Abstract
Objective
To evaluate the clinical efficacy of infusion of gemcitabine (GEM) and fluorouracil (5-FU)through the celiac artery and superior mesenteric artery in the treatment of pancreatic carcinoma (PC).
Methods
We analyzed 20 patients diagnosed clinically or pathologically with PC, without metastases, who had an estimated survival duration of >3 months in our department from May 2009 to December 2014. Nine patients were treated directly without surgical resection of the tumor, while the other 11 patients were treated after surgery. In all patients, the femoral artery was punctured using the Seldinger technique, and a catheter was placed in the opening of the celiac artery or the superior mesenteric artery. We administered 500 mg/m2 GEM and 500 mg/m2 5-FU. Observational data included data on clinical efficacy and survival rates during the follow-up period of 3–72 months.
Results
Twenty patients were treated 85 times with transcatheter arterial infusion chemotherapy (TAI). The survival rates were 80%, 40%, 35%, 20%, 10%, and 5% at 3, 6, 12, 24, and 72 months, respectively.
Conclusion
TAI chemotherapy with GEM and 5-FU may be a therapeutic option for the treatment of PC.
Keywords: Transcatheter arterial infusion, Chemotherapy, Pancreatic carcinoma
Introduction
Pancreatic carcinoma (PC) is an extremely aggressive cancer type and one of the leading causes of cancer-rekated death. Due to the anatomical position of the pancreas, physiological characteristics, and biological behavior, detecting it at an early stage is challenging. The five-year survival rate of PC is less than 9%,1 and the survival rate drops to 2.4%2 when there is metastases. Surgery remains the mainstay of the treatment, but its success depends on the disease stage at diagnosis.3,4 Chemoradiotherapy provides a favorable response for initially unresectable PC.5 Following the 2006 National Comprehensive Cancer Network guidelines, chemoradiotherapy with systemic fluorouracil (5-FU) and gemcitabine (GEM) plus radiation therapy are accepted treatments for locally advanced unresectable PC. With the development of interventional radiology, transcatheter arterial infusion chemotherapy (TAI) can be used to deliver these chemotherapeutic agents for PC treatment.
We analyzed the data of 20 patients with PC treated in our department by TAI through the celiac artery and the superior mesenteric artery.
Material and methods
Ethical approval
The study was approved by the ethics committee of The First Affiliated Hospital, Zhejiang University School of Medicine. All clinical practices and observations were conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from each patient before the study was conducted.
Study population
From, May 2009 to December 2014, 20 patients were diagnosed with PC in our department. Included patients had either undergone surgical resection for PC or had advanced PC that could not be resected with a general Karnofsky score (KPS) of ≥60 and an expected survival time of >3 months. Patients with liver and kidney dysfunction (ALT and AST >2.5 × the upper limit of normal, total bilirubin >51 μmol/L, serum Cr >104 μmol/L); or abnormal blood test results (WBC ≤4 × 10E9/L, PLTs ≤100 × 10E9/L, and Hb ≤ 90 g/L) were excluded. Patients were also excluded if they had undergone chemotherapy or radiotherapy 6 months prior to the study date, had other coexisting malignancies or metastases, or had severe heart and lung comorbidities or any active infection. All patients provided written informed consent before TAI. This study was approved by the Ethics Committee of Zhejiang University and was carried out in accordance with the Declaration of Helsinki.
Transcatheter arterial infusion chemotherapy (TAI)
TAI was performed using a standard procedure by radiologists trained and experienced in this technique. The perineum was disinfected, and the patient was positioned in the supine position. Under local anesthesia, the right femoral artery was punctured with a 5 F arterial sheath using the Seldinger technique. A 5 F hepatic artery catheter or Yashiro catheter was pushed into the celiac artery and superior mesenteric artery via the arterial sheath for selective angiography. After angiography, a bolus of 500 mg/m2 GEM and a bolus of 500 mg/m2 5-FU were pushed separately through the opening of the celiac artery and superior mesenteric artery (at half of the total perfusion flow tate for each vessel). All patients were treated once every 4 weeks. In patients in whom surgery was not performed, TAI was stopped when significant progress was observed or when TAI was not considered suitable. In surgically treated patients, with no recurrences or metastases, TAI was provided at least three times. In case of a recurrence or metastasis, TAI was discontinued.
