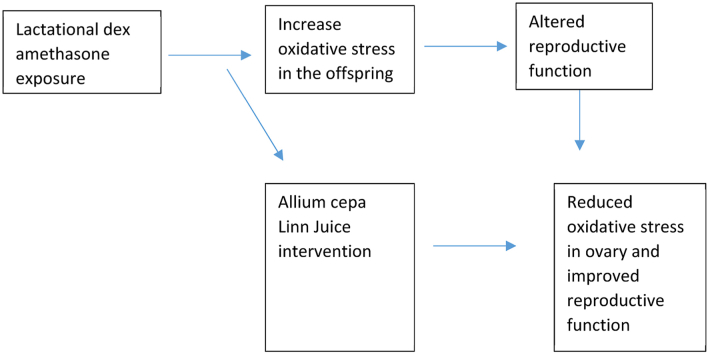
Abstract
Maternal treatment with dexamethasone induces oxidative stress in the reproductive structures of offspring. Consumption of Allium cepa Linn improves antioxidant status. This study was designed to evaluate the protective role of Allium cepa Linn juice on maternal dexamethasone induced alterations in reproductive functions of the female offspring of Wistar rats.
Twenty lactating dams (180–200 g) were randomly assigned into four groups (n = 5) on the day of parturition and treated as follows during lactation for 21 days: Control (5 ml/kg BW distilled water); Dexamethasone (60 μg/kg BW); Allium cepa (5 ml/kg BW); Dexamethasone + Allium cepa (60 μg/kg BW + 5 ml/kg BW). The female offspring were separated at birth. Days of vaginal opening and first oestrus cycle, length and frequency of estrous cycle as well as serum hormonal profiles were assessed as measure of reproductive functions. Ovarian superoxide dismutase (SOD) activity, catalase activity and malondialdehyde (MDA) level were measured as indices of oxidative stress.
Oestrous cycle length, frequencies of diestrus as well as the Ovarian MDA were significantly increased (p < 0.05) in dexamethasone (DEX) group relative to control group. Serum 17β-oestradiol and corticosterone level in addition to SOD and catalase activities were significantly reduced (p < 0.05) in DEX group relative to control. Co-administration of Dex with Allium cepa Linn juice reduced the oestrous length, frequency of diestrous as well as ovarian MDA. There was also a significant increase in serum 17β-oestradiol, ovarian SOD and catalase activity.
The results suggest that Allium cepa could protect against alterations in reproductive functions of offspring induced by maternal treatment with dexamethasone during lactation in Wistar rats. The flavonoid constituent of onion may also help in reducing oxidative stress in the offspring.
Keywords: Lactation, Dexamethasone, Allium cepa, Antioxidants, Reproductive dysfunctions
Graphical abstract
Highlights
-
•
Earlier reports have suggested that maternal exposure to Dexamethasone may affect reproductive function of offspring.
-
•
In this study, we assessed protective role of Allium cepa L on maternal dexamethasone induced reproductive toxicity in female offspring of Wistar rats.
-
•
We observed increase oxidative stress and altered female reproductive function following maternal dexamethasone treatment during lactation.
-
•
The alteration was reduced by Allium cepa L co-administration.
1. Introduction
Reports from epidemiological and clinical studies have shown that adverse early life events affect health trajectories in later life (Barker, 1995; Drake et al., 2005, 2007, 2007). In addition, many experimental studies in animal models have confirmed the fact that diseases in adult life can be induced by manipulating the environment around the foetus or the neonate (Bartol et al., 2009; Miller et al., 2013). Indeed, the evidence that the intrauterine ‘nature’ and postnatal ‘nurture’ program offsprings' physiology for adult diseases is overwhelming (Catalani et al., 2011; Cameron, 2011; Jeje et al., 2016). This programming occurs when the normal pattern of foetal/neonatal development is disrupted by an abnormal signal (insult) at a critical period during gestation or lactation causing a permanent alteration in the structure and function of developing tissues with consequent development of chronic health problems during postnatal life (Myatt, 2006).
There is evidence that during lactation, many factors such as homeostatic genes, transcription factors, growth factors, hormones, nutrients, maternal stress, infections, neonatal handling, synthetic glucocorticoids exposure, and breast milk microbiome could regulate the development of mammalian systems and, therefore, influence later pathophysiology (Bakker et al., 2001; Welberg and Seckl, 2001; Nilsson et al., 2002; Gohir et al., 2015; Perez-Munoz et al., 2017). A neonate can be exposed directly to synthetic glucocorticoids if the neonate or the mother is treated for medical conditions requiring the use of glucocorticoids such as dexamethasone as anti-inflammatory/immunosuppressant agents (WHO, 2003). Neonatal and maternal medical conditions that could necessitate glucocorticoid usage include asthma, colitis, croup, adrenal hormone deficiencies, allergies, anaphylaxis, bacterial meningitis, cancers, palliation of lymphoma, ulcerative colitis, thrombocytopenic purpura, multiple myeloma, and many other conditions (Grunwald and Rosner, 1984; Geelhoed et al., 1996; Ausejo et al., 1999; De Gans and van de Beek, 2002; WHO, 2003; Jeerakompassawat and Suprasert, 2017). Another possible exposure to exogenous glucocorticoids could be via maternal cosmetic abuse for weight gain and skin whitening (De Wasch et al., 2001; Amar et al., 2013).
Studies have shown that prenatal or lactational glucocorticoid excess, either from endogenous overproduction during maternal stress or through exogenous administration to the mother or neonate, could cause metabolic imbalances, lifelong hypertension, hyperglycaemia, behavioural abnormalities, reproductive dysfunction and infertility in offspring (Cameron, 2011; Jeje et al., 2016).
Glucocorticoids affect gonadal function at multiple levels in HPG axis by decreasing the synthesis and release of GnRH in the hypothalamus, inhibiting the synthesis and release of LH and FSH from the pituitary and modulating steroidogenesis and/or gametogenesis directly in the testis/ovary (Whirledge and Cidowski, 2010). Specifically, increased level of cortisol reduces oestradiol production by affecting the granulosa cell functions within the follicle, which results in deterioration in oocyte quality (Prasad et al., 2016).
Excess glucocorticoids exposure has been shown to increase the production of reactive oxygen species and reduce mitochondrial activities leading to oxidative stress (Tang et al., 2009). Experimental studies in the ovaries have confirmed that oxidative stress triggers apoptosis in most germ cells within the ovary and even in ovulated oocytes (Pandey et al., 2010). Both mitochondria as well as death-receptor pathways are involved in oocyte apoptosis (Tiwari et al., 2015, 2016, 2016; Prasad et al., 2016). Oxidative stress-induced mitochondria-mediated pathway plays a major role in the elimination of most germ cells from the ovary (Tiwari et al., 2016). Oxidative stress in the follicular fluid deteriorates oocyte quality and reduces reproductive outcome (Ebbesen et al., 2009). Indeed, oxidative stress is one of the major factors that have a direct negative impact on oocyte quality and limits female reproductive outcome in several mammalian species including human (Prasad et al., 2016). Hence there is a clear link between excessive production of glucocorticoids with oxidative stress and reproductive dysfunction in the female. Excess of cortisol (in humans but corticosterone in rodents) induces granulosa cell apoptosis and affects 17β-oestradiol biosynthesis in the ovary (Prasad et al., 2016). Reduced level of oestradiol in the ovary impairs growth and development of follicles and deteriorates oocyte quality by inducing apoptosis (Prasad et al., 2016). This could be one of the factors responsible for the increase in female related infertility and failure in assisted reproductive procedures, which have been overlooked (Demyttenaere et al., 1992; Kala et al., 2016).
Studies from our laboratory have shown that maternal dexamethasone treatment during gestation increased oxidative stress in the reproductive structure of the male offspring (Jeje et al., 2017). To minimize oxidative stress, antioxidants are useful since they scavenge free radicals and reduce reactive oxygen species level in the body (Agarwal et al., 2012). Available evidence confirms that antioxidant supplementation is beneficial to overcome deleterious effects of glucocorticoid-induced oxidative stress on oocytes (Lian et al., 2013). Supplementation of daily food practices with different antioxidant rich foods such as fresh green leafy vegetables, legumes, and other plant products may provide support to compensate oxidative stress (Asservatham et al., 2013), especially in pregnant and lactating women. One of such antioxidant rich and widely consumed food is Allium cepa, known commonly as onion (Slimestad et al., 2007; Izawa et al., 2008). It is very rich in flavonoids compound well known for its potent antioxidant activity (Maroon, 2009).
