHO Cambodia, personal communications). In-country partners such as Medecins sans Frontieres- France (MSF-F) have established simplified models of HCV care at community level since 2016 in two provinces (23,24), and provided testing for about 135,000 people and treatment for 18,000 people by the end of 2020 (MSF-F Cambodia, personal communications). The programme also provided training of healthcare workers and task-shifting to non-specialists and nurses, strengthening of laboratory capacity, active case finding as well as implementation research to provide evidence for national decision-making. In addition, financial support from The Global Fund to Fight AIDS, TB and Malaria (Global Fund) to support diagnosis and HCV cure of HIV-HCV co-infected patients in the national HIV programme, was secured by the National Centre for HIV/AIDS, Dermatology and STDs (NCHADS) as mortality of co-infected persons is significantly higher. Resourcing the national plan and scaling up to achieve hepatitis elimination is an ongoing challenge and especially amidst the COVID-19 pandemic. Although domestic financing allocation for HCV services would ensure sustainability, external donor support will likely be needed to achieve the 2030 elimination targets. Sustainable financing will also need to leverage more diverse sources of government revenues for health and include innovations and efficiencies such as cheaper generic DAAs, central or pooled procurement to enable lower bulk volume prices for medicines and diagnostics. There is also a need to empower communities in promoting health literacy to reduce transmission, address stigma and discrimination and optimize patient compliance with DAA treatment and achieve cure (25).
China
China has one of the largest burden of HCV with an estimated 7.6 million HCV infections at the start of 2016 (3). Investment cases for HCV elimination demonstrated good returns on investment and cost savings (26,27). The 2017-2020 national plan for prevention and control of viral hepatitis articulated a framework for action by multiple stakeholders. As availability of imported and domestically developed DAAs accelerated from 2015, attempts to improve patient access and mitigate the financial burden of these high-priced medicines were piloted in multiple provinces (28,29). Central price negotiations for hepatitis C medicines led by the National Healthcare Security Administration resulted in a more than 85% reduction for a three-month treatment course from US$ 10,000 to US$ 1-2,000 at the end of 2019 (30). This price reduction also enabled the inclusion of three DAA combinations (sofosbuvir/ledipasvir, sofosbuvir/ velpatasvir and elbasvir/grazoprevir) in the national health insurance package from January 2020, enabling further expansion of access to treatment. Development of the national HCV elimination plan 2021-2030 is in progress.
Malaysia
In its commitment to achieving UHC and elimination of viral hepatitis as a public health threat by 2030, Malaysia used compulsory licensing as a policy tool to improve access to affordable treatment (31). With access to cheaper DAA generics, prices have decreased by more than 50-80% since 2015, and the number of patients treated in the public sector have doubled. The country is rapidly expanding new clinical service capacity to deliver HCV testing, treatment and monitoring at primary and community care facilities (32). In addition, strategic partnerships between government and Drugs for Neglected Diseases initiative (DNDi) with the Foundation for Innovative New Diagnostics (FIND) have supported active research in diversification of HCV treatment options using a combination of sofosbuvir and ravidasvir as a new low-cost pan-genotypic regimen as well as the 'how' to deliver decentralized diagnosis, linkage to care, treatment initiation and cure at community level health facilities (33). Strong civil society input and engagement to support national expansion of services working with vulnerable groups and building empowered communities accelerates the country's UHC journey of getting to elimination (34).
Mongolia
Mongolia has the highest liver cancer incidence globally, mostly due to HBV and HCV, and was the first lower-middle-income country in the Asia and the Pacific region to commit to hepatitis elimination (35). The Healthy Liver Programme 2017-2020 strategy encompassed an ambitious plan to eliminate HCV and control HBV and hepatitis delta virus (HDV) by 2020 (36). A systematic phased approach with provision of care and treatment to priority populations through progressive service expansion was developed. The costs of HBV, HCV and HDV screening, diagnosis and treatment were covered under the national social health insurance programme. By 2019, the programme had screened over 1 million people and treated more than 30,000 through delivery of integrated services at primary care facilities across the country (37). Screening for liver cancer through ultrasonography is included as part of the essential package of primary healthcare services enabling decentralized access and routine surveillance across the country. Given the high burden of HBV, HCV and HDV, the national programme also offers, uniquely, routine antenatal HBV, HCV and HDV screening as part of integrated triple elimination of mother-to-child transmission of HIV, syphilis and viral hepatitis.
