Summary
In 2015, the World Health Organization (WHO) has set the goal of eliminating hepatitis C by reducing incidence of chronic viral hepatitis and related mortality by 2030 with the interim target of achieving 30% prevalence reduction by 2020. While The global prevalence of hepatitis C is known to be around 1.6%, the prevalence of hepatitis C in South Korea is 0.5-0.6% based on hepatitis C virus (HCV) antibody-positive rate. Although HCV antibody test has been included in the Annual National Health and Nutrition Survey in South Korea since 2012, a national initiative to eliminate hepatitis C was initiated by small clinic-related hepatitis C outbreaks in 2015-2016. These outbreaks caused by inappropriate use of syringes in 2015-2016 prompted the revision of hepatitis C reporting and control strategies in Korea following long-term discussion on including the HCV antibody test in the National Health Screening at a certain age. Since June 3, 2017, all hepatitis C cases should be reported to the Korea Disease Control Agency (KDCA). A pilot study for early detection of hepatitis C was conducted for the 56 years old population from September 1 to October 31 in 2020 by temporarily including HCV Ab in the National Health Screening followed by HCV RNA testing for HCV antibody positive cases. The final decision to include HCV antibody test in National Health Screening will be made based on results of the pilot study in 2020. To eliminate hepatitis B & C by 2030 in South Korea, the KDCA established a comprehensive viral hepatitis control and management system in 2020 with the interim goal of achieving an antibody positive rate of 0.3% and treatment rate of 90% by 2025.
Keywords: hepatitis C elimination, National Health and Nutrition Survey, hepatitis C virus antibody, hepatitis C virus RNA, treatment
Introduction
Viral hepatitis caused by hepatitis B virus (HBV) and hepatitis C virus (HCV) is the major cause of chronic liver disease in Korea. Hepatitis C remains a public health problem with low awareness resulting in low detection and treatment rate. WHO set the global target of eliminating hepatitis C by 2030 and adopted the WHO Global Health Sector Strategy on Viral Hepatitis 2016- 2021 which provides a roadmap for eliminating hepatitis B and C with 5 strategic directions in 2016 (1). Global hepatitis C elimination: an investment framework also provides an investment framework for global hepatitis C elimination to accelerate domestic and international financing and political commitment (2).
Dore and Bajis described some country examples of HCV elimination efforts in a recent paper (3). One example is Egypt that is an exemplar country demonstrating how national HCV screening and treatment strategy can contribute to HCV elimination (4). From October 2018 to April 2018, 5,800-8,000 teams consisting of a physician, nurse and a data-entry person were involved in screening of a 49.6 million population (around 80% of whole population in Egypt) (4). It is important to note that political will is a key success factor of the massive HCV screening and treatment program in Egypt (3). Dore and Bajis also emphasized that a potential impediment to HCV elimination is how to access highly maginalized people such as people who inject drugs and that innovative strategies are required to access those hard-to-reach populations (3) A strategy utilizing pharmacy known as pharmacist-led care proposed by Radley et al. to deliver an HCV care pathway made testing and treatment accessible for patients and maintained a high treatment success rate (5).
In this review, the current situation of hepatitis C in Korea, small clinic-related hepatitis C outbreaks in 2015- 2016 and national viral hepatitis C elimination strategy and action plan will be described.
Hepatitis C prevalence and current situation in Korea
Hepatitis C was classified as a designated infectious disease based on sentinel surveillance in 2000. It is estimated that there are around 300,000 hepatitis C cases in Korea (0.57%) as of 2020 (6). Among those estimated cases, only around 1/3-1/4 is being treated under the current national health insurance system as described by Jeong et al. (7).
Figure 1 shows annual reported cases of hepatitis C from 2011 to 2020. Since the reporting of hepatitis C cases changed from sentinel surveillance to mandatory reporting of all cases from June 3, 2017, annually reported HCV cases were dramatically reduced from 2017. Figure 2 shows the age distribution of reported hepatitis C cases in Korea. The analysis of age distribution shows that more than 94% of reported cases are > 40 years old (6).
Figure 1.

Annually reported hepatitis C cases in Korea, 2011-2020. Data source: Reference 14, Infectious Disease Portal, KDCA.
Figure 2.

Age distribution of reported hepatitis C cases in Korea (2017 June-2019). Data source: Reference 6.
Small clinic-related hepatitis C outbreaks in Korea
During 2015-2016, healthcare-associated outbreaks of HCV were detected in Korea. Epidemiological investigation revealed that reuse of needles or syringes was related to outbreaks. Briefly, samples from 1,721 patients who attended one clinic were tested for HCV antibody and HCV RNA followed by sequencing and 96 samples were positive for HCV Immunoglobulin(Ig) G. Among 96 HCV IgG positive samples, 70 were positive for HCV RNA. Interestingly, HCV genotype 1a sequences were detected from most cases. As genotype 1a is very rare in Korea, it was concluded that IV injection at this clinic was a source of HCV infection (8). From HCV RNA testing of additional environmental samples such as multi-dose vials and medical apparatus from this clinic, HCV RNA was also detected indicating HCV transmission occurred in the medication room due to reuse of syringes and contaminated multi-dose vials (8). Investigation of another HCV outbreak in an orthopedic clinic in 2015 also confirmed that medical procedures within the clinic were the source of HCV transmission.
