Abstract
This study investigated the influence of heat pre-conditioning on the recovery of muscle torque, microvascular function, movement economy and stride mechanics following exercise-induced muscle damage (EIMD). Twenty male participants were equally assigned to a control (CON) and an experimental group (HEAT), and performed a 30-min downhill run (DHR) to elicit EIMD. HEAT group received three consecutive days of heat exposure (45.1 ± 3.2 min of hot water immersion at 42 °C) prior to DHR. Microvascular function (near-infrared spectroscopy), maximal voluntary contraction (MVC) torque of the knee extensors, as well as two treadmill-based steady-state runs performed below (SSR-1) and above (SSR-2) the first ventilatory threshold were assessed prior to DHR and repeated for four consecutive days post-DHR (D1-POST to D4-POST). The decline in MVC torque following EIMD was attenuated in HEAT compared with CON at D1-POST (p = 0.037), D3-POST (p = 0.002) and D4-POST (p = 0.022). Muscle soreness increased in both CON and HEAT, but was significantly attenuated in HEAT compared with CON at D2-POST (p = 0.024) and D3-POST (p = 0.013). Microvascular function decreased in CON from D1-POST to D3-POST (p = 0.009 to 0.018), and was lower compared with HEAT throughout D1-POST to D3-POST (p = 0.003 to 0.017). Pre-heat treatment decreased the magnitude of strength loss and muscle soreness, as well as attenuated the decline in microvascular function following EIMD. Heat treatment appears a promising pre-conditioning strategy when embarking on intensified training periods or competition.
Keywords: Microvascular function, Skeletal muscle recovery, Near-infrared spectroscopy, Spring mass model, Heat therapy, Heat shock protein
Graphical abstract
Highlights
-
•
Three days of heat pre-conditioning decreases the extent of strength loss, soreness and microvascular function after EIMD.
-
•
Pre heat treatment might be a promising preconditioning tool prior to intensified training periods or competition.
-
•
Heat tretament may positively impact activities of daily living, training quality and adherence to training programs.
1. Introduction
Exercise-induced muscle damage (EIMD) is typically experienced following eccentric muscle loading (Byrne et al., 2004), and seems a common occurrence following prolonged activities such as marathon running (Bernat-Adell et al., 2021), as well as following high-intensity or collision sport such as soccer (Silva et al., 2018) and Rugby (Tavares et al., 2017). The decline in muscle force and the development of muscle soreness are arguably key features characterizing EIMD (Peake et al., 2017), whilst other studies have also reported disrupted microvascular structure and function resulting in compromised matching between oxygen delivery and utilization during contractions (Kano et al., 2004, 2005; Vernillo et al., 2017).
The secondary effects resulting from EIMD substantially influences physical and physiological function. For instance, the decline in muscle strength and increased soreness have been purported to in-part alter running kinematics, resulting in decreased stride length and increased stride frequency (Chen et al., 2007), coupled with increased stiffness (i.e., reduced center of mass vertical oscillation and/or leg length), and reduced ground reaction forces (Morin et al., 2011; Degache et al., 2013; Tsatalas et al., 2013). While these mechanical changes minimize the impact encountered by the musculoskeletal system during locomotion, the adoption of such strategies likely decreases movement economy (Chen et al., 2007, 2008) and exacerbates the increased metabolic cost (i.e., higher oxygen consumption during steady-state exercise) already incurred though alterations in microvascular function (Kano et al., 2005; Vernillo et al., 2017). Such impairments negatively influence athletic performance, reduce training quality or adherence to training regimens. Developing and optimizing recovery and conditioning programs are therefore fundamental such that the physical demands associated training and competition are well tolerated.
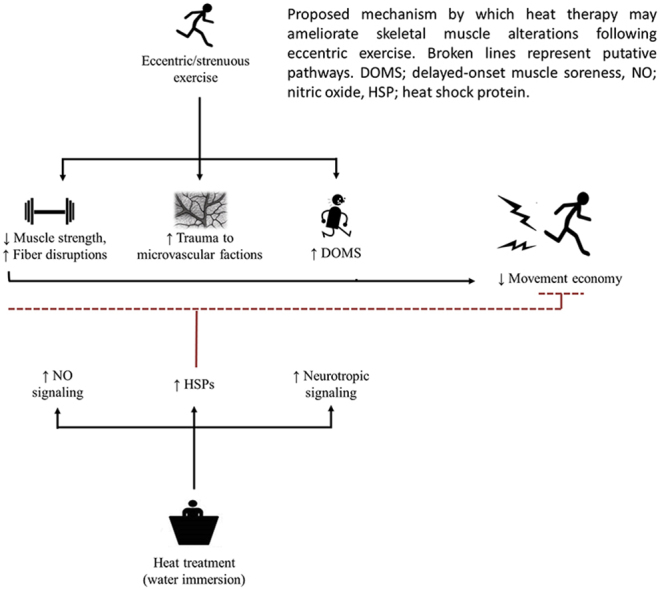
Heat treatment has been suggested as a potential therapeutic intervention to limit the extent of EIMD. Based on the underpinning mechanism that elevated muscle tissue temperatures may upregulate heat shock proteins (HSP) and cyto-protective signaling (Morton et al., 2009; Hafen et al., 2018; Ihsan et al., 2020a), studies have demonstrated improved recovery of muscle strength, muscle endurance and sensation of muscle soreness following heat treatment administered either prior to or following eccentric contractions (Nosaka et al., 2007; Kim et al., 2019). While the effect of heat on the recovery of muscle force within EIMD has been somewhat elucidated, there is limited information on how this translates to movement mechanics and economy. Moreover, there seems to be potential for heat therapy to improve microvascular function and alterations in muscle oxygen transport and utilization within an EIMD model, given the emerging reports demonstrating enhanced mitochondrial signaling, angiogenic signaling and improved vascular function/adaptation following acute or longer term heat exposure (Ihsan et al., 2014, 2020a; Kim et al., 2020; Brunt et al., 2016a, 2016b).
The purpose of this study was therefore to investigate the influence of repeated heat pre-conditioning on the recovery of muscle torque, microvascular function, movement economy and stride mechanics following EIMD. It is hypothesised that heat pre-conditioning will attenuate the EIMD-induced decline in the aforementioned parameters.
2. Methods
2.1. Participants
Twenty healthy, active males participated in this study, and were divided equally into a control (CON; age = 23.9 ± 1.6 years, stature = 170 ± 8 cm, mass = 64.9 ± 9.3 kg, maximal oxygen uptake = 47.1 ± 5.3 ml·kg-1·min-1) and experimental group receiving repeated heat treatment (HEAT; age = 23.4 ± 1.6 years, stature = 176 ± 8 cm, mass = 69.3 ± 10.7 kg, maximal oxygen uptake = 45.1 ± 5.6 ml·kg-1·min-1). They were informed of the study requirements and a written informed consent was signed prior to the commencement of data collection. Participants were not under any medication, had no history of lower limb musculoskeletal injuries and refrained from physical activity throughout the duration of the study. Moreover, participants abstained from prolonged thermal exposures (e.g., baths, saunas, steam rooms, and tanning devices) for four weeks prior to the preliminary testing and throughout the study duration. This study was approved by the local institutional review board, in accordance with the Declaration of Helsinki.
