Abstract
Purpose
The aim of the study was to evaluate the feasibility of a new venous-thrombus aspiration and autologous blood (auto-blood) reinfusion system.
Materials and methods
We constructed the venous model from polyvinyl chloride (PVC) tubes and three-way unions using a fresh clot of chicken blood as the venous thrombus. Eight French and 12F aspiration catheters were used to aspirate the thrombus in the right–pulmonary-artery model, 8 French and 14F aspiration catheters were used in the inferior–vena cava model, and 8 French and 10F aspiration catheters were used in the left–iliofemoral-vein model. A thrombus filtration and auto-blood reinfusion bottle was used to filter the thrombus and re-infuse auto-blood. We evaluated the thrombus aspiration capability of each catheter by comparing pre-aspirated with the post-aspirated thrombus volume, and we evaluated the difference in aspiration capability between the two catheters in each model by comparing their thrombus aspiration rates. We used Student's t-test for statistical analysis.
Results
Differences between pre-aspirated and post-aspirated thrombus volumes for each catheter were insignificant, as were those between the thrombus aspiration rates of the two catheters in each venous model. Using the thrombus aspiration and auto-blood reinfusion system, each aspiration catheter could fluently aspirate the thrombus out of the venous model.
Conclusion
In this study, we designed a new venous-thrombus aspiration system. This system could be used to aspirate acute venous thrombi and re-infuse autologous blood.
Keywords: Deep-venous thrombosis, Manual aspiration thrombectomy, Percutaneous mechanical thrombectomy, Autologous blood reinfusion, Aspiration catheter
1. Introduction
Deep-vein thrombosis (DVT) is well recognized as a cause of pulmonary embolism (PE) in the short term and post-thrombus syndrome (PTS) in the long term.1 Standard anticoagulation and catheter-directed thrombolysis (CDT) can relieve the acute symptoms. However, in one study, the incidence of PTS after 5 years was 71% with anticoagulation and 43% with CDT.2 Therefore, an acute venous thrombus should be removed as early and as radically as possible.
Recently, manual aspiration thrombectomy (MAT) has come into wide practice by many physicians as the first-line treatment method for its rapidity, effectiveness and excellent performance-to-price ratio.3, 4, 5, 6, 7, 8 However, the devices available for MAT have many shortcomings. In this study, we designed a new thrombus aspiration and autologous blood (auto-blood) reinfusion system to overcome these limitations.
2. Methods and materials
2.1. Model of venous system
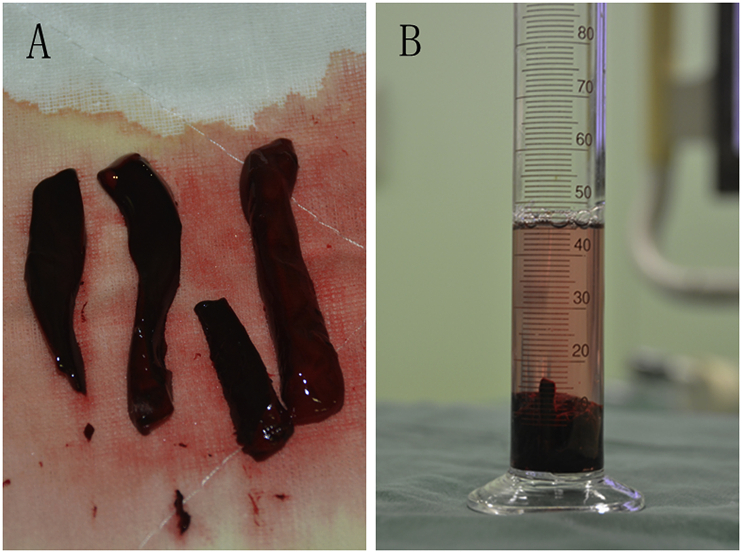
We constructed the venous model out of PVC tubes and 3-way unions (Tianjin Plastic Research Institute Co., Ltd., Tianjin, China). The model was built to simulate bilateral pulmonary arteries and iliofemoral veins, as well as the inferior vena cava. The diameter of the pulmonary-artery/iliofemoral-vein model was 10 mm compared to that of the inferior–vena cava model, which was 12 mm. We fixed the venous model to the bottom of a plastic box (Fig. 1) and immersed it in water. A fresh chicken blood clot, cut into small pieces and put into the venous model, was used as the acute–venous-thrombus model (Fig. 2A). We measured thrombus volume using a cylinder; volume was equal to that of displaced water (Fig. 2B).
Fig. 1.

