Summary
Viral hepatitis poses a major public health problem in Japan. Chronic viral hepatitis is a progressive liver disease that eventually develops into liver cirrhosis and liver cancer. Since nucleic acid analog therapy for hepatitis B and interferon-free therapy for hepatitis C have made it possible to control the disease status or eliminate the viruses, it is very important that more people receive hepatitis virus tests to confirm the presence of infection at an early stage, and that patients with hepatitis detected by the tests receive appropriate medical care. Currently, the government of Japan is implementing comprehensive measures for hepatitis control based on five key strategies. Moreover, the goal listed in the Basic Guidelines on Hepatitis Measures is to reduce the frequency of progression of hepatitis to cirrhosis or liver cancer through a scheme consisting of testing people for hepatitis, getting those who test positive to visit a medical institution and receive treatment, and providing appropriate and high-quality hepatitis care through specialized medical institutions and regional core centers for the management of liver disease. To achieve the goal, various subsidy programs including an expense subsidy system for hepatitis treatment have been implemented in Japan. It is important for healthcare professionals to have sufficient knowledge of public support for efficient hepatitis C virus (HCV)-related liver disease detection and care.
Keywords: Basic Guidelines on Hepatitis Measures, viral hepatitis policy, public support, subsidy program, hepatitis
Introduction
In Japan, more than 3 million people are infected with hepatitis B or C virus (1), making them among the most common infectious diseases in the country. Chronic hepatitis B and C, if left untreated, can progress from chronic hepatitis to more serious conditions such as liver cirrhosis and cancer. Moreover, hepatitis patients may not be aware of their infection until the advanced stage of disease, or they may not recognize the need for medical attention even after learning of their infection. Now that nucleic acid analog therapy for hepatitis B and interferon-free therapy for hepatitis C have made it possible to control the disease status or eliminate the viruses, it is very important that more people receive hepatitis virus tests to confirm the presence of infection at an early stage, and that patients with hepatitis detected by the tests receive appropriate medical care. Thus, viral hepatitis is an important public health issue for the Japanese people, and comprehensive measures for hepatitis control are currently implemented in Japan (2) based on the following five key strategies (3) : i) promotion of liver disease treatment; ii) promotion of hepatitis virus testing and prevention of progression to severe disease; iii) reinforcement of regional cooperative systems for liver disease treatment; iv) dissemination of accurate information about hepatitis to the public; and v) promotion of hepatitis research. The Basic Guidelines on Hepatitis Measures, revised in 2016, aim to reduce the number of people who progress to liver cirrhosis/ cancer through early detection and treatment of hepatitis. To accomplish the mission, various public supports have been implemented in Japan (Figure 1).
Figure 1.
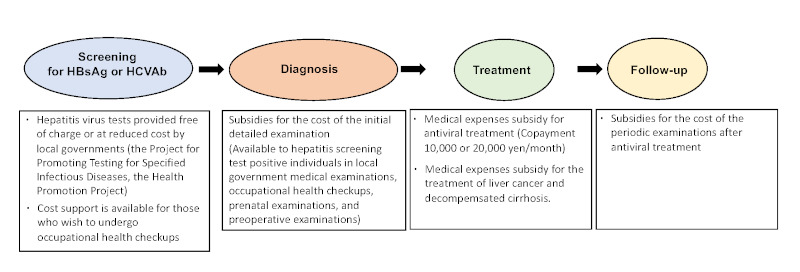
Seamless public support from screening to anti-HCV treatment and HCC/decompensated cirrhosis. HBsAg: hepatitis B surface antigen, HCC: hepatocellular carcinoma, HCV: hepatitis C virus, HCVAb: hepatitis C antibody.
This article reviews the subsidy programs for efficient hepatitis C virus (HCV)-related liver disease detection and care in Japan and public support for these programs.