2.2. Experimental design
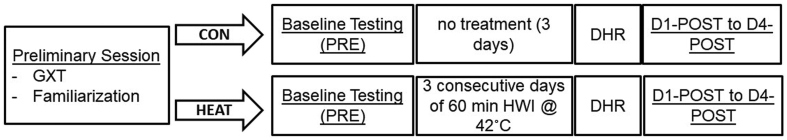
Fig. 1 illustrates the experimental design of this study. On their first visit to the laboratory, participants performed a graded treadmill test to exhaustion, following which, they were familiarized with all experimental procedures. They were then divided equally into CON and HEAT groups, matched for their maximal oxygen uptake (VO2max) obtained during the graded treadmill test. Four to seven days following their familiarization/incremental test session, both CON and HEAT groups reported to the laboratory for baseline testing (PRE) that consisted of the following measures in order; ratings of delayed-onset muscle soreness (DOMS), assessment of microvascular function using near-infrared spectroscopy (NIRS) and knee extensors strength using an isokinetic dynamometer. Participants then performed two 6-min steady-state running (SSR) bouts at velocities corresponding to 90% of their individual first ventilatory threshold (SSR-1), and at 50% of the difference between their VO2max and first ventilatory threshold (SSR-2), respectively. During both SSR bouts, pulmonary gas exchange and stride kinematics were examined. Three days following PRE, the CON group performed a 30-min downhill run (DHR) designed to elicit muscle damage (Chen et al., 2007). Test measures administered during PRE were then repeated for four consecutive days separated by 24 h each (D1-POST to D4-POST) thereafter. The order of events was identical in the HEAT group, except that participants were administered 60 min of hot water immersion for three consecutive days prior to performing the DHR the day after. We chose 3 days as it seemed a practical duration easily combined with heat acclimation protocols. All experimental sessions were undertaken at the same time of the day for each participant. For each experimental session, participants consumed their last meal 3 h before and 300–500 ml of water 2 h prior to their arrival. Meals were documented and participants were told to replicate their meals as best as possible (staples kept consistent, eg., amount of noodles, rice etc.) throughout, with slight variations permitted (eg., grilled or broiled variations in meat preparation). Caffeine containing beverages such as coffee were avoided for 18–24 h prior to their visit.
Fig. 1.
Schematic representation of the experimental design. GXT; graded exercise test, DHR; downhill running, HWI; hot water immersion.
2.2.1. Graded treadmill test
The test was performed on a motor-driven treadmill (HP Cosmos Venus, Traunstein, Germany). The test commenced at 8 km·h-1 with velocity increments of 1 km·h-1 every 2 min until volitional exhaustion ensued (Chen et al., 2007). Treadmill gradient was set at 1% throughout the test. Gas exchange was measured using an automated system (Parvo Medics Trueone 2400, Utah, USA). Prior to each test, the oxygen and carbon dioxide analyzers and ventilometer of the system were calibrated using gases of known concentrations and a 3-L syringe (Series 5530; Hans Rudolph, Inc.), respectively. Participant's VO2max was defined as the highest 30-s mean value recorded before the participant's volitional termination of the test, whilst ventilatory thresholds was determined using the ventilatory equivalents method (Shimizu et al., 1991).
2.2.2. Hot water immersion
HEAT group's participants were immersed in a semi-reclined position up to the sternum in a water bath maintained at 42.0 °C. We chose water immersion as it is more practical, accessible (e.g., bath tubs at home, easily implemented in the field, able to accommodate multiple athletes/patients) and cost-effective compared with other methods of passive heating, and can target multiple muscle groups at the same time (McGorm et al., 2018). Moreover, immersion temperatures of 42 °C aligns well with previous work (McGorm et al., 2018; Rodrigues et al., 2020). Pilot work in our laboratory with four male participants (age = 29.0 ± 5.4 years, stature = 175 ± 3 cm, mass = 71.5 ± 13.6 kg) not included in the main study resulted in approximately 2.5 °C increase in core temperature (37.00 °C ± 0.13 °C–39.43 °C ± 0.86 °C) using this immersion protocol. Core temperature during the pilot trials were determined using ingestible telemetric temperature sensor (VitalSense, Mini Mitter Co., Inc., Bend, Oregon, USA). Fluid ingestion (at room temperature, 24–25 °C) was allowed ad libitum throughout the immersion protocol. During immersion, ratings of thermal comfort, ranging from 1 (comfortable) to 10 (extremely uncomfortable) was obtained from the participants every 5 min. Termination criteria for the immersion included either one of the following: 1) the completion of the 60 min immersion duration, 2) tympanic temperatures >40.0 °C or 3) volitional termination by the participants which was supported by a thermal comfort rating of 10. Immersion was also terminated if the participants complained of substantial dizziness or nausea, or if extensive slurring was observed. An immersion time of 45.1 ± 3.2 min was achieved by our experimental cohort.
2.2.3. Downhill running
The 30-min DHR protocol used in this study was previously described by Chen et al. (2007, 2008). Briefly, participants first performed level running at a velocity corresponding to 70% of their pre-determined VO2max as a 5-min warm-up, and then commenced DHR at 70% VO2max at a treadmill gradient set at −16%. The treadmill velocity was adjusted in the first 5 min to obtain the pre-determined 70% VO2max, following which no changes in velocity was initiated for the remainder of the DHR bout. Participants’ gas exchange was monitored during the warm-up and for the first 10 min of DHR to ensure a relative intensity of 70% VO2max was achieved. After which the mouthpiece and headgear were removed from the participants.
2.2.4. Delayed-onset muscle soreness
Participants performed a step-up and step-down from a 40-cm box using their dominant leg, and indicated their ratings of DOMS (on their anterior thigh region) on a 10-cm line visual analogue scale with “no pain” and “extremely painful” anchored at either end of the scale (Chen et al., 2007, 2008). The DOMS measurements were performed twice on each occasion (average score used for analysis), and obtained before the start of the experimental procedures during each visit (i.e., PRE, D1-POST to D4-POST).
2.2.5. Assessment of Microvascular Function
Microvascular function was assessed by the vascular occlusion test coupled with a NIRS system (McLay et al., 2016a; Ihsan et al., 2020b). This system (Oxymon-MK III, Artinis Medical Systems, Netherlands) provides concentration changes in oxygenated hemoglobin (ΔO2Hb), deoxygenated hemoglobin (ΔHHb) and total hemoglobin (ΔtHb = ΔO2Hb + ΔHHb). Data analysis was performed using Hb difference (Hbdiff = ΔO2Hb - ΔHHb) as it has been shown to provide better signal to noise output (Southern et al., 2014). The NIRS probe unit consisted of 1 detector and 3 laser-emitting diodes, held 4 cm apart by specialized probe holders. The probe unit was affixed to the quadriceps vastus lateralis muscle belly, 10–12 cm from the patella border. The probe area was marked with a surgical marker to ensure accurate probe repositioning on subsequent visits. Prior to probe placement, the area of investigation was assessed for subcutaneous tissue thickness using Harpenden skinfold calipers (British Indicators Ltd, UK). The NIRS probe unit was secured using an adhesive tape and covered a layer of black cloth before being lightly reinforced with an elasticated bandage to prevent artefacts from movement and external light sources. A blood pressure cuff (SC10D, Hokanson, Bellevue, USA) connected to a rapid inflation system (Hokanson E20 Rapid Cuff Inflator and Hokanson AG-101 Air Source, Washington, USA) was attached to the participants' upper thighs (i.e., inguinal region). A 5-min period was allowed for NIRS signals to stabilize, during which participants lay rested in a supine position. Microvascular function was assessed according to McLay and Colleagues (McLay et al., 2016a, 2016b), which involved a 1-min baseline period, 5 min of arterial occlusion (280 mmHg) and 3 min of post-occlusion monitoring. All NIRS data was collected through the manufacturer's software (Oxysoft, Artinis Medical Systems, Netherlands) at 10 Hz and subsequently down-sampled to 1 Hz (i.e., 1-s averages) for further analysis.