Allium cepa has been used in traditional medicine for decades and is prominent for its reproductive health boosting properties (Griffiths et al., 2002). It also possess antithrombotic, hypolipidemic, antihypertensive, antibiotic, antidiabetic, antiatherogenic and anti-cancer properties (Augusti, 1996; Lee et al., 2013, 2017, 2017; Ndoye-Foe et al., 2016). Allium cepa bulb is a very rich source of dietary quercetin; a strong antioxidant flavonoid with the ability to chelate metals and scavenge free radicals, which in turn inhibits lipid peroxidation (Griffiths et al., 2002; Slimestad et al., 2007; Izawa et al., 2008). Recent studies reported that oral administration of fresh Allium cepa juice significantly improves serum testosterone levels, protects against testicular damage, and restores normal sexual behaviour in sexually dysfunctional male rats (Ola-Mudathir et al., 2008; Khaki et al., 2009; Allouh et al., 2013; Malviya et al., 2013). In male rats exposed to the toxic effects of aluminum, Ige and Akhigbe (2012) reported that Allium cepa bulb extract antagonizes the toxic effects of aluminium and improves the antioxidant status and sperm quality of male rat.
Therefore, this study was designed to evaluate effects of maternal administration of dexamethasone and Allium cepa bulb juice during lactation on reproductive functions in the female offspring of Wistar rats.
2. Materials and methods
2.1. Experimental animals
Adult male and nulliparous female Wistar rats weighing 200–230 g and 180–200 g respectively were used for the study. The rats were housed in well aerated plastic cages and allowed access to rodent's pelletized feed (Ladokun Feed Mill, Ibadan. Feed Composition: 26.5% protein, 40% carbohydrates, 29% Fat and 4.5% crude fibre) and water ad libitum. All animals were acclimatized to the environmental conditions of the laboratory for two weeks before the commencement of the study. All animal experiments were conducted in accordance with the International Ethical Norms on Animal Care and Use as contained in NIH publication/85–23, revised in 1985. The study was approved by Ethical committee on the use of laboratory animals, Department of Physiology, Cross River University of Technology, Calabar, Nigeria.
2.2. Preparation of dexamethasone
Dexamethasone-BP (DEX) tablets (Xasthen®, Jiangsu Penyao Pharmaceuticals Ltd, China) was suspended in distilled water and administered at an oral dose of 60 μg/kg body weight daily.
2.3. Collection and preparation of Allium cepa bulb juice
Allium cepa bulb juice was prepared according to the method described by Ola-Mudathir et al. (2008). The Kano Red Creole variety of fresh Allium cepa bulbs was purchased from Bodija market in Ibadan. It was identified at the herbarium of Botany Department, University of Ibadan and National Institute of Horticultural Studies (NIHORT), Ibadan. The Kano Red Creole species was selected as studies have reported its high antioxidant potency (Denton and Ojeifo, 1990). The fresh Allium cepa bulbs were washed, cut into small pieces and homogenized in a blender. The resultant slurry was squeezed and filtered through a fine cloth and the filtrate was used. The Allium cepa bulb extract was administered to the rats with the aid of a flexible oral gavage tube throughout the course of the study. Fresh juice was prepared daily in the morning.
2.4. Determination of oestrous cycle pattern
The determination of oestrous cycle was done using the Marcondes' technique (Marcondes et al., 2002) Oestrous pattern was studied by determining the oestrous phase of each animal every morning between 7:00 am and 8:00 am throughout the study. Vaginal content was collected with a Pasteur pipette containing about 0.1 mL of normal saline (0.9% NaCl) by gently inserting the tip of the pipette into the rat's vagina. The pipette was pressed to release the fluid content 2 or 3 times in order to make a vaginal lavage which contained some of the vaginal cells of the rat. The fluid and its content were thereafter siphoned with pipette and the content was placed on a glass slide. A new and clean glass slide was used for each animal. The slide was viewed using the ×10 and ×40 magnification objective lens of the microscope (Olympus, Japan). The cell types and the proportion among them were used to determine the oestrous cycle phase of the rat.
2.5. Co-habitation and confirmation of mating
Female rats were paired with male rats at ratio 2:1 (female: male) during the proestrus phase of the female rats' cycle. On the morning after pairing, vaginal lavage was collected with Pasteur pipette filled with about 0.1 mL of normal saline (0.9% NaCl) by gently inserting the tip of the pipette into the rat's vagina. The withdrawn vaginal content was dropped on a glass slide and the smear was spread out evenly. The glass slide was examined using the ×40 objective lens of the light microscope (Olympus, Japan) to determine the presence of spermatozoa. Mating was confirmed by the presence of spermatozoa in the vaginal smear.
2.6. Parturition/pup morphometry
Confirmed pregnant dams were transferred into nursing cages. Pregnant dams were allowed to litter naturally, and the day of parturition was designated as Post-Natal Day (PND) 1. The litter size was standardized to six pups per litter. Birth weight was measured within 24 h of postnatal life. They were weighed on a sensitive electronic scale (Lisay, China).
2.7. Grouping of lactating dams/pups
Twenty (20) lactating dams were divided into four groups (I-IV) of five rats each on PND1 and treated as follows:
Group I was administered 5 ml/kg body weight distilled water orally.
Group II received 5 ml/kg body weight Allium cepa bulb juice orally (Lee et al., 2013).
Group III received 60 μg/kg body weight dexamethasone orally (Amar et al., 2013).
Group IV received 60 μg/kg body weight dexamethasone and 5 ml/kg body weight Allium cepa bulb juice orally.
Administration was done in each group in the morning within 8–10am daily, and pups were fed by dams ad libitum.
Administration was done using an orogastric gavage method with an oral cannula. Administration lasted for 21 days (lactation period) after which the female pups were weaned and transferred into separate cages where they were allowed to mature.
2.8. Post-weaning handling
The female offspring were weaned on PND 28 (Post-Natal Week [PNW] 4 and pooled into groups depending on maternal lactational treatment. The weaned offspring were fed standard rat chow (Ladokun Feed Limited, Ibadan) and allowed access to water ad libitum. No administration was done on the randomly selected offspring. Body weight was recorded on post-natal weeks 4, 8, and 12.
2.8.1. Determination of vaginal opening and first oestrus
Sexual maturity of the female rodents was determined by vaginal opening. This heralds the attainment of puberty and sexual maturity. The first oestrus is an indication of the ability of the animal to conceive. Beginning from PND 40, the female rats were checked for vaginal opening and commencement of oestrous cycle was monitored. Date of vaginal opening (VOD), first oestrus, oestrous pattern (frequency, length), were monitored for 21 days.
2.9. Blood collection and serum preparation
On the 14th week of postnatal life during proestrus, the rats were euthanized under sodium thiopental anaesthesia (50 mg/kg, i.p.). They were surgically opened at the linea alba of the anterior abdominal wall to the thoracic cavity to expose the heart and other organs. Using a 5 mL syringe and needle, blood was collected into plain serum bottles through cardiac puncture. The blood was allowed to clot for at least 60 min after which it was centrifuged at 3000 rpm for 10 min. The serum portion which is the supernatant was decanted from the centrifuged blood and stored at −20 °C for hormonal assay. Serum levels of Follicle Stimulating Hormones (FSH), Luteinizing Hormones (LH), oestradiol, and corticosterone were assayed in the female offspring. The ovaries and uteri were harvested and freed of adherent tissues. All harvested organs were fixed in 10% formalin for histological examination.
2.9.1. Serum hormonal concentration level by enzyme-linked immunosorbent assay (ELISA) method
The serum corticosterone, FSH, LH and estradiol levels were determined per animal using the ELISA kit ((Fortress Diagnostics, UK) according to the protocol in respective manufacturer's manual.
2.10. Determination of ovarian redox status
One of the harvested ovaries was homogenized in phosphate buffer (pH 7.4) and the homogenate was centrifuged at 10,000 g × 15 min at 4 °C. The supernatant was collected for the assay of total protein, malondialdehyde (for lipid peroxidation), superoxide dismutase, catalase, and glutathione peroxidase concentration in the ovaries. All biochemical assays were done within 48 h of sample collection.
2.10.1. Assessment of ovarian lipid peroxidation
Ovarian lipid peroxidation was determined by measuring the Thiobarbituric Acid Reactive Substances (TBARS) produced during lipid peroxidation (Ohkawa et al., 1979). This method is based on the reaction between thiobarbituric acid (TBA) and malondialdehyde (MDA), an end product of lipid peroxide during peroxidation.
2.10.2. Assessment of superoxide dismutase (SOD) activity
The level of SOD activity was determined by the method of Misra and Fridovich (1972). The ability of SOD to inhibit the autoxidation of epinephrine at pH 10.2 makes this reaction a basis for a simple assay for this dismutase. Superoxide radical generated by the xanthine oxidase reaction caused the oxidation of epinephrine to adrenochrome and the yield of adrenochrome produced per superoxide introduced increased with increasing pH and also increased with increasing concentration of epinephrine.
