Abstract
Objective
The aim of this study was to compare the efficacy, safety and treatment costs of superficial femoral artery revascularization (SFA) with drug-coated balloon(DCB) versus avoiding revascularization strategy for the treatment of symptomatic SFA disease.
Methods
This retrospective single-center study reviewed 96 patients (113 limbs) with severe stenosis and occlusive SFA disease. All patients underwent either DCB(Group 1: n = 55 limbs) or nonrevascularization (Group 2: n = 58 limbs) between March 2015 and June 2019. The improvement of Rutherford class, walking impairment questionnaire score(WIQ), target limb reintervention, perioperative major adverse events, the catheterization laboratory cost and length of hospital stay were compared. The limb salvage and survival rates were calculated using the Kaplan-Meier method. Univariate and multivariate logistic regression analysis were performed to assess the association between factors and the improvement of Rutherford category at 12 months.
Results
The median follow-up time of Groups 1 and 2 was 17 and 33 months, respectively. At 12 months, the Rutherford category significantly decreased in both groups (P < 0.001), with no significant difference (79.7% vs. 64.3%, P = 0.074). Furthermore, multivariate analysis showed that the selected therapeutic method was not an influential factor for the improvement of Rutherford class at 12 months. The WIQ overall score as well as three subscales scores (distance, speed and stair-climbing), the survival rate, limb salvage rate and the length of stay between the two groups were comparable. The perioperative adverse events rate and catheterization laboratory cost in Group 2 was significantly lower compared to Group 1 [(34253.69 ± 28172.87) yuan vs. (56936.76 ± 41278.36) yuan, P = 0.001].
Conclusions
This study suggests that avoiding superficial femoral artery revascularization strategy has favorable efficacy and safety outcomes compared to combining revascularization with DCB in selected patients.
Keywords: Drug-coated balloon, Superficial femoral artery, Peripheral artery disease
Highlights
-
•
Home-based walking excersie is the recommended treatment for patients with PAD.
-
•
The patent profunda femoral artery plays an important role in the case of SFA occlusion.
-
•
SFA nonrevascularization strategy may be a therapeutic option for chronic obstructive SFA disease.
Introduction
Among patients with peripheral arterial disease (PAD), which affects more than 202 million individuals worldwide,1 superficial femoral artery (SFA) occlusion is more commonly involved. However, the appropriate strategy for the treatment of SFA remains controversial.2 Recently, the introduction of the drug-coated balloon (DCB), which provides a therapy combining mechanical balloon dilation and antiproliferative drug delivery,3 has consistently demonstrated superior anatomical outcomes including primary patency, binary restenosis, late lumen loss, and target lesion revascularization, compared to percutaneous old balloon angioplasty (POBA).2,4, 5, 6 Nonetheless, DCB provides no advantage in clinical outcomes, including amputation, mortality, and changes in the ankle-brachial index and Rutherford category.5,6 Moreover, endovascular treatment of complex SFA lesions with DCB may be unsuccessful, associated with provisional stenting rates as high as 40.4–46.8%,7,8 and may be correlated with increased all-cause mortality, as reported by a recent meta-analysis.9
Previous studies have demonstrated that endovascular treatments have greater long-term primary patency for iliac-femoral deep artery (IFA) compared to SFA disease and that the iliac femoral deep artery segment is less susceptible to atherosclerosis than the SFA segment in patients with PAD.10,11 The profunda femoris artery (PFA) plays an important role in the irrigation of the limbs when the SFA is severely stenosized or occluded.12 Previous studies reported the therapeutic value of isolated PFA revascularization with concomitant PFA and severe SFA stenosis or occlusive disease in patients with chronic limb ischemia.12,13 Furthermore, moderate exercise promoted the establishment of collateral circulation and accelerated the restoration of limb blood supply.14,15 Therefore, avoiding SFA revascularization; namely, the combination of patent IFA with exercise therapy, might be an important therapeutic option in selected patients with PAD.
Despite the above-mentioned evidence, few studies have evaluated the feasibility of avoiding SFA revascularization in the treatment of severe SFA stenosis or occlusive disease. Thus, this study compared the efficacy, safety, and treatment cost of SFA revascularization with DCB versus SFA nonrevascularization for the treatment of PAD.
Methods
This retrospective cohort study was approved by the medical ethics committee of Peking University Third Hospital (approval number: IRB00006761-M2019124), and the patients provided written informed consent. Ninety-six consecutive adult patients with severe stenosis and occlusive SFA, and with Rutherford categories of 2–5 were included. All patients underwent either SFA revascularization with DCB (DCB group) or SFA nonrevascularization (nSFA group) at the Department of Intervention Vascular Surgery, Peking University Third Hospital, Beijing, between March 2015 and June 2019. Concomitant endovascular strategies for iliac or infrapopliteal lesions were allowed. The patients were required to have at least one healthy outflow tract of the infrapopliteal artery (either pre-existing or re-established by POBA). The exclusion criteria were nonatherosclerotic disease such as acute embolism or infections; pregnancy or lactation; contraindications for aspirin, clopidogrel, heparin, and paclitaxel; bypass graft anastomosis lesions; heart failure; and significant renal or liver insufficiency.