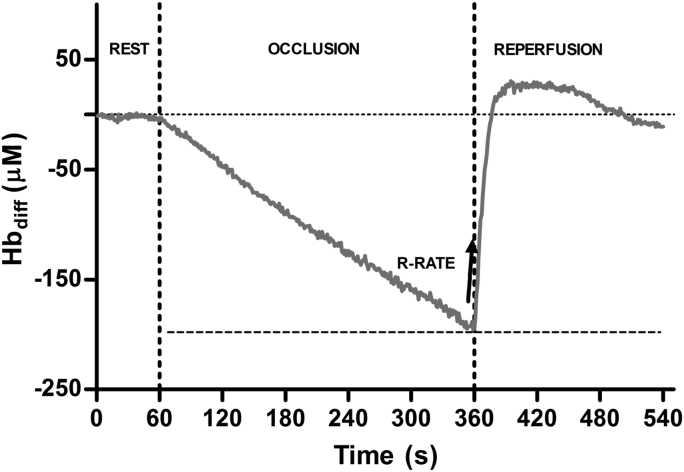
Typical Hbdiff changes during rest, arterial occlusion and reperfusion from a representative participant are presented in Fig. 2. Briefly, supra-systolic cuff inflation resulted in a steady decline in Hbdiff, reaching a nadir prior to cuff release. Upon cuff release, rapid reperfusion ensued, followed by a period where Hbdiff was elevated above baseline, after which a steady decline back to baseline levels was observed. The reperfusion rate (R-RATE) was determined on the initial 10-s window following cuff release, and taken as the index representing changes in microvascular function (McLay et al., 2016a). The R-RATE were determined using a linear function (McLay et al., 2016a): Hbdiff = a × t + b, where a is the slope (μM·s−1), t is the time (s) and b is the y-intercept (μM). This measure has been shown to correlate with ultrasound flow mediated dilation (r = 0.63, p = 0.003), with intraday and interday measurement reliability (coefficient of variation) reported to be between 9 and 14% (McLay et al., 2016a, 2016b).
Fig. 2.
Typical changes in Hbdiff profile in a representative participant during 5 min of arterial occlusion and subsequent recovery. R-RATE; Hbdiff reperfusion rate (μM·s−1) following cuff release.
2.2.6. Strength assessment of the knee extensors
Participants' isometric maximal voluntary contraction (MVC) torque of the dominant knee extensor was assessed at 90°, on a calibrated isokinetic dynamometer (Biodex System 3, New York, USA). Participants were seated upright, with the hip flexion at approximately 90°, with appropriate restraining straps fastened over them. The knee joint was then placed in line with the dynamometer's axis of the rotation, and the leg was secured to the dynamometer arm at the ankle. For all tests, participants were asked to perform five 3-s maximal isometric contractions with a 1-min rest period between contractions (Chen et al., 2007). Each contraction was analyzed for peak torque (plateau over 500 ms), and the average of the best 3 contractions were included for analysis.
2.2.7. Running economy, kinematics and spring mass characteristics during steady-state runs
Participants performed two 6-min SSR bouts 10 min apart, at velocities corresponding to 90% of their individual first ventilatory threshold (i.e., SSR-1), and at 50% of the difference between their VO2max and first ventilatory threshold (i.e., SSR-2), respectively. Treadmill gradient was set at 1%. Gas exchange was monitored throughout both bouts using the aforementioned system. Briefly, participants were instrumented with the one-way mouthpiece and a 2-min period was allowed for the gas exchange variables to stabilize before commencing the first SSR bout. During the 10-min recovery period, participants were relieved of the mouthpiece and assumed seated rest before being re-instrumented for the second SSR, which was also preceded by a 2-min baseline period. The average VO2 in the last minute (i.e., the 6th min) was taken as a measure of running economy.
Throughout the SSR bouts, both sides of the treadmill were instrumented with a sensor system (OptoJump, Microgate, Bolzano, Italy) which allowed for the determination of ground contact (tc) and flight (tf) time. A linear spring-mass model of running was used to investigate the main mechanical integrative parameters characterizing the lower limbs behavior during running. Due to equipment faults, data from CON is limited to n = 8 for all stride kinematic data. Vertical stiffness (Kvert = Fzmax·Δz−1) was calculated as the ratio of peak vertical forces (Fzmax = mg (π/2)·[(tf/tc)+1)]) to the maximal vertical downward displacement of center of mass (Δz = −(Fzmax/m)·(tc2/π2)+g (tc2/8)), which was determined by double integration of vertical acceleration of center of mass over time during ground contact. Leg stiffness (Kleg = Fzmax·ΔL−1) was calculated as the ratio of Fzmax to the maximum leg spring compression (ΔL = Δz + L0−L02−(0.5·running velocity·tc)2], both occurring at mid-stance. Initial leg length (L0, i.e., greater trochanter to ground distance in a standing position) was determined from participant's stature as L0 = 0.53·stature.
2.3. Statistics
Data distribution was assessed using the Shapiro-Wilk test, which demonstrated no deviations from normality in all variables. All variables were analyzed using a two-way ANOVA, where the within participant factor was time (i.e., PRE, D1-POST to D4-POST), and the between participant factor was condition (HEAT vs. CON). Where significant main effects (p ≤ 0.05) were evident, pairwise comparisons using Bonferroni correction were undertaken. Partial eta square (ηp2) was utilized to assess the magnitude of main effects, with ηp2 > 0.01, ηp2 > 0.06 and ηp2 > 0.14 indicating small, moderate and large effects. Smallest worthwhile change (0.2 x SD) for each variable was determined from pooled HEAT and CON groups using data from PRE. All statistical analysis was performed using SPSS version 19 (IBM SPSS, Chicago, IL).
