Abstract
Objective
To compare the efficacy of sorafenib plus hepatic arterial infusion chemotherapy (HAIC) with oxaliplatin to that of sorafenib alone in patients with advanced hepatocellular carcinoma (HCC).
Methods
This was a retrospective, single-center trial. Between April 3, 2017 and July 2, 2018, 104 patients with Child-Pugh A and advanced HCC received either 400 mg of sorafenib orally twice daily plus HAIC with oxaliplatin (oxaliplatin 85 mg/m2, every 3 weeks via repetitive catheterization) (n = 46, soraOXA group) or 400 mg of only sorafenib orally twice daily (n = 58, sorafenib group). Overall survival, progression-free survival, objective response rate, and treatment-related adverse events were compared.
Results
The median overall survival was 9.37 months (95% CI, 7.05–11.68) in the soraOXA group versus 4.8 months (95% CI, 2.98–6.62) in the sorafenib group (HR 0.46 [95% CI, 0.29–0.72]; P < 0.001). The soraOXA group also showed a higher objective response rate (16 [34.8%] vs 1 [1.7%]; P < 0.001) and a longer progression-free survival rate (5.5 months [95% CI, 2.32–8.68] vs 2.4 months [95% CI, 1.65–3.15], HR 0.54 [95% CI, 0.36–0.81], P = 0.003) than the sorafenib group. There was no significant difference in the overall incidence of any grade adverse events, grade 3/4 adverse events, serious adverse events, or incidence of treatment termination due to adverse events between the two groups.
Conclusion
Compared with sorafenib alone, sorafenib plus HAIC with oxaliplatin showed favorable treatment outcomes in patients with advanced HCC. The merits of this approach need to be established with a prospective trial.
Keywords: Hepatocellular carcinoma, Barcelona clinic liver cancer stage C, Sorafenib, Hepatic arterial infusion chemotherapy, Oxaliplatin
Introduction
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer around the world.1 About 50% of patients are diagnosed with Barcelona Clinic Liver Cancer (BCLC) stage C (advanced stage),2 with symptoms and/or macrovascular invasion or extrahepatic spread.3 These patients have an extremely poor prognosis, with a median survival of 4.2–7.9 months with supportive care.4,5 Sorafenib is the current standard treatment for these patients.4,5 However, the outcome of these patients treated with sorafenib remains poor, with a median survival time of 6.5–10.7 months.4,5
Cisplatin-based hepatic arterial infusion chemotherapy (HAIC) has been widely used in patients with advanced HCC in Japan.6 This procedure offers a feasible approach to elicit a greater antitumor effect than systemic chemotherapy and can reduce toxicity against other systemic organs, which significantly provides a better response rate (20.8–52%)7,8 than that of systemic chemotherapy (8%)9 and sorafenib monotherapy (2–3.3%).4,5 However, the disease commonly begins to progress again even after HAIC shrinks the tumor. Since sorafenib improved survival rates through disease stabilization and has been shown to exert a synergistic anticancer effect with chemotherapeutic agents in preclinical research studies,10, 11, 12 sorafenib combined with HAIC might benefit patients with advanced HCC more than either of the treatments alone. A phase 2 trial in patients with advanced HCC showed better outcomes with single-dose cisplatin arterial infusion chemotherapy plus sorafenib than with sorafenib monotherapy,13 but a randomized phase 3 trial showed that the combination of sorafenib and HAIC with cisplatin and 5-fluorouracil failed to demonstrate survival superiority over sorafenib.14 In summary, sorafenib plus cisplatin-based HAIC does not provide survival benefits for advanced HCC compared to sorafenib alone.
