Introduction
This study investigated the effects of a beet nitric oxide enhancing (NOE) supplement comprised of nitrite and nitrate on cycling performance indices in trained cyclists. Methods: Subjects completed a lactate threshold test and a high-intensity interval (HIIT) protocol at 50% above functional threshold power with or without oral NOE supplement. Results: NOE supplementation enhanced lactate threshold by 7.2% (Placebo = 191.6 ± 37.3 W, NOE = 205.3 ± 39.9; p = 0.01; Effect Size (ES) = 0.40). During the HIIT protocol, NOE supplementation improved time to exhaustion 18% (Placebo = 1251 ± 562s, NOE = 1474 ± 504s; p = 0.02; ES = 0.42) and total energy expended 22.3% (Placebo = 251 ± 48.6 kJ, NOE = 306.6 ± 55.2 kJ; p = 0.01; ES = 1.079). NOE supplementation increased the intervals completed (Placebo = 7.00 ± 2.5, NOE = 8.14 ± 2.4; p = 0.03; ES = 0.42) and distance cycled (Placebo = 10.9 ± 4.0 km, NOE = 13.5 ± 3.9 km; p = 0.01; ES = 0.65). Also, target power was achieved at a higher cadence during the HIIT work and rest periods (p = 0.02), which enhanced muscle oxygen saturation (SmO2) recovery. Time-to-fatigue was negatively correlated with the degree of SmO2, desaturation during the HIIT work interval segment (r = −0.67; p 0.008), while both SmO2 desaturation and the SmO2 starting work segment saturation level correlated with a cyclist's kJ expended (SmO2 desaturation: r = −0.51, p = 0.06; SmO2 starting saturation: r = 0.59, p = 0.03). Conclusion: NOE supplementation containing beet nitrite and nitrate enhanced submaximal (lactate threshold) and HIIT maximal effort work. The NOE supplementation resulted in a cyclist riding at higher cadence rates with lower absolute torque values at the same power during both the work and rest periods, which in-turn delayed over-all fatigue and improved total work output.
Keywords: Nitrate/nitrite supplementation, Nitric oxide, Lactate threshold, High intensity interval training, Cycling
Graphical abstract
Highlights
-
•
Beet nitric oxide enhancing supplementation comprised of inorganic nitrite and nitrate, but relying predominately on nitrite for nitric oxide production, improved lactate threshold power by 7.2% compared to placebo in trained cyclists and triathletes.
-
•
Beet nitric oxide enhancing supplementation increased the total number of work intervals completed while improving time to exhaustion 18%, the total energy expenditure expended during high intensity intervals 22.3%, and the total cycling distance completed 23.9% compared to placebo.
-
•
Beet nitric oxide enhancing supplementation improved performance by allowing each subject to maintain interval power targets at higher cadences and lower absolute torque which in turn enhanced SmO2 recovery during the rest intervals.
-
•
A beet nitric oxide enhancing supplement with an appropriate concentration of inorganic nitrite can be an effective ergogenic aid, and response faster than a nitrate supplement.
1. Introduction
Over the last decade, studies have shown that oral beet nitrate supplementation improves performance between 2 and 16% across various exercise modalities (Cermak et al., 2012; Lansley et al., 2011; Murphy et al., 2012). Oral beet nitrate supplementation elevates plasma nitrate [NO3−] and nitrite [NO2−] consequently enhancing nitric oxide (NO) production using an O2− independent stepwise reduction of inorganic NO3− to NO2− and then to NO (Lundberg et al., 2008; Wylie et al., 2013). Previous health and sports performance research show oral nitrate supplementation enhances skeletal muscle blood flow, lowers blood pressure, improves vascular conductance, increases metabolic efficiency, and improves oxygen diffusive conductance in exercising muscles (Breese et al., 2013; Ferguson et al., 2013; Larsen et al., 2006; Larsen et al., 2011; Larsen et al., 2010). In turn, nitrate supplementation appears to improve exercise performance and metabolic efficiency across various exercise durations, intensities, and populations (Aucouturier et al., 2015; Bailey et al., 2015; Bailey et al., 2009; Coggan and Peterson, 2016; Lansley et al., 2011; Lee et al., 2015; Rimer et al., 2016; Shannon et al., 2017; C. Thompson et al., 2017; Wylie et al., 2016).
While many studies show nitrate supplementation improves exercise performance and metabolic efficiency, meta-analysis research also suggests that non-elite athletes are most likely to experience positive physiological and performance benefits (Braakhuis and Hopkins, 2015). In addition, most studies focus on a specific performance target (i.e., time trial performance, high intensity interval work, etc.) in a specific population (i.e., young highly trained, young active, elite athlete, etc.) versus studying a given group of athletes across a majority of that sport's metabolic demands required for success. For example, road cycling requires an athlete to maintain excellent conditioning levels both aerobically and anaerobically (Allen et al., 2019). Cyclists are required to maintain high levels of sustainable power while also being able to generate peak force and power very quickly for short periods of time followed by various durations of steady-state recovery work for success. Previous research shows the primary determinants of endurance cycling performance include peak oxygen uptake, peak power output, gross efficiency, and numerous lactate markers representing distinct changes in aerobic metabolism (Coyle et al., 1988). Interestingly, as stated above, nitrate supplementation enhances many of these cycling related performance areas. However, there have been no studies on the effect of a nitric oxide enhancing (NOE) supplement comprised of both inorganic nitrite and nitrate on physical performance. Thus, this study investigated what effects an oral beet NOE supplement comprised of nitrite and nitrate had on lactate threshold, cycling performance metrics during submaximal workloads, and anaerobic high-intensity interval work (HIIT) in a group of trained cyclists and triathletes.
2. Methods
2.1. Design
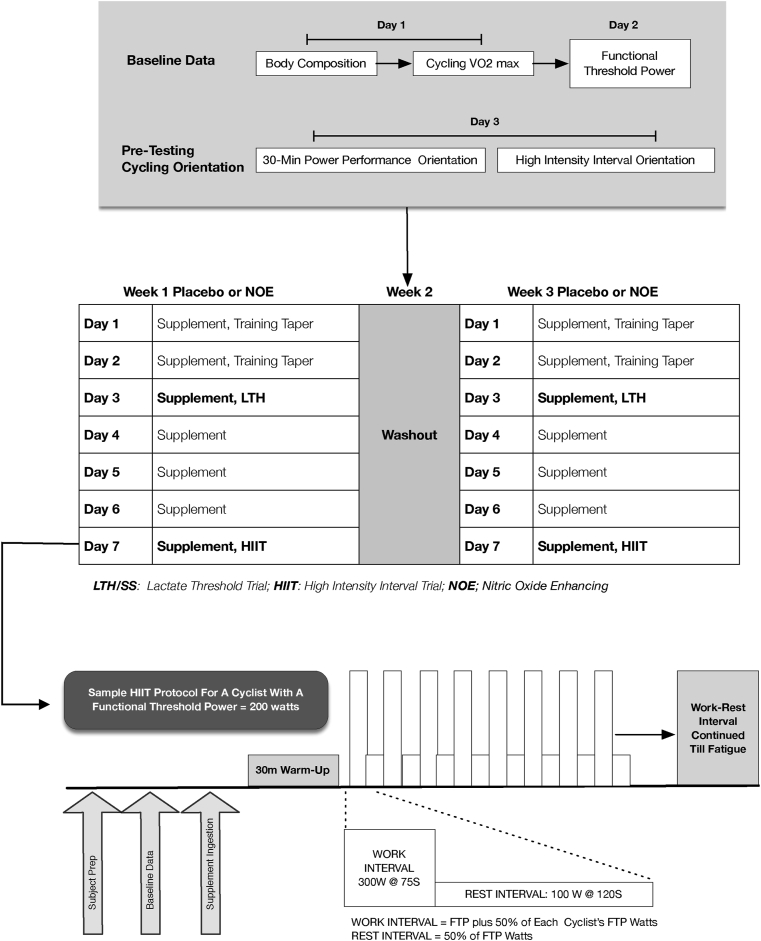
This study used a randomized, double-blind, crossover study design (Fig. 1). All procedures were approved by the Institutional Review Board at Northern Illinois University, Dekalb, IL. Prior to subject testing, each volunteer met a research coordinator for a detailed explanation of the study. The research coordinator explained the study design, the risks associated with the study, as well as the benefits for participating.