2.10.3. Assessment of catalase activity
Catalase activity was determined according to the method of Sinha (1972). This method is based on the fact that dichromate in acetic acid is reduced to chromic acetate when heated in the presence of H2O2, with the formation of perchromic acid as an unstable intermediate. The chromic acetate then produced is measured colorimetrically at 570–610 nm. Since dichromate has no absorbency in this region, the presence of the compound in the assay mixture does not interfere at all with the colorimetric determination of chromic acetate. The catalase preparation is allowed to split H2O2 for different periods of time. The reaction is stopped at a particular time by the addition of dichromate/acetic acid mixture and the remaining H2O2 is determined by measuring chromic acetate colorimetrically after heating the reaction mixture.
2.10.4. Assessment of glutathione peroxidase activity
Glutathione peroxidase (GPx) catalyzes the redox reaction between reduced glutathione (GSH) and hydrogen peroxide (H2O2). The amount of GSH utilized is estimated by measuring it in the assay mixture before and after the enzyme activity (Rotruck et al., 1973). GSH reacts with Ellman's reagent (5, 5′-dithiobis-2-nitrobenzoic acid or DTNB) to give a yellow colour which was then measured at 412 nm.
2.10.5. Histological assessment of the organs
Histological assessments were conducted following heamatoxylin and eosin staining techniques.
2.11. Statistical analysis
Data are expressed as mean ± Standard Error of Mean (SEM). Differences between mean values were evaluated by analysis of variance (ANOVA), followed by Tukey's post-hoc test for pairwise comparisons. Values of P < 0.05 were considered statistically significant. GraphPad Prism 7.0 software (GraphPad Inc, USA) was used for the statistical analysis.
3. Results
3.1. Effects of maternal administration of dexamethasone and Allium cepa bulb juice during lactation on oestrous pattern of the female offspring of Wistar rats
Diestrus frequency significantly increased (p < 0.05) in the female offspring of dexamethasone treated group when compared with control group. There was also a significant increase (p < 0.05) in the diestrus phase frequency in female offspring of dexamethasone treated group when compared with Dex + Allium cepa treated group (Table 1). There was no significant difference in the proestrus phase frequency across all groups (Table 1). There was no significant difference in the oestrus phase frequency across all groups (Table 1). Metestrus frequency significantly decreased (p < 0.05) in the female offspring of dexamethasone treated group when compared with control group. The metestrus phase frequency was significantly decreased (p < 0.05) in the female offspring of dexamethasone treated group when compared to Dex + Allium cepa treated group (Table 1).
Table 1.
Percentage frequency of Estrous Cycles phases in the female offspring of dexamethasone (DEX) and Allium cepa (A.cepa) treated lactating Wistar rats.
| Diestrous frequency (%) | Proestrous (%) | Estrous (%) | Metestrous (%) | |
|---|---|---|---|---|
| Control | 34.24 ± 1.76 | 20.98 ± 1.15 | 22.86 ± 0.94 | 21.92 ± 1.15 |
| A.cepa | 35.17 ± 1.89 | 20.04 ± 0.94 | 23.81 ± 1.50 | 21.93 ± 1.89 |
| Dex | 52.38 ± 4.26 a,b | 15.26 ± 1.79 | 19.06 ± 21.3 | 14.30 ± 1.52a,b |
| Dex + A.cepa | 38.16 ± 4.65c | 19.01 ± 2.10 | 21.91 ± 2.42 | 20.98 ± 1.15 |
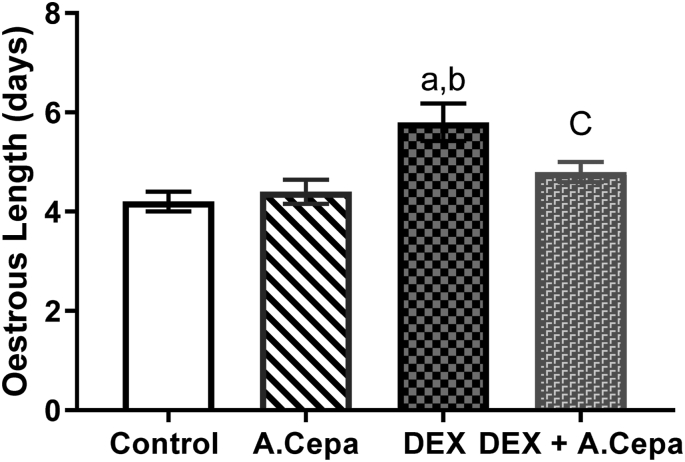
Oestrous cycle length significantly increased (p < 0.05) in the female offspring of dexamethasone treated group when compared with control group. The oestrous cycle length was significantly increased (p < 0.05) in the female offspring of dexamethasone treated group when compared with Dex + Allium cepa treated group (Fig. 1).
Fig. 1.
Oestrous cycle length in the female offspring of dexamethasone (DEX) and Allium cepa (A.cepa) treated lactating Wistar rats. Columns represent mean ± SEM. n = 5. ap<0.05 when compared with the control group. bp < 0.05 when compared with the Allium cepa treated group. cp < 0.05 when compared with the dexamethasone treated group. Analysis was based on one-way analysis of variance (ANOVA) and Turkey post hoc test.
Data represent mean ± SEM. n = 5. ap<0.05 when compared with the control group. bp < 0.05 when compared with the Allium cepa treated group. cp < 0.05 when compared with the dexamethasone treated group. Analysis was based on one-way analysis of variance (ANOVA) and Turkey post hoc test.
3.2. Effect of maternal administration of dexamethasone and Allium cepa during lactation on day of vaginal opening (DVO) and day of first oestrus (DFO)
There was no significant difference in the day of vaginal opening across all groups (Table 2). In addition, there was no significant difference in the day of first oestrus across all groups (Table 2).
Table 2.
Days of Vaginal opening, First estrous cycle and Weight at vaginal opening in the female offspring of dexamethasone (DEX) and Allium cepa (A.cepa) treated lactating Wistar rats.
| Days of Vaginal Opening (Post natal Days) | Day of First Estrous cycle (Postnatal Days) | Weight at Vaginal Opening (g) | |
|---|---|---|---|
| Control | 53.20 ± 1.12 | 54.40 ± 1.83 | 101.8 ± 1.39 |
| A.cepa | 51.20 ± 1.36 | 52.40 ± 1.17 | 86.40 ± 2.42 a |
| Dex | 54.00 ± 1.30 | 54.80 ± 1.32 | 88.20 ± 4.40 |
| Dex + A.cepa | 52.20 ± 1.59 | 53.40 ± 1.72 | 93.20 ± 4.89 |
Weight at vaginal opening was significantly decreased (p < 0.05) in the female offspring of Allium cepa treated group when compared with control group. Weight at vaginal opening was not significantly different in female offspring of dexamethasone and dexamethasone + Allium cepa treated group when compared with control group (Table 2).
Data represent mean ± SEM. n = 5. ap<0.05 when compared with the control group. Analysis was based on one way analysis of variance (ANOVA) and Turkey post hoc test.
3.3. Effect of maternal administration of dexamethasone and Allium cepa during lactation on serum reproductive hormones and corticosterone in female offspring of Wistar rats
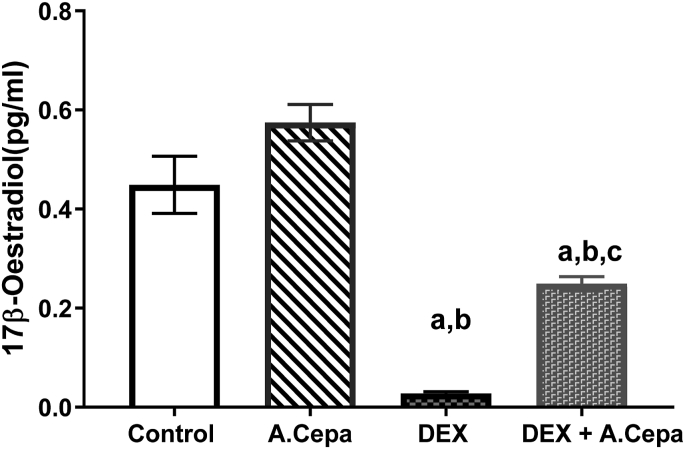
Serum concentration of 17β-estradiol significantly decreased (p < 0.05) in the female offspring of dexamethasone only and dexamethasone + Allium cepa treated group when compared with control group. Serum concentration of 17β-estradiol significantly decreased (p < 0.05) in the female offspring of dexamethasone and dexamethasone + Allium cepa treated group when compared with Allium cepa treated group (Fig. 2). In addition, co-administration of Dex with Allium cepa significantly raised the serum 17β-estradiol when compared with Dex treated group. There was no significant difference in the serum concentration of luteinizing hormone and follicle stimulating hormone across all groups (Table 3).
Fig. 2.