Compared with cisplatin, oxaliplatin has distinct pharmacokinetic, biochemical, cytotoxic, and immunological properties.15, 16, 17 Our previous phase 2 trial demonstrated the efficacy and safety of combined treatment with sorafenib and HAIC with FOLFOX (oxaliplatin 85 mg/m2, leucovorin 400 mg/m2 5-fluorouracil 400 mg/m2 on day 1, and 5-fluorouracil 2400 mg/m2 for 46 hours) in patients with HCC and major portal vein tumor thrombus (PVTT).18 However, some patients cannot tolerate infusion chemotherapy in bed for 2 days, and administration of 5-fluorouracil for a short time is useless as its action on tumor cells is time-dependent,19 and so these patients received arterial infusion of oxaliplatin (OXA) without leucovorin and 5-fluorouracil. In addition, one phase I study showed that HAI-oxaliplatin is a feasible, well tolerated, and demonstrated activity in patients with advanced HCC.20
Herein, we aimed to compare the efficacy of sorafenib plus HAIC with oxaliplatin to that of sorafenib monotherapy in patients with advanced HCC.
Methods
Study design and participants
Ethical approval was obtained from the Ethical Review Committee of Sun Yat-sen University Cancer Center for this retrospective study, and informed consent was obtained from all patients before conducting the treatment. The study was conducted in accordance with the principles of the Declaration of Helsinki. This study was conducted in 104 consecutive patients with advanced HCC who were treated with sorafenib plus HAI-OXA therapy (soraOXA group, n = 46) or sorafenib monotherapy (sorafenib group, n = 58) between April 3, 2017 and July 2, 2018. All recruited patients with hepatitis B virus-related HCC received preemptive antiviral therapy.
The inclusion criteria for this study were: 18 years or older, at least one complete cycle (3 weeks) of sorafenib plus HAIC or sorafenib alone, biopsy-confirmed HCC, BCLC stage C (advanced-stage), Child-Pugh A class liver function, Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–1, no previous treatment for HCC, at least one measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1,21 and adequate organ function (white blood cell count ≥3.0 × 10⁹ per L, absolute neutrophil count ≥ 1.5 × 10⁹ per L, platelet count ≥75 × 10⁹ per L, aspartate transaminase and alanine transaminase levels of ≤5 × upper limit of the normal, creatinine clearance rate of ≤1.5 × upper limit of the normal, and left ventricular ejection ≥45%). The exclusion criteria were: hepatic decompensation, including esophageal or gastric variceal bleeding or hepatic encephalopathy, central nervous system metastases, a known history of human immunodeficiency virus (HIV) infection, pregnancy or breastfeeding, a secondary malignancy, patients who received a treatment crossover, and patients lost to follow-up.
Sorafenib cohort
All patients initially received the standard 400 mg dose of sorafenib twice daily. Treatment interruptions and dose reductions (to 400 mg once daily or to 400 mg every other day) were permitted in cases of adverse drug reactions.4 If further dose reductions were required, patients were withdrawn from the study.
Sorafenib plus HAIC cohort
Patients in the soraOXA group were treated with 400 mg sorafenib orally twice daily every day, and the HAIC regimen was administered every 3 weeks. The start time of sorafenib and HAIC was within 1 week of each other.
Sorafenib was administered as above. For the HAIC, a 3.5 French catheter was inserted into the celiac trunk or superior mesenteric artery for arteriography, and both the gastroduodenal artery and the right gastric artery were embolized with a metallic coil. Then, a 2.7 French microcatheter was super selectively inserted and located in the feeding hepatic artery. The following regimen was administered via the hepatic artery: oxaliplatin 85 mg/m2 for 2 hours. After HAIC was completed, the catheter and sheath were removed. Repetitive catheterization was performed in the next HAIC cycle.
HAIC was delayed until recovery if the neutrophil counts were less than 1200 cells/μL; platelet counts, less than 60 000 platelets/μL; total bilirubin levels, >30 mmol/L; albumin levels <3.0 mg/dL; or serum creatinine levels up to 1.5 times the institutional upper limit of normal. Dose reduction (to 65 mg/m2) was allowed in cases of intolerable (grade 3 or 4) drug-related toxicities.