Fig. 1.
Study design & high intensity interval protocol.
2.2. Subjects
Ten men between the ages of 18 and 55 years old volunteered from local cycling and triathlon race clubs. All subjects were normotensive with no history of cardiovascular disease. To be eligible for the study, the following criteria were required: 1) cycling and race experience ≥ two years; and 2) needed to be at the 60th percentile for aerobic fitness based on the ACSM guideline standards (ACSM, 2018). Ten subjects qualified to participate. Eight subjects completed all aspects of the study. Study exclusion criteria included erectile dysfunction medications, self-reported allergies to beets, and individuals with a history of kidney stones.
2.3. Baseline testing
Baseline testing included a body composition assessment, an assessment of maximal aerobic capacity (VO2 max), and a functional threshold power (FTP) assessment. Body composition was measured using InBody's 570 multi-frequency bioelectrical impedance (BIA) system (InBody, Cerritos, CA). Each subject was instructed to abstain from solid food 6 h prior to testing. Subjects were instructed to drink 12–16 oz of water 2 h before testing to assure proper hydration status as recommended. If testing took place in the morning, subjects did not strenuously exercise the evening prior. If testing took place in the afternoon or evening, subjects did not strenuously exercise the day of testing. Finally, prior to each person's BIA test, subjects were asked to void and urinate if needed. Previous research has shown the In-Body BIA system to be both reliable and valid when compared to hydrostatic weight and DEXA measurements (Demura et al., 2004).
VO2 max was determined using a standardized linear ramp protocol with 1-min stages. For stage one, the starting workload was 75 W. Each subsequent stage, the workload increased 25 W until a subject reached volitional fatigue. VO2 max was defined as a plateau in oxygen uptake (≤1–2 ml/kg) with increasing workloads, RER ≥ 1.1, and a maximal heart rate ± five bpm of each subject's age predicted max heart rate (Tanaka et al., 2001). Maximal work output was measured in watts using a weighted mean that included the last stage a cyclist completed and the fraction of the next stage just prior to the final end point (e.g., Stage 10–300 W at 60s and Stage 11–325 W at 20s = 306 W at VO2 max). For the VO2 max test, each subject placed his bike on a Cyclus2 ergometer system, which uses a direct drive resistance set-up. Previous research comparing this ergometer system with SRM and Lode cycle ergometers showed the Cyclus2 system was a valid cycle ergometer (Reiser et al., 2000; Rodger, Plews, P., & Driller, 2017). The system's calibration curve verification from 100 W to 900 W equaled 0.5% across all measurements (Range: 0%–1%). The Cyclus2 system has been used in previous oral nitrate cycling studies (Masschelein et al., 2012). To assure accurate cycling performance data, each subject's personal demographics (age, gender, height (m), weight (kg)) and each cyclist's bike set-up (crank length (mm), front chain ring configuration, rear-cluster gearing (11-speed: 11/28 tooth configuration), and the bike weight (kg)) were entered and stored into the Cyclus2 system. This information was then used to determine key performance data: power (watts), force (N), gear ratio (m/pedal stroke), cadence (rpm), speed (kph), and distance (km). Oxygen uptake was measured using a breath-by-breath metabolic cart system (COSMED, Chicago, IL). Prior to each trial and after the metabolic cart was on for a minimum of 30 min; volume, oxygen, and carbon dioxide gas calibrations were performed as recommended by the manufacturer. Each pretest calibration was cross checked prior to starting a given test trial against the system's previous calibration logs to assure consistent pre-testing baseline calibrations.
Finally, FTP testing is a standard performance assessment technique currently used worldwide in cyclist and triathletes. FTP is defined as the highest normative watts a cyclist can maintain without fatiguing for 1-h (Allen and Coggan, 2010). In 2010, Allen and Coggan developed the FTP testing protocol that is now used by non-professional and professional cyclists who use power meters in their training program (Allen and Coggan, 2010). Previous research by Miller et al. (Miller et al., 2014) showed FTP expressed in W/kg was a valid predictor of cycling performance. In the current study, after a 30-min standardized warm-up, each cyclist was asked to maintain the highest possible watts while cycling for 20 min over a 0% grade racecourse. As previously defined by Allen and Coggan (Allen and Coggan, 2010), ninety-five percent of each cyclist's 20-min power average was recorded as the cyclist's FTP. Each cyclist's FTP was then used to calculate that cyclist's specific high-intensity interval work (HIIT) and recovery interval watts (See the HIIT protocol section below for how the FTP test specifically was used to set trial workloads). Table 1 shows the subjects' physical characteristics and baseline cycling performance results. The cycling and/or triathlon race experience of the subjects ranged from 2.5 to 27 yrs. For the eight subjects completing the study, four subjects competed in triathlons only, two subjects regularly participated in sportive events (several metric, full century or double century rides per year), and two cyclists were active category 2 bike racers. All subjects met the ACSM 60th percentile aerobic fitness criteria (i.e., VO2 max = 50.6 ml kg−1 • min−1 equals the 90th percentile). Mean percent body fat was 21.7 ± 0.05% and according to the ACSM guidelines, this value was at the 55th percentile. Max watts, watts/kg, and FTP watts/kg all were within the expected ranges based on age and cycling experience.
Table 1.
Subject physical and performance characteristics.
| Characteristic | Mean ± SD | Minimum | Maximum |
|---|---|---|---|
| Age | 41.4 ± 9.1 | 29.0 | 54 |
| Body Weight (kg) | 83.3 ± 9.6 | 70.8 | 102.2 |
| % Body Fat | 21.7 ± 0.05 | 11.0 | 28.0 |
| Lean Body Mass (kg) | 65.2 ± 6.2 | 52.3 | 75.0 |
| Fat Mass (kg) | 18.1 ± 5.8 | 8.5 | 27.2 |
| VO2max (mls•kg−1•min−1) | 50.6 ± 6.3 | 42.9 | 61.7 |
| VO2max (liters • min−1) | 4.20 ± 0.58 | 3.31 | 4.87 |
| Max Heart Rate | 181.6 ± 10.4 | 168.0 | 202.0 |
| Max Watts | 342.9 ± 60.6 | 245.0 | 400.0 |
| Max Watts/kg | 4.11 ± 0.60 | 3.30 | 5.16 |
| FTP† | 245.4 ± 43.6 | 165.0 | 299.0 |
| FTP in Watts/kg | 2.95 ± 0.49 | 2.33 | 3.86 |
†- FTP is defined as the maximum normalized watts a cyclist can sustain without fatiguing for 1-h, Hunter Allen and Andy Coggan, 2010 (Velopress, Boulder, CO).
Study Treatments - Both study treatments (Placebo and the NOE supplement) were provided by HumanN (Austin, Tx). Supplementation was counter-balanced so that treatment order for half the subjects was placebo/NOE supplement and the other half NOE supplement/placebo. The placebo was similar in nutrient content to the NOE supplement while also containing natural beet flavoring (containing sodium, potassium, carbohydrate, protein and magnesium), unsweetened caffeine free Kool-aid with vitamin C (60 mg), sodium (20 mg), and Sweet & Low but void of the NOE supplement. The study trial supplement was HumanN's BeetElite concentrated beet crystals. BeetElite contained beet nitrate (125 mg) and nitrite (20 mg), sodium (130 mg), potassium (320 mg), carbohydrate (8 g), protein (1 g), magnesium (20 mg), and vitamin C (100 mg). It should be noted that approximately 75% of nitrate consumed is immediately removed by the kidney. Of the 25% that remains, approximately 20% is converted to nitrite by commensal bacteria resulting in only 5% of the nitrate consumed converted to nitrite (Bryan and Ivy, 2015; Lundberg and Govoni, 2004). Therefore, the 20 mg of nitrite in BeetElite is equivalent to approximately 400 mg of nitrate.