Promotion of hepatitis virus testing (subsidies for testing costs)
A national survey conducted in 2011 showed that about 57% of the Japanese population had been tested for hepatitis B virus (HBV) and 48% for HCV, revealing that about half had not been tested for these viruses (4). With the goal of having all citizens undergo hepatitis virus testing at least once in their lifetime, measures have been implemented to establish testing systems, subsidize testing costs, and encourage the public to undergo testing. As for subsidies for testing costs, prefectural governments provide hepatitis viral tests, whereas municipal governments provide hepatitis virus screening. Furthermore, to encourage the incorporation of hepatitis virus testing in occupational health checkups, the following three measures have been implemented since FY2017 in order to raise awareness at workplaces in collaboration with health insurance associations and the Japan Health Insurance Association.
Hepatitis virus tests provided by local governments (the Project for Promoting Testing for Specified Infectious Diseases, the Health Promotion Project)
Prefectural governments conduct hepatitis virus tests (as part of the Project for Promoting Testing for Specified Infectious Diseases), whereas municipal governments provide hepatitis screening (as part of the Health Promotion Project); these are provided free of charge or at reduced cost. In all prefectures (including cities with public health centers and special wards), the Project for Promoting Testing for Specified Infectious Diseases is implemented at public health centers and at contract medical institutions for residents of all ages, and 95% of municipalities provide follow-up services for individuals who test positive for hepatitis virus. The Health Promotion Project is implemented in 1,656 municipalities, of which 1,543 (93%) provide the hepatitis screening service free of charge to eligible individuals aged 40 years or older. Hepatitis screening is conducted at contract medical institutions, group health checkups, public health centers, and health centers. In FY2018, about one million four thousand people received HCV tests under the Project for Promoting Testing for Specified Infectious Diseases and the Health Promotion Project, respectively (Figure 2).
Figure 2.
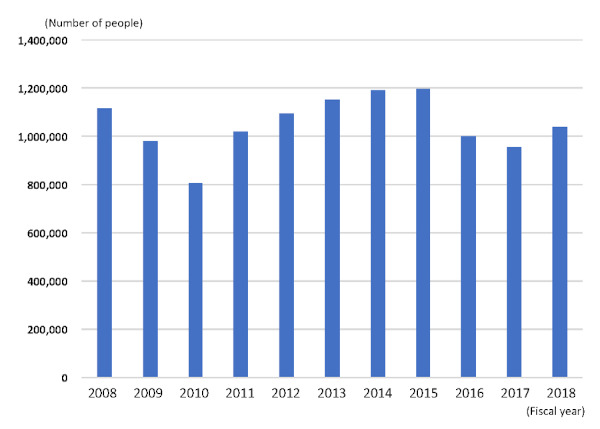
Development of the number of people who received HCV screening tests at local governments. The total number of the Project for Promoting Testing for Specified Infectious Diseases and the Health Promotion Project. HCV: hepatitis C virus.
Various efforts are being made by local governments to encourage the public to undergo hepatitis virus testing. The most common way of raising public awareness of the tests is posting information on websites and distributing informational pamphlets and newsletters within the prefecture, as well as providing guidance and encouragement to individual residents to undergo testing in municipalities (1,470 municipalities) (Figure 3). As for efforts to increase the convenience of hepatitis virus testing, simultaneous testing along with other tests is the most common approach among municipalities (1,590 municipalities) and is also increasingly being adopted in prefectural programs. In addition, 1,026 municipalities offer after-hours hepatitis virus testing at night, on weekends, and on holidays (5).
Figure 3.
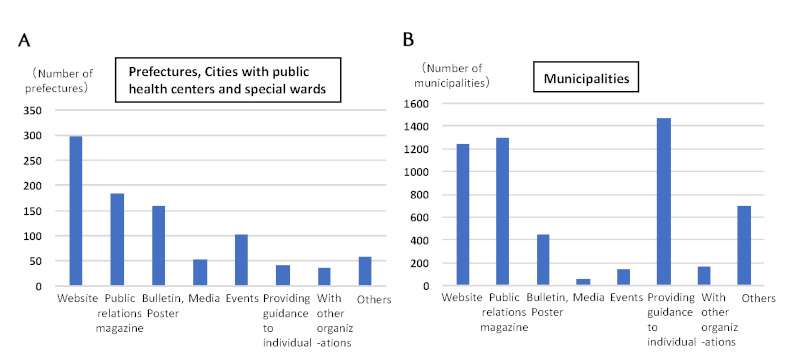
Efforts for raising public awareness of the hepatitis screening tests of the local governments. (A) The means used to make screening tests widely-known in Prefectures, Cities with public health centers and special wards. (B) The means used to make screening tests widely-known in municipalities.