3. Results
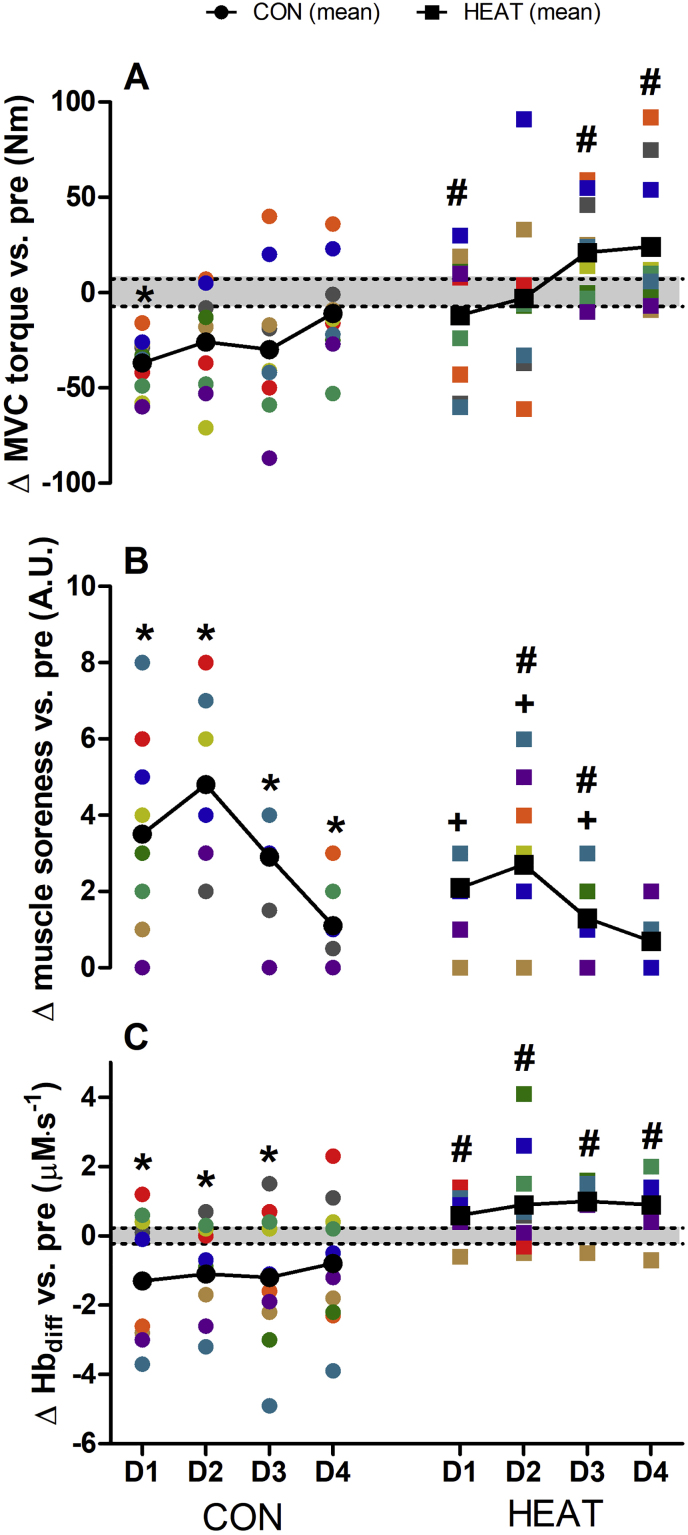
Changes in the MVC torque (Fig. 3A) of the knee extensors showed a significant main effect for time (p = 0.001, ηp2 = 0.229), condition (p = 0.003, ηp2 = 0.393) and interaction (p = 0.025, ηp2 = 0.142). Within CON, MVC torque was significantly depressed at D1-POST (p = 0.002), whilst no significant decrement in MVC torque was observed in HEAT between D1-POST to D4-POST (p = 0.250 to 1.000). Between conditions, MVC torque was significantly different between CON and HEAT at D1-POST (p = 0.037), D3-POST (p = 0.002) and D4-POST (p = 0.022). Changes in DOMS (Fig. 3B) showed significant main effects for time (p < 0.0001, ηp2 = 0.675), condition (p = 0.030, ηp2 = 0.236) and interaction (p = 0.018, ηp2 = 0.151). Compared with PRE, an increase in DOMS was observed within CON, between D1-POST to D4-POST (p < 0.0001 to 0.011), whilst the increase DOMS within HEAT was observed between D1-POST to D3-POST (p = 0.003 to 0.041). Moreover, the increase in DOMS within CON was significantly greater compared with HEAT at D2-POST (p = 0.024) and D3-POST (p = 0.013). Changes in R-RATE (Fig. 3C) demonstrated no changes over time (p = 0.594, ηp2 = 0.032), but demonstrated significant condition (p = 0.004, ηp2 = 0.378) and interaction effects (p = 0.003, ηp2 = 0.238). Pairwise comparisons revealed a significant decrease in R-RATE within CON over D1-POST (p = 0.009) up to D3-POST (p = 0.018), whilst no changes within HEAT were observed. Moreover, changes CON was significantly greater compared with HEAT throughout D1-POST to D4-POST (p = 0.003 to p = 0.017).
Fig. 3.
Changes in MVC torque (panel A), muscle soreness (panel B) and R-RATE (panel C) one to four days D1 to D4) following downhill running. Colored symbols denote individual plots. Black symbols with connecting lines denote the mean response. Shaded portion indicate the smallest worth change (derived from pooled data from CON and HEAT at PRE). ∗significantly different vs. PRE within CON (p < 0.05). +significantly different vs. PRE within HEAT (p < 0.05). #significantly different between CON and HEAT (p < 0.05).
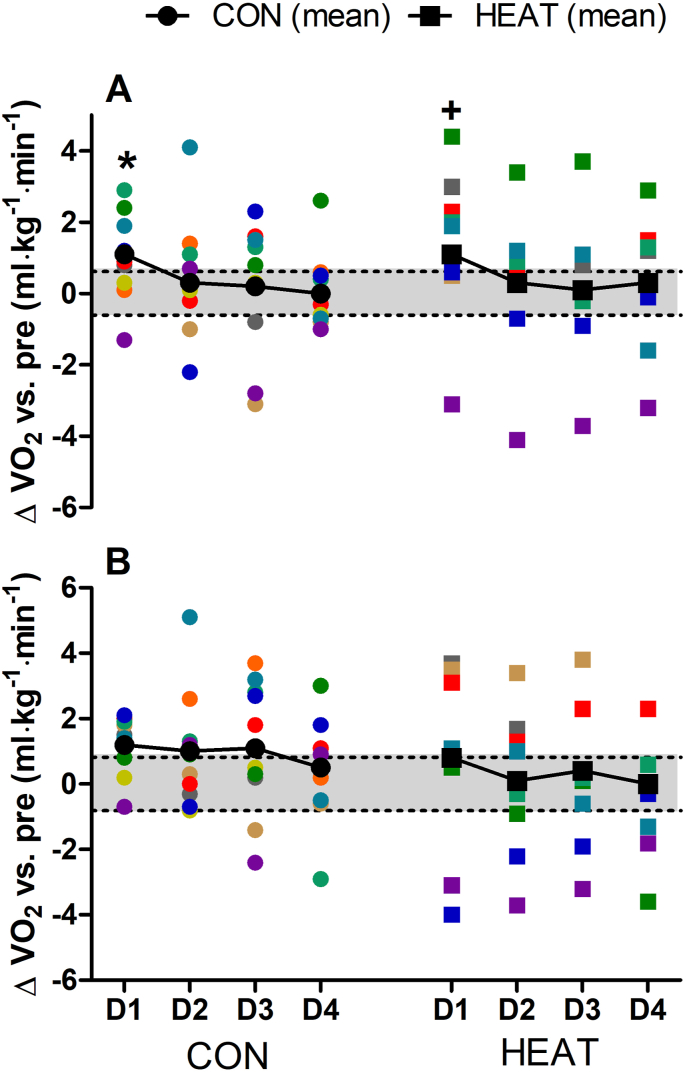
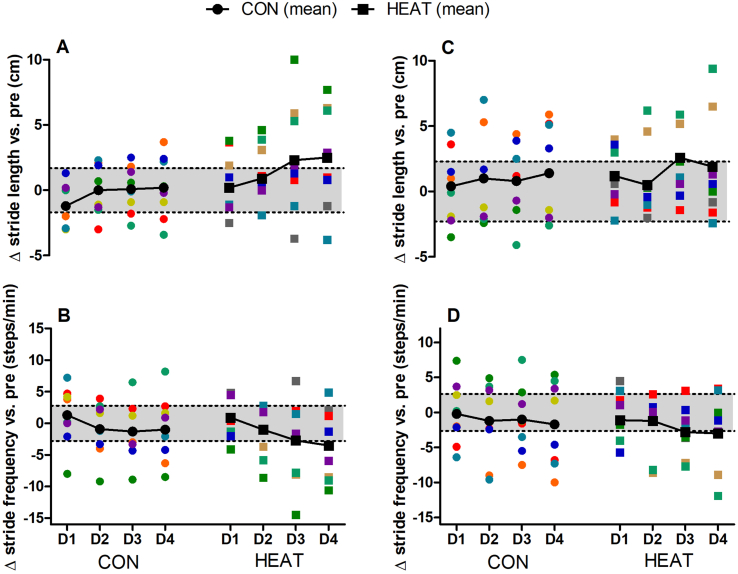
Changes in VO2 during SSR-1 (Fig. 4A) demonstrated significant main effects for time (p = 0.023, ηp2 = 0.144) but not for condition (p = 0.928, ηp2 < 0.0001) or interaction (p = 0.989, ηp2 = 0.004). An increase in VO2 (decreased economy) was observed in both conditions at D1-POST, with similar values compared to PRE at all subsequent time-points (p > 0.05). Changes in VO2 during SSR-2 (Fig. 4B) showed no time (p = 0.190, ηp2 = 0.081), condition (p = 0.396, ηp2 = 0.040) or interaction effects (p = 0.886, ηp2 = 0.016). Significant time effects were observed for stride length (p = 0.035, ηp2 = 0.653) and stride frequency (p = 0.025, ηp2 = 0.696) during SSR-1, with no condition (all p > 0.155) or interaction effects (all p > 0.291). However, pairwise comparisons showed no changes in stride length or frequency across POST-D1 to POST-D4 in CON or HEAT (Fig. 5A and B). No significant main effects were observed for changes in stride length (all p > 0.074) or stride frequency (all p > 0.121) during SSR-2 (Fig. 5C and D). Additional running kinematics data, as well as spring-mass characteristics during SSR-1 and SSR-2 are presented in Table 1, where likewise no significant main effects were observed for any of these variables (all p > 0.103).