Follow-up and assessments
Sorafenib or sorafenib plus HAIC was continued as long as possible until one of the following criteria for cessation of therapy were met: death, adverse events that required termination of treatment, the need for another anticancer treatment (such as surgery), no benefit from treatment, or withdrawal of consent. When disease progression was observed and doctors considered that patients can benefit from treatment, sorafenib or sorafenib plus HAIC would be continued. Before treatment discontinuation, patient visits were scheduled every 3 weeks to monitor safety and clinical and biological parameters. Additionally, computed tomography scans were performed every 6 weeks.
Overall survival was defined as the time from commencement of treatment to death from any cause, and progression-free survival was defined as the time from commencement of treatment to progression by RECIST criteria or death from any cause. Objective response rate was the proportion of patients with complete response or partial response according to RECIST version 1.1, and adverse events were according to the National Cancer Institute Common Terminology Criteria for adverse events version 4.03.
Statistical analyses
Because this was a retrospective study, no sample size calculations were performed. The results were compared using Student's t-tests or chi-square tests. Survival outcomes were calculated with the Kaplan-Meier method and compared by log-rank tests. Any factors that were statistically significant with a P value less than 0.10 in the univariate analysis were candidates for entry into a multivariable Cox proportional hazards model. All P values were two-sided, with P values less than 0.05 considered significant. The statistical package used to perform analyses was SAS, version 9.0 (SAS Institute).
Results
Patient characteristics and treatment
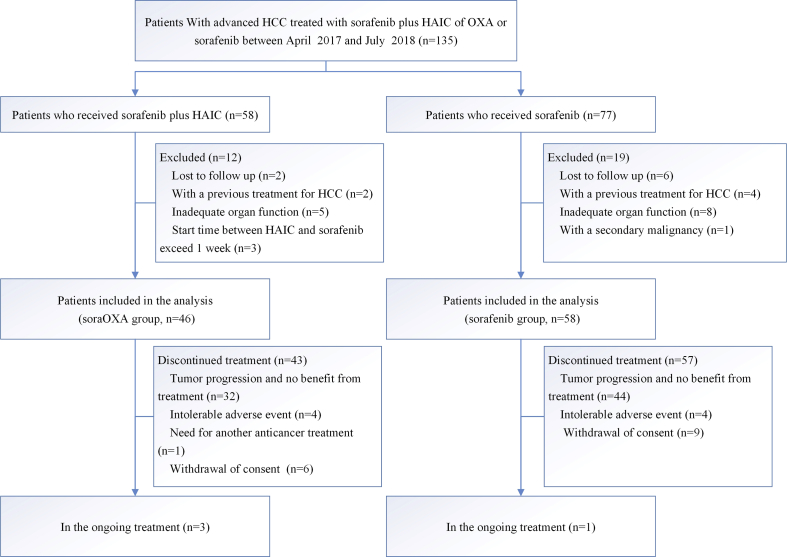
Between April 3, 2017 and July 2, 2018, 135 consecutive patients with advanced HCC were treated using either sorafenib plus HAI-OXA or sorafenib, and 104 patients met the inclusion criteria in this analysis: 46 patients underwent sorafenib plus HAIC and 58 patients underwent sorafenib (Fig. 1). The follow-up ended on April 1, 2019. The baseline characteristics of all patients included in the analysis are described in Table 1. There were no significant differences between groups. Of the 104 patients, 91 (87.5%) were infected with hepatitis B virus, 87 (83.7%) had multiple lesions, and 90 (86.5%) had PVTT that extended into the main portal vein (Vp4) or the first branch portal vein (Vp3).
Fig. 1.
Patients selection flow. HCC, hepatocellular carcinoma. HAIC, hepatic arterial infusion chemotherapy. OXA, oxaliplatin. SoraOXA group = sorafenib plus hepatic arterial infusion of oxaliplatin. Sorafenib group = Sorafenib monotherapy group.
Table 1.