DCB group. For secondary thrombosis of chronic occlusive disease of the SFA, a mechanical thrombectomy (Rotarex catheter, Straub Medical AG, Wangs, Switzerland) was performed before SFA revascularization to avoid distal embolism caused by thrombus detachment during the procedure. Pre-dilatation was performed with a plain balloon (0.5–1.0 mm smaller than the target lesion diameter), followed by the insertion of paclitaxel-coated balloons. The inflation time of DCBs was 2 min at 6–8 atm to dilate the lesion. DCBs of appropriate size and length (balloon/vessel diameter ratio of 1:1) were used. The choice of the DCB type (Passeo 18 Lux [Biotronik, Berlin, Germany] or Orchid [Acotec Scientific, Beijing, China]) was left to the discretion of the operator. Post-dilation was performed using balloons with a nominal diameter equal to that of the implanted devices. Self-expanding nitinol stents were implanted in cases with flow-limiting dissection and residual stenosis >30%. Unfractionated heparin (5000 units) was administered on postoperative day 1 to maintain an activated coagulation time of approximately 70–90 s.
nSFA group. The strategy of avoiding SFA revascularization was based on the patency of the iliofemoral deep artery and at least one healthy inferior genicular artery (either pre-existing or reestablished by POBA), as well as prescribed home-based walking exercise therapy and medical treatment. The participants were instructed to conduct above-ground walking exercise to a maximum pain threshold for at least three sessions weekly and to improve their walking duration gradually from 30 to 60 min per session (excluding rest time).
Pharmacotherapy therapy. All participants without contraindications at our institution received postprocedural antiplatelet therapy with aspirin (100 mg/day) and cilostazol (100 mg twice per day) for 6 months, and aspirin (100 mg/day) for life.
Study follow-up and assessment definitions. Patients were followed at 2–6 months post-discharge through clinic visits or telephone contact and annually thereafter. The patients were photographed each time and wound management was conducted at our wound care center. The follow-up endpoint was defined as the occurrence of amputation/death or the last follow-up in February 2020. Effectiveness and functional outcome measures were assessed by improvement in Rutherford category and walking impairment questionnaire (WIQ) scores, respectively. The safety endpoints were perioperative major adverse events, all-cause mortality, limb salvage rate, and target limb reintervention. The differences in catheterization laboratory costs and average length of hospital stay were also calculated.
Statistical analysis. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, NY, USA) and plotted using GraphPad Prism 8.0 (GraphPad, San Diego, CA). P < 0.05 was considered statistically significant. The quantitative data were expressed as means and standard deviations for normally distributed variables, as medians (quartiles) for non-normally distributed variables, and were assessed by t- and Mann–Whitney U tests, respectively. The qualitative data were described as proportions and compared using chi-square or Fisher’s exact tests. The limb salvage and survival rates were calculated using the Kaplan–Meier method and were compared using log-rank tests. Univariate and multivariate logistic regression analyses were performed to assess the associations between factors and improvement in Rutherford category at 12 months. Potentially significant predictors (P < 0.1) from the univariate analysis were integrated into a forward stepwise multivariate logistic regression model that was validated by a test of parallel lines.
Results
Baseline patient and lesion characteristics. Between March 2015 and February 2019, 96 inpatients (113 lesions) were observed according to the inclusion and exclusion criteria. The DCB group included 51 subjects (55 lesions) (37 men [40 lesions]; mean age 68.02 ± 7.02 years) while the nSFA group included 45 subjects (58 limbs) (33 males [43 limbs]; mean age 68.29 ± 8.83 years). The baseline patient and lesion characteristics (Table 1, Table 2) were similar between the two groups; however, a higher occurrence of concomitant iliac artery disease, multiple lesions, total SFA occlusion rate, and longer occluded SFA lesion length were observed in the nSFA group.
Table 1.