Hospitals that offer hepatitis virus testing can be searched from the Hepatitis Care Navigation System (https://kan-navi.ncgm.go.jp), which is provided by the Hepatitis Information Center of the National Center for Global Health and Medicine (Ichikawa, Chiba, Japan). The schedule, location, and sign-up procedures for hepatitis virus testing vary by municipality. Those who wish to be tested must either contact the municipality (city, town, or village) where they live or the appropriate health center, or check their websites.
Hepatitis virus testing at workplaces (Project to Promote Hepatitis Screening at Workplaces)
Hepatitis virus testing at workplaces may be useful for detecting latent hepatitis virus carriers and guiding them to seek medical attention. In a pilot study conducted in Hiroshima Prefecture, Sugiyama et al. found that a certain percentage of hepatitis-positive individuals were detected by occupational health screenings, and that nearly half of them were found to be hepatitis positive for the first time (6).
To encourage the implementation of consultation services addressing hepatitis B and C virus infections as well as the incorporation of hepatitis virus testing in occupational health screenings, the Project to Promote Hepatitis Screening at Workplaces has been implemented since FY2017. The project's aim is to raise awareness at workplaces in collaboration with health insurance associations and the Japan Health Insurance Association. Posters and leaflets are the most common means for raising awareness among municipalities. In FY2018, the project was implemented in 15 prefectures and 5 cities with public health centers in order to encourage the public to undergo hepatitis virus testing in conjunction with occupational health screenings.
Existence of people unrecognized receiving hepatitis screening test
Among the people who had been tested, the percentage of those who were aware that they had been tested for hepatitis B and C ("recognized persons") was about 17.8% and 17.7%, respectively (4). Other test recipients may have been tested prior to major surgery, childbirth, or blood donation, but they were not aware of the test or its results ("unrecognized persons"). Problems concerning unrecognized persons include that they do no seek medical attention even if their test results are positive, and that they may receive hepatitis virus testing repeatedly. To ensure that all hepatitis test recipients are notified of test results, regardless of the purpose or results of the test, the Ministry of Health, Labour and Welfare (MHLW) issued a notice titled "Notification of the results of hepatitis virus tests conducted before surgery, etc." (Notice No. 0423-1 issued by the director of the Specific Diseases Control Division, Health Service Bureau, MHLW) in April 2014. In addition, as part of the MHLW Scientific Research, an attempt to encourage patients to receive medical attention using the alert function of electronic medical records is being conducted mainly at core hospitals for collaborative liver disease care.
Promoting prevention of hepatitis progression (subsidies for the cost of the initial detailed examination and periodic examinations)
Despite the availability of effective treatments and medical expense subsidies, some people who test positive for hepatitis do not receive treatment. A national survey conducted in 2011 showed that only about 60% of hepatitis-positive individuals sought medical attention. In an awareness/trend survey of people who tested positive for hepatitis conducted by Kaishima et al. (2016), the most common reasons for not seeking medical attention were "I thought there was no need to visit a hospital" (35.2%), "The doctor said there was no need to visit a hospital" (20.7%), "There is nothing wrong with my liver function or my condition" (15.9%), and "I did not receive any information about what hospital I should visit" (13.1%) (4).
Based on these results, local governments are encouraging hepatitis-positive individuals to visit specialized medical institutions and are subsidizing the cost for the first detailed examination and periodic examinations at specialized medical institutions. The aim is to facilitate the early detection and treatment of hepatitis.