Subjects were asked to limit their intake of high concentrated nitrate containing foods (e.g., arugula, beet juice, beets, rhubarb, butter leaf lettuce etc.) two days prior to starting a given study treatment period (i.e., the start of pre-treatment week supplementation day 1 as shown in Fig. 1). For each treatment condition, subjects began taking that week's respective treatment two days prior to the first lab test day (e.g., for a first lab test day Monday start, supplementation began the Saturday morning prior). Supplementation continued each morning throughout a given treatment week. Thus, for the lactate threshold test, subjects were taking a given treatment for 3 consecutive days and for the HIIT trials, a total of seven consecutive days. Subjects were instructed not to use antibacterial toothpaste or mouthwash prior to consuming a given treatment supplement throughout the study. In the lab, matching the loading supplement treatment, either 10 g of placebo or BeetElite were mixed with 4 oz of water and given to each subject immediately after taking baseline data, which was 45 min prior to starting each subject's trial. During the remaining test weekdays, subjects continued to take a given treatment each morning as instructed.
Experimental Trial Controls - All subjects were tested under the exact same test conditions across each treatment (23–24 °C and 20–30% relative humidity), on the same test days within a given treatment week and the same test time ± 30 min. Prior to starting the data collection period on each test day, researchers maintained the same pre-testing set-up process and preparation timeframe (See Fig. 1). After resting baseline data were collected and the subject was given the respective treatment for that day's trial, the subject completed a standardized warm-up. The warm-up protocol was as follows: From 0 to 20 min, the cyclist pedaled at his usual cadence while the Cyclus2 system automatically adjusted the cyclist's workload using a linear ramp protocol starting at 75 W and ending at 50% of the cyclist's VO2 max watts (e.g., VO2 max watts = 350 W; peak warm-up watts = 175 W). Between 20 and 25 min, the cyclist continued cycling at 50% of his VO2 max watts but was asked at the start of each minute (20th, 21st etc.) to increase his pedaling cadence 10–15 rpms above his normal cadence for 10-secs. The Cyclus2 system continually measures cadence and automatically lowered the cyclist's torque so the target watts remained constant at 50% of VO2 max. Between minutes 25–30, the cyclist continued riding at his usual cadence while the workload decreased linearly from 50% to 30% of VO2 max watts. All subjects started a given test trial within 10–15 min of completing the warm-up period.
Lactate Threshold Testing – Testing consisted of five submaximal stages set at 40%, 50%, 60%, 70%, and 80% of each subject's VO2 max based watts. Relative to VO2 max, 40% of max watts at stage one produced a mean VO2 for all subjects of 52.4 ± 4.1% while the 80% of max watts resulted in a VO2 equal to 88.3 ± 4.9% of VO2 max. Each stage was 5 min long. Blood lactate was taken in duplicate from whole blood the last 15-secs of each stage using a Lactate Plus meter (Nova Biomedical, MA). Prior to each trial, the lactate meter's accuracy was verified and calibrated using two quality control solutions (Level 1: 1.0–1.6 mM; Level 2: 4.0–5.4 mM). When duplicate lactate measures for a given time-point were greater than 0.5 mmol, a third measurement was taken and the mean of the two closest values were averaged. Tanner et al. (Tanner et al., 2010) showed the Lactate Plus portable meter to be an accurate and reliable device compared to a laboratory standard system (Radiometer ABL 700, Copenhagen, Denmark). Lactate threshold was defined as the break-point watts just prior to a greater than 1 mmol increase in blood lactate accumulation above baseline (Smith, Skelton, D.E., Pascoe and Gladden, 1997). All tests were evaluated by the same researcher, who was blinded as to the subject and treatment the subject was on.
HIIT Trial Test Day – The HIIT test consisted of each person attempting to perform as many interval segments as possible. Fig. 1 shows a sample HIIT interval workout for a subject with a 200-W FTP. Each HIIT interval workload was set at a wattage 1.5 times greater than each person's baseline FTP, e.g., FTP = 200 W would equal a HIIT work interval of 300 W. Workloads were set using an ERGO mode setting, which kept each rider at the target watts even if the rider's cadence changed during the HIIT workout. Using an ERGO mode setting allowed us to record and observe initial evidence of leg fatigue (i.e., reductions in cadence for a given work interval) before complete fatigue occurred. Each HIIT segment lasted 75-secs and was followed by a 2-min recovery at 50% of FTP (100 W in the above example). During the trial, the following cycling data were recorded; power, force, cadence, speed, and distance completed. Additional physiological and performance data recorded included: lactate, muscle oxygen saturation (SmO2), total hemoglobin (THb), regional oxygenated hemoglobin (OxyHb) and deoxygenated hemoglobin (DeOxyHb) content, heart rate, rating of perceived exertion (RPE), total number of HIITs completed, time to fatigue (sec), total kJ expended, SmO2% desaturation drop per each completed HIIT interval, and starting SmO2% for each HIIT interval.
SmO2Measurements – SmO2 was determined using the Moxy monitor (US Patent No. 8,941,830 B2), a portable wireless ANT+ near-infrared device developed by Fortiori Design LLC (Hutchinson, MN). The Moxy monitor contains four light emitting diodes that sequentially sends light waves (630–830 nm) into the tissues just below the emitters. The Moxy monitor uses Beer-Lambert law principles to collect and process the amount of returning scattered light with two detectors placed at 12.5 and 25 mm from the light source. Moxy monitors have been shown to be both reliable and valid using a 0%–100% scaling model using arterial occlusion (Feldmann et al., 2019). Moxy monitors also have shown acceptable tissue oxygen saturation indexes (TSI) during supine rest and demonstrated similar trends during dynamic exercise (McManus et al., 2018). The Moxy monitor uses a mathematical proprietary calibration model based on how light propagates through tissues to allow consistent measurements during physical activities like cycling. Crum et al. (Crum, O'Connor, Van Loo, Valckx and Stannard, 2017) showed that Moxy monitors are a valid SmO2 measurement device during cycling when compared to other physiological parameters (heart rate and oxygen uptake). Based on previous pilot data showing consistent SmO2 measurements across all trial aspects of the current study, the Moxy monitor was placed directly over the vastus intermedius on the right leg. The Moxy monitor and its external light wave shielding device was secured using a Moxy supplied adhesive pad and medical tape to prevent unwanted sensor movements while maintaining external sensor pressures below 20 mmHg and minimize TSI pressure related artifacts. Once secure, the device's position (mm) relative to the top of the patella at the lateral mid-point was recorded. The Moxy monitor positioning remained consistent for all trials. After the warm-up period was completed, SmO2 and THb were recorded, and cross checked to assure similar pre-trial starting values.
2.4. Statistics
All statistical analyses were run using JMP statistical software (Version 13, SAS). Based on doubly blinded pilot data, a pre-study power analysis using 10 subjects with a p-value = 0.05 produced a power analysis value = 0.82 for kJs of HIIT work/hr. For the lactate threshold trials, a matched paired t-test was used. A matched paired t-test was also used for the over-all HIIT trial summary data (i.e., total secs completed under the placebo versus beet supplementation conditions), HIIT trial total work data, and HIIT trial total recovery data. When a significant difference was observed, Cohen's d effect size (ES) procedures were used to determine the magnitude. Cohen's d measures the difference between two means divided by the pooled standard deviation of each mean. Effect size descriptors used were as follows: very small = 0.01; small = 0.20; medium = 0.50; large = 0.80; very large = 1.20; and huge = 2.0 (Cohen, 1992; Sawilowsky, 2009). Additionally, a repeated-measures ANOVA was used to analyze the SmO2, OxyHb, and DeOxyHb measurements looking at those intervals subjects completed across all time points. (Treatment = 2 X HIIT Measurement Time Points = 6 (@ 0s of Work Interval, @ 65s of Work Interval, @ 10s Rest Interval, @ 60s Rest Interval, @ 90s Rest Interval, @ 120s Rest Interval). When a significant difference was found, orthogonal paired contrasting was used to identify where the difference was located. Finally, for the HIIT performance data, a correlation matrix and stepwise multiple regression analyses were used to determine which cycling performance and physiological variables accounted for the observed results across both treatments.