Baseline patient characteristics.a.
| Variables | DCB (n = 55 lesions) | nSFA (n = 58 lesions) | Statistics (Z/t/χ2) | P |
|---|---|---|---|---|
| Age,y | 68.02 ± 7.02 | 68.29 ± 8.83 | −0.184 | 0.855 |
| Sex | 0.029 | 0.865 | ||
| Female | 15(27.3%) | 15(25.9%) | ||
| Male | 40(72.7%) | 43(74.1%) | ||
| BMI(kg/m2) | 24.22(21.78,26.77) | 24.67(23.39,27.31) | −0.695 | 0.487 |
| Smoker | 0.115 | 0.944 | ||
| Current smoker | 23(41.8%) | 26(44.8%) | ||
| Former somkerb | 4(7.3%) | 5(8.6%) | ||
| Never smoker | 28(50.9%) | 27(48.3%) | ||
| Diabetes mellitus | 35(63.6%) | 32(55.2%) | 0.838 | 0.360 |
| Hypertension | 42(76.4%) | 47(81.0%) | 0.368 | 0.544 |
| Dyslipidemia | 22(40%) | 33(56.9%) | 3.226 | 0.072 |
| Coronary artery disease | 13(23.6%) | 18(31.0%) | 0.776 | 0.378 |
| Cerebrovascular accident | 15(27.3%) | 20(34.5%) | 0.686 | 0.407 |
| Previous TLR | 15(27.3%) | 5(8.6%) | 6.742 | 0.009 |
| Baseline Rutherford category | 7.556 | 0.056 | ||
| 2 | 12(21.9%) | 10(17.3%) | 0.377 | 0.539 |
| 3 | 18(32.7%) | 31(51.7%) | 4.935 | 0.026 |
| 4 | 13(23.6%) | 13(24.1%) | 0.024 | 0.877 |
| 5 | 12(21.8%) | 4(6.9%) | 5.171 | 0.023 |
Abbreviations: DCB = drug-coated balloon; nSFA = superficial femoral artery nonrevas cularization; TLR = target limb revascularization; BMI=Body mass index.
Normally distributed data were described as mean ± standard deviation, whereas skewed data as medians (quartiles). The qualitative data were described as counts (percentage).
Former smoker was the patients who had stopped smoking for at least six month.
Table 2.
Baseline lesion characteristics.a.
| Variables | DCB (n = 55 lesions) | nSFA n=(58 lesions) |
Statistics t/χ2 | P |
|---|---|---|---|---|
| Iliac artery disease | 6(10.9%) | 46(79.3%) | 53.17 | <0.001 |
| Popliteal artery disease | 28(50.9%) | 20(34.5%) | 3.117 | 0.077 |
| Runoff vessels | 6.821 | 0.060 | ||
| 0 | 3(5.5%) | 0(0.0%) | / | 0.112 |
| 1 | 17(30.9%) | 12(20.6%) | 1.545 | 0.214 |
| 2 | 23(41.8%) | 23(39.7%) | 0.055 | 0.815 |
| 3 | 12(21.8%) | 23(39.7) | 4.201 | 0.040 |
| Lesion type | 4.446 | 0.072 | ||
| De novo | 41(74.6%) | 52(89.7%) | ||
| Restenotic (nonstented) | 2(3.6%) | 1(1.7%) | ||
| In-stent restenosis | 12(21.8%) | 5(8.6%) | ||
| Lesion location | 5.468 | 0.019 | ||
| Single lesion | 29(52.7%) | 18(31.0%) | ||
| Multiple lesions | 26(47.3%) | 40(69.0%) | ||
| Stenosed lesion length(mm) | 87.89 ± 80.67 | 100.48 ± 55.92 | −0.661 | 0.511 |
| Lesion stenosis,% | 0.65 ± 0.16 | 0.70 ± 0.13 | −1.311 | 0.195 |
| Total occlusions,% | 28(50.9%) | 42(72.4%) | 5.538 | 0.019 |
| Occluded lesion length(mm) | 101.67 ± 69.97 | 183.11 ± 100.52 | −3.996 | <0.001 |
| Lesion calcification | 3.047 | 0.382 | ||
| Grade 0 | 6(10.9%) | 3(5.2%) | 0.606 | 0.436 |
| Grade 1 | 33(60%) | 38(65.5%) | 0.368 | 0.544 |
| Grade2 | 15(27.3%) | 13(22.4%) | 0.358 | 0.550 |
| Grade3 | 1(1.8%) | 4(6.9%) | / | 0.365 |
| Grade4 | 0(0.0%) | 0(0.0%) | / | / |
Abbreviations: DCB = drug-coated balloon; nSFA = superficial femoral artery nonrevas cularization.
The quantitative data were expressed as means and standard deviations. The qualitative data were described as counts (percentage).
Procedural outcomes. Initial technical success (residual stenosis ≤30%) was achieved in all subjects. More patients in the nSFA group received iliac artery reconstruction (65.5% vs. 10.9%, P < 0.001) compared to the DCB group. However, lower provisional stenting (36.4% vs. 0.0%) and adverse event rates were observed in the DCB group (12.7% vs. 1.7%, P = 0.029). The perioperative complications included six distal thromboses resolved with guiding catheter, one fibular artery arteriovenous fistula successfully treated with microcoil embolization in the DCB group, and one patient in the nSFA group with cardiac insufficiency that improved after conservative treatment.