Philippines
With over 600,000 people infected with chronic HCV, the Philippines embarked on piloting models of service delivery to enhance reaching those most affected including people who inject drugs and those incarcerated (38). Supported by WHO and funded by government, a pilot was launched mid-2019 in Cebu, Central Visayas Region, providing community-based HCV services offering free screening, viral load testing and treatment as a one-stop approach (39). Early experience from this and other pilot sites highlighted the need to accelerate universal offer of screening to all at-risk populations including people living with HIV, individuals who have ever injected drugs and incarcerated people. Expansion of availability of testing and treatment is now planned for other regions of the country beyond the current pilot sites and to engage private providers to significantly enhance service access.
Viet Nam
With liver cancer as the top cause of cancer deaths (40), Viet Nam established a 2015-2019 national plan outlining the core areas for prevention, testing and treatment for viral hepatitis which was updated for 2020-2024. Entry of DAAs in 2017 through fast-track drug registration mechanisms and importation of generic options has improved affordability (41). In 2019, social health insurance included coverage of four DAAs at a reimbursement rate of 50% of drug costs, significantly improving access and reducing financial burden to patients (42). However, DAA prices remain high because of in-country mark-ups which may include shipping, insurance, import duties and in-country taxes, and storage etc. (43).
What's next at the cusp of the decade of elimination by 2030
With the exception of certain early adopter countries such as Malaysia and Mongolia, many of the HCV elimination initiatives in the region were first established in 2019 and therefore the majority of services for screening, diagnosis, treatment and care are still in the initial phases of expansion. Discussions from the Expert Consultation on Viral Hepatitis Elimination in the Western Pacific held in December 2020, noted the tremendous progress made and recommended acceleration of coordinated integrated programming needed to achieve elimination. This includes immunization, maternal and child health, HIV/sexually transmitted infections, noncommunicable diseases and cancer control (44). A multi-dimensional, public health approach for prevention and treatment of viral hepatitis and chronic liver disease alongside addressing harmful use of alcohol and obesity (non-alcoholic fatty liver disease, NAFLD) is also required (45). There is need to strengthen national responses in access to hepatitis testing and care and achieve more systematic testing of the adults, adolescents and children in high-burden countries or high priority populations, ensure screening for liver cancer particularly among those with cirrhosis even following cure, simplify models of care and delivery, deliver decentralized and integrated services at primary care, task-shift routine care to non-specialists, and promote HCV-self testing use and multiplex rapid diagnostics in different population groups, as well as strategic placement of point-of-care HCV viral load platforms (11,46-49). Increasing use of pooled procurement will also be important to reduce costs of tests and medicines, as well as improved governance over existing data systems to enable better reporting to the Global Reporting Systems for Hepatitis (GRSH) (50).
COVID-19 has significantly strained health systems in all countries across the world as countries prioritize their response to the pandemic and to providing universal vaccination against SARS-CoV-2 (51). Public health interventions to control the pandemic such as physical distancing and re-organization of services and resources have affected the full continuum of HCV services, but especially routine testing, testing campaigns, clinics visits for treatment initiation, monitoring and cancer care (52). Community outreach, integral to providing care for the most vulnerable and at-risk individuals, were suspended in most countries (52,53). Studies documenting the impact of COVID-19 on essential service delivery including hepatitis care indicate acute and critical reduction of hepatitis services, but also suggested that many countries had implemented mitigation strategies to protect patients and providers (54). Innovations arising from COVID-19 response include increasing use of digital and e-health with tele-consultations, multi-month prescriptions, more flexible provision of opiate substitution therapy, and greater use of multi-disease diagnostic platforms - both high throughput lab-based and point-of-care. There is also an increasing recognition that investment in health and ensuring UHC (and so in turn, greater economic and national security) can provide opportunities and lessons to support a "building forward better" future for hepatitis elimination in the coming decade.