Fig. 4.
Changes in VO2 during steady-state running at 90% VT1 (panel A) and 50% between VT1 and VO2max (panel B) one to four days (D1 to D4) following downhill running. Colored symbols denote individual plots. Black symbols with connecting lines denote the mean response. Shaded portion indicate the smallest worth change (derived from pooled data from CON and HEAT at PRE). ∗significantly different vs. PRE within CON (p < 0.05). +significantly different vs. PRE within HEAT (p < 0.05).
Fig. 5.
Changes in stride kinematics during steady-state running at 90% VT1 (panel A & B) and 50% between VT1 and VO2max (panel C & D) one to four days (D1 to D4) following downhill running. CON; n = 8 and HEAT; n = 10 for both velocities. Colored symbols denote individual plots. Black symbols with connecting lines denote the mean response. Shaded portion indicate the smallest worth change (derived from pooled data from CON and HEAT at PRE).
Table 1.
Running mechanics during SSR-1 and SSR-2 in CON and HEAT at baseline and at four consecutive days (D1-POST to D4-POST) following exercise-induced muscle damage.
| Baseline |
D1-POST |
D2-POST | D3-POST |
D4-POST |
Main effects |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | HEAT | CON | HEAT | CON | HEAT | CON | HEAT | CON | HEAT | Time | Condition | Interaction | |
| SSR-1 | |||||||||||||
| tc (ms) | 340 ± 65 | 359 ± 36 | 342 ± 43 | 358 ± 38 | 344 ± 40 | 360 ± 42 | 341 ± 42 | 353 ± 50 | 344 ± 41 | 360 ± 37 | 0.737 | 0.727 | 0.971 |
| tf (ms) | 49 ± 33 | 32 ± 13 | 37 ± 22 | 32 ± 13 | 41 ± 24 | 36 ± 14 | 44 ± 24 | 42 ± 35 | 40 ± 26 | 34 ± 22 | 0.386 | 0.274 | 0.537 |
| Fzmax (kN) | 1.22 ± 0.11 | 1.16 ± 0.20 | 1.18 ± 0.15 | 1.16 ± 0.20 | 1.19 ± 0.15 | 1.18 ± 0.21 | 1.20 ± 0.15 | 1.21 ± 0.30 | 1.19 ± 0.15 | 1.18 ± 0.23 | 0.432 | 0.272 | 0.511 |
| Δz (cm) | 14.6 ± 5.4 | 15.9 ± 3.5 | 14.6 ± 3.8 | 15.8 ± 3.5 | 14.7 ± 3.5 | 16.0 ± 3.8 | 14.4 ± 3.6 | 15.5 ± 4.4 | 14.6 ± 3.6 | 16.2 ± 3.3 | 0.766 | 0.968 | 0.996 |
| ΔL (cm) | 24.5 ± 7.9 | 26.3 ± 4.3 | 24.4 ± 5.1 | 26.1 ± 4.6 | 24.8 ± 4.9 | 26.5 ± 5.1 | 24.3 ± 4.7 | 25.3 ± 6.3 | 24.6 ± 4.8 | 26.3 ± 4.9 | 0.770 | 0.931 | 0.997 |
| Kvert (kN m−1) | 9.8 ± 5.1 | 7.5 ± 1.6 | 8.6 ± 2.3 | 7.6 ± 1.7 | 8.5 ± 2.3 | 7.6 ± 2.0 | 8.8 ± 2.2 | 8.7 ± 4.7 | 8.5 ± 2.3 | 7.7 ± 2.3 | 0.543 | 0.311 | 0.474 |
| Kleg (kN m−1) | 5.7 ± 2.8 | 4.5 ± 0.7 | 5.0 ± 1.1 | 4.5 ± 0.9 | 5.0 ± 1.1 | 4.6 ± 1.0 | 5.1 ± 1.0 | 5.2 ± 2.8 | 5.0 ± 1.0 | 4.6 ± 1.3 | 0.549 | 0.315 | 0.486 |
| SSR-2 | |||||||||||||
| tr (ms) | 299 ± 40 | 299 ± 26 | 292 ± 41 | 298 ± 24 | 294 ± 41 | 295 ± 22 | 294 ± 44 | 296 ± 23 | 293 ± 43 | 280 ± 23 | 0.298 | 0.240 | 0.747 |
| tf (ms) | 64 ± 20 | 62 ± 29 | 72 ± 21 | 65 ± 28 | 72 ± 23 | 69 ± 30 | 72 ± 28 | 70 ± 33 | 74 ± 25 | 70 ± 33 | 0.103 | 0.753 | 0.915 |
| Fzmax (kN) | 1.29 ± 0.16 | 1.28 ± 0.16 | 1.33 ± 0.20 | 1.30 ± 0.19 | 1.33 ± 0.19 | 1.32 ± 0.24 | 1.33 ± 0.20 | 1.32 ± 0.22 | 1.34 ± 0.20 | 1.31 ± 0.21 | 0.156 | 0.759 | 0.902 |
| Δz (cm) | 11.1 ± 3.2 | 11.2 ± 1.8 | 10.5 ± 3.3 | 10.9 ± 1.9 | 10.9 ± 3.2 | 10.7 ± 0.2 | 10.7 ± 3.4 | 10.9 ± 0.2 | 10.8 ± 3.4 | 10.9 ± 0.2 | 0.325 | 0.692 | 0.785 |
| ΔL (cm) | 21.1 ± 5.9 | 22.2 ± 2.9 | 20.3 ± 6.2 | 21.8 ± 2.3 | 20.4 ± 6.0 | 21.5 ± 2.5 | 20.4 ± 6.1 | 21.7 ± 2.9 | 20.4 ± 6.3 | 22.1 ± 2.8 | 0.337 | 0.683 | 0.781 |
| Kvert (kN m−1) | 12.4 ± 3.1 | 11.8 ± 1.8 | 13.4 ± 3.7 | 12.1 ± 2.1 | 13.2 ± 3.4 | 12.5 ± 2.8 | 13.3 ± 4.0 | 12.4 ± 2.5 | 13.4 ± 3.9 | 12.2 ± 2.3 | 0.183 | 0.572 | 0.717 |
| Kleg (kN m−1) | 6.9 ± 3.2 | 5.8 ± 0.7 | 7.5 ± 3.6 | 5.9 ± 0.9 | 7.4 ± 3.7 | 6.2 ± 1.6 | 7.5 ± 4.1 | 6.2 ± 1.4 | 7.5 ± 3.9 | 6.0 ± 1.3 | 0.158 | 0.513 | 0.705 |
SSR-1 and SSR-2 were performed at running velocities corresponding to 90% of the first ventilatory threshold and 50% of the difference between their VO2max and first ventilatory threshold, respectively (CON; n = 8 and HEAT; n = 10 for both velocities). tc, contact time; tf, flight time; Fzmax, peak vertical ground reaction forces; Δz, center of mass vertical displacement; ΔL, maximum leg spring compression; Kvert, vertical stiffness; Kleg, leg stiffness.