Serum concentration of 17β-oestradiol in female offspring of dexamethasone (DEX) and Allium cepa (A.cepa) treated lactating Wistar rats. Columns represent mean ± SEM. n = 5. ap<0.05 when compared with the control group. bp < 0.05 when compared with the Allium cepa treated group. cp < 0.05 when compared with the dexamethasone treated group. Analysis was based on one-way analysis of variance (ANOVA) and Turkey post hoc test.
Table 3.
Serum concentration of LH and FSH in female offspring of dexamethasone (DEX) and Allium cepa (A.cepa) treated lactating Wistar rats.
| LH mlU/ml | FSH ml/U/ml | |
|---|---|---|
| Control | 1.71 ± 0.17 | 0.48 ± 0.01 |
| A.cepa | 1.91 ± 0.17 | 0.53 ± 0.04 |
| Dex | 1.93 ± 0.16 | 0.51 ± 0.03 |
| Dex + A.cepa | 1.86 ± 0.07 | 0.51 ± 0.02 |
Data represent mean ± SEM. n = 5.
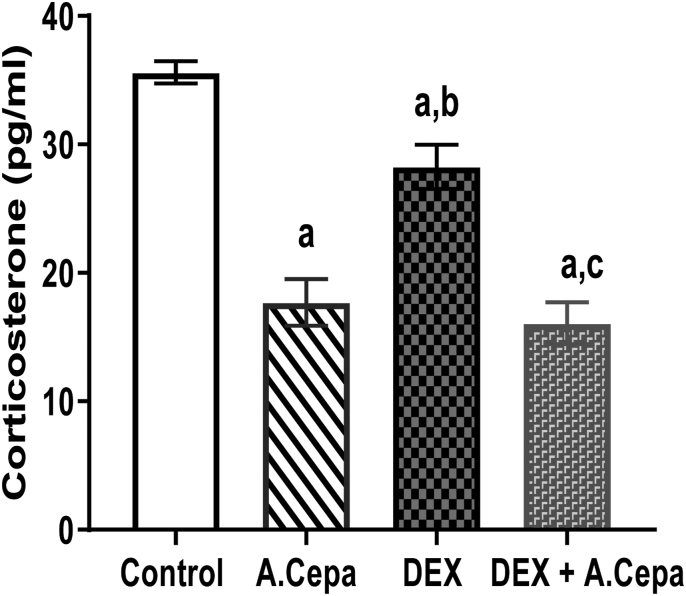
Serum corticosterone level significantly decreased (p < 0.05) in the female offspring of dexamethasone only and dexamethasone + Allium cepa treated group when compared with control group. However, serum corticosterone level significantly increased (p < 0.05) in the female offspring of dexamethasone treated group when compared with Dex + Allium cepa treated group (Fig. 3).
Fig. 3.
Serum concentration corticosterone in female offspring of dexamethasone (DEX) and Allium cepa (A.cepa) treated lactating Wistar rats. Columns represent mean ± SEM. n = 5. ap<0.05 when compared with the control group. bp < 0.05 when compared with the Allium cepa treated group. cp < 0.05 when compared with the dexamethasone treated group. Analysis was based on one-way analysis of variance (ANOVA) and Turkey post hoc test.
3.4. Effect of maternal administration of dexamethasone and Allium cepa during lactation on antioxidant enzymes activities in female offspring of Wistar rats
The ovarian superoxide dismutase (SOD) activity was significantly decreased (p < 0.05) in the female offspring of dexamethasone only and dexamethasone + Allium cepa treated group when compared with control group. Also, SOD activity was significantly decreased (p < 0.05) in the female offspring of dexamethasone treated group when compared with Dex + Allium cepa treated group. The increase in the SOD activity in dexamethasone + Allium cepa treated group when compared with Allium cepa treated group was not statistically significant (Table 4).
Table 4.
Oxidative stress indices in the female offspring of dexamethasone (DEX) and Allium cepa (A.cepa) treated lactating Wistar rats.
| SOD activities (U/mg protein) | Catalase Activities (U/mg protein) | MDA level μM/mg protein |
|
|---|---|---|---|
| Control | 1342 ± 44.77 | 1.05 ± 0.08 | 25.28 ± 3.40 |
| A.cepa | 1101 ± 107.4 | 1.45 ± 0.09 | 15.75 ± 1.50 |
| Dex | 689.3 ± 100.3 a,b | 0.92 ± 0.11b | 48.70 ± 3.24a,b |
| Dex + A.cepa | 926.2 ± 36.36 a,c | 1.22 ± 0.03a,c | 39.33 ± 3.22a,b,c |
Data represent mean ± SEM. n = 5. ap<0.05 when compared with the control group. bp < 0.05 when compared with the Allium cepa treated group. cp < 0.05 when compared with the dexamethasone treated group. Analysis was based on one-way analysis of variance (ANOVA) and Turkey post hoc test.
The ovarian catalase activity was significantly decreased (p < 0.05) in the female offspring of dexamethasone treated group when compared with Allium cepa treated group. There is also a significant increase in the catalase activity in Dex + Allium cepa treated group when compared with Dex treated group (Table 4).
Ovarian MDA level was significantly increased (p < 0.05) in the female offspring of dexamethasone only and dexamethasone + Allium cepa treated group when compared with control group. Ovarian MDA level also was significantly increased (p < 0.05) in the female offspring of dexamethasone and Dexamethasone + Allium cepa treated group when compared with Allium cepa treated group (Table 4).
3.5. Effect of maternal administration of dexamethasone and Allium cepa during lactation on body weight in female offspring of Wistar rats
At birth (PND1), there was no statistically significant difference in the body weights of female pups from all four groups. At post-natal week (PNW 4), mean body weight of female offspring was significantly decreased (p < 0.05) in the dexamethasone and dexamethasone + Allium cepa treated group when compared with control. At PNW 8, mean body weight of female offspring was significantly decreased (p < 0.05) in the dexamethasone and dexamethasone + Allium cepa treated group when compared with control. At PNW 12, there was no statistically significant difference in the body weights of adult female offspring from all four groups (Table 5).
Table 5.
Effect of maternal administration of dexamethasone and Allium cepa during lactation on body weight in the female offspring of Wistar rats.
| AT BIRTH | PNW4 | PNW 8 | PNW12 | |
|---|---|---|---|---|
| Control | 5.51 ± 0.28 | 43.72 ± 2.92 | 107.2 ± 2.78 | 129.8 ± 4.12 |
| A.ceparowhead | 5.61 ± 0.37rowhead | 43.22 ± 3.18 | 87.2 ± 2.154 | 126.4 ± 2.62 |
| DEX | 5.57 ± 0.30 | 23.23 ± 1.02a | 81.4 ± 5.15a,b | 122.0 ± 0.95 |
| DEX + A. cepa | 5.77 ± 0.22 | 29.87 ± 3.144a | 90.4 ± 6.79a,b | 132.4 ± 4.13 |
Data represent mean ± SEM. n = 5. ap<0.05 when compared with the control group. bp < 0.05 when compared with the Allium cepa treated group. PNW: post natal week; Analysis was based on one way analysis of variance (ANOVA) and Turkey post hoc test.
3.6. Effect of maternal administration of dexamethasone and Allium cepa during lactation on absolute organ weight in the female offspring of Wistar rats
The absolute weight of the adrenal gland, ovaries, and uteri was not significantly different in the female offspring of Allium cepa, dexamethasone, and dexamethasone + Allium cepa treated group when compared with control group (Table 6).
Table 6.
Effect of maternal administration of dexamethasone and Allium cepa during lactation on relative organ weight in the female offspring of Wistar.
|
Group |
Ovaries (%) |
|---|---|
|
Control |
0.05 ± 0.01 |
|
A. cepa |
0.06 ± 0.01 |
|
DEX |
0.07 ± 0.01 |
|
DEX+ A. cepa |
0.05 ± 0.01 |
Data are expressed as mean ± SEM. n = 5. Analysis was based on one-way analysis of variance (ANOVA) and Turkey post hoc test.