In 2022, WHO will launch the next Global Health Strategy for Viral Hepatitis 2022-2030, alongside those for HIV and syphilis, and work is also in progress on the WHO framework for multi-disease elimination. The updated strategy will include new epidemiological data and 2025 targets to bridge the gap between the 2020 targets and 2030, alignment with new political commitments including those on primary health care and on universal health coverage, shifts towards domestic rather than donor-driven funding, technological advances and innovations, and shift to increasing community delivery and differentiated services, while highlighting areas where responses have stagnated and the need to deliver more regionalized and population-specific responses (55). WHO has recently developed interim guidance with criteria and measurement approaches for countries seeking validation of elimination of viral hepatitis as a public health threat (56). Overall, the guidance proposes the use of absolute impact targets (rather than the relative reduction targets as originally defined in the 2016 global health sector strategy for viral hepatitis for validation of HCV elimination. These include an absolute annual HCV incidence of ≤ 5 per 100,000 persons and of ≤ 2 per 100 people who inject drugs, and an HCV-related annual mortality rate of ≤ 2 per 100,000 persons (56).
The Western Pacific Region has a shared vision for WHO's work with countries and partners outlined in the "For the Future: towards the healthiest and safest region" (57). This vision requires transformation and future-proofing health systems taking a whole-of-systems approach for health and wellbeing. To get to hepatitis elimination in the next decade, there is need to recognize that COVID-19 will continue to impact the region for a very long time. This also confers opportunities to pivot, adapt and re-design investments in health systems and services to benefit all populations beyond COVID-19 while preparing for future public health emergencies (58). Consolidated efforts of governments, donors, partners and communities will be needed to shape this future and build the common vision towards hepatitis elimination by 2030. The future is yet to be written.
Funding: None.
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- 1. World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016-2020. https://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06-eng.pdf;jsessionid=5BF52293B12E4CCFB85FCE3FDE10440A?sequence=1 (accessed May 20, 2021).
- 2. World Health Organization. Progress report on HIV, viral hepatitis and sexually transmitted infections 2019. Accountability for the global health sector strategies, 2016-2021. https://apps.who.int/iris/bitstream/handle/10665/324797/WHO-CDS-HIV-19.7-eng.pdf?ua=1 (accessed May 20, 2021).
- 3. World Health Organization. Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. https://apps.who.int/iris/rest/bitstreams/1348210/retrieve (accessed May 26, 2021).
- 4. UNITAID. Technology and market landscape: hepatitis C medicines. https://unitaid.org/assets/HCV-Medicines-Landscape_Aug-2017.pdf (accesssed May 20, 2021) .
- 5. AASLD-IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015; 62:932-954. [DOI] [PubMed] [Google Scholar]
- 6. AASLD-IDSA HCV Guidance Panel. Hepatitis C guidance 2018 update: AASLD-IDSA recommendations for testing, managing, and treating hepatitis C virus infection. Clin Infect Dis. 2018; 67:1477-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ghany MG, Morgan TR; AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C guidance 2019 update: American Association for the Study of Liver Diseases- Infectious Diseases Society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology. 2020; 71:686-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Omata M, Kanda T, Wei L, et al. APASL consensus statements and recommendation on treatment of hepatitis C. Hepatol Int. 2016; 10:702-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization. Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection. https://apps.who.int/iris/bitstream/handle/10665/273174/9789241550345-eng.pdf?ua=1 (accessed May 20, 2021). [PubMed]
- 10. Bemelmans M, van den Akker T, Ford N, Philips M, Zachariah R, Harries A, Schouten E, Hermann K, Mwagomba B, Massaquoi M. Providing universal access to antiretroviral therapy in Thyolo, Malawi through task shifting and decentralization of HIV/AIDS care. Trop Med Int Health. 2010; 15:1413-1420. [DOI] [PubMed] [Google Scholar]
- 11. Oru E, Trickey A, Shirali R, Kanters S, Easterbrook P. Decentralisation, integration, and task-shifting in hepatitis C virus infection testing and treatment: a global systematic review and meta-analysis. Lancet Glob Health. 2021; 9:e431-e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shiha G, Soliman R, Mikhail NNH, Easterbrook P. An educate, test and treat model towards elimination of hepatitis C infection in Egypt: Feasibility and effectiveness in 73 villages. J Hepatol. 2020; 72:658-669. [DOI] [PubMed] [Google Scholar]
- 13. Shiha G, Soliman R, Mikhail NNH, Easterbrook P. Reduced incidence of hepatitis C in 9 villages in rural Egypt: Progress towards national elimination goals. J Hepatol. 2021; 74:303-311. [DOI] [PubMed] [Google Scholar]
- 14. Shiha G, Soliman R, Serwah A, Mikhail NNH, Asselah T, Easterbrook P. A same day 'test and treat' model for chronic HCV and HBV infection: Results from two community-based pilot studies in Egypt. J Viral Hepat. 2020; 27:593-601. [DOI] [PubMed] [Google Scholar]
- 15. Mangia A, Cotugno R, Cocomazzi G, Squillante MM, Piazzolla V. Hepatitis C virus micro-elimination: Where do we stand? World Journal of Gastroenterology. 2021; 27:1728-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Cancer Research Fund. Liver cancer statistics. https://www.wcrf.org/dietandcancer/liver-cancer-statistics/ (accessed May 22, 2021).