Patient baseline demographics and clinical characteristics.
| Characteristics | Baseline characteristics analysis |
Univariable analysis |
|||
|---|---|---|---|---|---|
| SoraOXA group (n = 46) | Sorafenib group (n = 58) | P1 value | Median survival time, months | P2 value | |
| Age, years | 0.55 | 0.38 | |||
| ≤ 50 | 27 | 30 | 4.8 | ||
| > 50 | 19 | 28 | 7.33 | ||
| Sex | 0.5 | 0.51 | |||
| Male | 41 | 54 | 7 | ||
| Female | 5 | 4 | 6.57 | ||
| ECOG | 0.77 | 0.015 | |||
| 0 | 7 | 7 | 11.17 | ||
| 1 | 39 | 51 | 5.53 | ||
| HBsAg | 0.56 | 0.91 | |||
| Negative | 7 | 6 | 5.5 | ||
| Positive | 39 | 52 | 6.83 | ||
| Tumor size (cm) | 0.69 | 0.13 | |||
| ≤ 10 | 28 | 33 | 6.13 | ||
| > 10 | 18 | 25 | 7.23 | ||
| Tumor number | 0.26 | 0.78 | |||
| Single | 9 | 18 | 7.13 | ||
| Multiple | 37 | 40 | 6.57 | ||
| PVTT degree | 0.76 | 0.007 | |||
| Vp0-2 | 5 | 9 | 8.2 | ||
| Vp3 | 23 | 29 | 7.13 | ||
| Vp4 | 18 | 20 | 5.13 | ||
| Extrahepatic sites, n (%) | 0.37 | 0.026 | |||
| Absent | 37 | 42 | 7.13 | ||
| Present | 9 | 16 | 4.5 | ||
| AFP, ng/ml | 0.4 | 0.57 | |||
| ≤ 400 | 13 | 22 | 7.13 | ||
| > 400 | 33 | 36 | 6.17 | ||
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group Performance Status; HBsAg, surface antigen of the hepatitis B virus; AFP, alpha-fetoprotein; PVTT, portal vein tumor thrombus; Vp4, main portal vein invasion; Vp3, first branch portal vein invasion; Vp2, second branch portal vein invasion; Vp1, third branch portal vein invasion.
SoraOXA group = sorafenib plus hepatic arterial infusion of oxaliplatin. Sorafenib group = Sorafenib monotherapy group.
P1 value was calculated by a two-sided Chi-square test.
P2 value was calculated with two-sided log-rank test.
After the termination of treatment, some patients received the following other subsequent therapies: resection (1 patient), PD-1 inhibitor treatment (6 patients), lenvatinib (4 patients), and TACE (2 patients) in the soraOXA group, and D-1 inhibitor treatment (8 patients), lenvatinib (5 patients), systemic chemotherapy (1 patient), and TACE (4 patients) in the sorafenib group.
Efficacy
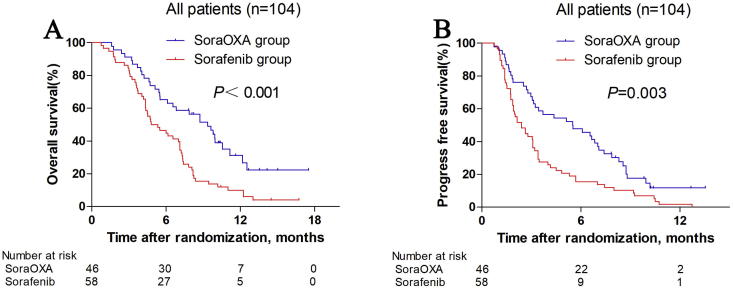
Patients in the soraOXA group had a median overall survival of 9.37 months (95% confidence interval [CI], 7.05–11.68) compared with 4.8 months (95% CI, 2.98–6.62) in the sorafenib group (hazard ratio [HR] 0.46 [95% CI, 0.29–0.72]; P < 0.001; Fig. 2A). The results of the univariable survival analysis are listed in Table 1, and treatment allocation, ECOG score, PVTT degree, and the presence or absence of extrahepatic sites were found to be statistically significant at P < 0.05 on univariate analysis. In the multivariable analysis, independent risk factors of survival were treatment allocation (HR 0.38 [95% CI, 0.24–0.62], P < 0.001), ECOG score (HR 0.45 [95% CI, 0.22–0.94], P = 0.033), and portal vein invasion grade Vp0-2 vs Vp4 (HR 0.33 [95% CI, 0.16–0.67], P = 0.002).