3. Results
3.1. Lactate Threshold Testing
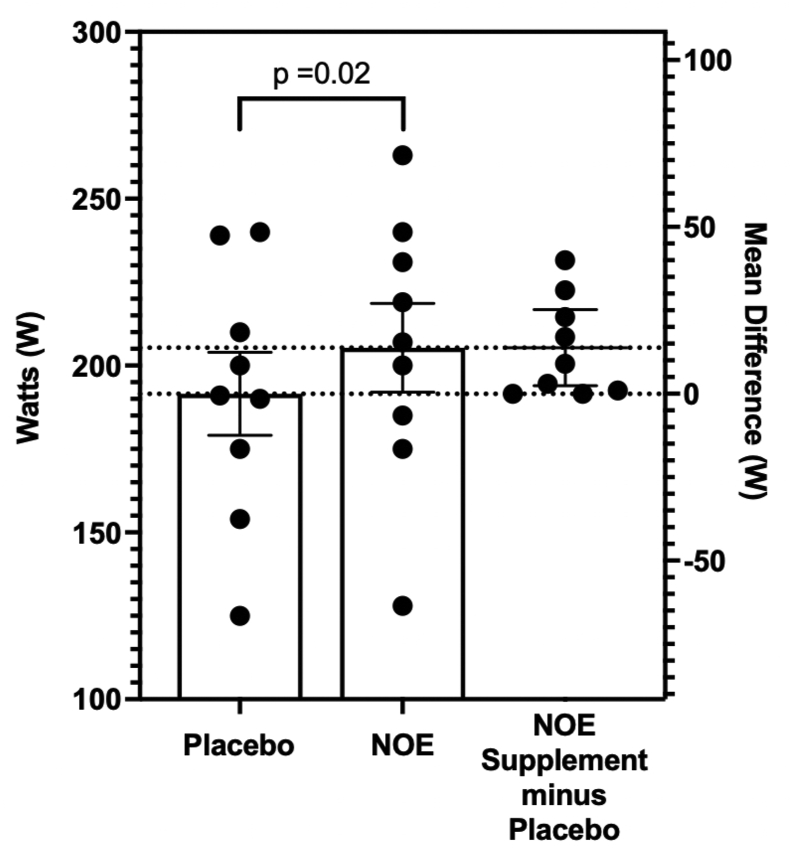
Fig. 2 shows that lactate threshold significantly increased by 7.2% from 191.6 ± 37.3 W during the placebo trial to 205.3 ± 39.9 W while on NOE supplementation (p = 0.02, ES = 0.40). Using FTP based training zone principles outlined by Allen et al. (2019), a cyclist's tempo training zone ranges between 76% and 90% of a cyclist's FTP sustainable power. Tempo is considered the watts most age group triathletes and cyclists sustain for a half-Ironman event or a cyclist maintains sitting in a fast road race peloton (Friel, 2012). Thus, under placebo conditions, lactate threshold watts were 78.4% of FTP compared to the NOE supplementation trials, which increased to 83.7% of each cyclist's FTP.
Fig. 2.
Effect of Nitric Oxide Enhancing Supplementation on Lactate Threshold. Presented as mean ± sem.(NOE = nitric oxide enhancing).
3.2. HIIT results
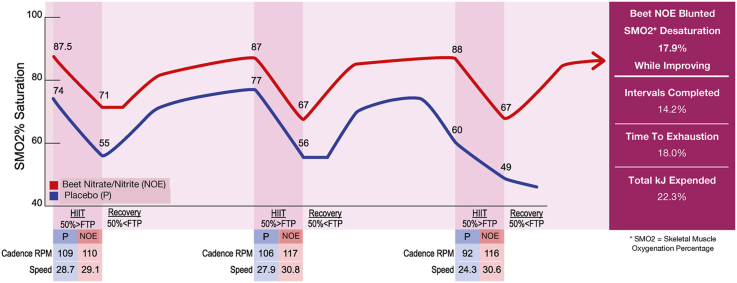
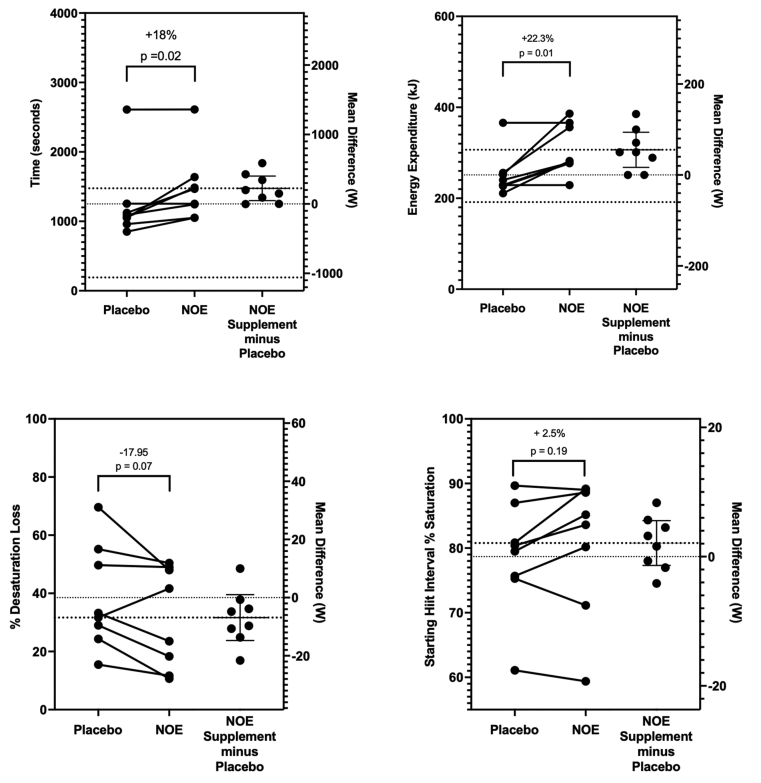
The HIIT analyses showed that NOE supplementation significantly improved time to exhaustion (Placebo: 1251 ± 562 s, NOE: 1,4767 ± 504 s; p = 0.02; ES = 0.423) and total energy expended (Placebo: 251.3 ± 48.6 kJ, NOE: 306.6 ± 55.2 kJ; p = 0.01; ES = 1.079) compared to placebo conditions (See Fig. 3). Subjects during the NOE trials completed more intervals (NOE = 8.14 ± 2.4, Placebo = 7.00 ± 2.5, p = 0.03, ES = 0.42) and cycled 23.9% further (NOE = 13.5 ± 3.9 km, Placebo = 10.9 ± 4.0 km, p = 0.01, ES = 0.65).