- 17. World Health Organization Regional Office for the Western Pacific. Regional Action Plan for Viral Hepatitis in the Western Pacific 2016-2020: a priority action plan for awareness, surveillance, prevention and treatment of viral hepatitis in the Western Pacific Region. https://apps.who.int/iris/bitstream/handle/10665/208337/97892906177617_eng.pdf?sequence=1&isAllowed=y (accessed May 22, 2021).
- 18. World Health Organization Regional Office for the Western Pacific. Regional Framework for the Triple Elimination of Mother-to-Child Transmission of HIV, Hepatitis B and Syphilis in Asia and the Pacific, 2018-2030. https://apps.who.int/iris/bitstream/handle/10665/274111/9789290618553-eng. pdf?sequence=1&isAllowed=y (accessed May 23, 2021).
- 19. World Health Organization Regional Office for the Western Pacific. Western Pacific Regional Strategy for Health Systems Based on the Values of Primary Health Care. https://apps.who.int/iris/handle/10665/207483 (accessed May 22, 2021).
- 20. World Health Organization Regional Office for the Western Pacific. Implementation progress of the regional action plan for viral hepatitis in the Western Pacific 2016- 2020: progress report, June 2019. https://www.who.int/westernpacific/health-topics/hepatitis/implementation-progress-of-the-regional-action-plan-for-viral-hepatitis-in-the-western-pacific-2016-2020 (accessed May 23, 2021).
- 21. Coalition Plus. The essential role of communities in HCV elimination: A summary of the three-part virtual meeting held December 8-10, 2020. https://www.coalitionplus.org/wordpress/wp-content/uploads/2021/05/Activist-Meeting-Summary-Final-Version.pdf (accessed May 24, 2021).
- 22. World Health Organization Regional Office for the Western Pacific. Health financing regional profile 2018: transitioning to integrated financing and service delivery of priority public health services. https://iris.wpro.who.int/bitstream/handle/10665.1/14332/9789290618638-eng.pdf (accessed May 24, 2021).
- 23. Medecins Sans Frontieres. Cambodia: MSF provides first free Hep C care in the country. https://msf-seasia.org/MSF-provides-first-free-Hepatitis-C-care-in-Cambodia (accesssed May 23, 2021).
- 24. Zhang M, O'Keefe D, Craig J, Samley K, Bunreth V, Jolivet P, Balkan S, Marquardt T, Dousset JP, Le Paih M. Decentralised hepatitis C testing and treatment in rural Cambodia: evaluation of a simplified service model integrated in an existing public health system. Lancet Gastroenterol Hepatol. 2021; 6:371-380. [DOI] [PubMed] [Google Scholar]
- 25. Clinton Health Access Initiative. Cambodia experience: developing a hepatitis C financing strategy. https://www.hepatitisfinance.org/wp-content/uploads/2020/12/Cambodia_Financing_reportFINAL.pdf (accessed May 23, 2021).