Effectiveness outcomes. During the 12-months of follow-up, one patient died in each of the two groups and one subject in the nSFA group was lost to follow-up at 2 months after discharge. Thus, the remaining 50 patients (54 limbs) in the DCB group and 43 patients (56 limbs) in the nSFA group were eligible for evaluation.
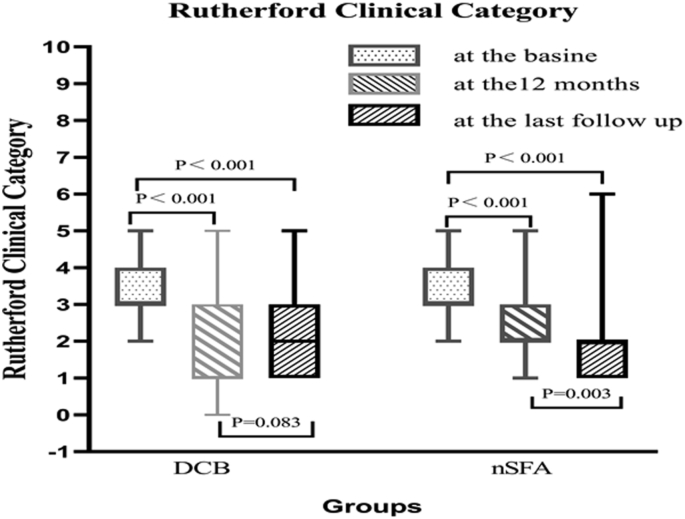
At 12 months, the Rutherford categories had significantly decreased in both groups (P < 0.001; Fig. 1) and, while the total effective rate of the nSFA group was higher than that of the DCB group, the difference was not statistically significant (79.7% vs. 64.3%, P = 0.074; Table 3). In the univariate analysis, the choice of therapeutic method was associated with a decrease in Rutherford category at 12 months. However, multivariate analysis showed that the choice of therapeutic method was not an influential factor for the improvement of Rutherford category at the 12-month follow-up (odds ratio [OR] = 0.42, 95% confidence interval [CI][-1.804, 0.077], P = 0.072; Fig. 2). The effective rate was significantly higher in the DCB group at 12 months.
Fig. 1.
Comparison of Rutherford categories prior to intervention, at 12-month and at last follow-up. DCB, drug-coated balloon; nSFA, superficial femoral artery nonrevascularization strategy.
Table 3.
Clinical effectiveness outcomes at the 12 months and last follow-up.a.
| DCB | nSFA | Z/χ2 | P | |||
|---|---|---|---|---|---|---|
| At baseline | 3(3,4) | 3(3,4) | −1.316 | 0.188 | ||
| At the 12-months | N = 54 limbs | N = 56 limbs | ||||
| Change in Rutherford class | ||||||
| marked improvement(-4) | 7(13.0%) | 0(0.0%) | / | 1.000 | ||
| moderate improvement (−3) | 8(14.8%) | 2(3.6%) | 2.955 | 0.086 | ||
| mild improvement(-2) | 13(24.1%) | 13(23.2%) | 0.011 | 0.915 | ||
| minimal improvement(-1) | 15(27.8%) | 21(37.5%) | 1.180 | 0.277 | ||
| no change(0) | 10(18.4%) | 16(28.6%) | 1.539 | 0.215 | ||
| mildly worse(+1) | 1(1.9%) | 4(7.1%) | / | 0.364 | ||
| moderately worse(+2) | 0(0.0%) | 0(0.0%) | / | / | ||
| Improvement of Rutherford | ||||||
| markedly effective rate | 28(51.9%) | 15(26.8%) | 7.254 | 0.007 | ||
| the effective rate | 15(27.8%) | 21(37.5%) | 1.180 | 0.277 | ||
| total effective rate | 43(79.7%) | 36(64.3%) | 3.198 | 0.074 | ||
| ineffective rate | 10(18.4%) | 16(28.6%) | 1.539 | 0.215 | ||
| deterioration rate | 1(1.9%) | 4(7.1%) | / | 0.364 | ||
| At the last follow up | N = 54 limbs | N = 56 limbs | ||||
| Change in Rutherford class | ||||||
| marked improvement(-4) | 6(11.1%) | 2(3.6%) | 1.334 | 0.248 | ||
| moderate improvement (−3) | 6(11.1%) | 6(10.7%) | 0.004 | 0.947 | ||
| mild improvement(-2) | 9(16.6%) | 19(33.9%) | 4.317 | 0.038 | ||
| minimal improvement(-1) | 12(22.2%) | 12(21.4%) | 0.010 | 0.920 | ||
| no change(0) | 19(35.2%) | 15(26.8%) | 0.908 | 0.341 | ||
| mildly worse(+1) | 1(1.9%) | 1(1.8%) | / | 1.000 | ||
| moderately worse(+2) | 1(1.9%) | 1(1.8%) | / | 1.000 | ||
| Improvement of Rutherford | ||||||
| markedly effective rate | 21(38.8%) | 27(48.2%) | 0.972 | 0.324 | ||
| the effective rate | 12(22.2%) | 12(21.4%) | 0.010 | 0.920 | ||
| total effective rate | 33(61.0%) | 39(69.6%) | 0.885 | 0.347 | ||
| ineffective rate | 19(35.2%) | 15(26.8%) | 0.908 | 0.341 | ||
| deterioration rate | 2(3.8%) | 2(3.6%) | / | 1.000 | ||
Abbreviations: DCB = drug-coated balloon; nSFA = superficial femoral artery nonrevas cularization.