3.7. Effect of maternal administration of dexamethasone and Allium cepa during lactation on histology of ovaries in the female offspring of Wistar rats
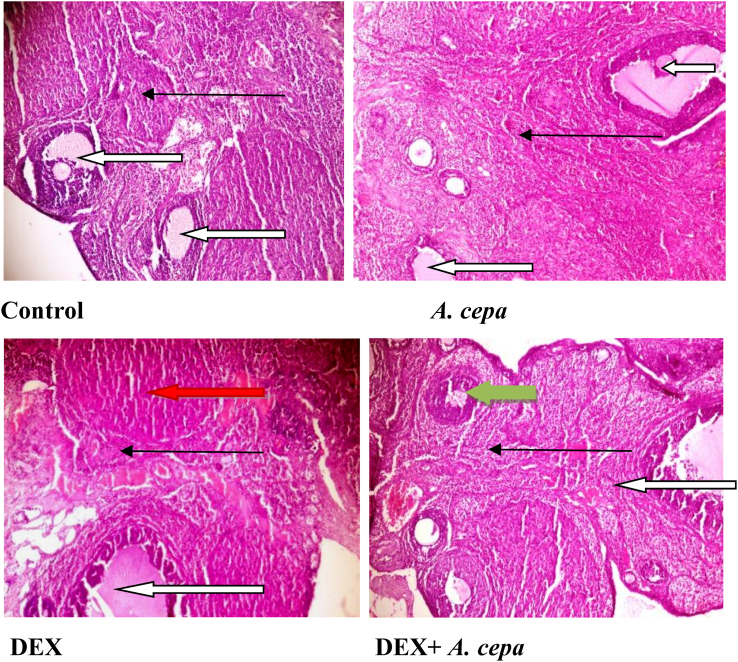
Female offspring from the control and Allium cepa treated dams had normal ovarian stroma with luteinization within the granular cells. Female offsprings from the dexamethsone treated dam had poor ovarian stroma with increased fibroblast. There is severe vascular congestion with perivascular infiltration with few Graafian follicles noted. Female offsprings from the dexamethasone + Allium cepa treated dams had normal ovarian stroma with matured Graafian follicles (with thick theca cells) (Fig. 4).
Fig. 4.
Photomicrographs of ovarian sections from female offspring of dexamethasone (DEX) and Allium cepa (A.cepa) treated lactating Wistar rats. Sections were stained with H&E presented at ×100 magnification. Mature Graafian follicles (white arrow); mild inflammatory cells (slender arrow); follicles with thick theca cells (green arrow); Severe vascular congestion with perivascular infiltration (red arrow).
4. Discussion
Exposure to stressful stimulus early in life could disrupt the differentiation of reproductive organs in both male and female leading to adverse outcomes which may culminate in reproductive dysfunction in adult life. Consumption of natural products has been shown to ameliorate the deleterious effects of the aforementioned (Brummelte et al., 2006; Khaki et al., 2009; Allouh et al., 2013; Kala et al., 2016). In the present study, the protective role of Allium cepa on maternal dexamethasone induced alterations in reproductive functions of female offspring were investigated.
The results from this study showed that maternal treatment with dexamethasone significantly reduced the body weight in the female offspring. Co-administration with Allium cepa was able to improve the body weight at 12 weeks of postnatal life. This observation is in agreement with previous study that maternal exposure to dexamethasone during lactation or early neonatal life reduce growth rate (Wang et al., 2010; Jeje and Raji, 2015).
In both rodents and humans, sexual maturity is reached earlier in females than in males. In male rats, puberty is assessed by the day of preputial separation and in female rats, complete vaginal perforation or opening and commencement of regular oestrous cycle following the first oestrus (Marcondes et al., 2002; Goldman et al., 2007) indicates puberty. Among other factors, body weight has been associated with the attainment of sexual maturity in both human (Ganong, 2005) and rats (Rivier and Rivest, 1991). In this study, the mean age of vaginal opening was not significantly different in all the treated rats. This disagrees with reports from other study, that dexamethasone treatment during gestation or lactation may increase pubertal timing in the offspring (Smith and Waddell, 2000; Jeje et al., 2016). However, these previous studies use higher doses (100 μg/Kg bwt). Therefore, the resultant effect could be dose dependent. It is also evident from this study that offspring of dams treated with Allium cepa attained sexual maturity at a lower weight than offspring from the other groups.
It has been reported in many reproductive toxicity studies that following vaginal patency, few pubertal rats initially show some irregular cycles, before settling down to recurrently synchronized patterns of ovulatory cycles of 4 or 5 days (Goldman et al., 1985). However, exposure to toxic agents could cause misalignment in cycle pattern, occurrence and length resulting in irregularities, which could be of significance. It is established that when female rodents spend 50% of their time on a phase or have an average cycle length of 7 days or longer, they are considered to have irregular oestrous patterns (Goldman et al., 2007; Li et al., 2017). The findings of this study showed that offspring of the dexamethasone treated dams exhibited prolonged and irregular oestrous cycle pattern in comparison with the control group and the Allium cepa treated group. In the view of Ryan and Schwart (1997), rats who have prolonged diestrus may indicate acyclical periods. Although maternal exposure to dexamethasone did not adversely affect sexual maturity adjudged from day of vaginal opening, however, adult offspring showed acyclical patterns later in life. Notwithstanding, the fact that exposure to certain xenobiotic compounds at low dose could cause disruption in the cyclicity of the oestrous phases, however, despite continuous dosing, the system is able to adapt after a period and resume cyclicity and normal ovulation (Ryan and Schwart, 1997). These facts are consistent with the observation of this study, as the aberration observed early after puberty was only short lived as most of the offspring resumed regular cycles later in life before they were euthanatized. Another interesting finding of this study was, the fact that offspring of Allium cepa treated dams showed regular oestrous patterns gives credence to the reproductive functions promoting the effect of Allium cepa in rats (Khaki et al., 2012). Additionally, even offspring from dexamethasone + Allium cepa treatment group even showed regular oestrous cycle patterns similar to offspring from control and Allium cepa treatment groups. It is therefore evident that Allium cepa administration was able to ameliorate the negative effect of maternal dexamethasone treatment on offspring's oestrous cyclicity. Estrous cyclicity is highly dependent on production and release of essential reproductive hormones including both gonadal and pituitary hormones.
The result from this study, showed that maternal dexamethasone administration during lactation reduced serum oestradiol concentration in the female offspring at adulthood. However, co-administration with Allium cepa significantly increased the serum level of estradiol at adulthood. Allium cepa Linn has been reported to increase oestrogenic function (Ahrafaie et al., 2011). This could be responsible for increased levels of estrogen level observed in the offspring. It has been reported that dexamethasone reduced oestrogen level by inhibiting the substrates used for oestrogen synthesis such as DHEA and DHEAs. In a study among women with polycystic ovarian syndrome, DHEAs–an androgen precursor produced by the adrenal gland was reduced by 46% in dexamethasone treated group (Vanky et al., 2004). This indicates that low dose dexamethasone could suppress adrenal androgen synthesis. In another study, dexamethasone influence ovarian functions by inhibiting the enhancement of FSH in granulosa cells as well as estrone cells in-vitro (Suzuki et al., 1999). In another study, dexamethasone inhibits FSH induced estrogen synthesis in cultured rat granulosa cells (Hsueh and Erickson, 1978). Yet another study concluded that a single dose of dexamethasone could disrupt gonadal functions in female rats and could lead to infertility (Illera et al., 2005). The fact that offspring of dams from the dexamethasone + Allium cepa treatment group had reduced oestradiol levels when compared with offspring from the control group shows that Allium cepa showed a weak action against effects of dexamethasone exposure. This reduction did not cause any obvious effect on the oestrous cycle pattern of the rats, showing that this reduction may rather be multifactorial.
During the oestrous cycle, the levels of the sex hormones vary. FSH mimics the pattern of LH, with the highest levels peaking during late proestrus and again early in oestrus (Goldman et al., 2007). However, in this study, the serum levels of FSH and LH were not significantly different in each group. The findings of the present study agree with other studies who did not find any adverse effect on FSH and LH release following the administration of synthetic glucocorticoids (Rockwell and Koos, 2009; Amorim et al., 2011; Dalatabadi and Zarchii, 2015) and Allium cepa juice administration in female and male rats (Khaki et al., 2009, 2012). Since the FSH/LH surge is necessary for ovulation to occur. The preovulatory surge is triggered by LH releasing hormone (LHRH) which is dependent on estradiol levels (Khaki et al., 2009) since the levels were low in the dexamethasone group, this could be associated with the acyclical oestrus patterns observed in the dexamethasone treated group. The result of this study suggest a neonatal programming of the pituitary gland by dexamethasone as reduction in estradiol levels should normally be followed by a feedback increase in FSH or LH.
In this study, corticosterone level significantly decreased in the female offspring of dexamethasone only and dexamethasone + Allium cepa treated group when compared with control group. Also, corticosterone level significantly increased in the offspring of dexamethasone treated group when compared with Dex + Allium cepa and Allium cepa treated groups.