These outbreaks prompted the change of hepatitis C surveillance from sentinel surveillance to mandatory reporting in 2017.
National viral hepatitis C elimination strategy and action plan
Availability of effective direct-acting antiviral agents (DAA) for treating hepatitis C allows global elimination efforts feasible. If cases are detected and treated early, hepatitis C- related morbidity and mortality can be significantly reduced. A review by Shahid et al. describes that the advancement and implementation of state-of-the-art diagnostic platforms in low-to-middle income countries as well as high income countries allowed identification of millions of undiagnosed hepatitis C-infected individuals (9). With the availability and the national health insurance coverage of DAA that can cure HCV infection, Jeong et al. (7) stressed the importance of HCV screening test in conjunction with national health examination as a cost-effective strategy for HCV elimination and eradication. A preprint submitted by Tatara et al. re-iterates the importance of reaching out to a hard-to-reach population emphasizing that re-treatment with DAA would be needed to achieve hepatitis C elimination especially among individuals who inject drugs (10).
The Korea Disease Control Agency (KDCA) is the national public health institute with responsibilities for management and control of human diseases that include infectious diseases. It establishes and implements national polices on infectious diseases including viral hepatitis. KDCA set a vision of eliminating hepatitis B and C by 2030 with an integrated viral hepatitis control and management system.
For prevention of HCV transmission, reuse of disposable syringes and medical supplies are strictly prohibited and relevant guidelines were revised and distributed (10). KDCA is also planning to strengthen research on epidemiology, disease burden, treatment strategies as well as prevention and control policies to control viral hepatitis. Improving the proportion of timely reporting of hepatitis C among all reported cases was included as one criterion for the hepatitis C control program. Implementation of the Integrated Viral Hepatitis Control and Management System shown in Figure 3 and national 5- year plan for integrated management and control of viral hepatitis shown in Figure 4 by KDCA will accelerate the process to achieve hepatitis C elimination by 2030 (11,12).
Figure 3.
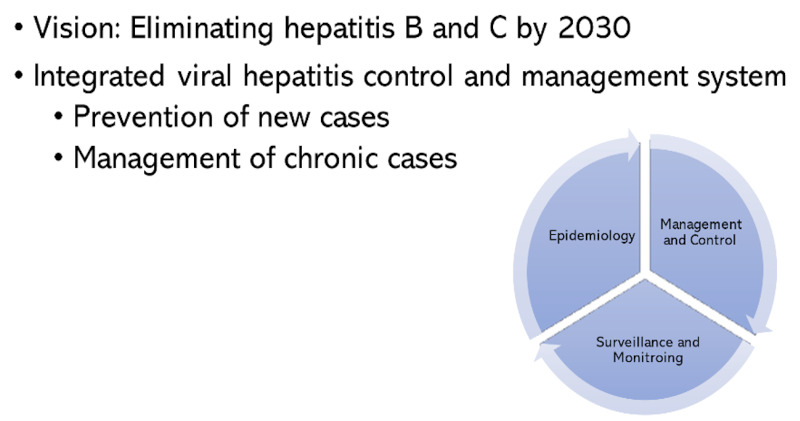
Integrated Viral Hepatitis Control and Management System. Integrated Viral Hepatitis Control System of Epidemiology, Management and Control and Surveillance and Monitoring to prevent new cases and to manage chronic cases. Data source: Reference 12.
Figure 4.

National target to control and eliminate viral hepatitis B and C by 2025 and 2030. National targets to control and eliminate viral hepatitis B and C by 2025 and 2030 are proposed. Data source: Reference 12.
For early detection and treatment of hepatitis C cases, KDCA is planning to introduce HCV antibody testing as a life cycle-based screening in the National Health Screening and to expand the insurance benefit for treatment of hepatitis C. Life cycle-based HCV screening strategy to include HCV antibody testing at the certain age of the National Health Screening was proposed by the Korean Association for Study of the Liver.
During liver week 2020, the Korean Association for Study of the Liver (KASL) proposed 4 strategies to eliminate hepatitis C: i) establishing designated division for viral hepatitis in the Korea Disease Control Agency, ii) increasing hepatitis C research fund, iii) conducting a study on reviewing cost-effectiveness of introducing HCV antibody testing in National Health Screening as a life cycle approach, and iv) integrated control of chronic viral hepatitis B and C. KASL announced the vision, strategies and goal of eliminating hepatitis C by 2030 by improving awareness from 30% to 90%, increasing hepatitis C laboratory testing rate from < 10% to 90% and case treatment rate from 60% to 90% by 2028 (13).