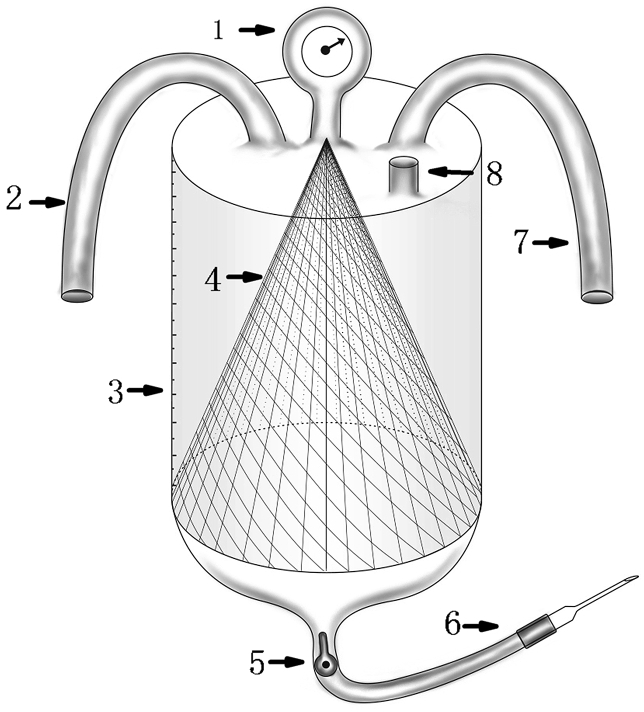
Model of the venous system. 1. Pulmonary artery. 2. Inferior vena cava. 3. Iliofemoral vein.
Fig. 2.
(A) The thrombus model, a fresh chicken blood clot, was cut into small pieces. (B) Volume of pre-aspirated thrombus was measured using a cylinder.
2.2. Aspiration catheters
We modified standard catheters (Tuoren Medical Instrument Co., Ltd, Changyuan, China) into aspiration catheters for our thrombus aspiration and auto-blood reinfusion system. Catheter diameter was 8F, 10F, 12F or 14F; the diameter of each end of each catheter was 6 mm (Fig. 3A). These dimensions were chosen to match that of the thrombus aspiration pipe (Fig. 3B). The design ensured that once the venous thrombus was aspirated into the catheter, it would not be blocked.
Fig. 3.

(A) Tip and end of the aspiration catheter. (B) The end of the aspiration catheter was designed with a diameter matching that of the thrombus aspiration pipe. 1. End of aspiration catheter. 2. Tip of aspiration catheter. 3. Thrombus aspiration pipe.
2.3. Thrombus filtration and auto-blood reinfusion bottle
The bottle contained a filtration net that was cone-shaped in order to increase filtration efficiency (Fig. 4A). The net was made of polyethylene terephthalate and polyamide fabric, with mesh apertures of approximately 200 μm.
Fig. 4.

(A) Cone filtration net. (B) Thrombus filtration and autologous blood reinfusion bottle. 1. Piezometer. 2. Thrombus aspiration pipe. 3. Bottle. 4. Cone filtration net. 5. Negative-pressure pipe.
The upper and lower mouths of the bottle were stopped with rubber plugs. The upper plug had 3 holes, designed to accommodate the thrombus aspiration pipe, negative-pressure pipe and piezometer. An infusion apparatus was inserted into the lower plug to re-infuse autologous blood (Fig. 4B). The thrombus aspiration pipe was connected to the aspiration catheter, the negative-pressure pipe to the medical center's suction system.
The thrombus filtration and auto-blood reinfusion bottle for clinical application was designed as an integrated and sterile device. The bottle was made of polycarbonate, which was hard and transparent. Heparin sodium chloride could be injected into the bottle to prevent blood clotting (Fig. 5).
Fig. 5.
Thrombus filtration and autologous-blood reinfusion bottle. 1. Piezometer. 2. Thrombus aspiration pipe. 3. Scale of the bottle. 4. Cone filtration net. 5. Tap. 6. Infusion apparatus. 7. Negative-pressure pipe. 8. Heparin input.
2.4. Thrombus filtration and auto-blood reinfusion procedures
We placed the plastic box containing the venous model on an angiographic table (Allura Xper FD20; Philips Medical Systems, Bothell, Washington, USA) and filled it with water, driving air out of the model. We put the measured thrombus into the right–pulmonary-artery model and introduced the 8F aspiration catheter into the same artery along the guiding wire. Pre-aspirated venography was performed through the catheter (Video 1). The negative-pressure pipe was connected to the medical center's suction system to maintain negative pressure in the venous system. The thrombus aspiration pipe was connected to the aspiration catheter. During the process of thrombus aspiration, negative pressure in the system was maintained at 40–50 kPa. Under fluoroscopy, the venous thrombus was sucked into the thrombus filtration and auto-blood reinfusion bottle (Videos 2 and 3). Then we performed post-aspirated venography (Video 4). The filtered water was discharged through the infusion apparatus. We measured the volumes of pre-aspirated and post-aspirated thrombus by cylinder. We performed these procedures 10 times, and then we repeated all procedures 10 times using the 12F aspiration catheter.
