Abstract
Background
Fetal balloon atrial septoplasty (BAS) is performed through the restrictive foramen ovale in fetal cases with established hypoplastic left heart syndrome (HLHS) and an intact or highly restrictive atrial septum (RAS).
Methods
In the current report, we present a case of high position BAS in a fetus with HLHS/RAS.
Results
Echocardiography confirmed an adequate atrial opening above the foramen ovale and fetal pleural effusion resolved spontaneously 1 day after the procedure.
Conclusion
To the best of our knowledge, the creation of a high position hole in the thinner part of the atrial septum, instead of the restrictive tiny hole, has not been reported in fetal cases with HLHS/RAS.
Keywords: Congenital heart disease, Fetal balloon atrial septoplasty, Hypoplastic left heart syndrome, Intact or highly restrictive atrial septum
Introduction
Most congenital heart diseases (CHDs) are curable after birth. However, hypoplastic left heart syndrome (HLHS) with an intact or highly restrictive atrial septum (RAS) is still associated with high neonatal mortality, although the survival rate has improved in recent years. The fetal left atrial (LA) hypertension associated with this condition is believed to be the cause of the pulmonary abnormalities including arterialization of the pulmonary vein and lymphangiectasia, which jointly contribute to poor postnatal outcomes. Thus, many fetal medicine centers attempt to open the atrial septum in order to decompress the fetal left atrium.
To date, in fetal cases with HLHS/RAS, balloon atrial septoplasty (BAS) has been performed through the fetal restrictive foramen ovale via an 18–20 G introducer cannula, or together with stent implantation. From our experience, the small balloon size limited by the luminal constraints of the introducer cannula is unlikely to be successful due to atrial recoil in the thick part of the atrial septum. The administration of aspirin as a prophylactic measure against thromboembolic complications to the pregnant women is still a problem after stent implantation. Here, we report a new method of high position fetal BAS in a fetus with HLHS/RAS.
Methods
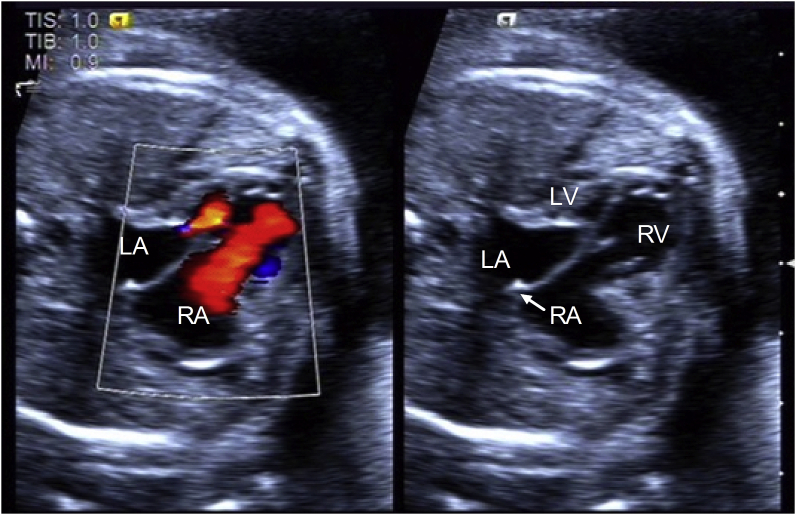
We report on a pregnant woman referred to our center at 25+3 weeks of gestation due to fetal HLHS. Comprehensive echocardiography showed HLHS with RAS. The atrial septum was bulging into the right atrium (RA) and the foramen ovale diameter was 2.2 mm with restrictive right-to-left shunting (Fig. 1). Weekly ultrasound follow-up showed severe fetal pleural effusion at 27 weeks’ gestational age.
Fig. 1.
The hypoplastic left heart syndrome (HLHS) with a highly restrictive atrial septum (RAS) was shown by Color Doppler and 2-dimensional imaging. The initial echocardiogram showed a 2.2-mm atrial communication. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fetal chromosome abnormalities were excluded via chromosomal microarray analysis of fetal amniotic fluid cells after the diagnosis of HLHS/RAS. A multidisciplinary team including the maternal-fetal medicine specialist, pediatric cardiologist, ultrasonologist, and anesthetist provided detailed counseling about all treatment options, including pregnancy termination, fetal BAS, palliative care, and postnatal treatments. The BAS procedure was approved by the hospital ethics committee. The parents were informed of the maternal and fetal benefits and risks of the intervention and written informed consent was obtained.