Fig. 2.
Kaplan-Meier curves for overall survival (A), and progression-free survival (B). SoraOXA group = sorafenib plus hepatic arterial infusion of oxaliplatin. Sorafenib group = Sorafenib monotherapy group.
Patients in the soraOXA group had a significantly longer median progression-free survival (5.5 months [95% CI, 2.32–8.68]) than those in the sorafenib group (2.4 months [95% CI, 1.65–3.15]; HR 0.54 [95% CI, 0.36–0.81], P = 0.003; Fig. 2B). On the basis of RECIST, the overall response was significantly higher in the soraOXA group (34.8%, 16 of 46 patients) than in the sorafenib group (1.7%, 1 of 58 patients; Table 2; P < 0.001).
Table 2.
Best tumor responses by RECIST criteria.
| SoraOXA group (n = 46) | Sorafenib group (n = 58) | P value | |
|---|---|---|---|
| Complete response | 0 | 0 | |
| Partial response | 16 (34.8%) | 1 (1.7%) | <0.001 |
| Stable disease | 15 (32.6%) | 29 (50%) | 0.11 |
| Progressive disease | 14 (30.4%) | 27 (46.6%) | 0.11 |
| Not evaluable* | 1 (2.2%) | 2 (3.4%) | 1.00 |
| Objective response | 16 (34.8%) | 1 (1.7%) | <0.001 |
| Disease control rate | 31 (67.4%) | 30 (51.7%) | 0.12 |
Abbreviations: RECIST, Response Evaluation Criteria in Solid Tumors.
SoraOXA group = sorafenib plus hepatic arterial infusion of oxaliplatin. Sorafenib group = Sorafenib monotherapy group.
*There were 3 patients who could not be evaluated for treatment response because of death, poor performance status, or patients' refusal of computed tomography scanning.
Statistical significance was assessed with the chi-square test.
Safety
Treatment-related adverse events, which occurred in ≥10% of patients, are shown in Table 3. The overall incidence of treatment-related adverse events was similar between the soraOXA group and the sorafenib group (any grade: 40 [86.96%] vs 49 [84.48%], P = 0.79; grade 3/4: 20 [43.48%] vs 27 [46.55%], P = 0.84). Only “any grade sensory neuropathy” was higher in the soraOXA group (P < 0.001). Serious adverse events were reported in 5 (10.87%) of 46 patients in the soraOXA group (2 gastrointestinal bleeding, 2 renal failures, and 1 ascite) and 8 (13.79%) of 58 patients in the sorafenib group (2 gastrointestinal bleeding, 3 ascites, 1 hepatic encephalopathy, and 2 diarrhea) (P = 0.77).
Table 3.
Treatment-related adverse events*.