It is recommended to undergo an initial detailed examination as soon as possible to prevent progression to severe conditions, including chronic hepatitis, cirrhosis, and liver cancer. Early treatment and prevention of progression are facilitated through periodic interventions for these patients. The subsidy for the initial detailed examination was previously available to only hepatitis-positive individuals detected by hepatitis virus testing provided by local governments. However, the scope has been expanded to include cases detected at workplaces since FY2019, as well as cases detected during prenatal checkups or preoperative hepatitis screening since FY2020. Patients with chronic hepatitis, cirrhosis, or liver cancer resulting from hepatitis virus infection (including those under post-treatment follow-up) can apply for the subsidy for periodic examinations up to twice a year, and this subsidy is being increased every year. In FY2017, the copayment to be paid by individuals whose annual municipal inhabitant tax is less than 235,000 JPY was reduced to 2,000 JPY per examination for those with chronic hepatitis and to 3,000 JPY for those with liver cirrhosis/cancer (free of charge for low-income households that do not pay municipal inhabitant tax).
Hyogo Prefecture has the largest number of users of the subsidy program for the initial detailed examination, followed by Tokyo, Fukuoka Prefecture, and Chiba Prefecture. The number of users of the subsidy for periodic examinations is highest in Saitama Prefecture, followed by Hiroshima, Fukuoka, and Ehime Prefectures (FY2018 results) (5).
Local governments regularly contact hepatitis-positive individuals, with their consent, to check whether they have received medical attention and treatment. Those who have not sought medical attention are encouraged to do so by phone or other means, as necessary. Individuals who apply for the subsidies to cover the cost of the initial detailed examination and periodic examinations must consent to the follow-up project.
Medical expense subsidy system for hepatitis treatment
Rapid progress has been made in hepatitis therapeutics. For hepatitis C, interferon-free therapy not only eliminates viruses at a high rate, but also allows for relatively safe treatment in elderly patients and patients with compensated cirrhosis, who tend not to tolerate interferon therapy. For hepatitis B, the advent of nucleic acid analogue therapy has enabled better disease control. However, because of increased monthly medical expenses or increased cumulative medical expenses from longer-term treatment, the Medical Expense Subsidy System for Hepatitis Treatment was launched in 2008 to facilitate early treatment, and the system has been expanded along with rapid progress in hepatitis treatments. The MHLW and prefectural governments provide medical subsidies for interferon therapy for hepatitis B and C, interferon-free therapy for hepatitis C, and nucleic acid analogue therapy for hepatitis B (7,8). Specifically, the subsidies are provided so that the copayment for medical treatment, including the cost of medicines and tests, does not exceed 10,000 JPY or 20,000 JPY per month, depending on the annual household income subject to municipal inhabitant tax. Applicants to this system need to submit their application to the appropriate office (e.g., a public health center) of the prefecture where their residency is registered. As of the end of FY2017, 269 patients receiving interferon therapy (for hepatitis B or C), 31,507 patients receiving interferon-free therapy, and 79,817 patients receiving nucleic acid analogue therapy have been issued hepatitis treatment beneficiary certificates (Figure 4). Moreover, 57.6% of patients treated for liver disease caused by HBV infection received subsidies for nucleic acid analogue therapy, and 9.3% of patients treated for liver disease caused by HCV infection received subsidies for interferon-free therapy.
Figure 4.
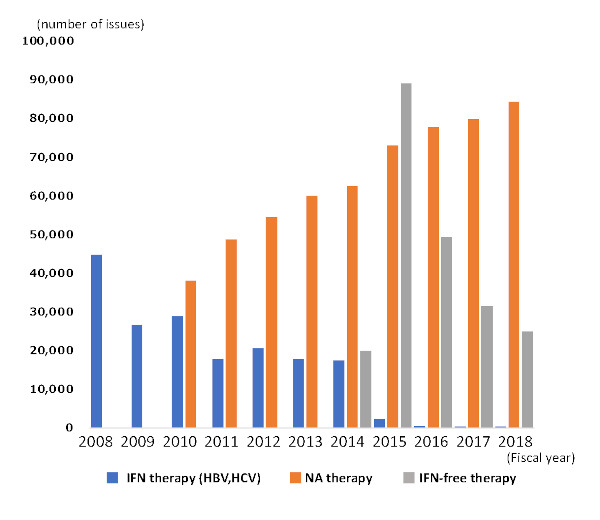
Development of the number of issued hepatitis treatment beneficiary certificates. In the roughly 5 years since interferon-free therapy was approved in Japan for chronic hepatitis C, the number of certificates regarding IFN-free therapy have decreased. IFN: interferon, NA: nucleic acid analogue.