- 26. Adee M, Zhuo Y, Zhan T, Chen Q, Toumi A, Ayer T, Nwankwo C, Zhong H, Puenpatom A, Chhatwal J. A tool to inform hepatitis C elimination: A case for hepatitis C elimination in China. Clin Liver Dis (Hoboken). 2021; 17:99-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heffernan A, Ma Y, Nayagam S, Chan P, Chen Z, Cooke GS, Guo Y, Liu C, Thursz M, Zhang W, Zhang X, Zhang X, Jia M, Hallett TB. Economic and epidemiological evaluation of interventions to reduce the burden of hepatitis C in Yunnan province, China. PLoS One. 2021; 16:e0245288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang P, Guo R, Lian J, Zhi M, Lu C, Wu W, Wang L, Chan P, Chen Z, Sun J. Unblocking barriers of access to hepatitis C treatment in China: Lessons learned from Tianjin. Ann Glob Health. 2020; 86:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou HY, Liu S, Zheng SJ, Peng XX, Chen Y, Duan C, Zheng QF, Wang Z, Duan ZP. Coverage of different health insurance programs and medical costs associated with chronic hepatitis C infection in mainland China: a cross-sectional survey in 20 provinces. Hepatol Med Policy. 2016; 1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. World Health Organization Regional Office for the Western Pacific. Minimising the financial burden of hepatitis C. https://www.who.int/china/activities/minimising-the-financial-burden-of-hepatitis-c (accessed May 24, 2021).
- 31. World Health Organization Regional Office for South East Asia. UHC technical brief: Country experiences in using TRIPS safeguards: Part 1. https://apps.who.int/iris/rest/bitstreams/1140143/retrieve (accessed May 23, 2021).
- 32. Chan H, Hassali MA, Md Said R, Abu Hassan MR. Treatment coverage and drug expenditure in hepatitis C patients from 2013 to 2019: A journey of improving treatment accessibility in Malaysia through government-led initiatives. Hepatitis Monthly. 2020; 20:e107372. [Google Scholar]
- 33. FIND and DNDi team up to support Malaysian MOH efforts to simplify and decentralize hepatitis C screening & treatment. https://dndi.org/press-releases/2018/find-dndi-malaysianmoh-efforts-hepatitisc-screening-treatment/ (accessed May 25, 2021).
- 34. Positive Malaysian Treatment Access and Advocacy Group (MTAAG+) and Treatment Action Group (TAG). Hepatitis C virus diagnostics advocacy workshop: summary report. https://hepcoalition.org/IMG/pdf/malaysia_summary_report_hcv_diagnostics_advocacy_workshop.pdf (accessed May 24, 2021).
- 35. Urgent need to increase hepatitis testing and treatment: Mongolia showcases impressive progress, inspiring hope and opportunities. https://www.who.int/westernpacific/news/detail/26-07-2018-urgent-need-to-increase-hepatitis-testing-and-treatment (accessed May 27, 2021).
- 36. World Health Organization Regional Office for the Western Pacific. Action on hepatitis in Mongolia. https://www.who.int/westernpacific/news/feature-stories/detail/action-on-hepatitis-in-mongolia (accessed May 26, 2021).
- 37. World Health Organization. Accelerating access to hepatitis C diagnostics and treatment: Overcoming barriers in low- and middle-income countries. https://apps.who.int/iris/rest/bitstreams/1328465/retrieve (accessed May 25, 2021).
- 38. Center for Disease Analysis. Polaris Observatory: Philippines profile: hepatitis C. https://cdafound.org/dashboard/polaris/dashboard.html (accessed May 23, 2021).
- 39. Belarmino ZC, Carbajosa JC. Center offers free treatment for hepatitis. https://www.pressreader.com/philippines/the-freeman/20190626/281676846446060 (accessed May 24, 2021).
- 40. Nguyen TP, Luu HN, Nguyen MVT, Tran MT, Tuong TTV, Tran CTD, Boffetta P. Attributable causes of cancer in Vietnam. JCO Glob Oncol. 2020; 6:195-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. WHO welcomes progress in access to Hepatitis C treatment in Viet Nam. https://www.who.int/vietnam/news/detail/10-05-2017-who-welcomes-progress-in-access-to-hepatitis-c-treatment-in-viet-nam (accessed May 25, 2021).
- 42. Boeke CE, Adesigbin C, Agwuocha C, et al. Initial success from a public health approach to hepatitis C testing, treatment and cure in seven countries: the road to elimination. BMJ Glob Health. 2020; 5:e003767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Clinton Health Access Initiative. Hepatitis C market report. https://3cdmh310dov3470e6x160esb-wpengine.netdna-ssl.com/wp-content/uploads/2020/05/Hepatitis-C-Market-Report_Issue-1_Web.pdf (accessed May 23, 2021).
- 44. World Health Organization Regional Office for the Western Pacific. Expert consultation on viral hepatitis elimination in the Western Pacific Region, 1-3 December 2020 Virtual meeting. https://iris.wpro.who.int/bitstream/handle/10665.1/14717/RS-2020-GE-36-virtual-eng.pdf (accessed May 23, 2021).