| Adverse event | SoraOXA group (n = 46) |
Sorafenib group (n = 58) |
P value |
|||
|---|---|---|---|---|---|---|
| Any grade | Grades 3–4 | Any grade | Grades 3–4 | Any grade | Grades 3–4 | |
| Overall incidence | 40 | 20 | 49 | 27 | 0.79 | 0.84 |
| Blood/bone marrow suppression | ||||||
| Neutropenia | 19 | 3 | 17 | 2 | 0.67 | 0.65 |
| Thrombocytopenia | 21 | 4 | 25 | 2 | 0.84 | 0.4 |
| Anemia | 22 | 1 | 21 | 1 | 0.32 | 0.75 |
| Systemic toxicity | ||||||
| Hypertension | 10 | 1 | 16 | 2 | 0.65 | 1.00 |
| Edema | 7 | 1 | 5 | 1 | 0.36 | 1.00 |
| Fatigue | 27 | 1 | 25 | 1 | 0.17 | 1.00 |
| Weight loss | 14 | 0 | 19 | 1 | 0.84 | 1.00 |
| Sensory neuropathy | 15 | 0 | 2 | 0 | <0.001 | – |
| Dermatologic events | ||||||
| Hand–foot skin reaction | 18 | 4 | 24 | 7 | 0.84 | 0.75 |
| Alopecia | 6 | 0 | 10 | 0 | 0.6 | – |
| Rash | 7 | 0 | 11 | 1 | 0.8 | 1.00 |
| Gastrointestinal events | ||||||
| Nausea | 21 | 3 | 19 | 1 | 0.23 | 0.32 |
| Vomiting | 16 | 1 | 12 | 0 | 0.12 | 0.44 |
| Diarrhea | 12 | 3 | 17 | 6 | 0.83 | 0.73 |
| Hepatic function | ||||||
| Elevated ALT | 27 | 5 | 36 | 7 | 0.84 | 1.00 |
| Elevated AST | 30 | 5 | 33 | 8 | 0.43 | 0.77 |
| Hyperbilirubinemia | 21 | 1 | 31 | 1 | 0.55 | 1.00 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
SoraOXA group = sorafenib plus hepatic arterial infusion of oxaliplatin. Sorafenib group = sorafenib monotherapy group.
P value was calculated by a two-sided chi-square test.
*Listed are adverse events, as defined by the National Cancer Institute Common Terminology Criteria (version 4.03), that occurred in at least 10% of patients in either study group.
Patients in the soraOXA group were treated with a total of 126 cycles of HAIC, and the median number of HAIC treatments administered to each patient was 3 (range, 1 to 6 cycles). In the soraOXA group, a dose reduction of oxaliplatin was performed in 3 (4.3%) patients because of grade 4 bone marrow suppression. There was no difference in the dose reduction of sorafenib (17 of 46 patients vs 20 of 58 patients, P = 0.84) and the interruption of sorafenib (13 of 46 patients vs 19 of 58 patients, P = 0.67) due to adverse events between the two groups. Reasons for treatment discontinuation are shown in Fig. 1, and there was no difference in the rates of permanent discontinuation due to adverse events between the two groups (4 of 46 patients vs 4 of 58 patients, P = 0.73).
Discussion
This is the first retrospective study to compare the efficacy and safety of OXA-based HAIC versus sorafenib in patients with advanced HCC. Compared with patients who received sorafenib alone, patients who received sorafenib plus HAIC with oxaliplatin had a significantly longer overall survival (9.37 vs 4.8 months), a markedly longer median progression-free survival (5.5 vs 2.4 months), and a significantly higher radiologic response rate (34.8% vs 1.7% by RECIST criteria). In addition, both sorafenib plus HAIC with oxaliplatin and sorafenib were well tolerated and had manageable side effects.
The survival benefit demonstrated in this study may be partly due to the synergistic antitumor effect of sorafenib and chemotherapeutic agents. Sorafenib has multiple targets, including Raf-1, Braf, VEGFR-1-3, and PDGFR-β, and the inhibition of Raf-1 by sorafenib can induce apoptosis and help overcome resistance to chemotherapeutic agents.10 Moreover, sorafenib may induce vessel normalization in HCC and increase drug delivery.12 Besides, sorafenib has been shown to interact with platinum transporter proteins.22 Finally, compared with an implanted port catheter system, repetitive catheterization and digital subtraction angiography before starting each session of HAIC is more reliable for concentrating the chemotherapy dose in the targeted area and avoiding anticancer drug exposure in other organs.