Project to promote research on treatment of liver cancer and severe cirrhosis
This project was launched in December 2018 and aims to reduce the burden of medical costs for patients with liver cancer or severe (decompensated) cirrhosis caused by hepatitis B or C virus infection. It also aims to collect clinical data and thereby promote research on treatment for liver cancer and severe cirrhosis with the aim of improving prognosis, enhancing quality of life, and reducing the recurrence of liver cancer.
The beneficiaries of this project are patients with liver cancer or severe cirrhosis caused by hepatitis B or C virus infection with an annual income of less than about 3,700,000 JPY. Applicants are required to complete and submit a clinical research form and an informed consent form. Medical expenses for treatment of liver cancer and severe cirrhosis are paid from public funds for patients who meet specified criteria, such as exceeding the maximum amount of high-cost medical care expenses a certain number of times in the past year.
Because this project is implemented primarily by prefectural governments, medical institutions through which patients can receive support for treatment costs (designated medical institutions) are determined by the prefectural governments. The designated medical institutions for this project can be searched for in the Hepatitis Medical Navigation System (https://kan-navi. ncgm.go.jp). As of June 2020, 1,393 institutions have been registered in this system. For more information about the project, visit the Ministry of Health, Labor and Welfare (MHLW) website (https://www.mhlw. go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/ kekkaku-kansenshou/kanen/kangan/index.html).
As of December 2019, 391 people have been certified as eligible for the project and the cumulative total number of people who have received medical cost subsidies was 743 (9). There are continued efforts to make the operation more flexible and to publicize the project so that patients eligible for these subsidies can be smoothly guided to the system. Specifically, at the beginning of the project, all inpatient care from the 1st to 4th month of hospitalization had to be provided by designated medical institutions. However, from January 2020, 3 months of hospitalization, which is a requirement for certification of eligible patients, can be provided by non-designated medical institutions. Furthermore, since FY2021, the requirements have been relaxed as follows: i) molecular targeted therapy in outpatient as well as inpatient settings is covered; and ii) if the maximum amount of high-cost medical care expenses was exceeded in 2 or more months in the past year, medical expenses in the third and subsequent months exceeding the maximum amount of high-cost medical care expenses are paid from public funds (Figure 5). In other efforts, non-designated medical institutions are encouraged to apply for the designation when patients who have been hospitalized at the institutions apply for certification of eligibility in the project. In addition to posters and leaflets for patients, leaflets encouraging application for the designated medical institution status are also used to publicize the project.
Figure 5.
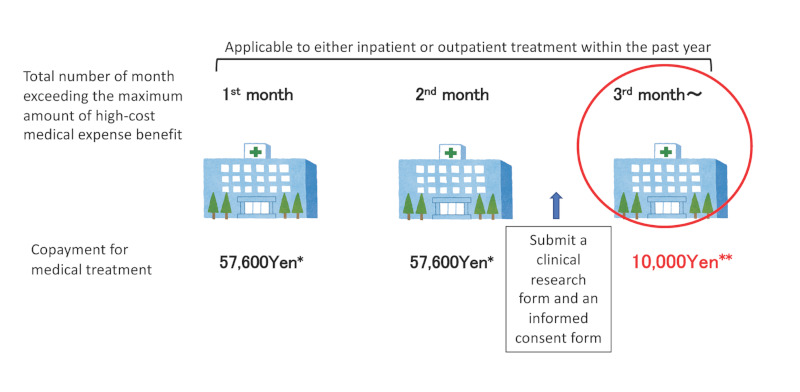
The image of project to promote research on treatment of liver cancer and severe cirrhosis. *Maximum amount of high-cost medical expense benefit. **Treatment should be done at designated medical institutions to receive support for treatment costs.