Non-normally distributed data were described as medians (quartiles). The qualitative data were described as counts (percentage).
Fig. 2.
Summarizes the results of influential factor of the improvement of Rutherford category at 12-month identified in multivariate analysis.
At the last follow-up visit, sustained improvement of the Rutherford category from the 12-month follow-up visit was observed in the nSFA group (Fig. 2). However, there was no significant change in the DCB group (Fig. 2). The total effective rates at the last follow-up were similar between the DCB and nSFA groups (61.0% vs. 69.6%, P = 0.347). A significantly higher (yet mild) improvement in Rutherford category was detected in the nSFA group at the last follow-up visit (Table 3).
Safety outcomes and treatment costs. The median follow-up times of the DCB and nSFA groups were 17 and 33 months, respectively. The limb salvage rates (as estimated by Kaplan–Meier method) of the DCB and nSFA groups at 54 months were similar (76.5% vs. 85.6%, P = 0.114; Fig. 3). The survival rates at 54 months were also similar between the DCB and nSFA groups (76.6% vs. 81.4%, P = 0.380; Fig. 4). The lengths of hospital stay were also comparable between the groups. The catheterization laboratory cost and perioperative adverse events rate of the nSFA group were significantly lower than those of the DCB group (34253.69 ± 28172.87 yuan vs. 56936.76 ± 41278.36 yuan, P = 0.001; Table 4). Five target limb reinterventions were performed in the DCB group.
Fig. 3.
The limb salvage rate at 54 months for drug-coated balloon (DCB) angioplasty vs superficial femoral artery nonrevascularization strategy (nSFA). the log-rank p value was 0.114.
Fig. 4.
Kaplan-Meier estimates of the survival rate at 54-month the log-rank p value was 0.380. DCB, drug-coated balloon; nSFA, superficial femoral artery nonrevascularization strategy.
Table 4.
Safety outcomes and treatment cost.a.
| DCB | nSFA | t/χ2 | P | |
|---|---|---|---|---|
| Major adverse events | 7/55(12.7%) | 1/58(1.7%) | / | 0.029 |
| target limb distal embolision | 6/55(10.9%) | 0/58(0.0%) | / | 1.000 |
| cardiac insufficiency | 0/55(0.0%) | 1/58(1.7%) | / | 1.000 |
| arteriovenous fistula | 1/55(1.8%) | 0/58(0.0%) | / | 1.000 |
| Target limb reintervention | 5/55(9.1%) | |||
| Length of hospital stay | 7.00(5.00,9.00) | 7.0(5.00,10.25) | −0.937 | 0.349 |
| Treatment cost(yuan) | 56936.76± | 34253.69± | 3.373 | 0.001 |
| 41278.36 | 28172.87 |
Abbreviations: DCB = drug-coated balloon; nSFA = superficial femoral artery nonrevas cularization.
Normally distributed data were described as mean ± standard deviation, whereas skewed data as medians (quartiles). The qualitative data were described as counts (percentage).
Functional outcomes. After excluding the data of patients lost to follow-up or death, 46 patients (50 limbs) in the DCB group and 38 patients (48 limbs) in the nSFA group were included in the analysis. Both groups showed improvement in the overall WIQ score as well as its three subscales (distance, speed, and stair) from baseline to the last follow-up visit, with no significant difference between the two groups (P > 0.05; Table 5).
Table 5.
Functional outcomes at the last follow-up.a.
| WIQ | DCB | nSFA | Z/t | P | |
|---|---|---|---|---|---|
| Total score | at admission | 0.34 ± 0.26 | 0.36 ± 0.16 | −0.612 | 0.542 |
| at the last follow up | 0.51 ± 0.28 | 0.52 ± 0.21 | −0.237 | 0.813 | |
| T | −3.233 | −4.243 | |||
| P | 0.002 | <0.001 | |||
| Distance | at admission | 0.30(0.04,0.68) | 0.45(0.20,0.57) | −1.229 | 0.219 |
| at the last follow up | 0.86(0.19,1.00) | 0.89(0.48,1.00) | −0.955 | 0.340 | |
| Z | −3.551 | −4.512 | |||
| P | <0.001 | <0.001 | |||
| Speed | at admission | 0.25(0.10,0.33) | 0.26(0.23,0.28) | −0.290 | 0.772 |
| at the last follow up | 0.31(0.24,0.43) | 0.28(0.25,0.34) | −0.376 | 0.707 | |
| Z | −2.277 | −2.682 | |||
| P | 0.023 | 0.007 | |||
| Stair-climbing | at admission | 0.25(0.04,0.67) | 0.42(0.17,0.53) | −0.096 | 0.923 |
| at the last follow up | 0.61(0.17,0.88) | 0.50(0.23,0.88) | −0.272 | 0.786 | |
| Z | −2.262 | −2.895 | |||
| P | 0.024 | 0.004 |
Abbreviations: DCB = drug-coated balloon; nSFA = superficial femoral artery nonrevas cularization; WIQ = walking impairment questionnaire.