The finding is consistent with results from previous studies (Catalani et al 2000, 2011, 2011; Casolini et al., 2007). According to Catalani et al. (2000), the female offspring from dams who drank water supplemented with low dose of corticosterone (200 μg/mg of corticosterone hemisuccinate) from post-natal day 1 to weaning exhibited: (i) lower stress induced corticosterone secretion; (ii) higher number of corticosteroid receptors in the hippocampus; (iii) better performance in conditioned learning; (iv) reduction in fearfulness in two conflict situations. In their viewpoint, a moderate increase in corticosterone in infancy provides the offspring a better coping strategy to deal with during stressful situations later in life. The possible explanation given is that rise in corticosterone in dams could induce pup over care in the mother that in turn triggers off a chain of events of beneficial outcomes (Catalani et al., 2011). It is reported that maternal care goes a long way to influence the development of the offspring even later in life. Even among humans, the nature of maternal care that an infant receives affects child's emotional and cognitive development and better shapes that neonate to cope with challenges later in life (Catalani et al., 2011). Although, this observation, contradict our observation in male offspring. We have observed, an increased in corticosterone level in the male offspring following maternal treatment with dexamethasone during lactation (Jeje et al., 2020). The reason for this is not known but however, cases of sex dependent response of offspring to maternal exposure to endocrine disrupters is not uncommon.
Another significant finding in this study was the reduction in serum corticosterone levels in offspring from dams treated with Allium cepa when compared with offspring of dams treated with dexamethasone and dams of the control group. Although it is a claim in alternative medical practices that consumption of Allium cepa could help relieves stress, there are no experimental studies reporting this. Nevertheless, this effect could be attributed to its Allium cepa's rich quercetin content (Griffith et al., 2002). In their study, Nagaraja et al. (2009) found that quercetin supplementation caused a significant reduction in corticosterone levels in rats exposed to forced swimming test. Corroboratively, Kawabata et al. (2010) reported that quercetin suppresses CRF mRNA in the hypothalamus. Since hypothalamic CRF is the most important component of the HPA hormonal cascade, there may be a decrease in CRF and ACTH after quercetin exposure in the lactating pups, and in turn, it decreased the corticosterone levels in later life. However, in the light of this present study, there is a need for further mechanistic research into how and where the chemical constituents in Allium cepa influence corticosterone release, especially during neonatal development.
The increased level of MDA in the female offspring of dexamethasone treated group clearly indicates that the offspring of dexamethasone treated dams were prone to oxidative stress even though they could cope with physical and psychological stress. It is now known that synthetic glucocorticoids treatment has been shown to increase the production of reactive oxygen species and reduce mitochondrial activities leading to oxidative stress (Tang et al., 2009). So far, studies have opined that administration of synthetic glucocorticoids can be associated with oxidative stress owing to its ability to reduce the activities of the antioxidant enzymes (superoxide dismutase, catalase, and glutathione peroxidase) both in vivo and in vitro (Zafir and Banu, 2009). In this study, offspring from dams treated with dexamethasone had a significant reduction in ovarian superoxide dismutase and catalase levels. This finding is similar to that reported by McIntosh et al. (1998) and Jeje et al. (2017).
On the contrary, MDA levels were significantly low in offspring of Allium cepa treated dams. Other studies have extensively reported on the antioxidant properties of Allium cepa in the gonads following direct administration (Olamudathir et al., 2008; Khaki et al., 2009; Allouh et al., 2013; Malviya et al., 2013) but this the first study to report on the effect on offspring's ovarian tissue following maternal exposure. It is therefore obvious that Allium cepa did not only promote lactation but decrease the tissue markers of oxidative stress on the offspring's ovaries. On the other hand, in offspring of Allium cepa treated dams, superoxide dismutase activities were similar with; catalase activities were increased, while glutathione peroxidase activities were reduced in comparison with offspring of the dams in the control group.
The levels of superoxide dismutase and catalase both serve as lines of defense against reactive oxygen and nitrogen species and could confer a measure of protection on the offspring ovarian tissues against being overwhelmed by oxidative stress. Experimental studies in the ovaries have confirmed that oxidative stress triggers apoptosis in the majority of germ cells within the ovary and even in ovulated oocytes (Pandey et al., 2010). Although both mitochondria-as well as death-receptor pathways are involved in oocyte apoptosis, oxidative stress induced mitochondria-mediated pathway plays a major role in the elimination of the majority of germ cells from the ovary (Tiwari et al., 2016). Oxidative stress in the follicular fluid deteriorates oocyte quality and reduces reproductive outcome (Ebbesen et al., 2009). Indeed, oxidative stress is one of the major factors that have a direct negative impact on oocyte quality and limits female reproductive outcome in several mammalian species including human. This was confirmed in histological sections of the ovaries and uteri from offsprings of the dams from all the groups in this study. Photomicrograph of ovaries of offsprings from control and Allium cepa treated dams showed normal ovarian stroma with luteinization within the granular cells. Several developing and mature graffian follicles were noted. In contrast, the photomicrograph of ovarian section from offspring of dexamethasone treated dams showed poor ovarian stroma with increased fibroblast. There was also severe vascular congestion with perivascular infiltration while few few graffian follicles were noted. According to the study by Ristic et al. (2008), exposure to dexamethasone decreased the pool of non-growing follicles in the ovary without affecting the processes of folliculogenesis and atresia. Also, dexamethasone induces germ cell apoptosis in neonatal ovary (Poulain et al., 2012), Reduction in estradiol observed in the group could have effects on granulosa cell functions resulting in deterioration in oocyte quality (Prasad et al., 2016). The biochemical assay also showed increased MDA levels which is a clear indication of oxidative stress and this could be responsible for poor ovarian stroma and vascular congestion on the histological sections. On the reversed side, photomicrograph of ovarian tissue from offspring of dams treated with dexamethasone + Allium cepa showed normal graffian follicles development. The observed follicles had thick theca cells and normal stroma with fibroblastic tissues but infiltration of inflammatory cells was not observed. There was also moderate vascularization without congestion in the tissues. This showed that even in the face of dexamethasone induced ovarian toxicity, Allium cepa was able to profer some measure of protection and maintain a healthy outlook in the ovaries. This could be atrributed to its rich antioxidant properties.
In conclusion, the results suggest that Allium cepa ameliorated deleterious reproductive alterations and ovarian oxidative stress in offspring of lactating Wistar rat dams treated with dexamethasone.
CRediT authorship contribution statement
S.O. Jeje: Conceptualization, and designed the experiments, Formal analysis, Perform the experiments, Writing – original draft. E.E. Akpan: ceived and designed the experiments, Performed the experiments, Formal analysis, Contributed reagents, materials, analysis tools or data, Writing – original draft. O.T. Kunle-Alabi: Performed the experiments, Contributed reagents, materials, analysis tools or data. O.O. Akindele: Performed the experiments, Contributed reagents, materials, analysis tools or data. Y. Raji: Conceptualization, and designed the experiments, Writing – original draft.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Agarwal A., Aponte-Mellado A., Premkumar B., Shaman A., Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod. Biol. Endocrinol. 2012;10 doi: 10.1186/1477-7827-10-49. 49.l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrafaie Z., Amin H., Elgayad S. Estrogenecity of outer scales of onions on the uteri of immature mice. Can. J. Physiol. Pharmacol. 2011;89(11):829–835. doi: 10.1139/y11-085. [DOI] [PubMed] [Google Scholar]
- Allouh M., Daradka H., Al Barbarawi M., Mustafa A. Behavior in male rats with and without Paroxetine-induced sexual dysfunction. Exp. Biol. Med. 2013;239(2):177–182. doi: 10.1177/1535370213508360. [DOI] [PubMed] [Google Scholar]
- Amar M., Adam Shama I., Enaia A., Hind A., Hager A. Effects of various levels of oral doses of Dexamethasone (Al-Nagma) abused as cosmetic by Sudanese women on wistar rats. J. Med. Sci. 2013;13:432–438. [Google Scholar]
- Amorim J., Chuffa L., Teixeira G., Mendes L., Fioruci B. Variation in maternal care alters corticosterone and 17β estradiol, estrous cycle and folliculogenesis and stimulate estrogen receptors alpha and beta in the ovaries of Uch rats. Reprod. Biol. Endocrinol. 2011;9:160. doi: 10.1186/1477-7827-9-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aseervatham G., Sivasudha T., Jeyadevi R., Arul A. Environmental factors and unhealthy lifestyle influence oxidative stress in humans-an overview. Environ Sci Pollution Res Int. 2013;20:4356–4369. doi: 10.1007/s11356-013-1748-0. [DOI] [PubMed] [Google Scholar]