Results
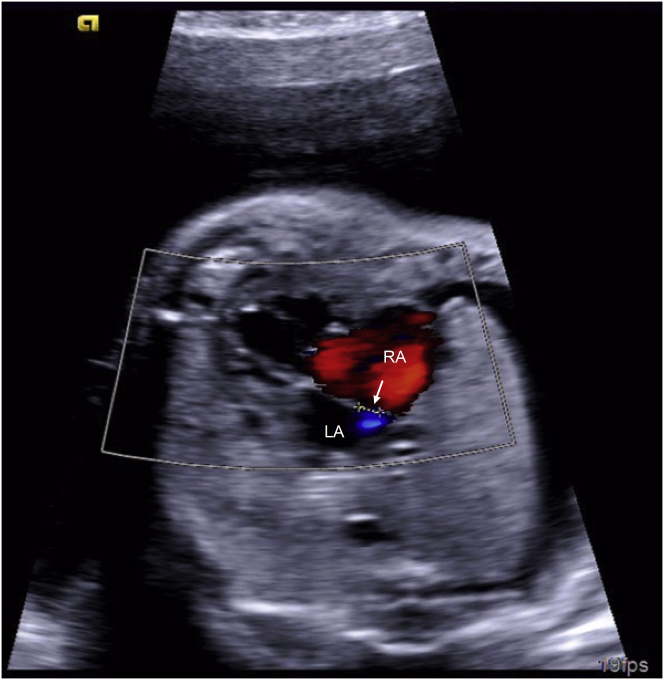
The fetal BAS was performed at 27+5 weeks of gestation. The mother received general anesthesia with inhaled and intravenous agents. After the fetal position was optimal, an 18G trocar needle was advanced into the RA, through the inter-atrial septum above the foramen ovale and then positioned into the LA, close to the mouth of the left pulmonary vein. After the guide wire (0.014″, Conquest Pro, Asahi) was inserted into the pulmonary vein, the coronary angioplasty balloon (2.5 mm*10 mm, Boston Scientific) was advanced across the atrial septum, and inflated to 6.0 atm. Afterwards, the balloon was deflated and retracted within the 18G needle, and the whole ensemble was withdrawn from the heart. The procedure lasted about 15 min. No significant fetal pericardial effusion or arrhythmia was observed during the procedure. Echocardiography confirmed an adequate atrial opening above the foramen ovale and fetal pleural effusion resolved spontaneously 1 day after the procedure (Fig. 2).
Fig. 2.
The echocardiogram after the high position fetal balloon atrial septoplasty showed a 2.9-mm opening above the foramen ovale. LA, left atrium; RA, right atrium.
Discussion
RAS occurs in around 22% of HLHS cases.1 These neonatal patients become severely hypoxic and acidotic, sometimes requiring emergent transcatheter septoplasty and support by extracorporeal membrane oxygenation. HLHS/RAS patients represent a high-risk subset with nearly 50% neonatal mortality, despite aggressive neonatal intervention.2
The ongoing mortality might be due to the irreversible lung injury attributed to the LA hypertension during fetal life. Intrauterine LA hypertension is thought to cause the edema, lymphangiectasia, muscularized pulmonary veins, and increased resistance of the pulmonary vasculature that characterize this clinical situation.3 These pathological features are indications for an attempt to decompress fetal LA pressure by BAS or stent placement.
Based on the limited volume of literature on fetal HLHS/RAS, BAS was performed in the center of the atrial septum through the fetal restrictive foramen ovale. Several unsuccessful cases reported were due to atrial recoil.4,5 The size of the defect is limited by the balloon diameter, which itself is limited by the luminal constraints of the introducer needle. But the application of a larger introducer might increase the risk of fetal complications including fetal pericardial effusion and arrhythmia. Therefore, stent implantation of the fetal atrial septum was considered to increase the success rate of an intrauterine procedure.6 Nevertheless, there are several technical challenges including malposition and embolization.
In the present case, the high position fetal BAS for HLHS/RAS was technically feasible. We created a high position hole in the thinner part of the atrial septum. We avoided the position below the foramen ovale because we presumed that intervention near the atrioventricular node might increase the risk of fetal atrioventricular block. Although several issues regarding the potential effects of this procedure remain poorly understood, we believe this simpler management might be superior to the alternatives.
Financial support
This work was supported by grants from National Natural Science Foundation of China (NO: 81770316), Mount Tai Scholarship Project and Qingdao Outstanding Health Professional Development Fund (2017).
Declaration of competing interest
There are no conflicts of interest.
References
- 1.Rychik J., Rome J.J., Collins M.H. The hypoplastic left heart syndrome with intact atrial septum: atrial morphology, pulmonary vascular histopathology and outcome. J Am Coll Cardiol. 1999;34:554–560. doi: 10.1016/s0735-1097(99)00225-9. [DOI] [PubMed] [Google Scholar]
- 2.Goltz D., Lunkenheimer J.M., Abedini M. Left ventricular obstruction with restrictive inter-atrial communication leads to retardation in fetal lung maturation. Prenat Diagn. 2015;35:463–470. doi: 10.1002/pd.4559. [DOI] [PubMed] [Google Scholar]
- 3.Olivieri L., Ratnayaka K., Levy R.J. Hypoplastic left heart syndrome with intact atrial septum sequelae of left atrial hypertension in utero. J Am Coll Cardiol. 2011;57:e369. doi: 10.1016/j.jacc.2010.10.064. [DOI] [PubMed] [Google Scholar]
- 4.Jantzen D.W., Moon-Grady A.J., Morris S.A. Hypoplastic left heart syndrome with intact or restrictive atrial septum: a report from the international fetal cardiac intervention registry. Circulation. 2017;136:1346–1349. doi: 10.1161/CIRCULATIONAHA.116.025873. [DOI] [PubMed] [Google Scholar]
- 5.Marshall A.C., Levine J., Morash D. Results of in utero atrial septoplasty in fetuses with hypoplastic left heart syndrome. Prenat Diagn. 2018;28:1023–1028. doi: 10.1002/pd.2114. [DOI] [PubMed] [Google Scholar]
- 6.Chaturvedi R.R., Ryan G., Seed M. Fetal stenting of the atrial septum: technique and initial results in cardiac lesions with left atrial hypertension. Int J Cardiol. 2013;168:2029–2036. doi: 10.1016/j.ijcard.2013.01.173. [DOI] [PubMed] [Google Scholar]