Percutaneous vertebroplasty (PVP) is an interventional radiological procedure involving the injection of bone cement (polymethylmethacrylate) into a fractured vertebral body to remodel the vertebral body and relieve lumbar pain. This procedure has become an increasingly recommended therapeutic intervention due to its high efficacy and safety.1,2
Nevertheless, in some cases, the leakage of bone cement can result in lethal complications such as cardiac and pulmonary cement embolus. However, recent descriptions of cardiac and pulmonary cement embolus in the clinical literature are limited to a few case reports, and the management associated with such embolisms is not well established.3, 4, 5 Here, we present a patient who suffered from cardiac and pulmonary embolus caused by cement leakage after PVP, which was successfully managed by percutaneous endovascular retrieval via a modified wire-loop snare technique.
A 70-year old woman diagnosed with vertebral body compression fractures of T10 and L4 caused by trauma underwent uneventful percutaneous vertebroplasty under fluoroscopic guidance (Fig. 1a). The patient was asymptomatic after the procedure. However, prevertebral leakage of cement was detected on spine X-ray performed on postoperative day 1 (Fig. 1b) and confirmed with subsequent emergency chest and abdomen CT (Fig. 1c, d and 1e, cement leakage into the paravertebral vein and inferior vena cava). Also, a chest CT scan revealed a pulmonary embolus was also detected in the segmentary pulmonary arteries of the right inferior lobe (Fig. 1f).
Fig. 1.
1a.MRI image shows vertebral body compression fractures of T10 and L4 (arrows); 1b. Post PVP spine X-ray shows prevertebral leakage of cement (arrow); 1c-d. Post PVP chest and abdomen CT shows leakage of bone cement into a paravertebral vein (arrow); 1e.Post PVP chest and abdomen CT shows cement leakage into inferior vena cava (arrow); 1f. Post PVP chest and abdomen CT shows small cement fragment within the segmentary pulmonary arteries of the right inferior lobe (arrow); 1 g. Post PVP transthoracic echocardiogram shows an about 6-cm long hyperechogenic thread-like cement (thick arrow) running from the lateral wall of the right atrium (RA) to the right ventricle (RV) through the tricuspid valve (thin arrow); 1 h. Post PVP CTPA shows a high-attenuation thread-like cement of 6 cm length from the right atrium to the right ventricle (arrow).
The patient was treated conservatively due to the small volume of cement and because the condition was asymptomatic. Unfortunately, a 6 cm long hyperechogenic thread-like structure running from the lateral wall of the right atrium (RA) to the right ventricle (RV) through the tricuspid valve (Fig. 1g) was detected on transthoracic echocardiogram performed on postoperative day 4, and a valve malfunction was also detected. An emergent CT pulmonary angiogram (CTPA) was immediately performed. On the CTPA, there was a high-attenuation thread-like structure 6 cm in length from the right atrium to the right ventricle (Fig. 1h). After consideration of our patient's history and thoughtful interpretation of the CTPA scan, the thread-like structure was identified as the bone cement.
The tip of the cement embolus was inside the myocardium of the lateral wall of the right atrium, with a high risk of cardiac perforation. Therefore, it was clear that the cement embolus should be removed to avoid potentially catastrophic consequences. Given the complexity of this uncommon complication, a multidisciplinary team involving cardiologists, cardiothoracic surgeons, interventional radiologists, orthopedic surgeons, vascular surgeons, and an anesthetist was assembled to deal with this problem. The team initially planned open heart surgery to remove the cement. After carefully balancing the risks and benefits of interventional therapy versus open surgery, we postponed the open surgery since the risk of surgery was high and the potential benefit from the surgery was low. However, adequate preparation for open surgery was required in case interventional radiologists failed to retrieve the cement embolus in the right heart. After the treatment plan was made, the patient was immediately transferred to the angiography room for removal of the cement.