In this trial, overall survival in the soraOXA group was lower than that observed in our phase 2 trial with sorafenib plus FOLFOX-based HAIC (overall survival: 9.37 months [95% CI, 7.05–11.68] vs 13.2 months [95% CI, 5.4–21]),18 but the objective response rate was similar between the two studies (34.8% vs 40% using RECIST criteria). The survival difference may be related to the lack of infusion of 5-fluorouracil in this study, and experimental data showed synergistic activity of the oxaliplatin/5-fluorouracil combination.23,24 The similar response might be explained by the fact that oxaliplatin played a major role in tumor reduction, and two randomized trials about colorectal cancer showed that the objective response rate was more than two times higher in patients who received oxaliplatin plus 5-fluorouracil than in patients who received 5-fluorouracil alone.25,26 Whether sorafenib plus HAIC with FOLFOX is superior to sorafenib plus HAIC with oxaliplatin needs to be assessed in further randomized prospective trials. In addition, the overall survival among participants in the sorafenib group in this study appeared to be less satisfactory than that in previous studies,4,5 because this trial enrolled patients with more advanced tumors; 86.5% (90 of 104 patients) had advanced PVTT (Vp3 or Vp4).
Moreover, the adverse events in the soraOXA group were slightly lower than those observed in our phase 2 study,18 and all sorafenib-related adverse events were consistent with those in previous trials of sorafenib.4,5 Although patients treated with sorafenib plus HAIC had significantly elevated frequencies of any grade sensory neuropathy that is related to oxaliplatin, these adverse events were not unexpected and were manageable by treatment interruption or dose modification. In addition, there was no significant difference in the overall incidence of any grade or grade 3/4 adverse events between groups.
The present study has some limitations. First, the study included only a small number of patients and was retrospective in nature. A large-scale prospective trial should be performed in the future to verify the efficacy of sorafenib plus oxaliplatin in patients with advanced HCC. Second, this study was performed in an endemic area. The predominant etiology of HCC in China was hepatitis B virus. Therefore, whether the results could be applied to western countries, where the etiology of hepatocellular carcinoma is mainly hepatitis C virus, remains to be elucidated.
In summary, sorafenib plus HAIC with oxaliplatin showed a favorable efficacy and safety in patients with advanced HCC, resulting in a significantly higher objective response rate, longer progression-free survival, and longer overall survival than those with sorafenib alone. Further prospective studies are needed to clarify the results.
Funding
This work was supported by National Key R&D Program of China (2017YFA0505803), National Natural Science Foundation of China (No. 81625017, No.81572385), National Science and Technology Major Project of China (2018ZX10302205).
References
- 1.Global Burden of Disease Cancer C. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol 3. 2017:524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cabibbo G., Enea M., Attanasio M. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51:1274–1283. doi: 10.1002/hep.23485. [DOI] [PubMed] [Google Scholar]
- 3.Forner A., Reig M.E., de Lope C.R. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 4.Llovet J.M., Ricci S., Mazzaferro V. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Cheng A.L., Kang Y.K., Chen Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 6.Kudo M., Matsui O., Izumi N. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver canc. 2014;3:458–468. doi: 10.1159/000343875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ando E., Tanaka M., Yamashita F. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: analysis of 48 cases. Cancer. 2002;95:588–595. doi: 10.1002/cncr.10694. [DOI] [PubMed] [Google Scholar]
- 8.Park J.Y., Ahn S.H., Yoon Y.J. Repetitive short-course hepatic arterial infusion chemotherapy with high-dose 5-fluorouracil and cisplatin in patients with advanced hepatocellular carcinoma. Cancer. 2007;110:129–137. doi: 10.1002/cncr.22759. [DOI] [PubMed] [Google Scholar]