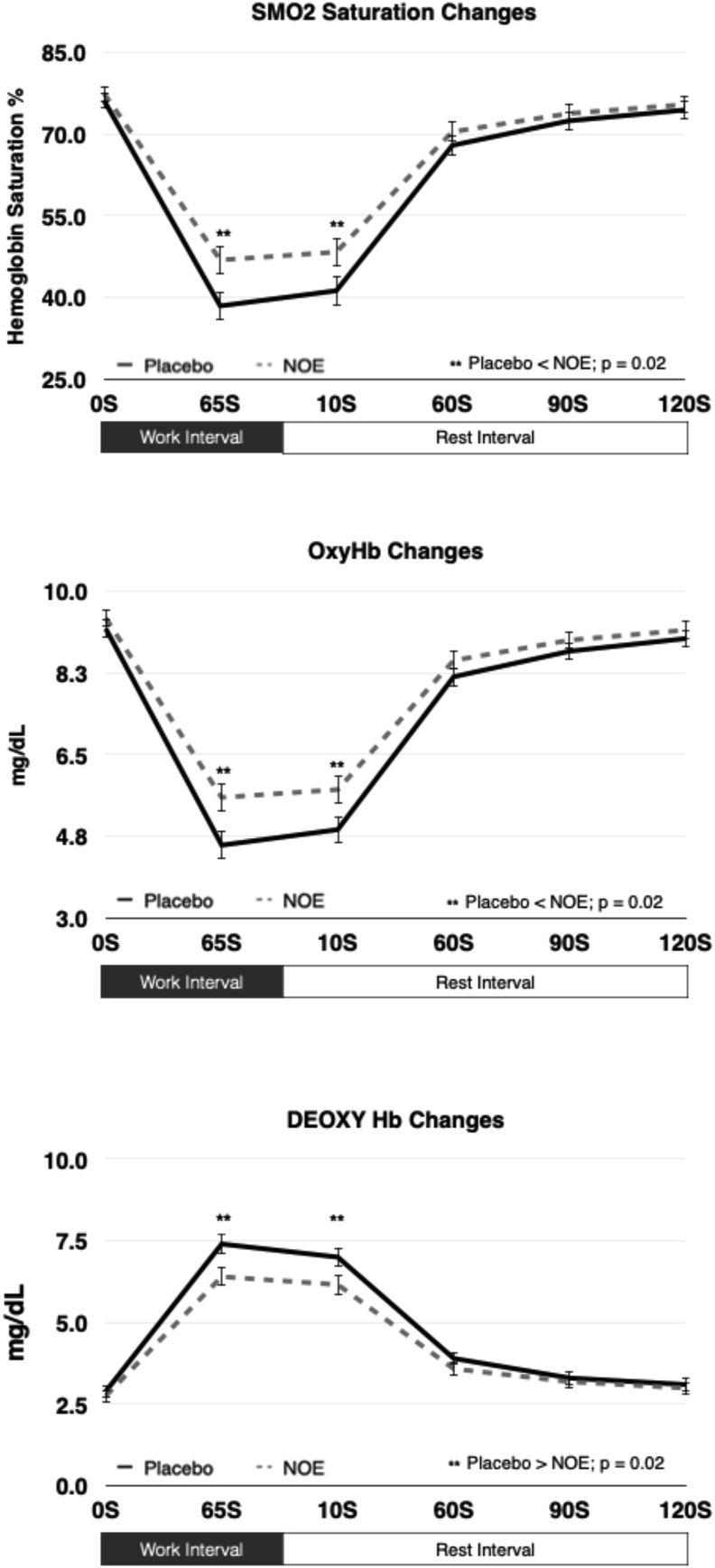
Fig. 3.
Effects of Nitric Oxide Enhancing Supplementation and High Intensity Interval Training On Performance, Energy Expenditure and Skeletal Muscle Saturation Changes. Data Presented as mean ± sem.(NOE = nitric oxide enhancing).
When the mean SmO2 desaturation drop was averaged for each HIIT work interval, the SmO2 desaturation drop was 17.9% less during the NOE trials compared to placebo but did not reach significance (p = 0.07, ES = 0.385). However, time to fatigue was negatively correlated with the degree of SmO2 desaturation that occurred per work interval (R-value = −0.67; p = 0.008) while both SmO2 desaturation and the SmO2 starting work segment saturation level were correlated with a cyclist's total kJ expended (SmO2 desaturation: r-value = −0.51, p = 0.06; SmO2 starting saturation: r-value = 0.59, p = 0.03). Additionally, stepwise regression showed the best predictors for the total HIIT time in seconds were power at 4 mmol lactate (p = 0.0001), power at lactate threshold (p = 0.0001), degree of SmO2 HIIT desaturation (p = 0.0078), and starting interval SmO2 saturation (p = 0.03). This stepwise regression model produced a r2 value = 0.965 ± 49.2s (3.6% of HIIT trial time mean in seconds). For the total HIIT kJs expended, the main factors entering into the stepwise regression model were power at 4 mmol lactate (p = 0.004), starting interval SmO2 saturation (p = 0.01), and power at lactate threshold (p = 0.02). This model produced an r2 value = 0.759 ± 29.6 kJ (10.6% of HIIT kJ expended).
Additionally, pedal force, cadence, speed, SmO2, OxyHb, and DeOxyHb were analyzed for the HIIT work and recovery interval sections separately. These data indicate that NOE supplementation significantly improved several cycling and SmO2 related metrics. Table 2 shows that during the HIIT work interval, force was not significantly different between the treatments, but cadence and speed were significantly higher during the NOE trials by 2.0% and 2.5%, respectively (Cadence: p = 0.02; ES = 0.20; Speed; p = 0.02; ES = 0.20). Correspondingly, SmO2 and OxyHb were significantly greater during the NOE trial compared to placebo (SmO2 6.5%, p = 0.001, ES = 0.16; OxyHb = 8.2%, p = 0.001, ES = 0.20). Accordingly, DeOxyHb was significantly lower during the NOE trial by 8.7% (p = 0.001, ES = 0.13). During the recovery periods, comparing the NOE trial to placebo, force was significantly lower (Placebo: 68.2 ± 15.6 N, NOE: 65.5 ± 13.7 N, p = 0.01, ES = 0.23), cadence was significantly higher (Placebo: 91.8 ± 10.9 rpm, NOE: 93.9 ± 7.4 rpm, p = 0.01, ES = 0.23), and speed was significantly greater (Placebo: 30.7 ± 3.3 kph, NOE: 31.4 ± 1.9 kph, p = 0.02, ES = 0.27). Similar to the work interval results, NOE supplementation enhanced SmO2 saturation levels by 4.1% (Placebo: 67.2 ± 18.6%; NOE: 70.4 ± 18.6%; p = 0.001, ES = 0.15), maintained OxyHb better by 2.5% (Placebo: 7.9 ± 2.9 mg/dL; NOE: 8.1 ± 2.3 mg/dL; p = 0.01, ES = 0.10), and lowered DeOxyHb by 7.7% (Placebo: 3.9 ± 2.2 mg/dL, NOE: 3.6 ± 2.3 mg/dL, p = 0.01, ES = 0.13). Finally, Fig. 4 illustrates, at each time point, the mean value ± sem for all time points completed that were equal for all subjects (each time point represents the mean value for 40 measurements across all subjects - interval n = 5 X n = 8 subjects). A repeated measures ANOVA analysis showed that while SmO2 and OxyHb were higher throughout all the time segments during the NOE trial, only the 65-sec (p = 0.02) and 10-sec post (p = 0.04) interval time points were significantly greater than the placebo trial. Similarly, DeOxyHb was significantly lower than placebo during the 65-sec (p = 0.02) and 10-sec post interval (p = 0.02) time points.
Table 2.
HIIT interval performance and SMO2 data.
| HIIT Interval Data† | ||||
|---|---|---|---|---|
| Performance Marker | Placebo Mean ± SD | NOE¥ Supplement Mean ± SD | Percent Difference |
p-value |
| Pedal Force (N) | 184.8 ± 36.5 | 185.7 ± 38.6 | 0% | NS |
| Cadence (rpms) | 97.5 ± 10.1 | 99.4 ± 8.7 | 2.0% | p = 0.02 |
| Speed (kph) | 32.6 ± 3.9 | 33.4 ± 4.0 | 2.5% | p = 0.02 |
| SMO2% | 61.3 ± 24.5 | 65.3 ± 24.1 | 6.5% | p = 0.001 |
| OxyHb (mg/dL) | 7.3 ± 3.0 | 8.2 ± 2.9 | 7.0% | p = 0.001 |
| DeOxyHb (mg/dL) | 4.6 ± 2.93 | 4.2 ± 2.91 | 8.7% | p = 0.001 |
| HIIT Recovery Data† | ||||
|---|---|---|---|---|
| Pedal Force (N) | 68.2 ± 15.6 | 65.5 ± 13.7 | 4.0% | p = 0.01 |
| Cadence (rpms) | 91.8 ± 10.9 | 93.9 ± 7.4 | 2.0% | p = 0.01 |
| Speed (kph) | 30.7 ± 3.3 | 31.4 ± 1.9 | 2.2% | p = 0.02 |
| SMO2% | 67.6 ± 18.6 | 70.4 ± 18.6 | 4.1% | p = 0.001 |
| OxyHb (mg/dL) | 7.9 ± 2.9 | 8.1 ± 2.3 | 2.5% | p = 0.001 |
| DeOxyHb (mg/dL) | 3.9 ± 2.2 | 3.6 ± 2.3 | 7.7% | p = 0.001 |
† Shows only intervals that all subjects completed. ¥ NOE - Nitric Oxide Enhancing.