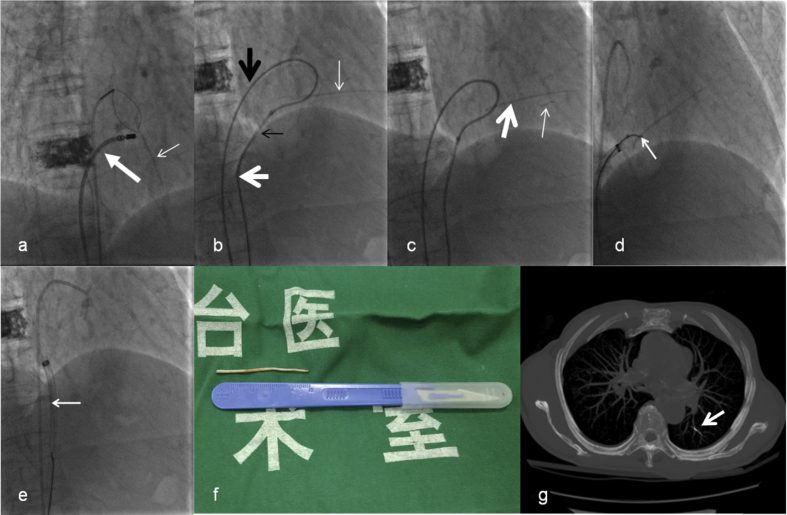
Access was gained into the right femoral vein, and a 6-F vascular sheath (Terumo Corporation, Shibuya-ku, Japan) was placed. A6-F MPA2 catheter (Cordis Corporation, Miami Lakes, USA) was advanced into the right atrium, and then a 5- F loop snare catheter (Shanghai Shape Memory Alloy Corporation, Shanghai, China) was manipulated to snare the thread-like cement. However, fluoroscopy showed thread-like cement from the right atrium to the right ventricle without accessible free end. Thus, the thread-like cement could not be snared. To solve this dilemma, a new access point via the right femoral vein was obtained, and an 8-F vascular sheath (Cordis Corporation, Miami Lakes, USA) was placed. Then we introduced an 8-F ablation catheter (Biosense Webster Ltd, Yokneam, Israel) through the 8-F vascular sheath and attempted to relocate the thread-like cement but failed (Fig. 2a).
Fig. 2.
2a. Fluoroscopy shows thread-like cement from the right atrium to the right ventricle without accessible free end (thin arrow). Therefore, the thread-like cement could not be snared. Then we introduce an ablation catheter (thick arrow) to relocate the thread-like cement but fail to release an accessible free end; 2b. TIG catheter (thick black arrow), guidewire, snare loop catheter (white thick arrow) and MPA2 catheter (black thin arrow)are manipulated to form a loop, and then the loop is used to hook thread-like cement (white thin arrow) slightly and try to release an accessible free end; 2c. The tiny bit of the thread-like cement (thin arrow) is broken off, whereas the major part of thread-like cement (thick arrow) is relocated and an accessible free end is released; 2d.The snare loop catheter snares the free end of the thread-like cement (arrow); 2e.Thread-like cement is successfully extracted through 12-F long sheath (arrow); 2f. The extracted thread-like cement with length of 5 cm; 2 g. Post-operative chest CT confirms the presence of broken cement fragment migrated into the segmentary pulmonary arteries of left inferior lobe (arrow).
A 0.035-inch soft guidewire (Terumo Corporation, Shibuya-ku, Japan) and a 5-F TIG catheter (Terumo Corporation, Shibuya-ku, Japan) were subsequently introduced to the right atrium through the 8-F vascular sheath. Then we manipulated the soft guide wire to encompass the thread-like cement; afterward, the soft guidewire was advanced back into the snare. The leading end of the wire was subsequently snared and withdrawn into the MPA2 catheter to form a loop. After that, the loop was used to hook the thread-like cement slightly and attempt to release an accessible free end (Fig. 2b). Unfortunately, the tiny bit of the thread-like cement was broken off and migrated into the pulmonary artery. However, the major part of thread-like cement was relocated, and an accessible free end was released (Fig. 2c). Then a 12-F long sheath (Life tech Scientific Corporation, Shenzhen, China) was placed and advanced into the right atrium by exchanging with the previous 8-F vascular sheath. Afterward, the snare loop catheter was introduced to the right atrium through the sheath. The snare loop catheter finally snared the free end of the thread-like cement (Fig. 2d), and then the thread-like cement was successfully extracted through the 12-F long sheath (Fig. 2e). The extracted thread-like cement was approximately 5 cm long (Fig. 2f).
Compared to the pre-operative CTPA, the post-operative chest CT scan revealed no remnants of the cement embolus in the right atrium and confirmed the presence of a broken cement fragment that migrated into the segmentary pulmonary arteries of the left inferior lobe (Fig. 2g). Finally, the patient had an uncomplicated recovery and was discharged one week later.