Supplementary video related to this article can be found at https://doi.org/10.1016/j.jimed.2019.05.004.
The following are the supplementary data related to this article:
Pre-aspirated venography in the right–pulmonary-artery model.
Aspirating the thrombus out of the right–pulmonary-artery model.
Filtering the thrombus.
Post-aspirated venography in the right–pulmonary-artery model.
We aspirated the thrombus out of the inferior–vena cava model using 8 French and 14F aspiration catheters and in the left–iliofemoral-vein model using 8 French and 10F aspiration catheters. The procedures were repeated 10 times for each aspiration catheter.
2.5. Statistical methods
The thrombus aspiration capability of each aspiration catheter was evaluated by comparing volumes of pre-aspirated and post-aspirated thrombus. To compare the thrombus aspiration capability of the 2 aspiration catheters in each model, we used the thrombus aspiration rate, which was equal to the volume of post-aspirated thrombus divided by that of pre-aspirated thrombus. For statistical analysis, we carried out a Student's t-test using SPSS statistical software version 22.0 (SPSS, Inc., Chicago, Illinois, USA). A P-value of <0.05 was deemed to indicate statistical significance.
3. Results
In the right–pulmonary-artery model, the average volumes of pre-aspirated and post-aspirated thrombus for the 8F aspiration catheter were 9.80 ± 2.23 and 9.75 ± 2.09 ml, respectively. The difference between these measurements was insignificant (P > 0.05). The average volumes of pre-aspirated and post-aspirated thrombus for the 12F aspiration catheter were 7.66 ± 1.53 and 7.60 ± 1.53 ml, respectively; the difference, here, was also insignificant (P > 0.05; Table 1). Thrombus aspiration rates for the 8F and 12F aspiration catheters were 0.998 ± 0.024 and 0.993 ± 0.043, respectively; again, the difference was insignificant (P > 0.05; Table 2). The thrombus aspiration capability of the 8F catheter was the same as that of the 12F catheter.
Table 1.
Average volumes of pre-aspirated and post-aspirated thrombus for each aspiration catheter. We evaluated the thrombus aspiration capability of each catheter by comparing volumes of pre-aspirated and post-aspirated thrombus via a Student's t-test.
| x | Catheter (F) | Volume (ml) |
Volume (ml) |
P Value |
|---|---|---|---|---|
| (Pre-aspirated) | (Post-aspirated) | |||
| Pulmonary Artery | 8 | 9.80 ± 2.23 | 9.75 ± 2.09 | 0.959 |
| 12 | 7.66 ± 1.53 | 7.60 ± 1.53 | 0.931 | |
| Inferior Vena Cava | 8 | 16.68 ± 4.56 | 16.65 ± 4.66 | 0.989 |
| 14 | 17.78 ± 2.08 | 17.75 ± 2.14 | 0.975 | |
| Iliofemoral Vein | 8 | 8.94 ± 1.84 | 8.92 ± 1.95 | 0.616 |
| 10 | 8.67 ± 1.64 | 8.66 ± 1.57 | 0.989 |
Table 2.
Thrombus aspiration rate of each aspiration catheter. The rate was equal to the volume of post-aspirated thrombus divided by that of pre-aspirated thrombus (Student's t-test). We compared the thrombus aspiration rates of the two catheters in each model.
| Venous Model | Catheter (F) | Thrombus Aspiration Rate (%) | P Value |
|---|---|---|---|
| Pulmonary Artery | 8 | 0.998 ± 0.024 | 0.756 |
| 12 | 0.993 ± 0.043 | ||
| Inferior Vena Cava | 8 | 0.996 ± 0.022 | 0.330 |
| 14 | 0.896 ± 0.315 | ||
| Iliofemoral Vein | 8 | 0.995 ± 0.033 | 0.619 |
| 10 | 1.001 ± 0.028 |
In the inferior–vena cava model, for the 8F and 14F aspiration catheters, the difference between average pre-aspirated and post-aspirated thrombus volumes was insignificant (Table 1), as was that between the catheters’ thrombus aspiration rates (Table 2). In the left–iliofemoral-vein model, statistical results were similar for the 8F and 10F aspiration catheters (Table 1, Table 2). Using the thrombus aspiration and auto-blood reinfusion system, each aspiration catheter could fluently aspirate thrombus out of the venous model.