Fig. 4.
How Regional Skeletal Muscle Oxygenation Is Affected By Nitric Oxide Enhancing Supplementation. Data Presented as mean ± sem. (NOE = nitric oxide enhancing).
4. Discussion
This study was designed to determine how oral beet NOE supplemention affected cycling performance and related variables (pedal force, cadence, speed, time to fatigue, SMO2% changes) during submaximal (lactate threshold) and high intensity interval training. The study's results showed oral beet NOE supplementation enhanced lactate threshold and enhanced a cyclist's ability to sustain repeated supra-maximal anaerobic interval work while also improving regional oxygenation during and in recovery following high intensity anaerobic work rates. A unique feature of this study is that the NOE supplement relied primarily on beet nitrite rather than nitrate to generate NO. Consumption of 1 g of nitrite is equivalent to consuming approximately 20 g of nitrate. A NOE supplement relying primarily on nitrite rather than nitrate has several advantages. First, the production of NO following the consumption of nitrite is 4–5 times faster than following the consumption of nitrate. Therefore, the supplement can be consumed within 30–40 min rather than 120–150 min before exercise. Second, consumption of nitrite eliminates the need for the conversion of nitrate to nitrite by commensal bacteria. There are a number of lifestyle habits, diets and medications that can reduce or eliminate the commensal bacteria required to convert nitrate to nitrite making any NOE supplement that relies exclusively on nitrate non-functional under such conditions (Bryan et al., 2017).
In the current study, lactate threshold improved 7.2% suggesting an attenuated glycolytic demand as reported by others (Ferguson et al., 2013; Masschelein et al., 2012). Interestingly, when we calculated the watts that produced lactate concentrations equal to 2, 3, and 4 mmol using an exponential curve modeling procedure (r-value = 0.99 for all trial models), the watts required to generate these lactate values, as observed for lactate threshold, were greater during the nitrate supplementation trials compared to placebo for the 2 and 3 mmol points but did not reach statistical significance (3.3% (p = 0.14), 1.2% (p = 0.65), respectively). These data support the concept that in order to generate the same degree of glycolytic metabolic demand observed under placebo conditions, additional power was required until the point in which the cyclists reached their upper sustainable aerobic power requirements, i.e., FTP or 4 mmol lactate concentration. This concept is supported by previous cycling related studies (Lansley et al., 2011; Lee et al., 2015). In the study by Lansley et al. (2011), they observed during both a 4 k and 16 k simulation time trial races that nitrate supplementation improved performance by 2.4% and 2.7%, respectively. They also observed that power was significantly greater by 13 W during both events. In a study by Lee et al. (2015) evaluating time trial cycling performance (21 k), the results showed that the time to complete the distance improved by 2.1% with oral nitrate supplementation compared to placebo. Several previous studies also support this hypothesis showing nitrate supplementation delays fatigue across a variety of exercise durations and intensities (Aucouturier et al., 2015; Bailey et al., 2010; Bailey et al., 2009; Lansley et al., 2011; K. G. Thompson et al., 2014).
The current results agree well with previous research investigating the benefits of nitrate supplementation on performance during high intensity exercise (Aucouturier et al., 2015; Bailey et al., 2009). The HIIT trial results showed that NOE supplementation enhanced several cycling performance outcomes compared to placebo conditions. During the HIIT work segments, while cyclists maintained the same pedal force between the treatment conditions (placebo = 184.8 ± 36.5 N; NOE = 185.7 ± 38.6 N, NS), cyclists showed a significant 2.0% increase in cadence and a 2.5% increase in speed during the work intervals with NOE supplementation. In a study by Husmann et al. (Husmann et al., 2019), it was shown that nitrate supplementation improved time-to-exhaustion resulting in a reduced maximum voluntary torque change during high intensity dynamic exercise. Additionally, for the first time, the Husmann et al. study (Husmann et al., 2019) showed nitrate supplementation blunted muscle fatigue by possibly reducing impairment in muscle excitation-contraction coupling.
During the HIIT rest intervals under NOE supplementation, cyclists recovered at faster cadence rates and over-all speed while producing the required fixed recovery power (watts) at a lower force load (N) compared to the placebo condition (See Table 2). Consequently, throughout the work and recovery periods, SmO2% and OxyHb concentrations were maintained significantly higher while DeOxyHb concentrations were lower during the NOE supplementation trials compared to placebo. Accordingly, compared to placebo, NOE supplementation significantly delayed fatigue (18.0%, p = 0.02, ES = 0.423), increased over-all energy expenditure (22.3%, p = 0.01, ES = 1.079), allowed the cyclists to finish more intervals (16.2%, p = 0.03, ES = 0.42), and cycle further (23.9%, p = 0.01, ES = 0.65) over the entire HIIT session. At the same time, NOE supplementation partially blunted the degree of SmO2% desaturation that occurred per HIIT work interval by 17.9% (p = 0.07) while slightly increasing each interval's starting SmO2% saturation level (p = 0.19) (See Fig. 3). These results indicate that supplementation had a powerful effect on enhancing high intensity exercise performance compared to placebo. The results suggest that NOE supplementation had a moderate to large effect size in relation to enhancing high intensity interval work tolerance.
In a study by Aucouturier et al. (2015), it was found that 3 days of oral nitrate supplementation improved supra-maximal intensity intermittent exercise performance (15s sprints @ 170% of maximal aerobic power followed by 30s passive recovery) by 19.7% compared to placebo. In the current study, the HIIT work was fixed at 1.5 times greater than each cyclist's FTP wattage or maximal aerobic sustainable power. Thus, compared to the study by Aucouturier et al. (2015), the HIIT work segment of the current study was approximately 10–20% greater than each cyclist's maximal aerobic power. At the same time, the HIIT interval duration was 5-times longer (75s) while the recovery period was 4-times longer (120s) and again set at a fixed wattage (50% of FTP watts for each cyclist). Bailey et al. (2009) found that during severe exercise (70% of the difference between the measured power at the gas exchange threshold and VO2 peak), oral nitrate supplementation significantly increased time to exhaustion by 15.8% (Placebo: 583 ± 145s; Nitrate: 675 ± 203s, p < 0.05). Interestingly, in the current study, when we looked at the total amount of time in seconds done for only the HIIT work segments and not the recovery time, NOE supplementation increased the total HIIT work segment duration by 16.1% (p = 0.03). Because the over-all time to fatigue including the rest segments was 18.0%, the above data suggest that oral NOE supplementation had a complimentary recovery effect that enhanced the cyclist's over-all HIIT work segment tolerance.
In the current study, it is important to understand that both the HIIT work segments and recovery watt loads were fixed at a rider's target power levels as described in the methods section. Therefore, as a rider's cadence either increased or decreased during a trial, the Cyclus2 system automatically adjusted the rider's resistive load (Pedal force) so the rider remained at the target watts until they reached complete fatigue. Previous research shows that performing a fixed watt-based workload at low versus high cadence rates played a role in the effectiveness oral nitrate supplementation had on time-to-exhaustion and phase II VO2 kinetics (Bailey et al., 2015). For example, Bailey et al. (2015), looked at short-term dietary nitrate supplementation on exercise tolerance while performing the same relative workload at two distinct pedal cadence rates (Very low = 35 rpms; Very high = 115 rpms). The study design created a muscular work relationship where the same cycle power output was generated under a low force/high cadence with a potentially better maintenance of blood flow during exercise, and a high force/low cadence requiring a greater degree of muscular contractile force and theoretically a greater blood flow occlusion during exercise. They reported that during the low cadence-high force work state, there were no physiological or performance differences found between oral nitrate supplementation and placebo. However, when their subjects completed the same relative cycling workload at a high cadence but low muscular force, skeletal muscle oxygenation was higher throughout the exercise, phase II VO2 kinetics improved, and time to exhaustion increased 21.9% as a result of oral nitrate supplementation. These findings agree well with the results of the current study. We observed during NOE supplementation, the cyclists maintained a higher cadence at the same or higher muscular force (HIIT work segments), which in turn allowed regional SmO2 muscle saturation and OxyHb concentrations to be better maintained throughout the entire HIIT exercise session. It is noteworthy to point out that as each cyclist neared his individual fatigue point (last sustainable HIIT work interval), the SmO2 saturation reached a value that was similar between both treatment conditions. In addition, all subjects showed regional THb concentration increases just prior (1 or 2 intervals) to the final HIIT work segment completed. These findings suggest that HIIT fatigue occurred when energy demand could not be met coupled with a decline in muscle blood flow due to a drop in cycling cadence and increase in muscle force.