Normally distributed data were described as mean ± standard deviation, whereas skewed data as medians (quartiles).
Discussion
Although previous studies recommended isolated PFA revascularization for the treatment of concomitant PFA and significant SFA significant atherosclerotic disease,12,13 the PFA is less involved than SFA. For most patients with PAD with patent PFA and occluded SFA, iliac angioplasty and/or exercise therapy were applicable in the nSFA group. Sagar et al.16 identified high performers as those with combined WIQ subscale scores for walking distance and stair-climbing of >75.5 (out of 100). In the current study, patients in the DCB and nSFA groups showed evidence of high walking performance at the last follow-up visit, as shown by the combined WIQ subscale scores. This finding may be related to the collateral pathways, which are compensated following severe stenosis or occlusive SFA disease. In the presence of a patent iliofemoral deep artery and at least one healthy outflow tract of the infrapopliteal artery (either pre-existing or re-established by POBA), adequate collateral flow helps maintain downstream perfusion pressure, relieving limb symptoms (such as intermittent claudication or ischemic resting pain), and improving walking performance in patients with PAD. This further facilitates the development of follow-up home-based walking exercise. In turn, collateral vessels tend to increase in number with home-based walking exercise.17 Moreover, walking exercise can also improve the ankle plantar flexor and hip extension muscle strength.18 Cases classified as Rutherford category 5 generally require revascularization of the affected limb.19 SFA revascularization was performed in the DCB group in the present study, while iliac/PFA angioplasty and exercise therapy were applied in the nSFA group. At least one vessel inflow to the wound was achieved in both groups. All patients with tissue loss were treated with dressing change or vacuum sealing drainage. A previous study showed that walking exercise may accelerate wound healing.20 However, the number of patients with Rutherford 5 grade in the present study was too small for statistical analysis and definitive conclusions.
In the current study, a significantly higher number of total occlusive lesions, longer lesion length, and multiple segments of SFA disease were observed in the nSFA group compared to those in the DCB group. Endovascular interventions for long SFA occlusive lesions have risks of acute distal thrombus embolism, longer operation time, and endometrial damage at both ends of the SFA occlusive segment.8 Therefore, it is preferable to avoid SFA reconstruction, especially for older people with underlying diseases and good runoff vessels. In this retrospective study, selection bias was inevitable and theoretically decreased the clinical efficacy in the nSFA group. However, at the 12-month follow-up visit, total effective rates did not differ significantly between the two groups. In addition, multivariate analysis showed that the therapeutic method was not an influential factor for the improvement of Rutherford category at 12 months; this finding further suggests that the effectiveness of avoiding SFA reconstruction in PAD patients is not inferior to SFA revascularization with DCB from the perspective of improving the Rutherford category. Similar to previous research results, the Rutherford category improvement rates did not differ significantly between SFA revascularization with POBA and SFA nonrevascularization strategies at 12 months.21 Furthermore, a meta-analysis suggested no significant difference between DCB versus POBA in terms of improvement in Rutherford category at 12 months.6
At present, when evaluating the therapeutic effects of PAD, the patency of the target vessels is no longer the main concern. The improvement in clinical symptoms and limb salvage rates have also received attention because these factors are the ultimate treatment goals. The 54-month follow-up of this study showed no significant differences in the limb salvage rates between the DCB and nSFA groups (P = 0.114). Furthermore, the effective rate was significantly higher in the DCB group at 12 months. Conversely, a significantly higher (yet mild) improvement in Rutherford category was detected in the nSFA group at the last follow-up. This may imply that SFA revascularization with DCB can restore the blood supply of the lower extremities rapidly and alleviate clinical symptoms in the short-term. As such, this treatment approach is suitable for addressing acute ischemic events of the lower extremities. While the SFA nonrevascularization strategy enhances the collateral vessel flow slowly and continuously, it may be more applicable for chronic obstructive SFA disease.