Other social security systems (Certification of hepatic impairment under the Act on Welfare of Physically Disabled Persons and the disability pension)
Since April 2010, people with hepatic impairment who meet certain criteria have been issued a Physical Disability Certificate, which makes them eligible for partial reduction of medical expenses, depending on the severity of disability. In April 2016, the scope of certification was expanded and the requirements in the disability classification table were relaxed. The Physical Disability Certificate is issued by local governments including prefectures, designated cities, and core cities. Consultation and application for the certificate can be made at the office in charge of disability welfare (welfare office or section) in the municipality of residence.
Patients with hepatitis/cirrhosis who meet the eligibility criteria for the disability pension certified and provided by the Japan Pension Service can also apply for the pension. Inquiries and requests can be made to the municipality of residence or the Japan pension service (JPS) branch office for the disability basic pension, or to the JPS branch office for the disability employees' pension.
Establishment of medical care and support systems for liver diseases
Hepatitis care network in Japanese prefectures
In 2007, the Guidelines for the Establishment of Post- Hepatitis Test Care System for Liver Diseases in Prefectures was released in a report of the National Medical Conference on Hepatitis C Countermeasures on January 26, 2007, in which it listed three roles required of medical institutions for liver disease care: primary care physicians, specialized institutions for liver diseases, and medical institutions that provide highly advanced medical care for liver diseases (regional core centers for the management of liver disease, hereinafter referred to as "regional core centers"). Based on these guidelines, the MHLW issued the "Notice on the Establishment of Liver Disease Care Systems" (Notice No. 0419001 issued by the Director of the Health Service Bureau, MHLW) in April 2007. In response to this, the construction of liver disease care systems began nationwide, starting with regional core centers and specialized institutions.
Regional core centers are designated by the Council for Hepatitis Measures in each prefecture from among medical institutions that currently play a central role in the liver disease care network in each prefecture. Regional core centers are required to i) provide general medical information on liver diseases; ii) collect information on medical institutions in the prefecture and refer patients to these institutions; iii) provide training for healthcare professionals and education for community residents; iv) set up opportunities for consultation with specialized medical institutions for liver diseases; and v) provide multidisciplinary treatment for liver cancer. Currently, at least one regional core center is designated in each prefecture, with a total of 71 institutions nationwide, all of which have established liver disease consultation and support centers to provide support to patients and their families. Specialized institutions are selected by the Council for Promotion of Hepatitis Measures in each prefecture and are required to i) make a diagnosis and determine a treatment strategy, as recommended by specialists; ii) appropriately implement antiviral therapy; and iii) identify groups at high risk of liver cancer and perform early diagnosis. Over 3,000 specialized medical institutions have been designated nationwide.
In the process of developing regional liver disease care systems, the environment surrounding liver disease care has also changed, including the improvement of hepatitis control measures and development of new treatments. Against this background, in March 2017, the MHLW issued the "Notice on the Establishment of Liver Disease Care Systems and Support Systems for Liver Disease Patients" (10) (Notice No. 0331-8 issued by the Director of the Health Service Bureau, MHLW; hereafter referred to as the "new notice"). The new notice, while maintaining the previous basic policies, sets out the following new basic approaches: i) setting of goals and milestones; ii) establishment of a system to ensure the smooth linkage of hepatitis screening, examination, treatment, and follow-up; iii) realization of patient-centered liver disease care; iv) improvement of and elimination of disparities in liver disease care; and v) provision of consultation and appropriate support for hepatitis patients. The aim is to establish hepatitis care networks based on these approaches and thereby promote hepatitis control measures that are tailored to local needs.