- 9.Qin S., Bai Y., Lim H.Y. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol : Off. J. Am. Soc. Clin. Oncol. 2013;31:3501–3508. doi: 10.1200/JCO.2012.44.5643. [DOI] [PubMed] [Google Scholar]
- 10.Ma S.Q., Cao B.R., Zhang H. The lack of Raf-1 kinase feedback regulation enhances antiapoptosis in cancer cells. Oncogene 36. 2017:2014–2022. doi: 10.1038/onc.2016.384. [DOI] [PubMed] [Google Scholar]
- 11.Malofeeva E.V., Domanitskaya N., Gudima M. Modulation of the ATPase and transport activities of broad-acting multidrug resistance factor ABCC10 (MRP7) Cancer Res. 2012;72:6457–6467. doi: 10.1158/0008-5472.CAN-12-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewin M., Fartoux L., Vignaud A. The diffusion-weighted imaging perfusion fraction f is a potential marker of sorafenib treatment in advanced hepatocellular carcinoma: a pilot study. Eur Radiol. 2011;21:281–290. doi: 10.1007/s00330-010-1914-4. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda M., Shimizu S., Sato T. Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin versus sorafenib for advanced hepatocellular carcinoma: randomized phase II trial. Ann Oncol. 2016;27:2090–2096. doi: 10.1093/annonc/mdw323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kudo M., Ueshima K., Yokosuka O. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, phase 3 trial. The lancet Gastroenterol. Hepatol. 2018;3:424–432. doi: 10.1016/S2468-1253(18)30078-5. [DOI] [PubMed] [Google Scholar]
- 15.Bruno P.M., Liu Y., Park G.Y. A subset of platinum-containing chemotherapeutic agents kills cells by inducing ribosome biogenesis stress. Nat Med. 2017;23:461–471. doi: 10.1038/nm.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dzodic R., Gomez-Abuin G., Rougier P. Pharmacokinetic advantage of intra-arterial hepatic oxaliplatin administration: comparative results with cisplatin using a rabbit VX2 tumor model. Anti Cancer Drugs. 2004;15:647–650. doi: 10.1097/01.cad.0000131684.06390.fe. [DOI] [PubMed] [Google Scholar]
- 17.Tesniere A., Schlemmer F., Boige V. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29:482–491. doi: 10.1038/onc.2009.356. [DOI] [PubMed] [Google Scholar]
- 18.He M.K., Zou R.H., Li Q.J. Phase II study of sorafenib combined with concurrent hepatic arterial infusion of oxaliplatin, 5-fluorouracil and leucovorin for unresectable hepatocellular carcinoma with major portal vein thrombosis. Cardiovasc Interv Radiol. 2018;41:734–743. doi: 10.1007/s00270-017-1874-z. [DOI] [PubMed] [Google Scholar]
- 19.Calabro-Jones P.M., Byfield J.E., Ward J.F. Time-dose relationships for 5-fluorouracil cytotoxicity against human epithelial cancer cells in vitro. Cancer Res. 1982;42:4413–4420. [PubMed] [Google Scholar]
- 20.Rathore R., Safran H., Soares G. Phase I study of hepatic arterial infusion of oxaliplatin in advanced hepatocellular cancer: a brown university oncology group study. Am J Clin Oncol. 2010;33:43–46. doi: 10.1097/COC.0b013e31819d8668. [DOI] [PubMed] [Google Scholar]
- 21.Eisenhauer E.A., Therasse P., Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Heim M., Scharifi M., Zisowsky J. The Raf kinase inhibitor BAY 43-9006 reduces cellular uptake of platinum compounds and cytotoxicity in human colorectal carcinoma cell lines. Anti Cancer Drugs. 2005;16:129–136. doi: 10.1097/00001813-200502000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Raymond E., Buquet-Fagot C., Djelloul S. Antitumor activity of oxaliplatin in combination with 5-fluorouracil and the thymidylate synthase inhibitor AG337 in human colon, breast and ovarian cancers. Anti Cancer Drugs. 1997;8(9):876–885. doi: 10.1097/00001813-199710000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Mathe G., Kidani Y., Segiguchi M. Oxalato-platinum or 1-OHP, a third-generation platinum complex: an experimental and clinical appraisal and preliminary comparison with cis-platinum and carboplatinum. Biomed Pharmacother. 1989;43:237–250. doi: 10.1016/0753-3322(89)90003-6. [DOI] [PubMed] [Google Scholar]
- 25.de Gramont A., Figer A., Seymour M. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 26.Giacchetti S., Perpoint B., Zidani R. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18:136–147. doi: 10.1200/JCO.2000.18.1.136. [DOI] [PubMed] [Google Scholar]