4. Discussion
DVT is well recognized as a cause of PE in the short term and PTS in the long term.1 Standard anticoagulation and CDT can relieve the acute symptoms. However, in one study, the incidence of PTS after 5 years was 71% with anticoagulation and 43% with CDT.2 Therefore, acute DVT should be removed as early and as radically as possible. Open venous thrombectomy has been shown to achieve 84% vein patency at 10 years, but >90% of the patients suffered from PTS at follow-up.9, 10, 11 Recently, open v2enous thrombectomy has been replaced by percutaneous treatment methods, which include MAT3, 4, 5, 6, 7, 8 and percutaneous mechanical thrombectomy (PMT).12, 13, 14, 15
Currently, MAT is widely practiced by many physicians as the first-line treatment for DVT due to its rapidity, effectiveness and excellent performance-to-price ratio.3, 4, 5, 6, 7, 8 However, the instruments available for MAT have many shortcomings. First, the diameter of the ends of all available catheters is the same as the tips of syringes, meaning that the thrombus will be occluded in the small lumen of the syringe tip. Second, the blood aspirated out of the thrombus cannot be re-infused. Third, there is no automatic negative-pressure power.
The thrombus filtration and auto-blood reinfusion system designed in this study have overcome these shortcomings. First, the diameter of the ends of all thrombus aspiration catheters was 6 mm to match the thrombus aspiration pipe. This was large enough to permit the thrombus to be aspirated out fluently; once it was sucked into the catheter, it was not occluded. Second, the thrombus filtration and auto-blood reinfusion bottle could filter out the thrombus and re-infuse auto-blood. Third, negative pressure in the venous system was maintained by the medical center's suction system and could also be maintained by another negative-pressure-generating device.
PMT is also an attractive method for treatment of acute DVT because of its rapidity and effectiveness. Available devices include the Rotarex thrombectomy system (Rotarex Meditec, Lingten, Luxembourg), Aspirex thrombectomy system (Straub Medical AG, Wangs, St. Gallen, Switzerland), AngioJet devices (Boston Scientific, Marlborough, Massachusetts, USA) and AngioVac aspiration thrombectomy devices (AngioDynamics, Inc., Latham, New York, USA).12, 13, 14, 15 AngioVac stands out among these devices. It is not a rheolytic device that can cause significant haemolysis and arrhythmias.16 It consists of an aspiration line, a filter, a pump head and a reinfusion line; it can be used to remove thrombi, tumors and foreign bodies.17,18 However, this device has various shortcomings. First, the 22F suction cannula must be inserted through a 26F sheath.16 Under general anesthesia, the 26F sheath is inserted through the right internal jugular vein or common femoral vein. This may result in serious injury to these veins, especially the right internal jugular. Second, the 22F suction cannula cannot pass through the right ventricle to aspirate the thrombus from the pulmonary artery; this would displace the inferior-vena cava filter. Third, the filter, pump head and pump drive unit of the AngioVac device are very expensive.
In comparison, our thrombus filtration and auto-blood reinfusion system has some advantages. First, the diameter of the aspiration catheters, which included 8F, 10F, 12F and 14F, is much smaller than that of the AngioVac device's suction cannula. The catheters may safely be introduced from the internal jugular vein or common femoral vein. Second, it is safe for the 8F or 12F aspiration catheter to pass through the inferior-vena cava filter and right ventricle to aspirate the thrombus from the pulmonary artery. Third, no component in the system was expensive, and therefore the performance-to-price ratio of the system was excellent.
This study has some limitations. First, these results were obtained through in vitro testing. The efficiency and safety of our system must yet be demonstrated via in vivo and clinical experiments. Second, we did not model venous flow. Third, the system was designed to aspirate an acute venous thrombus; it might not be suitable for subacute venous and arterial thrombi.
5. Conclusion
We designed a venous-thrombus aspiration system that has overcome the shortcomings of extant MAT devices. This system, which has an excellent performance-to-price ratio, could be used to aspirate acute venous thrombi. However, further research is needed to overcome the limitations of this study.