4. Discussion
This study investigated the effect of 3 days of repeated heat pre-conditioning on the recovery of muscle torque, microvascular function, as well as running economy and stride kinematics following EIMD. Our main findings indicate that pre-heat treatment decreased the magnitude of knee extensor strength loss, and reduced the sensation of muscle soreness following EIMD. It also attenuated the decline in NIRS-derived microvascular function following EIMD, whilst no beneficial effects were observed in running economy. As such, repeated heat pre-conditioning may be a beneficial strategy to mitigate the decline in muscle strength and soreness, as well as microvascular function following EIMD.
Using an established EIMD model (Chen et al., 2007, 2008), we demonstrate consistent alterations across different outcome variables assessing physical performance, pain and physiological function. Specifically, DHR resulted in a 24% decline in MVC torque, which is comparable to previous studies reporting knee-extensor strength losses of 15–20% using similar DHR protocols (Chen et al., 2007, 2008). Moreover, in line with previous studies, the observed decline in MVC torque occurred in concert with increased DOMS, lasting at least 4 days following DHR. The current study also contributes to emerging data on the effect of EIMD on microvascular function in humans (Larsen et al., 2015, 2019; Caldwell et al., 2016). Within CON, we report a decrease in NIRS-derived index of microvascular function persistent up to 72 h post-DHR, consistent with data from animal models of EIMD showing disruptions within the capillary geometry, and consequently delayed hyperemic response and decreased microvascular oxygen pressures (Kano et al., 2004, 2005). However, it must be mentioned that findings from human models of EIMD are less consistent, with some studies reporting reduced hyperemic response and altered matching of oxygen delivery and utilization (Larsen et al., 2015, 2019), whilst others demonstrating impaired macrovascular but not microvascular reactivity (Caldwell et al., 2016).
We were unable to elucidate how EIMD (and by extension, heat pre-conditioning) might have influenced more functional measures such as whole-body running economy, or mechanical parameters. In the current study, a decrease in running economy (i.e., increased VO2) was observed only at POST-D1 in SSR-1, but not in SSR-2. Likewise, main time effects characterizing an initial decrease and subsequent recovery in stride length (with stride frequency demonstrating opposite trend) was observed for SSR-1 (5A & 5B) but not SSR-2 (Fig. 5C and 5D). However, no further changes in stride kinematics or spring mass model were observed following EIMD. This is in contrast to previous studies demonstrating impaired running economy, along with decreased stride length and increased stride frequency lasting 72 h following EIMD (Chen et al., 2007; Tsatalas et al., 2013). In addition, studies have also reported reduced vertical oscillation of the spring mass model, generally characterized by increased vertical stiffness in concert with an overall decrease in ground reaction forces 0–3 h following prolonged running (Morin et al., 2011; Degache et al., 2013). A possible explanation would be that the extent of muscle damage experienced by our participants may not have been severe enough to result in sustained decrease in running economy, or appreciably alter stride kinematics, given that such mechanical changes (and associated changes in VO2) are adopted to minimize pain during the eccentric phase and overall load experienced by locomotor system (Morin et al., 2011). Additionally, spring mass characteristics in the aforementioned studies were examined 0–3 h following prolonged running (Morin et al., 2011; Degache et al., 2013), which would have been influenced by both EIMD and fatigue. As such, further studies employing more aggressive muscle damage protocols are warranted to understand and profile the mechanical changes following EIMD.
The decline in MVC torque within the HEAT group seemed to be largely attenuated, and demonstrated only a 6% decrease at POST-D1 (vs. 24% in CON). These findings corroborate with the relatively small body of literature in humans that demonstrate a positive effect of heat on the attenuation of muscle damage. Specifically, prior localized heat treatment via microwave diathermy has been shown to enhance the recovery of elbow flexor strength following eccentric contractions (Nosaka et al., 2007). In a more recent study, localized heat treatment lasting 90 min/day applied throughout recovery (4 days) resulted in the faster recovery of quadriceps fatigue following muscle damaging eccentric contractions, although no differences in the recovery of peak knee extensor torque was observed (Kim et al., 2019). The mechanisms underpinning these findings likely involve the expression of HSPs, which have shown to be upregulated following passive heat exposure in skeletal muscles of rodents (Tamura et al., 2014) and humans (Hafen et al., 2018; Ihsan et al., 2020a), and importantly, implicated in a wide array of cytoprotective functions regulating protein homeostasis (Morton et al., 2009). In further support, studies employing transgenic approaches have demonstrated HSP overexpression to reduce the extent of necrosis, attenuate the decrease in muscle torque and facilitate recovery following eccentric contractions or cryolesions (McArdle et al., 2004; Miyabara et al., 2006).
In line with the MVC torque data, repeated heat pre-conditioning reduced the magnitude of muscle soreness. Our findings are similar to recent studies in humans, where heat treatment was shown to limit the development of muscle soreness following muscle damaging eccentric contractions (Nosaka et al., 2007; Kim et al., 2019). It is traditionally accepted that myofiber disruption and ensuing inflammatory response largely account for the development of muscle soreness. By reporting decreased magnitude of strength loss following heat treatment, and by extension lower myofiber disruption, our findings could in this regard account for the reduced magnitude of muscle soreness. Alternatively, there is emerging evidence implicating neurotropic pathways, namely the B2-bradykinin receptor-nerve growth factor pathway, and the COX-2-glial cell line-derived neurotrophic pathway as important mediators of muscle soreness following eccentric exercise (Peake et al., 2017). However, it is somewhat uncertain how heat exposure might mitigate muscle soreness through these pathways. One possibility might be that increases in blood flow and circulation following heat exposure might accelerate the removal of the above agents or other interacting co-factors that sensitize muscle nociceptors.