- Augusti K. Therapeutic values of onion (Allium cepa L.) and garlic (Allium sativum L.) Indian J. Exp. Biol. 1996;34:634–640. [PubMed] [Google Scholar]
- Ausejo M., Saenz A., Pham B. The effectiveness of glucocorticoids in treating croup: meta-analysis. Br. Med. J. 1999;319:595–600. doi: 10.1136/bmj.319.7210.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J., Van B., Heijnen C. Neonatal glucocorticoids and the developing brain: short-term treatment with life-long consequences? Trends Neurosci. 2001;24:649–653. doi: 10.1016/s0166-2236(00)01948-2. [DOI] [PubMed] [Google Scholar]
- Barker D. Fetal origins of coronary heart disease. Br. Med. J. 1995;311(6998):171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartol F., Wiley A., Bugneli C. Relaxing and maternal lactocrine programming of neonatal development. Ann. N. Y. Acad. Sci. 2009;116:158–163. doi: 10.1111/j.1749-6632.2008.03820.x. [DOI] [PubMed] [Google Scholar]
- Brummelte S., Pawluski J., Galea L. High post-partum levels of corticosterone given to dams influence postnatal hippocampal cell proliferation and behavior of offspring: a model of post-partum stress and possible depression. Horm. Behav. 2006;50(3):370–382. doi: 10.1016/j.yhbeh.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Cameron N. Maternal programming of reproductive function and behaviour in female rat. Frontiers in Evolutionary Science. 2011;3(1):1–10. doi: 10.3389/fnevo.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casolini P. Maternal exposure to low levels of corticosterone during lactation protects the adult offspring against ischemic brain damage. J. Neurosci. 2007;27(26):7041–7046. doi: 10.1523/JNEUROSCI.1074-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalani A., Casolini P., Scaccianoce S., Patacchioli F.R., Spinozzi P., Angelucci L. Maternal corticosterone during lactation permanently affects brain corticosteroid receptors, stress response and behaviour in rat progeny. Neuroscience. 2000;100:319–325. doi: 10.1016/s0306-4522(00)00277-3. [DOI] [PubMed] [Google Scholar]
- Catalani A., Alemà G., Cinque C., Zuena A., Casolini P. 2011. Maternal corticosterone effects on hypothalamus-pituitary-adrenal axis regulation and behavior of the offspring in. [DOI] [PubMed] [Google Scholar]
- Dalatabadi A., Zarchii S. The effect of prescription of different dexamethasone doses on the reproductive system. Biomed. Res. 2015;26(4):656–660. [Google Scholar]
- De Gans J., van de Beek D. 2002. For the European Dexamethasone in Adulthood Bacterial Meningitis Study Investigators. (Dexamethasone in Adults with Bacterial) [Google Scholar]
- De Wasch K., De Brabander H.F., van de Wiele M., Vercammen J., Courtheyn D. Differentiation between dexamethasone and betamethasone in a mixture using multiple mass spectrometries. J. Chromatogr. A. 2001;926:79–86. doi: 10.1016/s0021-9673(01)00744-0. [DOI] [PubMed] [Google Scholar]
- Demyttenaere K., Nijs P., Evers-Kiebooms G., Koninckx P. Coping and the ineffectiveness of coping influence the outcome of in vitro fertilization through stress responses. Psychoneuroendocrinology. 1992;17:655–665. doi: 10.1016/0306-4530(92)90024-2. [DOI] [PubMed] [Google Scholar]
- Denton L., Ojeifo I. Onion production practices and their improvement in Nigeria. Onion Newsletter Topics. 1990;2:10–13. [Google Scholar]
- Drake A., Walker B., Seckl J. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;1:R34–R38. doi: 10.1152/ajpregu.00106.2004. [DOI] [PubMed] [Google Scholar]
- Drake A.J., Tang J.I., Nyirenda M.J. Mechanisms underlying the role of glucocorticoids in early life programming of adult disease. Clin. Sci. 2007;113:219–232. doi: 10.1042/CS20070107. [DOI] [PubMed] [Google Scholar]
- Ebbesen S., Zachariae R., Mehlsen M., Thomsen D., Hojgaard A., Ottosen L. Stressful life events are associated with a poor in-vitro fertilization (IVF) outcome: a prospective study. Hum. Reprod. 2009;24:2173–2182. doi: 10.1093/humrep/dep185. [DOI] [PubMed] [Google Scholar]
- Ganong W. twenty-second ed. McGraw Hill; Boston: 2005. Review of Medical Physiology. [Google Scholar]
- Geelhoed G., Turner J., Macdonald W. Efficacy of a small single dose of oral dexamethasone for outpatient croup: a double blind placebo controlled clinical trial. Br. Med. J. 1996;313:140–142. doi: 10.1136/bmj.313.7050.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohir W., Ratelife E., Sloboda D. Of the bugs that shape us: maternal obesity, gut microbiome, and long-term disease risk. Paediatric Research. 2015;77:196–204. doi: 10.1038/pr.2014.169. [DOI] [PubMed] [Google Scholar]
- Goldman J., Murr A., Cooper R. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res. Part B. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Trueman L., Crowther T., Thomas B., Smith B. Onions-a global benefit to health. Phytother Res. 2002;16(7):603–615. doi: 10.1002/ptr.1222. [DOI] [PubMed] [Google Scholar]
- Grunwald H., Rosner F. Dexamethasone as an antiemetic during cancer chemotherapy. Ann. Intern. Med. 1984;101:398. doi: 10.7326/0003-4819-101-3-398_1. [DOI] [PubMed] [Google Scholar]
- Hsueh A., Ericksson G. Glucocorticiods inhibition of FSH- induced estrogen production in cultured rat granulosa cells. Steroids. 1978;32(5):639–648. doi: 10.1016/0039-128x(78)90074-0. [DOI] [PubMed] [Google Scholar]
- Ige S., Akhigbe R. The role of Allium cepa on aluminum-induced reproductive dysfunction in experimental male rat models. J. Hum. Reprod. Sci. 2012;5(2):200–205. doi: 10.4103/0974-1208.101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illera J., Sylvan G., Martinez M., Blass L. The effect of dexamethasone on the disruption of ovarian steroid and receptors in female rats. J. Physiol. Biochem. 2005;61:429. doi: 10.1007/BF03168449. [DOI] [PubMed] [Google Scholar]
- Izawa H., Kohara M., Aizawa K., Suganuma H., Inakuma T., Watanabe G., Taya K., Sagai M. Alleviative effects of Quercetin and Onion on male reproductive toxicity induced by Diesel exhaust particles. Biosc. Biotech. Biochem. 2008;72(5):1235–1241. doi: 10.1271/bbb.70705. [DOI] [PubMed] [Google Scholar]
- Jeerakornpassawat D., Suprasert P. Randomized, controlled trial of dexamethasone versus dexamethasone plus hydrocortisone as prophylaxis for hypersensitivity reactions due to paclitaxel treatment for gynecologic cancer. Int. J. Gynecol. Canc. 2017 doi: 10.1097/IGC.0000000000001069. [DOI] [PubMed] [Google Scholar]
- Jeje S., Raji Y. Effect of maternal dexamethasone exposure during lactation on metabolic imbalance and oxidative stress in the liver of male offspring of wistar rats. Niger. J. Physiol. Sci. 2015:131–137. [PubMed] [Google Scholar]
- Jeje S.O., Akindele O.O., Balogun M.E., Raji Y. Maternal treatment with dexamethasone during lactation delays male puberty and disrupts reproductive functions via hypothalamic–pituitary gonadal axis alterations. J. Pathophysiol. 2016;23:43–49. doi: 10.1016/j.pathophys.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Jeje S.O., Ola-Mudathir F.K., Raji Y. Experimental maternal treatment with dexamethasone during lactation induces neonatal testicular and epididymal oxidative stress;Implications for early postnatal exposure. Pathophysiology. 2017;24(4):261–265. doi: 10.1016/j.pathophys.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Jeje S.O., Adegbite L.O., Akindele O.O., Kunle-Alabi O.T., Raji Y. Allium cepa Linn juice protect against alterations in reproductive functions induced by maternal dexamethsone treatment during lactation in male offspring of Wistar rats. Heliyon. 2020;6(Issue 5) doi: 10.1016/j.heliyon.2020.e03872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kala M., Nivsarkar M. Role of cortisol and superoxide dismutase in psychological stress induced anovulation. Gen. Comp. Endocrinol. 2016;225:117–124. doi: 10.1016/j.ygcen.2015.09.010. [DOI] [PubMed] [Google Scholar]
- Khaki A., Fathiazad F., Nouri M., Khaki A., Khamenehi H., Hamadeh M. Evaluation of androgenic activity of Allium cepa on spermatogenesis in rat. Folia Morphol. (Wars.) 2009;68:45–51. [PubMed] [Google Scholar]
- Khaki A., Farnam A., DavatgarBadie A., Nikniaz H. Treatment effects of onion (Allium cepa) and ginger (Zingiber officinale) on sexual behavior of rat after inducing an antiepileptic drug (Lamotrigine) Balkan Med. J. 2012;29(3):236–246. doi: 10.5152/balkanmedj.2012.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Lee H., Cha Y., Joo W., Kang D., Moon J. In-vivo investigation of anti-diabetic properties of ripe onion juice in normal and streptozotocin-induced diabetic rats. Preventive Nutrition and Food Science. 2013;18(3):169–174. doi: 10.3746/pnf.2013.18.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Yun H., Kim C., Cho Y., Lee Y., Kim M. Prenatal exposure to dexamethasone in the mouse induces sex-specific differences in placental gene expression. Dev. Growth Differ. 2017 doi: 10.1111/dgd.12376. [DOI] [PubMed] [Google Scholar]
- Li J., Kim J., Abejuela V., Lamano J., Klein N., Christian C. Disrupted female estrous cyclicity in the intrahippocampal kainic acid mouse model of temporal lobe epilepsy. Epilepsia Open. 2017;2(1):39–47. doi: 10.1002/epi4.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian H., Gao Y., Jiao G., Sun M., Wu X., Wang T. Antioxidant supplementation overcomes the deleterious effects of maternal restraint stress-induced oxidative stress on mouse oocytes. Reproduction. 2013;146:559–568. doi: 10.1530/REP-13-0268. [DOI] [PubMed] [Google Scholar]
- Malviya N., Jain, Gupta V., Vyas S. Management of drug induced sexual dysfunction in male rats by ethyl acetate fraction of onion. Acta Pol. Pharm. 2013;70(2):317–322. [PubMed] [Google Scholar]
- Marcondes F., Bianchi F., Tanno A. Determination of the estrous cycle phases of rats: some helpful considerations. Braz. J. Biol. 2002;62(4a):609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- Maroon, Joseph. The Longevity Factor. New York : Atria Books, a division of Simon &.