Hepatitis medical care coordinator
Some local governments also began training hepatitis medical care coordinators in 2003 to facilitate the awareness-raising and education for residents as well as dissemination of information to patients and their families. However, there were large regional differences in these efforts, and after some debate, the basic roles and activities of hepatitis medical coordinators were defined in April 2017 (11) ("Notice on the Training and Utilization of Hepatitis Medical Care Coordinators" [Notice No. 0425-4 issued by the Director of the Health Service Bureau, MHLW]). Hepatitis medical care coordinators are located in local communities, workplaces, hospitals, and the like, and their activities include providing the basic knowledge and information on hepatitis that are required for each area, promoting understanding of hepatitis, providing assistance for consultation, guiding residents to the consultation centers, encouraging residents to receive hepatitis screening and examination, and providing explanations of the system. These coordinators are from various fields and include nurses, public health nurses, pharmacists, local government officials, and workplace representatives. Rather than one coordinator taking on all the roles, coordinators from various fields use their strengths to support patients from the general public and coordinate to ensure the appropriate promotion of hepatitis care. Cooperation and collaboration among hepatitis medical care coordinators from various fields is expected to facilitate hepatitis prevention, screening, diagnosis, treatment, and follow-up. It is also hoped that the activities of hepatitis medical care coordinators in local communities and workplaces will foster a foundation for spreading understanding of hepatitis in society, leading to the elimination of discrimination and prejudice against hepatitis patients. Hepatitis medical care coordinators are located at core hospitals, specialized medical institutions, public health centers, municipalities, pharmacies, and other facilities, although their main places of activity currently appear to be core hospitals and public health centers (12). An increasing number of prefectures are providing training to improve the skills of hepatitis medical care coordinators and conducting supportive activities to revitalize their efforts, such as publishing the list of institutions with coordinators, creating coordinator's badges, and establishing a consultation system for coordinators.
Consultation support system for patients with liver diseases
Consultations provided at the liver disease consultation and support centers of core hospitals cover a wide range of topics. The most common topics are medical expense subsidy systems, treatment, and the disease itself, and there has been recent growth in the number of consultations on things to keep in mind in daily life as well as medical institutions, livelihood support, and hepatitis-related lawsuits (13). In response to the need to improve the consultation system for hepatitis patients in various situations, the Consultation Support System for Patients with Liver Diseases was introduced at core hospitals across Japan in July 2018 to provide consultation support for hepatitis patients. This system is managed and operated by the Hepatitis Information Center, which is described below. This system is designed to create a database of consultations received by hepatitis patients; entries can be searched and edited by consultation staff at core hospitals and other related facilities. The database is shared among core hospitals and is utilized to ascertain the number and trends of consultations, search for model answers to consultations, and exchange opinions among consultation staff. This enables the hospitals to share the trends of diversifying consultation content and difficult-to-answer questions nationwide and to use the database as an auxiliary tool for responding appropriately to individual consultations. Thus, it is expected that these capabilities will help to alleviate hepatitis patients' concerns and improve their quality of life.
Conclusion
Now that advances in treatment have made it possible to control hepatitis or eliminate the viruses, it is critical that hepatitis patients detected by testing receive appropriate medical care as soon as possible. At the same time, many patients require long-term treatment due to the nature of the disease, and measures are needed to ensure that these patients can continue to receive treatment with peace of mind.
Hepatitis control measures in Japan have been developed mainly based on the Basic Act on Hepatitis Measures, and extensive efforts have been made accordingly. In addition to providing subsidies for testing and medical expenses, it is necessary to further strengthen cooperation among the national and local governments, Hepatitis Information Center, core hospitals, specialized medical institutions, primary care physicians, and others. Thereby, hepatitis control measures may be promoted in line with local needs, thus encouraging the public to receive hepatitis virus testing in various settings, including the workplace, and promoting follow-up of individuals who test positive for hepatitis virus infection.
Funding: The data included in this review article were partly granted by the Ministry of Health, Labour and Welfare, Japan (20HC2002).
Conflict of Interest
Tatsuya Kanto receives lecture fees from Gilead Sciences and Abbvie. Yasuhito Tanaka is currently conducting research sponsored by Fujifilim Corp., Janssen Pharmaceutical K.K, Gilead Sciences, GlaxoSmithKline Pharmaceuticals Ltd, and Stanford University. Lecture fees are as follows: Fujirebio Inc., Abbvie. and Gilead Sciences. Scholarship Donations from Abbvie. The other authors have no conflicts of interest to disclose.