The current study provides novel data demonstrating an attenuated EIMD-induced decrease in microvascular function following repeated heat pre-conditioning. Although a number of recent studies in humans have demonstrated beneficial effects of passive whole-body or localized heat therapy on vascular function, muscle capillarity or angiogenic regulation (Ihsan et al., 2020a; Kim et al., 2020; Brunt et al., 2016a, 2016b; Hesketh et al., 2019), we are only aware of one other study that has investigated the effect of heat exposure on angiogenic regulation following EIMD (Kim et al., 2019). In this study, localized heat treatment following eccentric contractions resulted in increased vascular endothelial growth factor and angiopoietin 1 mRNA expression, although capillary structure surrounding myofibre was not influenced (Kim et al., 2019). We have extended these findings to show that prior whole-body heat treatment can attenuate the decline in microvascular function following EIMD. The mechanisms underpinning the protective heat effect on microvascular dysfunction in EIMD models may be multi-faceted. For instance, apart from the likely involvement of HSPs in a cytoprotective role within the microvasculature, the increase in HSPs may also serve as precursor to increase nitric oxide bioavailability (Pritchard et al., 2001). The nitric oxide pathway in turn, has been shown to be an important mechanism underlying improved cutaneous microvascular and conduit artery function following regular heat therapy in healthy sedentary humans (Brunt et al., 2016a, 2016b; Hesketh et al., 2019). Alternatively, heat exposure has been shown to increase the vascular endothelial growth factor and angiopoietin 1, which could also account for the attenuated decrease in microvascular function following EIMD (Ihsan et al., 2020a; Kim et al., 2019; Liu and Brooks, 2012). Further research to elucidate the mechanisms underpinning (repeated) heat pre-conditioning and EIMD.
It must be mentioned that HWI protocol used in this study resulted in considerable thermal discomfort, and in some cases, dizziness and nausea. Waist level HWI could perhaps be a reasonable tradeoff between ensuring sufficient increases in body temperatures and improving thermal comfort. Additionally, practitioners may consider to personalize such treatments, and gather relevant data in a controlled setting (eg., immersion time and temperature, core temperature responses, subjective and objective thresholds associated with extreme discomfort etc.), before transitioning to the field.
In conclusion, the present study demonstrates the beneficial effect conferred by 3 days of repeated heat pre-conditioning on the magnitude of knee extensors’ strength loss, the development of DOMS, and the decline in NIRS-derived microvascular function following EIMD. Based on these findings, pre heat treatment might be a beneficial therapeutic strategy to mitigate the decline in muscle function and soreness following EIMD, and hence a promising pre-conditioning tool when embarking on intensified training periods or competition.
Author contribution statement
MI, FT, MS, SAH conceived and designed the research. MS, SAH, HJ, MI collected the data, MS, SAH, HJ, FB performed data analysis. MI and MS wrote the manuscript. SR, FB, FT edited and revised the manuscript. All authors approved the final version of the manuscript.
Funding and conflict of interest
No external funding was received for this study. The authors declare no conflicts of interest, financial or otherwise.
Data availabity statement
The raw data supporting the conclusions of this manuscript will be made available by the authors upon request, without undue reservation to any qualified researcher.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank the participants for their time and effort contributed to the study.
References
- Bernat-Adell M.D., Collado-Boira E.J., Moles-Julio P., Panizo-Gonzalez N., Martinez-Navarro I., Hernando-Fuster B., Hernando-Domingo C. Recovery of inflammation, cardiac, and muscle damage biomarkers after running a marathon. J. Strength Condit Res. 2021;35(3):626–632. doi: 10.1519/JSC.0000000000003167. [DOI] [PubMed] [Google Scholar]
- Brunt V.E., Eymann T.M., Francisco M.A., Howard M.J., Minson C.T. Passive heat therapy improves cutaneous microvascular function in sedentary humans via improved nitric oxide-dependent dilation. J. Appl. Physiol. 2016;121(3):716–723. doi: 10.1152/japplphysiol.00424.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt V.E., Howard M.J., Francisco M.A., Ely B.R., Minson C.T. Passive heat therapy improves endothelial function, arterial stiffness and blood pressure in sedentary humans. J. Physiol. 2016;594(18):5329–5342. doi: 10.1113/JP272453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne C., Twist C., Eston R. Neuromuscular function after exercise-induced muscle damage: theoretical and applied implications. Sports Med. 2004;34(1):49–69. doi: 10.2165/00007256-200434010-00005. [DOI] [PubMed] [Google Scholar]
- Caldwell J.T., Wardlow G.C., Branch P.A., Ramos M., Black C.D., Ade C.J. Effect of exercise-induced muscle damage on vascular function and skeletal muscle microvascular deoxygenation. Phys. Rep. 2016;4(22) doi: 10.14814/phy2.13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.C., Nosaka K., Tu J.-H. Changes in running economy following downhill running. J. Sports Sci. 2007;25(1):55–63. doi: 10.1080/02640410600718228. [DOI] [PubMed] [Google Scholar]
- Chen T.C., Nosaka K., Wu C.C. Effects of a 30-min running performed daily after downhill running on recovery of muscle function and running economy. J. Sci. Med. Sport. 2008;11(3):271–279. doi: 10.1016/j.jsams.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Degache F., Guex K., Fourchet F., Morin J.B., Millet G.P., Tomazin K., Millet G.Y. Changes in running mechanics and spring-mass behaviour induced by a 5-hour hilly running bout. J. Sports Sci. 2013;31(3):299–304. doi: 10.1080/02640414.2012.729136. [DOI] [PubMed] [Google Scholar]
- Hafen P.S., Preece C.N., Sorensen J.R., Hancock C.R., Hyldahl R.D. Repeated exposure to heat stress induces mitochondrial adaptation in human skeletal muscle. J. Appl. Physiol. 2018;125(5):1447–1455. doi: 10.1152/japplphysiol.00383.2018. [DOI] [PubMed] [Google Scholar]
- Hesketh K., Shepherd S.O., Strauss J.A., Low D.A., Cooper R.G., Wagenmakers A.J., Cocks M. Passive heat therapy in sedentary humans increases skeletal muscle capillarisation and eNOS content but not mitochondrial density or GLUT4 content. Am. J. Physiol. Heart Circ. Physiol. 2019;317(1):H114–H123. doi: 10.1152/ajpheart.00816.2018. [DOI] [PubMed] [Google Scholar]
- Ihsan M., Watson G., Abbiss C. PGC-1α mediated muscle aerobic adaptations to exercise, heat and cold exposure. Cell Mol Exerc Physiol. 2014;3(1):e7. [Google Scholar]
- Ihsan M., Deldicque L., Molphy J., Britto F., Cherif A., Racinais S. Skeletal muscle signaling following whole-body and localized heat exposure in humans. Front. Physiol. 2020;11:839. doi: 10.3389/fphys.2020.00839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihsan M., Watson G., Choo H.C., Govus A., Cocking S., Stanley J., Abbiss C.R. Skeletal muscle microvascular adaptations following regular cold water immersion. Int. J. Sports Med. 2020;41(2):98–105. doi: 10.1055/a-1044-2397. [DOI] [PubMed] [Google Scholar]