In conjunction with these findings, studies by Coggan et al. (2015) and Rimer et al. (2016) investigated the effects of oral nitrate supplementation on the knee extension force-velocity relationship and 3–4s peak power development over various isokinetic cadences, respectively. Coggan et al. (2015) found that oral nitrate supplementation significantly increased force/torque and power during isokinetic knee extensions at moderate-to-high angular velocities in healthy men and women. These findings reached a medium to large effect size (0.63–0.67). As a result, significant increases in maximal speed and power were reported. The results of the study by Rimer et al. (2016) indicated that oral nitrate supplementation significantly increased peak power at a cadence rate of 125 rpms, which was not observed during the placebo trials. Although not significant, power at cadences above 125 rpms were also greater during the oral nitrate trial while no differences were observed under placebo conditions. The results of these studies, and those of Bailey et al., 2009, 2010, Husmann et al. (2019), and the current study indicate that oral NOE supplementation enhances neuro-muscular work in a way that meaningfully impacts exercise performance across a wide array of metabolic domains.
In the current study, it was observed that the greater the SmO2 work interval desaturation in combination with how impaired the SmO2 re-saturation, the more likely fatigue would occur. The measurement of regional SmO2 saturation is thought to reflect how well oxygen delivery matches a given skeletal muscle's metabolic oxygen uptake needs at that given moment in time. In the current study, because NOE supplementation enhanced the maintenance of regional skeletal muscle SmO2 during the HIIT work and rest segments, these results suggest that NOE supplementation may have attenuated an imbalance in oxygen delivery to utilization that occurred during the repeated HIIT work intervals under the placebo condition. Based on previous research, reducing the imbalance in oxygen delivery to oxygen utilization could have been due to an increase in muscle blood flow as observed by Ferguson et al. (2013) in exercising rats, an increased mitochondrial efficiency reducing the O2 requirement for ATP production (Rodger et al., 2017), an increase in energy efficiency resulting in a reduced ATP turnover for a given work rate (Wylie et al., 2013) or a combination of these physiological alterations that have been previously observed.
These results also suggest that NOE supplementation improved submaximal work performance (i.e., lactate threshold), which is a significant performance factor in high intensity work efforts during cycling. Consequently, improvements in a cyclist's lactate threshold as a result of beet NOE supplementation, could have enhanced the recovery processes between HIIT intervals helping explain why during the HIIT sessions, NOE supplementation improved over-all time to fatigue, total work in kJ, increased total HIITs completed, and increased total cycle distance completed during the HIIT workout. These results indicate that NOE supplementation when provided appropriately should improve cycling performance requiring mixed periods of both moderate-to-high intensity aerobic and anaerobic metabolic domain work demands.
Finally, it is important to note that the NOE supplement was comprised of both beet nitrite and nitrate, and formulated so that the supplement's NO generating potential relied primarily on the nitrite content rather than its nitrate content. Therefore, these results indicate that an NOE supplement with an appropriate concentration of inorganic nitrite can be an effective ergogenic aid, which can respond faster than a nitrate supplement and not require commensal bacteria for NO production.
Author contribution
Craig E. Broeder: Principle investigator, Study Design, Data analysis, Primary manuscript. Author Frank Wojan: Project Coordinator, data collection, statistical data prep, biochemical assays. Victoria Flores: Data collection, blood and biochemical processing. Bill Julian: Data collection, blood and biochemical processing. Rachel Tauber: Data Collection, subject recruitment, subject scheduling and baseline data collection. Laurie Schubert: Data collection, blood and biochemical processing. Amanda Salacinski: Data collection, study design. John L. Ivy: Assisted in the study design, interpreting the results and writing the manuscript.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests. There are no actual or potential conflicts of interest for the authors on this paper with the exception for Dr. John L. Ivy from the University of Texas at Austin. Dr. Ivy is currently President of the Scientific Advisory Board of HumanN. Dr Ivy helped design the study, interpret the results and write the manuscript. He was not involved in data collection of analysis.
Acknowledgments
This research was supported by a grant from HumanN Corporation, Austin, TX.
References
- ACSM . tenth ed. Wolters Kluwer; Philadelphia: 2018. Guidelines for Exercise Testing and Prescription. [Google Scholar]
- Allen H., Coggan A. Velo Press; Boulder, CO: 2010. Training and Racing with a Power Meter. [Google Scholar]
- Allen H., Coggan A., McGregor S. third ed. Velo Press; Boulder, CO: 2019. Training and Racing with a Power Meter. [Google Scholar]
- Aucouturier J., Boissiere J., Pawlak-Chaouch M., Cuvelier G., Gamelin F.X. Effect of dietary nitrate supplementation on tolerance to supramaximal intensity intermittent exercise. Nitric Oxide. 2015;49:16–25. doi: 10.1016/j.niox.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Bailey S.J., Fulford J., Vanhatalo A., Winyard P.G., Blackwell J.R., DiMenna F.J., Jones A.M. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J. Appl. Physiol. 2010;109(1):135–148. doi: 10.1152/japplphysiol.00046.2010. 1985. [DOI] [PubMed] [Google Scholar]
- Bailey S.J., Varnham R.L., DiMenna F.J., Breese B.C., Wylie L.J., Jones A.M. Inorganic nitrate supplementation improves muscle oxygenation, O(2) uptake kinetics, and exercise tolerance at high but not low pedal rates. J. Appl. Physiol. 2015;118(11):1396–1405. doi: 10.1152/japplphysiol.01141.2014. 1985. [DOI] [PubMed] [Google Scholar]
- Bailey S.J., Winyard P., Vanhatalo A., Blackwell J.R., Dimenna F.J., Wilkerson D.P., Jones A.M. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J. Appl. Physiol. 2009;107(4):1144–1155. doi: 10.1152/japplphysiol.00722.2009. 1985. [DOI] [PubMed] [Google Scholar]
- Braakhuis A.J., Hopkins W.G. Impact of dietary antioxidants on sport performance: a Review. Sports Med. 2015;45(7):939–955. doi: 10.1007/s40279-015-0323-x. [DOI] [PubMed] [Google Scholar]
- Breese B.C., McNarry M.A., Marwood S., Blackwell J.R., Bailey S.J., Jones A.M. Beetroot juice supplementation speeds O2 uptake kinetics and improves exercise tolerance during severe-intensity exercise initiated from an elevated metabolic rate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305(12):R1441–R1450. doi: 10.1152/ajpregu.00295.2013. [DOI] [PubMed] [Google Scholar]
- Bryan N.S., Ivy J.L. Inorganic nitrite and nitrate: evidence to support consideration as dietary nutrients. Nutr. Res. 2015;35(8) doi: 10.1016/j.nutres.2015.06.001. Aug. 1879-0739 (Electronic)), 643-654. [DOI] [PubMed] [Google Scholar]
- Bryan N.S., Tribble G., Angelov N. Oral microbiome and nitric oxide: the missing link in the management of blood pressure. Curr. Hypertens. Rep. 2017;19(4):33. doi: 10.1007/s11906-017-0725-2. [DOI] [PubMed] [Google Scholar]