- Miller D., Willey A., Chen J., Bagnel C., Bartol F. Nursing for 48 hours from birth support porcine uterine gland and endometrial cell compartment-specific gene expression. Mol. Biol. Rep. 2013;88:4. doi: 10.1095/biolreprod.112.105056. [DOI] [PubMed] [Google Scholar]
- Misra H., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biolo Chem. 1972 [PubMed] [Google Scholar]
- Myatt L. Placental adaptive responses and fetal programming. J. Physiol. 2006;572(Pt 1):25–30. doi: 10.1113/jphysiol.2006.104968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraja H., Ammu R., Nagarajah L., Ponnusamy K. Flavonoid quercetin protects against swimming stress-induced changes in oxidative biomarkers in hypothalamus of rats. Eur. J. Pharmacol. 2009;621:46–52. doi: 10.1016/j.ejphar.2009.08.030. [DOI] [PubMed] [Google Scholar]
- Ndoye F., Tchinang T., Nyegue A., Abdou J., Yaya A., Tchinda A., Essame J., Etoa F. Chemical composition, in vitro antioxidant and anti-inflammatory properties of essential oils of four dietary and medicinal plants from Cameroon. BMC Compl. Alternative Med. 2016;16:117. doi: 10.1186/s12906-016-1096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson C., Jennische E., Ho H.P., Eriksson E., Bjorntorp P., Holmang A. Postnatal endotoxin exposure results in increased insulin sensitivity and altered activity of neuroendocrine axes in adult female rats. Eur. J. Endocrinol. 2002;146:251–260. doi: 10.1530/eje.0.1460251. [DOI] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anaytical Biochemistry. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Ola-Mudathir K., Suru S., Fafunso M., Obioha U., Faremi T. 2008. Protective Roles of Onions and Garlic Extract on Cadmium-Induced Changes in Sperm Characteristics and. [DOI] [PubMed] [Google Scholar]
- Pandey A., Tripathi A., Premkumar K., Shrivastav T., Chaube S. Reactive oxygen and nitrogen species during meiotic resumption from diplotene arrest in mammalian oocytes. J. Cell. Biochem. 2010;111:521–528. doi: 10.1002/jcb.22736. [DOI] [PubMed] [Google Scholar]
- Perez-Munoz M., Arieta M., Ramer-Tait A., Walter J. A critical assessment of the sterile womb and in utero colonization hypothesis: implications for research on the pioneer infant microbiome. Microbiome. 2017;5(48):3–19. doi: 10.1186/s40168-017-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain M., Frydman N., Duquenne C., N′Tumba-Byn T., Benachi A., Habert R., Rouiller-Fabre V., Livera G. Dexamethasone induces germ cell apoptosis in the human fetal ovary. J. Clin. Endocrinol. Metab. 2012;97(10):E1890–E1897. doi: 10.1210/jc.2012-1681. [DOI] [PubMed] [Google Scholar]
- Prasad S., Tiwari M., Pandey A., Shrivastav T., Chaube S. Impact of stress on oocyte quality and reproductive outcome. J. Biomed. Sci. 2016;23:36. doi: 10.1186/s12929-016-0253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristic N., Nestorovic N., Manojlovic-Stojanoski M., Filipovic B., Sosic-Jurjevic B., Milo Sevic V., Sekulic M. Maternal dexamethasone treatment reduces ovarian follicle number in neonatal rat offspring. J. Microsc. 2008;232(3):549–557. doi: 10.1111/j.1365-2818.2008.02117.x. [DOI] [PubMed] [Google Scholar]
- Rivier C., Rivest S. Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: peripheral and central mechanisms. Biol. Reprod. 1991;45(4):523–532. doi: 10.1095/biolreprod45.4.523. [DOI] [PubMed] [Google Scholar]
- Rockwell L.C., Koos R.D. Dexamethasone enhances fertility and preovulatory serum prolactin levels in eCG/hCG primed immature rats. J. Reprod. Dev. 2009;55(3):247–251. doi: 10.1262/jrd.20108. Epub 2009 Feb 6. PMID: 19202320. [DOI] [PubMed] [Google Scholar]
- Rotruck J., Pope A.L., Ganther H., Swanson A., Hafeman D., Hoekstra W. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179(4073):588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Ryan K., Schwatz N. Grouped female mice: demonstration of pseudopregnancy. Biol. Reprod. 1997;17:578–583. doi: 10.1095/biolreprod17.4.578. [DOI] [PubMed] [Google Scholar]
- Sinha A. Colorimetric assay of catalase. Anal. Biochem. 1972;47(2):389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- Slimestad R., Fossen T., Vågen I. Onions: a source of unique dietary flavonoids. J. Agric. Food Chem. 2007;55(25):10067–10080. doi: 10.1021/jf0712503. [DOI] [PubMed] [Google Scholar]
- Smith J.T., Waddell B.J. Increased fetal glucocorticoids exposure delays puberty onset in postnatal life. Endocrinology. 2000;141:2422–2428. doi: 10.1210/endo.141.7.7541. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Sugino N., Fukaya T., Sugiyama S., Uda T., Takaya R., Yajima A., Sasano H. Superoxide dismutase in normal cycling human ovaries: immunohistochemical localization and characterization. Fertil. Steril. 1999;72:720–726. doi: 10.1016/s0015-0282(99)00332-5. [DOI] [PubMed] [Google Scholar]
- Tang L., Carey L., Bi J., Valego N., Sun X., Deibel P., Perrott J., Figueroa J., Chappell M., Rose J. Gender differences in the effects of antenatal betamethasone exposure on renal function in adult sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R309–R317. doi: 10.1152/ajpregu.90645.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari M., Prasad S., Tripathi A., Pandey A., Ali I., Singh A. Apoptosis in mammalian oocytes: a review. Apoptosis. 2015;20:1019–1025. doi: 10.1007/s10495-015-1136-y. [DOI] [PubMed] [Google Scholar]
- Tiwari M., Prasad S., Tripathi A., Pandey A., Singh A., Shrivastav T., Chaube S. Involvement of reactive oxygen species in meiotic cell cycle regulation and apoptosis in mammalian oocytes. Reactive Oxygen Species. 2016;1:110–116. [Google Scholar]
- Vanky E., Salvesen K., Carlsen S. Six-month treatment with the low dose dexamethasone further reduces androgen levels in PCOS women treated with diet and lifestyle advice and metformin. Hum. Reprod. 2004;19(3):529–531. doi: 10.1093/humrep/deh103. [DOI] [PubMed] [Google Scholar]
- Wang Y., Huang C., Hsu K. The role of growth retardation in lasting effects of neonatal dexamethasone treatment on hippocampal synaptic function. PloS One. 2010;5(9):12809. doi: 10.1371/journal.pone.0012806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welberg L., Seckl J. Prenatal stress, glucocorticoids and the programming of the brain. J. Neuroendocrinol. 2001;13:113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- Whiledege S., Cidlowski J. Glucocorticiods, stress and fertility. Mineva Endocrinology. 2010;35(2):109–125. [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO . Department of Child and Adolescent Health and Development; Geneva: 2003. Recommendation for Drugs in the Eleventh WHO Model List of Essential Drugs in Breast Feeding and Maternal Medication; pp. 1–3. [Google Scholar]
- Zafir A., Banu N. Modulation of in vivo oxidative status by exogenous corticosterone and restraint stress in rats. Stress. 2009;12(2):167–177. doi: 10.1080/10253890802234168. [DOI] [PubMed] [Google Scholar]