- Kano Y., Sampei K., Matsudo H. Time course of capillary structure changes in rat skeletal muscle following strenuous eccentric exercise. Acta Physiol. Scand. 2004;180(3):291–299. doi: 10.1111/j.0001-6772.2003.01250.x. [DOI] [PubMed] [Google Scholar]
- Kano Y., Padilla D.J., Behnke B.J., Hageman K.S., Musch T.I., Poole D.C. Effects of eccentric exercise on microcirculation and microvascular oxygen pressures in rat spinotrapezius muscle. J. Appl. Physiol. 2005;99(4):1516–1522. doi: 10.1152/japplphysiol.00069.2005. [DOI] [PubMed] [Google Scholar]
- Kim K., Kuang S., Song Q., Gavin T., Roseguini B. Impact of heat therapy on recovery following eccentric exercise in humans. J. Appl. Physiol. 2019;126(4):965–976. doi: 10.1152/japplphysiol.00910.2018. [DOI] [PubMed] [Google Scholar]
- Kim K., Reid B.A., Casey C.A., Bender B.E., Ro B., Song Q., Trewin A.J., Petersen A.C., Kuang S., Gavin T.P., Roseguini B.T. Effects of repeated local heat therapy on skeletal muscle structure and function in humans. J. Appl. Physiol. 2020;128(3):483–492. doi: 10.1152/japplphysiol.00701.2019. [DOI] [PubMed] [Google Scholar]
- Larsen R.G., Hirata R.P., Madzak A., Frokjaer J.B., Graven-Nielsen T. Eccentric exercise slows in vivo microvascular reactivity during brief contractions in human skeletal muscle. J. Appl. Physiol. 2015;119(11):1272–1281. doi: 10.1152/japplphysiol.00563.2015. [DOI] [PubMed] [Google Scholar]
- Larsen R.G., Thomsen J.M., Hirata R.P., Steffensen R., Poulsen E.R., Frokjaer J.B., Graven-Nielsen T. Impaired microvascular reactivity after eccentric muscle contractions is not restored by acute ingestion of antioxidants or dietary nitrate. Phys. Rep. 2019;7(13) doi: 10.14814/phy2.14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.-T., Brooks G.A. Mild heat stress induces mitochondrial biogenesis in C2C12 myotubes. J. Appl. Physiol. 2012;112(3):354–361. doi: 10.1152/japplphysiol.00989.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle A., Dillmann W.H., Mestril R., Faulkner J.A., Jackson M.J. Overexpression of HSP70 in mouse skeletal muscle protects against muscle damage and age-related muscle dysfunction. Faseb. J. 2004;18(2):355–357. doi: 10.1096/fj.03-0395fje. [DOI] [PubMed] [Google Scholar]
- McGorm H., Roberts L.A., Coombes J.S., Peake J.M. Turning up the Heat: an evaluation of the evidence for heating to promote exercise recovery, muscle rehabilitation and adaptation. Sports Med. 2018;48(6):1131–1328. doi: 10.1007/s40279-018-0876-6. [DOI] [PubMed] [Google Scholar]
- McLay K.M., Fontana F.Y., Nederveen J.P., Guida F.F., Paterson D.H., Pogliaghi S., Murias J.M. Vascular responsiveness determined by near-infrared spectroscopy measures of oxygen saturation. Exp. Physiol. 2016;101(1):34–40. doi: 10.1113/EP085406. [DOI] [PubMed] [Google Scholar]
- McLay K.M., Nederveen J.P., Pogliaghi S., Paterson D.H., Murias J.M. Repeatability of vascular responsiveness measures derived from near-infrared spectroscopy. Phys. Rep. 2016;4(9) doi: 10.14814/phy2.12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyabara E.H., Martin J.L., Griffin T.M., Moriscot A.S., Mestril R. Overexpression of inducible 70-kDa heat shock protein in mouse attenuates skeletal muscle damage induced by cryolesioning. Am. J. Physiol. Cell Physiol. 2006;290(4):C1128–C1138. doi: 10.1152/ajpcell.00399.2005. [DOI] [PubMed] [Google Scholar]
- Morin J., Tomazin K., Edouard P., Millet G. Changes in running mechanics and spring–mass behavior induced by a mountain ultra-marathon race. J. Biomech. 2011;44(6):1104–1107. doi: 10.1016/j.jbiomech.2011.01.028. [DOI] [PubMed] [Google Scholar]
- Morton J.P., Kayani A.C., McArdle A., Drust B. The exercise-induced stress response of skeletal muscle, with specific emphasis on humans. Sports Med. 2009;39(8):643–662. doi: 10.2165/00007256-200939080-00003. [DOI] [PubMed] [Google Scholar]
- Nosaka K., Muthalib M., Lavender A., Laursen P.B. Attenuation of muscle damage by preconditioning with muscle hyperthermia 1-day prior to eccentric exercise. Eur. J. Appl. Physiol. 2007;99(2):183–192. doi: 10.1007/s00421-006-0331-5. [DOI] [PubMed] [Google Scholar]
- Peake J.M., Neubauer O., Della Gatta P.A., Nosaka K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. 2017;122(3):559–570. doi: 10.1152/japplphysiol.00971.2016. [DOI] [PubMed] [Google Scholar]
- Pritchard K.A., Jr., Ackerman A.W., Gross E.R., Stepp D.W., Shi Y., Fontana J.T., Baker J.E., Sessa W.C. Heat shock protein 90 mediates the balance of nitric oxide and superoxide anion from endothelial nitric-oxide synthase. J. Biol. Chem. 2001;276(21):17621–17624. doi: 10.1074/jbc.C100084200. [DOI] [PubMed] [Google Scholar]
- Rodrigues P., Trajano G.S., Wharton L., Minett G.M. Muscle temperature kinetics and thermoregulatory responses to 42 degrees C hot-water immersion in healthy males and females. Eur. J. Appl. Physiol. 2020;120(12):2611–2624. doi: 10.1007/s00421-020-04482-7. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Myers J., Buchanan N., Walsh D., Kraemer M., McAuley P., Froelicher V.F. The ventilatory threshold: method, protocol, and evaluator agreement. Am. Heart J. 1991;122(2):509–516. doi: 10.1016/0002-8703(91)91009-c. [DOI] [PubMed] [Google Scholar]
- Silva J., Rumpf M., Hertzog M., Castagna C., Farooq A., Girard O., Hader K. Acute and residual soccer match-related fatigue: a systematic review and meta-analysis. Sports Med. 2018;48(3):539–583. doi: 10.1007/s40279-017-0798-8. [DOI] [PubMed] [Google Scholar]
- Southern W.M., Ryan T.E., Reynolds M.A., McCully K. Reproducibility of near-infrared spectroscopy measurements of oxidative function and postexercise recovery kinetics in the medial gastrocnemius muscle. Appl. Physiol. Nutr. Metabol. 2014;39(5):521–529. doi: 10.1139/apnm-2013-0347. [DOI] [PubMed] [Google Scholar]
- Tamura Y., Matsunaga Y., Masuda H., Takahashi Y., Terada S., Hoshino D., Hatta H. Post-exercise whole-body heat stress additively enhances endurance training-induced mitochondrial adaptations in mouse skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;307(7):R931–R943. doi: 10.1152/ajpregu.00525.2013. [DOI] [PubMed] [Google Scholar]
- Tavares F., Smith T.B., Driller M. Fatigue and recovery in rugby: a review. Sports Med. 2017;47(8):1515–1530. doi: 10.1007/s40279-017-0679-1. [DOI] [PubMed] [Google Scholar]
- Tsatalas T., Giakas G., Spyropoulos G., Sideris V., Lazaridis S., Kotzamanidis C., Koutedakis Y. The effects of eccentric exercise-induced muscle damage on running kinematics at different speeds. J. Sports Sci. 2013;31(3):288–298. doi: 10.1080/02640414.2012.729135. [DOI] [PubMed] [Google Scholar]
- Vernillo G., Brighenti A., Limonta E., Trabucchi P., Malatesta D., Millet G.P., Schena F. Effects of ultratrail running on skeletal-muscle oxygenation dynamics. Int. J. Sports Physiol. Perform. 2017;12(4):496–504. doi: 10.1123/ijspp.2015-0745. [DOI] [PubMed] [Google Scholar]