- Cermak N.M., Gibala M.J., van Loon L.J. Nitrate supplementation's improvement of 10-km time-trial performance in trained cyclists. Int. J. Sport Nutr. Exerc. Metabol. 2012;22(1):64–71. doi: 10.1123/ijsnem.22.1.64. https://www.ncbi.nlm.nih.gov/pubmed/22248502 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Coggan A.R., Leibowitz J.L., Kadkhodayan A., Thomas D.P., Ramamurthy S., Spearie C.A., Peterson L.R. Effect of acute dietary nitrate intake on maximal knee extensor speed and power in healthy men and women. Nitric Oxide. 2015;48:16–21. doi: 10.1016/j.niox.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggan A.R., Peterson L.R. Dietary nitrate and skeletal muscle contractile function in heart failure. Curr. Heart Fail. Rep. 2016;13(4):158–165. doi: 10.1007/s11897-016-0293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol. Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Coyle E.F., Coggan A.R., Hopper M.K., Walters T.J. Determinants of endurance in well-trained cyclists. J. Appl. Physiol. 1988;64(6):2622–2630. doi: 10.1152/jappl.1988.64.6.2622. 1985. [DOI] [PubMed] [Google Scholar]
- Crum E.M., O'Connor W.J., Van Loo L., Valckx M., Stannard S.R. Validity and reliability of the Moxy oxygen monitor during incremental cycling exercise. Eur. J. Sport Sci. 2017;17(8):1037–1043. doi: 10.1080/17461391.2017.1330899. [DOI] [PubMed] [Google Scholar]
- Demura S., Sato S., Kitabayashi T. Percentage of total body fat as estimated by three automatic bioelectrical impedance analyzers. J. Physiol. Anthropol. Appl. Hum. Sci. 2004;23(3):93–99. doi: 10.2114/jpa.23.93. [DOI] [PubMed] [Google Scholar]
- Feldmann A., Schmitz R., Erlacher D. Near-infrared spectroscopy-derived muscle oxygen saturation on a 0% to 100% scale: reliability and validity of the Moxy Monitor. J. Biomed. Opt. 2019;24(11):1–11. doi: 10.1117/1.JBO.24.11.115001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S.K., Hirai D.M., Copp S.W., Holdsworth C.T., Allen J.D., Jones A.M., Poole D.C. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J. Physiol. 2013;591(2):547–557. doi: 10.1113/jphysiol.2012.243121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel J. Velo Press; Boulder, CO: 2012. The Power Meter Handbook: A User's Guide for Cyclists and Triathetles. [Google Scholar]
- Husmann F., Bruhn S., Mittlmeier T., Zschorlich V., Behrens M. Dietary nitrate supplementation improves exercise tolerance by reducing muscle fatigue and perceptual responses. Front. Physiol. 2019;10:404. doi: 10.3389/fphys.2019.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansley K.E., Winyard P.G., Bailey S.J., Vanhatalo A., Wilkerson D.P., Blackwell J.R.…Jones A.M. Acute dietary nitrate supplementation improves cycling time trial performance. Med. Sci. Sports Exerc. 2011;43(6):1125–1131. doi: 10.1249/MSS.0b013e31821597b4. [DOI] [PubMed] [Google Scholar]
- Larsen F.J., Ekblom B., Sahlin K., Lundberg J.O., Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N. Engl. J. Med. 2006;355(26):2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- Larsen F.J., Schiffer T.A., Borniquel S., Sahlin K., Ekblom B., Lundberg J.O., Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metabol. 2011;13(2):149–159. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Larsen F.J., Weitzberg E., Lundberg J.O., Ekblom B. Dietary nitrate reduces maximal oxygen consumption while maintaining work performance in maximal exercise. Free Radic. Biol. Med. 2010;48(2):342–347. doi: 10.1016/j.freeradbiomed.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Lee J., Kim H.T., Solares G.J., Kim K., Ding Z., Ivy J.L. Caffeinated nitric oxide-releasing lozenge improves cycling time trial performance. Int. J. Sports Med. 2015;36(2):107–112. doi: 10.1055/s-0034-1387762. [DOI] [PubMed] [Google Scholar]
- Lundberg J.O., Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic. Biol. Med. 2004;37(3):395–400. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Lundberg J.O., Weitzberg E., Gladwin M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008;7(2):156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- Masschelein E., Van Thienen R., Wang X., Van Schepdael A., Thomis M., Hespel P. Dietary nitrate improves muscle but not cerebral oxygenation status during exercise in hypoxia. J. Appl. Physiol. 2012;113:736–745. doi: 10.1152/japplphysiol.01253.2011. [DOI] [PubMed] [Google Scholar]
- McManus C.J., Collison J., Cooper C.E. Performance comparison of the MOXY and PortaMon near-infrared spectroscopy muscle oximeters at rest and during exercise. J. Biomed. Opt. 2018;23(1):1–14. doi: 10.1117/1.JBO.23.1.015007. [DOI] [PubMed] [Google Scholar]
- Miller M.C., Moir G.L., Stannard S.R. Validity of using functional threshold power and intermittent power to predict corss-country mountain bike race outcome. j Sci Cycling. 2014;3:16–20. [Google Scholar]
- Murphy M., Eliot K., Heuertz R.M., Weiss E. Whole beetroot consumption acutely improves running performance. J. Acad. Nutr. Diet. 2012;112(4):548–552. doi: 10.1016/j.jand.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Reiser M., Meyer T., Kindermann W., Daugs R. Transferability of workload measurements between three different types of ergometer. Eur. J. Appl. Physiol. 2000;82(3):245–249. doi: 10.1007/s004210050678. [DOI] [PubMed] [Google Scholar]
- Rimer E.G., Peterson L.R., Coggan A.R., Martin J.C. Increase in maximal cycling power with acute dietary nitrate supplementation. Int. J. Sports Physiol. Perform. 2016;11(6):715–720. doi: 10.1123/ijspp.2015-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger J., Plews D., P L., Driller M. The effects of an oral ß-hydroxybutyrate supplement on exercise metabolism and cycling performance. j Sci Cycling. 2017;6 [Google Scholar]
- Sawilowsky S. New effect size rules of thumb. J. Mod. Appl. Stat. Methods. 2009;8(2):467–474. [Google Scholar]
- Shannon O.M., Barlow M.J., Duckworth L., Williams E., Wort G., Woods D., O'Hara J.P. Dietary nitrate supplementation enhances short but not longer duration running time-trial performance. Eur. J. Appl. Physiol. 2017;117(4):775–785. doi: 10.1007/s00421-017-3580-6. [DOI] [PubMed] [Google Scholar]
- Smith E.W., Skelton L.S., K D.E., Pascoe D.D., Gladden L.B. Lactate distribution in the blood during progressive exercise. Med. Sci. Sports Exerc. 1997;29:654–660. doi: 10.1097/00005768-199705000-00011. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Monahan K.D., Seals D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001;37(1):153–156. doi: 10.1016/s0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- Tanner R.K., Fuller K.L., Ross M.L. Evaluation of three portable blood lactate analysers: lactate pro, lactate scout and lactate Plus. Eur. J. Appl. Physiol. 2010;109(3):551–559. doi: 10.1007/s00421-010-1379-9. [DOI] [PubMed] [Google Scholar]
- Thompson C., Wylie L.J., Blackwell J.R., Fulford J., Black M.I., Kelly J.…Jones A.M. Influence of dietary nitrate supplementation on physiological and muscle metabolic adaptations to sprint interval training. J. Appl. Physiol. 2017;122(3):642–652. doi: 10.1152/japplphysiol.00909.2016. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K.G., Turner L., Prichard J., Dodd F., Kennedy D.O., Haskell C., Jones A.M. Influence of dietary nitrate supplementation on physiological and cognitive responses to incremental cycle exercise. Respir. Physiol. Neurobiol. 2014;193:11–20. doi: 10.1016/j.resp.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Wylie L.J., Bailey S.J., Kelly J., Blackwell J.R., Vanhatalo A., Jones A.M. Influence of beetroot juice supplementation on intermittent exercise performance. Eur. J. Appl. Physiol. 2016;116(2):415–425. doi: 10.1007/s00421-015-3296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie L.J., Kelly J., Bailey S.J., Blackwell J.R., Skiba P.F., Winyard P.G., Jones A.M. Beetroot juice and exercise: pharmacodynamic and dose-response relationships. J. Appl. Physiol. 2013;115(3):325–336. doi: 10.1152/japplphysiol.00372.2013. 1985. [DOI] [PubMed] [Google Scholar]