Percutaneous vertebroplasty (PVP) with intravertebral injection of polymethylmethacrylate (PMMA) bone cement was first described in 1987 by Galiber and colleagues for treatment of patients with vertebral hemangiomas.6 PVP is considered a minimally invasive and relatively safe procedure. However, with increasing applications of PVP, the number of reports of complications is also rising. The reported complications include bleeding at the puncture site, inaccurate needle placement, pain exacerbation, local infection, leakage of cement into the spinal canal or paravertebral tissues, perivertebral venous leakage, pulmonary cement embolism, and cardiac cement embolism.7,8
The most lethal complications are cardiac and pulmonary cement embolus, which are caused by cement leakage migrating from the vertebral venous plexus to the inferior vena cava, right heart chambers, and pulmonary arteries.9 The presentations of cardiac and pulmonary cement embolus may range from asymptomatic to symptoms such as chest pain, dyspnea, syncope or life-threatening symptoms such as acute respiratory distress or cardiac tamponade.10
Although cement leakage from the treated vertebrae is the most common complication associated with PVP (with cement leakage reported in 20%–73% of treated vertebrae), pulmonary cement embolus was considered as a rare complication secondary to cement leakage.11,12 However, a recent systematic review of the literature shows the risk of pulmonary cement embolus ranging from 3.5% to 23%, suggesting that this is a more frequent complication than originally thought.13
Although the exact mechanism of how thread-like cement reached and perforated the RV in our patient is unknown, the low viscosity of cement, placement of a needle into the basivertebral vein, and overfilling of a vertebral body could all attribute to causes of cement embolus.
Until recently, the best treatment strategy for the management of pulmonary cement embolus remained controversial and was not well characterized. The choice of treatment for these conditions depends on the location of the cement and the severity of symptoms. The treatment for symptomatic or central pulmonary cement embolus is an open surgery or percutaneous removal, whereas conservative management (anticoagulation or careful observation) is recommended for smaller or peripherally located emboli.3,12 However, the benefits of anticoagulation for asymptomatic patients are still controversial. The rationale for anticoagulation is to reduce the risk of thrombus formation on the embolic material.13 However, no data support the formation of thrombi on the cement. On the contrary, the patients with pulmonary cement embolus in Du Hwan Choe's12 and Asem Mansour's study14 received no anticoagulation therapy and remained asymptomatic during a long-term follow-up period, which supported the conclusion that asymptomatic patients may be effectively managed conservatively with careful observation.
The early detected pulmonary cement embolus in our patient was only managed conservatively with careful observation due to the asymptomatic presentation and small emboli. However, intracardiac cement embolus detected later must be retrieved due to the high risk of cardiac rupture and cardiac tamponade.4 Open surgery and percutaneous retrieval are two feasible treatment options for the management of intracardiac cement embolus. Catherine Hatzantonis15 reviewed the published literature and reported that the majority (14/19) of intracardiac cement embolus cases were treated with open surgery; 4 cases were treated with percutaneous removal, and only 1 case was treated conservatively with anticoagulation and clinical follow-up. In the present study, the multidisciplinary team was established to ensure the optimal treatment for the patient. We initially planned open heart surgery to remove the cement in the right heart. However, given the high surgical risk and poor prognosis of an elderly patient (70-year-old) combined with diabetes, percutaneous retrieval was recommended. However, the retrieval of cardiac cement embolus in the present case was not uneventful because of thread-like cement was embed into the lateral wall of the RA. We tried many methods and finally successfully retrieved the embed thread-like cement via a modified wire-loop snare technique.
In conclusion, more attention should be paid to the possibility of the development of cardiac and pulmonary cement embolus caused by cement leakage following PVP. When considering the appropriate treatment strategy, percutaneous endovascular retrieval may provide a less invasive alternative to open surgery. Also, the multidisciplinary team meetings may be useful for helping guide the assessment and management of patients with this uncommon but potentially fatal complication.
Conflicts of interest
All authors have no conflict of interest regarding this paper.
Ethical standards
The authors declare that they complied with ethical standards and the study was in accord with the Helsinki Declaration.
We confirm that the research was not funded by any organization.
Contributor Information
Fei Teng, Email: tengfeinb2018@126.com.