References
- 1. Ministry of Health, Labour and Welfare. Promotion of comprehensive measures for hepatitis control - What is hepatitis -. http://www.mhlw.go.jp/bunya/kenkou/kekkaku-kansenshou09/hepatitis_about.html (accessed June 10, 2021). (in Japanese) .
- 2. Oza N, Isoda H, Ono T, Kanto T. Current activities and future directions of comprehensive hepatitis control measures in Japan: The supportive role of the Hepatitis Information Center in building a solid foundation. Hepatol Res. 2017; 47:487-496. [DOI] [PubMed] [Google Scholar]
- 3. Health, Labour and Welfare Statistics Association. Viral Hepatitis. In:Trends in national health, 2016/2017. Health, Labour and Welfare Statistics Association. Tokyo, Japan, 2016; pp.146-148. (in Japanese) [Google Scholar]
- 4. Kaishima T, Fujii T, Matsuoka T, et al. Study of the issues of receiving hepatitis screening and the rate of consulting hospitals - The rate of recognized receiving hepatitis screening and that of the unrecognized. Kanzo. 2016; 57:634-648. (in Japanese) [Google Scholar]
- 5. Ministry of Health, Labour and Welfare. The 25th Meeting of the Council for Promotion of Hepatitis Measures. Document 1. https://www.mhlw.go.jp/content/10901000/000719442.pdf (accessed June 10, 2021). (in Japanese) .
- 6. Sugiyama A, Fujii T, Nagashima S, Ohisa M, Yamamoto C, Chuon C, Akita T, Matsuo J, Katayama K, Takahashi K, Tanaka J. Pilot study for hepatitis virus screening among employees as an effective approach to encourage employees who screened positive to receive medical care in Japan. Hepatol Res. 2018; 48:E291-E302. [DOI] [PubMed] [Google Scholar]
- 7. Ministry of Health, Labour and Welfare. Promotion of comprehensive measures for hepatitis control - Medical expense subsidy system for hepatitis treatment -. http://www.mhlw.go.jp/bunya/kenkou/kekkaku-kansenshou09/080328_josei.html (accessed December 1, 2017). (in Japanese) .
- 8. Hepatitis Information Center, National Center for Global Health and Medicine. Medical expense subsidy system for hepatitis treatment. http://www.kanen.ncgm.go.jp/cont/040/zyosei.html (accessed June 10, 2021). (in Japanese) .
- 9. Hepatitis Information Center, National Center for Global Health and Medicine. The 1st liaison meetings among the regional core centers (FY2019). Document. http://www.kanen.ncgm.go.jp/archive/conference/council/20200710_kourou.pdf (accessed June 10, 2021). (in Japanese) .
- 10. Ministry of Health, Labour and Welfare. Promotion of comprehensive measures for hepatitis control - Notice on the Establishment of Liver Disease Care Systems and Support Systems for Liver Disease Patients -. http://www.mhlw.go.jp/bunya/kenkou/kekkaku-kansenshou09/pdf/hourei-170404-2.pdf (accessed June 10, 2021). (in Japanese) .
- 11. Ministry of Health, Labour and Welfare. Promotion of comprehensive measures for hepatitis control - Notice on the Training and Utilization of Hepatitis Medical Care Coordinators -. http://www.mhlw.go.jp/bunya/kenkou/kekkaku-kansenshou09/pdf/hourei-170425-1.pdf (accessed June 10, 2021). (in Japanese) .
- 12. Ministry of Health, Labour and Welfare. The 24th Meeting of the Council for Promotion of Hepatitis Measures. Document 1. https://www.mhlw.go.jp/content/10901000/000576275.pdf (accessed June 10, 2021). (in Japanese) .
- 13. Setoyama H, Korenaga M, Kitayama Y, Oza N, Masaki N, Kanto T. Nationwide survey on activities of regional core centers for the management of liver disease in Japan: Cumulative analyses by Hepatitis Information Center 2009-2017. Hepatol Res. 2020; 50:165-173. [DOI] [PMC free article] [PubMed] [Google Scholar]