PAD is a part of systemic atherosclerotic disease and is more likely to be accompanied by cardiovascular and cerebrovascular diseases. The cardiovascular and cerebral mortality rates in patients with PAD are higher than those in patients without PAD.22 Regarding PAD patients with cardio- and cerebrovascular diseases, researchers have expressed concern regarding the recurrence of cardio-/cerebrovascular events during supervised treadmill exercises.23 The current study used home-based walking exercise; although this simple and practicable approach (without relying on large equipment) is not as effective as supervised walking exercises, it can relatively decrease the risk of cardio-/cerebrovascular events.
Previous studies24 reported no significant difference in all-cause mortality rates between endovascular reconstruction and exercise therapy for patients with intermittent claudication. The all-cause mortality rates were also similar between the DCB and nSFA groups in this study. A higher incidence of perioperative major adverse events and target vessel revascularization was observed in the DCB group. However, selection bias occurred, including a lower proportion of total occlusive disease, multiple segments, and long length of SFA in the DCB group. Moreover, six lesions were treated with Rotarex catheters before DCB reconstruction of SFA occlusive lesions. These factors contributed to the reduced occurrence of perioperative adverse events in the DCB group.
The effectiveness and safety were comparable between the two cohorts in the present study. Cost-effectiveness should also be considered in the treatment decision; the catheterization laboratory cost of the nSFA group was significantly lower than that of the DCB group. The reasons for this result are as follows. First, 15 patients (16 lesions) in the nSFA group were treated with walking exercise and medication therapy only, meaning that there were no interventional treatment costs. Second, all 51 patients (55 lesions) in the DCB group were pre-dilated with a plain balloon and reconstructed with DCB, while only 33 patients (38 lesions) in the nSFA group were treated with POBA and the price of drug-coated balloons was higher than that of plain balloons. Furthermore, six patients (six lesions) received Rotarex catheters, which further increased the treatment cost of the DCB group. Finally, the selection bias in this study may have exaggerated the cost-effectiveness ratio of the DCB group. If these selection biases were eliminated, avoiding SFA reconstruction for the treatment of PAD will be more advantageous in terms of medical costs.
The current study had some limitations. First, the aforementioned selection and information biases due to the retrospective design. Furthermore, our study did not report ankle-brachial index test findings since some of these measures were lost at baseline. However, a previous meta-analysis25 reported that, while this index may be an effective screening tool for identifying patients with PAD, it may play only a minor role in guiding treatment decisions. Third, the main follow-up visit was conducted using structured telephone interviews. This study obtained at least three contact phone numbers for each patient, which may be related to the low rate of patients lost to follow-up. Another major limitation of this study was the small sample size. Thus, future randomized controlled studies are needed to further verify the findings of this study.
Conclusion
The results of this study suggest that avoiding SFA revascularization provides favorable efficacy and safety outcomes compared to SFA revascularization with DCB in selected patients. In cases with patent iliofemoral deep artery and at least one healthy run-off vessel of the infrapopliteal artery (which were either pre-existing or re-established by POBA), home-based walking exercise combined with medication therapy may be a therapeutic option for the treatment of chronic obstructive SFA disease.
Declaration of competing interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Contributor Information
Hongcheng Ren, Email: hongchengren@pku.edu.cn.
Jinman Zhuang, Email: zhuangjinman@sina.com.
Xuan Li, Email: xuanli@vip.sina.com.
Tianrun Li, Email: litianrun@163.com.
Jingyuan Luan, Email: drluan@139.com.
Changming Wang, Email: wcmwy@163.com.
References
- 1.Fowkes F.G., Rudan D., Rudan I. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 2.Tepe G., Laird J., Schneider P. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. 2015;131:495–502. doi: 10.1161/CIRCULATIONAHA.114.011004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katsanos K., Kitrou P., Spiliopoulos S. Comparative effectiveness of plain balloon angioplasty, bare metal stents, drug-coated balloons, and drug-eluting stents for the treatment of infrapopliteal artery disease: systematic review and bayesian network meta-analysis of randomized controlled trials. J Endovasc Ther. 2016;23:851–863. doi: 10.1177/1526602816671740. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfield K., Jaff M.R., White C.J. Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. N Engl J Med. 2015;373:145–153. doi: 10.1056/NEJMoa1406235. [DOI] [PubMed] [Google Scholar]