Xin jian Xu, Email: kg529452183@126.com.
Qiang Liu, Email: liuqiangdoc@126.com.
References
- 1.Mathis J.M., Barr J.D., Belkoff S.M. Percutaneous vertebroplasty: a developing standard of care for vertebral compression fractures. Am J Neuroradiol. 2001;22(2):373–381. [PMC free article] [PubMed] [Google Scholar]
- 2.Cotten A., Dewatre F., Cortet B. Percutaneous vertebroplasty for osteolytic metastases and myeloma: effects of the percentage of lesion filling and the leakage of methyl methacrylate at clinical follow-up. Radiology. 1996;200(2):525–530. doi: 10.1148/radiology.200.2.8685351. [DOI] [PubMed] [Google Scholar]
- 3.Tozzi P., Abdelmoumene Y., Corno A.F. Management of pulmonary embolism during acrylic vertebroplasty. Ann Thorac Surg. 2002;74(5):1706–1708. doi: 10.1016/s0003-4975(02)03962-0. [DOI] [PubMed] [Google Scholar]
- 4.Caynak B., Onan B., Sagbas E. Cardiac tamponade and pulmonary embolism as a complication of percutaneous vertebroplasty. Ann Thorac Surg. 2009;87(1):299–301. doi: 10.1016/j.athoracsur.2008.05.074. [DOI] [PubMed] [Google Scholar]
- 5.Lee V., Patel R., Meier P. Conservative management of inferior vena cava cement spike after percutaneous vertebroplasty causes fatal cardiac tamponade. J Rheumatol. 2014;41(1):141–142. doi: 10.3899/jrheum.130570. [DOI] [PubMed] [Google Scholar]
- 6.Galibert P., Deramond H., Rosat P. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie. 1987;33(2):166–168. [PubMed] [Google Scholar]
- 7.Park J.H., Choo S.J., Park S.W. Acute pericarditis caused by acrylic bone cement after percutaneous vertebroplasty. Circulation. 2005;111(6):e98. doi: 10.1161/01.CIR.0000155502.90653.A5. [DOI] [PubMed] [Google Scholar]
- 8.Cortet B., cotton A., Boutry N. Percutaneous vertebraplasty in the treatment of osteoporotic vertebral compression fractures: an open prospective study. J Rheumatol. 1999;26(10):2222–2228. [PubMed] [Google Scholar]
- 9.Groen R.J., Du T.D., Phillips F.M. Anatomical and pathological considerations in percutaneous vertebroplasty and kyphoplasty: a reappraisal of the vertebral venous system. Spine. 2004;29(13):1465–1471. doi: 10.1097/01.brs.0000128758.64381.75. [DOI] [PubMed] [Google Scholar]
- 10.Lee I.J., Choi A.L., Yie M.Y. CT evaluation of local leakage of bone cement after percutaneous kyphoplasty and vertebro plasty. Acta Radiol. 2010;51(6):649–654. doi: 10.3109/02841851003620366. [DOI] [PubMed] [Google Scholar]
- 11.Jensen M.E., Evans A.J., Mathis J.M. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. AJNR. 1997;18:1897–1904. [PMC free article] [PubMed] [Google Scholar]
- 12.Choe D.H., Marom E.M., Ahrar K. Pulmonary embolism of polymethyl methacrylate during percutaneous vertebroplasty and kyphoplasty. Ajr American Journal of Roentgenology. 2004;183(4):1097–1102. doi: 10.2214/ajr.183.4.1831097. [DOI] [PubMed] [Google Scholar]
- 13.Krueger A., Bliemel C., Zettl R. Management of pulmonary cement embolism after percutaneous vertebroplasty and kyphoplasty: a systematic review of the literature. Eur Spine J. 2009;18(9):1257–1265. doi: 10.1007/s00586-009-1073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mansour A., Abdel-Razeq N., Abuali H. Cement pulmonary embolism as a complication of percutaneous vertebroplasty in cancer patients. Cancer Image. 2018;18(1):5. doi: 10.1186/s40644-018-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatzantonis C., Czyz M., Pyzik R. Intracardiac bone cement embolism as a complication of vertebroplasty: management strategy. Eur Spine J. 2016:1–7. doi: 10.1007/s00586-016-4695-x. [DOI] [PubMed] [Google Scholar]