- 45. Kasai T. Time to act to make elimination of viral hepatitis a reality. Lancet Gastroenterol Hepatol. 2020; 5:102-103. [DOI] [PubMed] [Google Scholar]
- 46. Thu N, Thuy Cao, Huyen N, Dung N, Kinh N, Sacks J, Boeke C, Tebor J, Ramers C. Preliminary cure rates from the national HCV pilot program in Vietnam. Global Hepatitis Summit 2018; 14-17 June 2018; Toronto, Canada, 2018. [Google Scholar]
- 47. Lazarus JV, Pericas JM, Picchio C, Cernosa J, Hoekstra M, Luhmann N, Maticic M, Read P, Robinson EM, Dillon JF. We know DAAs work, so now what? Simplifying models of care to enhance the hepatitis C cascade. J Intern Med. 2019; 286:503-525 [DOI] [PubMed] [Google Scholar]
- 48. Government of South Australia. Nursing model of care for viral hepatitis management in South Australia. https://www.sahealth.sa.gov.au/wps/wcm/connect/f5dc9e63-ad02-4d51-801c-164efc46143d/NursingMOCViralHepatitisManagementSA-CDCB-04122017.pdf?MOD=AJPERES&CACHEID=ROOTWORKSPACE-f5dc9e63-ad02-4d51-801c-164efc46143d-nwL2B6x (accessed May 23, 2021).
- 49. Lloyd AR, Clegg J, Lange J, Stevenson A, Post JJ, Lloyd D, Rudge G, Boonwaat L, Forrest G, Douglas J, Monkley D. Safety and effectiveness of a nurse-led outreach program for assessment and treatment of chronic hepatitis C in the custodial setting. Clin Infect Dis. 2013; 56:1078-1084. [DOI] [PubMed] [Google Scholar]
- 50. World Health Organization. Global reporting system for hepatitis 2018. https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hepatitis/strategic-information/global-reporting-system/docs/default-source/hq-hiv-hepatitis-and-stis-library/ghrs---global-hepatitis-reporting-system/GRSH-Data_entry_manual (accessed July 2, 2021).
- 51. OECD/World Health Organization. Chapter 2. The impact of the COVID-19 outbreak on Asia-Pacific health systems. 2020. https://www.oecd-ilibrary.org/sites/aaa5448f-en/index.html?itemId=/content/component/aaa5448f-en (accessed August 3, 2021).
- 52. Laury J, Hiebert L, Ward JW. Impact of COVID-19 response on hepatitis prevention care and treatment: Results from global survey of providers and program managers. Clin Liver Dis (Hoboken). 2021; 17:41-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Care Consortium. Community-led rapid survey COVID-19 impact on key populations, people Living with HIV and Global Fund Sub-Recipient Organizations in Sri Lanka. https://apcaso.org/apcrg/wp-content/uploads/2020/07/English-Final_COVID-19-Impact-Survey-Report-1.pdf (accessed May 23, 2021).
- 54. World Health Organization. Second round of the National pulse survey on continuity of essential health services during the COVID-19, January-March 2021. https://apps.who.int/iris/rest/bitstreams/1343409/retrieve (accessed May 23, 2021).
- 55. World Health Organization. EB148/37 Global strategies and plans of action that are scheduled to expire within one year: The global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections, for the period 2016-2021, report by the Director-General. https://apps.who.int/gb/ebwha/pdf_files/EB148/B148_37-en.pdf (accessed May 28, 2021).
- 56. World Health Organization. Interim guidance for country validation of viral hepatitis elimination. https://www.who.int/publications/i/item/9789240028395 (accessed May 23, 2021).
- 57. World Health Organization Regional Office for the Western Pacific. For the future : towards the healthiest and safest Region. A vision for the WHO work with Member States and partners in the Western Pacific. https://iris.wpro.who.int/bitstream/handle/10665.1/14476/WPR-2020-RDO-001-eng.pdf (accessed May 24, 2021).
- 58. Kasai T. From the "new normal" to a "new future": A sustainable response to COVID-19. Lancet Reg Health West Pac. 2020; 4:100043. [DOI] [PMC free article] [PubMed] [Google Scholar]