- 5.Jongsma H., Bekken J.A., de Vries J.P. Drug-eluting balloon angioplasty versus uncoated balloon angioplasty in patients with femoropopliteal arterial occlusive disease. J Vasc Surg. 2016;64:1503–1514. doi: 10.1016/j.jvs.2016.05.084. [DOI] [PubMed] [Google Scholar]
- 6.Kayssi A., Al-Atassi T., Oreopoulos G. Drug-eluting balloon angioplasty versus uncoated balloon angioplasty for peripheral arterial disease of the lower limbs. Cochrane Database Syst Rev. 2016;8:CD011319. doi: 10.1002/14651858.CD011319.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brodmann M. IN.PACT global study: overview and 12-month outcomes of the long lesion and In-stent restenosis imaging cohorts. Endovasc Today. 2015;12:8–10. [Google Scholar]
- 8.Tepe G., Micari A., Keirse K. Drug-coated balloon treatment for femoropopliteal artery disease: the chronic total occlusion cohort in the IN.PACT global study. JACC Cardiovasc Interv. 2019;12:484–493. doi: 10.1016/j.jcin.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Katsanos K., Spiliopoulos S., Kitrou P. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.011245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallino A., Mahler F., Probst P. Percutaneous transluminal angioplasty of the arteries of the lower limbs: a 5 year follow-up. Circulation. 1984;70:619–623. doi: 10.1161/01.cir.70.4.619. [DOI] [PubMed] [Google Scholar]
- 11.Karnabatidis D., Spiliopoulos S., Pastromas G. Endovascular management of the arteria profunda femoralis: long-term angiographic and clinical outcomes. Cardiovasc Intervent Radiol. 2012;35:1016–1022. doi: 10.1007/s00270-011-0284-x. [DOI] [PubMed] [Google Scholar]
- 12.de Athayde Soares R., Matielo M.F., Brochado Neto F.C. The importance of the superficial and profunda femoris arteries in limb salvage following endovascular treatment of chronic aortoiliac occlusive disease. J Vasc Surg. 2018;68:1422–1429. doi: 10.1016/j.jvs.2018.02.052. [DOI] [PubMed] [Google Scholar]
- 13.Davies R.S., Rashid S.H., Adair W. Isolated percutaneous transluminal angioplasty of the profunda femoris artery for limb ischemia. Vasc Endovasc Surg. 2013;47:423–428. doi: 10.1177/1538574413491636. [DOI] [PubMed] [Google Scholar]
- 14.Hamburg N.M., Balady G.J. Exercise rehabilitation in peripheral artery disease: functional impact and mechanisms of benefits. Circulation. 2011;123:87–97. doi: 10.1161/CIRCULATIONAHA.109.881888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lejay A., Laverny G., Paradis S. Moderate exercise allows for shorter recovery time in critical limb ischemia. Front Physiol. 2017;8:523. doi: 10.3389/fphys.2017.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sagar S.P., Brown P.M., Zelt D.T. Further clinical validation of the walking impairment questionnaire for classification of walking performance in patients with peripheral artery disease. Int J Vasc Med. 2012;2012:190641. doi: 10.1155/2012/190641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDermott M.M., Carroll T.J., Kibbe M. Proximal superficial femoral artery occlusion, collateral vessels, and walking performance in peripheral artery disease. JACC Cardiovasc Imaging. 2013;6:687–694. doi: 10.1016/j.jcmg.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schieber M.N., Pipinos, Johanning J.M. Supervised walking exercise therapy improves gait biomechanics in patients with peripheral artery disease. J Vasc Surg. 2020;71:575–583. doi: 10.1016/j.jvs.2019.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji Donghua, Zhang Tao, Cheng Li. Evaluation of angiosome-targeted infrapopliteal endovascular revascularization in critical diabetic limb ischemia. J Intervent Med. 2018;1:176–181. doi: 10.19779/j.cnki.2096-3602.2018.03.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emery C.F., Kiecolt-Glaser J.K., Glaser R. Exercise accelerates wound healing among healthy older adults: a preliminary investigation. J Gerontol A Biol Sci Med Sci. 2005;60:1432–1436. doi: 10.1093/gerona/60.11.1432. [DOI] [PubMed] [Google Scholar]
- 21.Zhuang J.M., Li X., Li T.R. Randomized controlled trial to superficial femoral artery recanalization for lower extremity arteriosclerosis obliterans. Journal of Peking University Health sciences. 2017;49:153–157. [PubMed] [Google Scholar]
- 22.Diehm C., Allenberg J.R., Pittrow D. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation. 2009;120:2053–2061. doi: 10.1161/CIRCULATIONAHA.109.865600. [DOI] [PubMed] [Google Scholar]
- 23.Gommans L.N., Fokkenrood H.J., van Dalen H.C. Safety of supervised exercise therapy in patients with intermittent claudication. J Vasc Surg. 2015;61:512–518. doi: 10.1016/j.jvs.2014.08.070. [DOI] [PubMed] [Google Scholar]
- 24.Frans F.A., Bipat S., Reekers J.A. Systematic review of exercise training or percutaneous transluminal angioplasty for intermittent claudication. Br J Surg. 2012;99:880–881. doi: 10.1002/bjs.7656. [DOI] [PubMed] [Google Scholar]
- 25.Pandey A., Banerjee S., Ngo C. Comparative efficacy of endovascular revascularization versus supervised exercise training in patients with intermittent claudication: meta-analysis of randomized controlled trials. JACC Cardiovasc Interv. 2017;10:712–724. doi: 10.1016/j.jcin.2017.01.027. [DOI] [PubMed] [Google Scholar]