References
- 1.Sharifi M., Mehdipour M., Bay C. Endovenous therapy for deep venous thrombosis: the TORPEDO trial. Cathet Cardiovasc Interv. 2010;76(3):316–325. doi: 10.1002/ccd.22638. [DOI] [PubMed] [Google Scholar]
- 2.Haig Y., Enden T., Grøtta O. Post-thrombotic syndrome after catheter-directed thrombolysis for deep vein thrombosis (CaVenT): 5-year follow-up results of an open-label, randomised controlled trial. Lancet Haematol. 2016;3(2):e64–71. doi: 10.1016/S2352-3026(15)00248-3. [DOI] [PubMed] [Google Scholar]
- 3.Kwon S.H., Oh J.H., Seo T.S. Percutaneous aspiration thrombectomy for the treatment of acute lower extremity deep vein thrombosis: is thrombolysis needed? Clin Radiol. 2009;64(5):484–490. doi: 10.1016/j.crad.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Q.H., Zhou C.Y., Chen Y. Percutaneous manual aspiration thrombectomy followed by stenting for iliac vein compression syndrome with secondary acute isolated iliofemoral deep vein thrombosis: a prospective study of single-session endovascular protocol. Eur J Vasc Endovasc Surg. 2014;47(1):68–74. doi: 10.1016/j.ejvs.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 5.Lou W.S., Gu J.P., He X. Endovascular treatment for iliac vein compression syndrome: a comparison between the presence and absence of secondary thrombosis. Korean J Radiol. 2009;10(2):135–143. doi: 10.3348/kjr.2009.10.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia Z., Tu J., Zhao J. Aspiration thrombectomy using a large-size catheter for acute lower extremity deep vein thrombosis. J Vasc Surg: Venous and Lym Dis. 2016;4:167–171. doi: 10.1016/j.jvsv.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Kwon S.H., Park S.H., Oh J.H. Prophylactic placement of an inferior vena cava filter during aspiration thrombectomy for acute deep venous thrombosis of the lower extremity. Vasc Endovasc Surg. 2016;50(4):270–276. doi: 10.1177/1538574416644524. [DOI] [PubMed] [Google Scholar]
- 8.Kwon S.H., Ahn S.E., Shin J.S. A phantom model study to identify the most effective manual aspiration thrombectomy for acute deep-vein thrombosis of the lower extremity. Clin Radiol. 2016;71:321–327. doi: 10.1016/j.crad.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 9.Kuo W.T. Optimizing catheter-directed thrombolysis for acute deep vein thrombosis: validating the open vein hypothesis. J Vasc Interv Radiol. 2013;24(1):24–26. doi: 10.1016/j.jvir.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 10.Meissner M.H., Manzo R.A., Bergelin R.O. Deep venous insufficiency: the relationship between lysis and subsequent reflux. J Vasc Surg. 1993;18(4):596–598. discussion 606-8. [PubMed] [Google Scholar]
- 11.Juhan C.M., Alimi Y.S., Barthelemy P.J. Late results of iliofemoral venous thrombectomy. J Vasc Surg. 1997;25(3):417–422. doi: 10.1016/s0741-5214(97)70249-0. [DOI] [PubMed] [Google Scholar]
- 12.Delomez M., Beregi J.P., Willoteaux S. Mechanical thrombectomy in patients with deep venous thrombosis. Cardiovasc Interv Radiol. 2001;24(1):42–48. doi: 10.1007/s002700001658. [DOI] [PubMed] [Google Scholar]
- 13.Morgan R., Belli A.M. Percutaneous thrombectomy: a review. Eur Radiol. 2002;12:205–217. doi: 10.1007/s003300101014. [DOI] [PubMed] [Google Scholar]
- 14.Blackwood S., Dietzek A.M. Pharmacomechanical thrombectomy: 2015 update. Expert Rev Cardiovasc Ther. 2016;14(4):463–475. doi: 10.1586/14779072.2016.1140038. [DOI] [PubMed] [Google Scholar]
- 15.Murphy K.D. Mechanical thrombectomy for DVT. Tech Vasc Intervent Radiol. 2004;7(2):79–85. doi: 10.1053/j.tvir.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Behrens G., Bjarnason H. Venous thromboembolic disease: the use of the aspiration thrombectomy device AngioVac. Semin Intervent Radiol. 2015;32:374–378. doi: 10.1055/s-0035-1564792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nickel B., McClure T., Moriarty J. A novel technique for endovascular removal of large volume right atrial tumor thrombus. Cardiovasc Interv Radiol. 2015;38:1021–1024. doi: 10.1007/s00270-014-0986-y. [DOI] [PubMed] [Google Scholar]
- 18.Al-Hakim R., Patel K., Moriarty J.M. AngioVac aspiration for paradoxical emboli protection through a fenestrated fontan during central venous thrombus manipulation. Cardiovasc Interv Radiol. 2015;38:752–754. doi: 10.1007/s00270-014-0953-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pre-aspirated venography in the right–pulmonary-artery model.
Aspirating the thrombus out of the right–pulmonary-artery model.
Filtering the thrombus.
Post-aspirated venography in the right–pulmonary-artery model.