KDCA and KASL are collaborating to conduct a pilot study for early detection of hepatitis C among 56 years-old population in Korea from September to October 2020 by temporarily including anti-HCV antibody testing followed by HCV RNA testing in the National Health Screening (6). This pilot study is to confirm the prevalence and risk factors of hepatitis C and cost-effectiveness of including anti-HCV testing and HCV RNA testing in the National Health Survey. The results of the study will be utilized for the government's decision to include HCV antibody test in the National Health Screening and design of additional studies. The national strategy may also address strategic preparation for possible unification with North Korea in the future (6).
WHO Global Health Sector Strategy on Viral Hepatitis 2016-2021 emphasizes the importance of implementing evidence-based national hepatitis plans and priority actions such as establishing a national governance structure and coordination mechanism (1). It is urgently needed to set up an integrated governance structure for eliminating viral hepatitis in KDCA, to finalize and implement the comprehensive national hepatitis plan and to monitor progress of the national hepatitis plan in partnership with KASL.
Conclusion
To achieve the goal of elimination of hepatitis C and hepatitis B by 2030, KDCA's leadership in pursuing the goal and close partnership between KDCA and KASL would be essential. Partnering with WHO, Coalition for Global Hepatitis Elimination (CGHE) and other international public and private partners will facilitate the efforts to eliminate hepatitis C in Korea.
Funding: None.
Conflict of Interest
The author has no conflicts of interest to disclose.
References
- 1. World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016-2021. http://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06-eng.pdf;jsessionid=9A17B3F9C66A7A152BD4D5368FBB2524?sequence=1 (accessed May 16, 2021).
- 2. Pedrana A, Howell J, Scott N, et al. Global hepatitis C elimination: an investment framework. Lancet Gastroenterol Hepatol. 2020; 5:927-939. [DOI] [PubMed] [Google Scholar]
- 3. Dore GJ, Bajis S. Hepatitis C virus elimination: laying the foundation for achieving 2030 targets. Nat Rev Gastroenterol Hepatol. 2021; 18:91-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Waked I, Esmat G, Elsharkawy A, et al. Screening and treatment program to eliminate hepatitis C in Egypt. N Engl J Med. 2020; 382:1166-1174. [DOI] [PubMed] [Google Scholar]
- 5. Radley A, de Bruin M, Inglis SK, Donnan PT, Hapca A, Barclay ST, Fraser A, Dillon JF. Clinical effectiveness of pharmacist-led versus conventionally delivered antiviral treatment for hepatitis C virus in patients receiving opioid substitution therapy: a pragmatic, cluster-randomised trial. Lancet Gasteroenterol Hepatol. 2020; 5:809-818. [DOI] [PubMed] [Google Scholar]
- 6. Ministry of Health and Welfare of the Republic of Korea. Press Release "Launching a pilot study on early detection of hepatitis C cases". http://www.mohw.go.kr/react/al/sal0301vw.jsp?PAR_MENU_ID=04&MENU_ID=0403&page=1&CONT_SEQ=359673 (accessed May 16, 2021). (in Korean) .
- 7. Jeong SH, Jang ES, Choi HY, Kim KA, Chung W, Ki M. Current status of hepatitis C virus infection and countermeasures in South Korea. Epidemiol Health. 2017; 39:e2017017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chung YS, Choi JY, Han MG, Park KR, Park SJ, Lee H, Jee Y, Kang C. A large healthcare-associated outbreak of hepatitis C virus genotype 1a in a clinic in Korea. J Clin Virol. 2018; 106:53-57. [DOI] [PubMed] [Google Scholar]
- 9. Shahid I, Alzahrani AR, Al-Ghamdi SS, Alanazi IM, Rehman S, Hassan S. Hepatitis C diagnosis: simplified solutions, predictive barriers, and future promises. Diagnostics (Basel). 2021; 11:1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tatara E, Gutfraind A, Collier NT, Echevarria D, Cotler SJ, Major M, Ozik J, Dahari H, Boodram B. Re-treatment with direct-acting antivirals policy is needed to eliminate Hepatitis C among persons who inject drugs. bioRxiv. 2021. doi: https://doi.org/10.1101/653196. [Google Scholar]
- 11. 2020 National Hepatitis C Control Guideline by KDCA. http://www.kdca.go.kr/npt/biz/npp/portal/nppPblctDtaView.do?pblctDtaSeAt=8&pblctDtaSn=2081 (accessed May 16, 2021) (in Korean) .
- 12. Kim Y. Current status and countermeasures for hepatitis B and D in Korea. https://www.kasl.org/pdf/2016_fall/%ea%b9%80%ec%9c%a4%ec%a4%802.pdf (assessed May 16, 2021) (in Korean) .
- 13. Korean Association for the Study of the Liver. Press release "Elimination of hepatitis C by 2030". https://www.kasl.org/bbs/index.html?code=report&category=&gubun=&page=1&number=4207&mode=view&keyfield=&key= (assessed May 16, 2021) .
- 14. Infectious Disease Portal. Korea Disease Control Agency. http://www.kdca.go.kr/npt/biz/npp/ist/bass/bassDissStatsMain.do (assessed May 16, 2021) .