All patients were monitored after the procedure (vital signs, oxygen saturation, routine blood investigations, and liver and kidney function), with particular attention paid to the lower limb skin temperature and color, and dorsalis pedis pulses. Rehydration was considered paramount to protect the liver and prevent infection.
Statistical methods
This was an observational study, and thus, no power calculation was performed. Statistical analysis was performed using SPSS 18.0. Descriptive statistics were used for continuous variables (such as age and duration of follow-up) and are reported as means ± standard deviations (SD). Continuous variables were analyzed using the Kaplan–Meier survival analysis method.
Results
Patient characteristics
Table 1 presents the clinical characteristics of the patients. Our cohort included 13 men and 7 women, ranging from 36 to 77 years (mean age, 60.5 ± 10.79 years). The location of the PC was the head and neck of the pancreas in 16 cases, and the body and tail of the pancreas in the remaining 4 patients. Eleven cases involved surgical resection before TAI, while the other nine patients were treated only with TAI. The first symptom was abdominal pain in 9 cases and, jaundice in 6 cases, of which 2 patients passed clay-colored stools, one had itching, and three passed yellow urine. Two patients were diagnosed through physical examination, two patients were treated for waist and back pain, and the remaining patients were diagnosed whileendergoing examinations for weight loss. Two patients had comorbid hypertension, two had diabetes, and one had both hypertension and diabetes. Five patients showed close vascular invasion and lymph node metastasis.
Table 1.
Characteristics of the 20 patients with pancreatic carcinoma.
| Characteristic | N |
|---|---|
| Clinical presentation | |
| Abdominal pain | 9 |
| Jaundice | 6 |
| Clay stool | 2 |
| Itching | 1 |
| Yellow urine | 3 |
| Physical findings | 2 |
| Waist and back pain (radiating pain) | 2 |
| Weight loss | 1 |
| Location of pancreatic carcinoma | |
| Head and neck | 16 |
| Body and tail | 4 |
| Adjacent vascular invasion | 5 |
| Comorbid diseases | |
| Hypertension | 2 |
| Diabetes | 2 |
| Hepatitis B | 1 |
| Diarrhea | 1 |
| Intrahepatic metastasis after TAI | 4 |
| Fever after TAI | 3 |
| No significant complications | 17 |
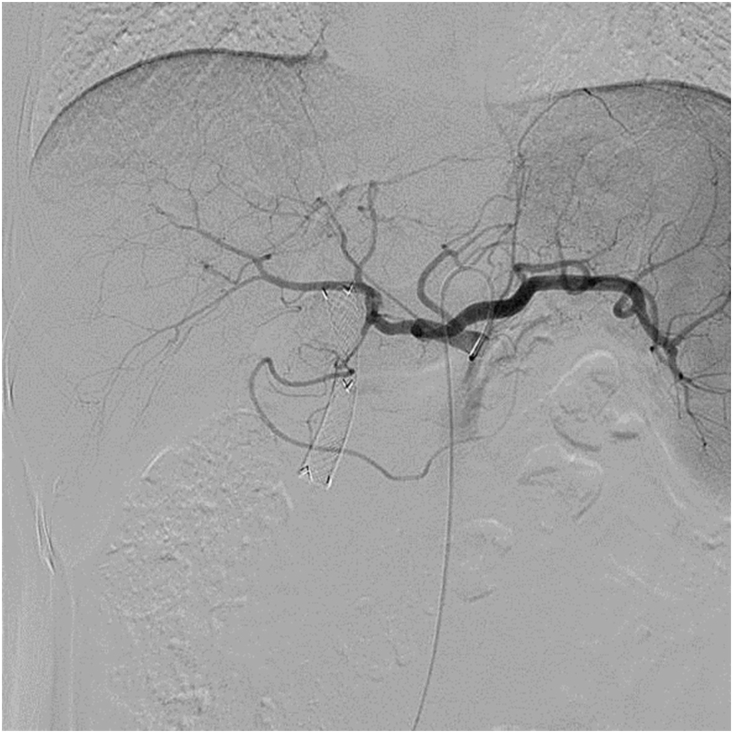
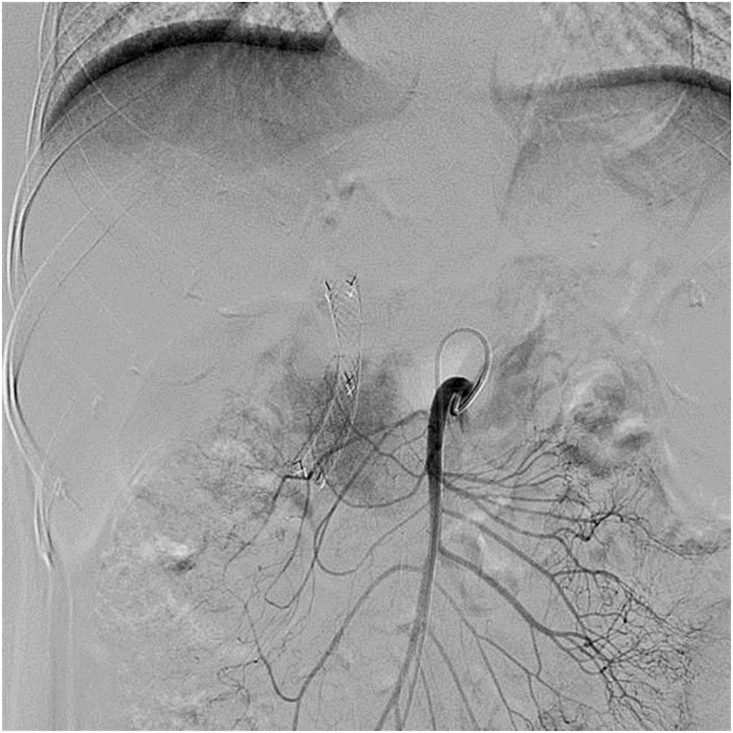
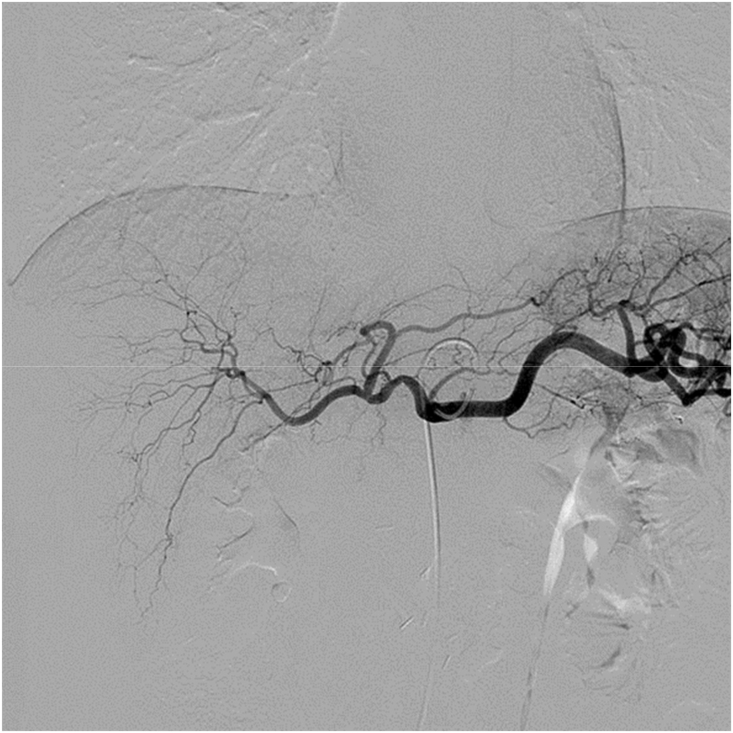
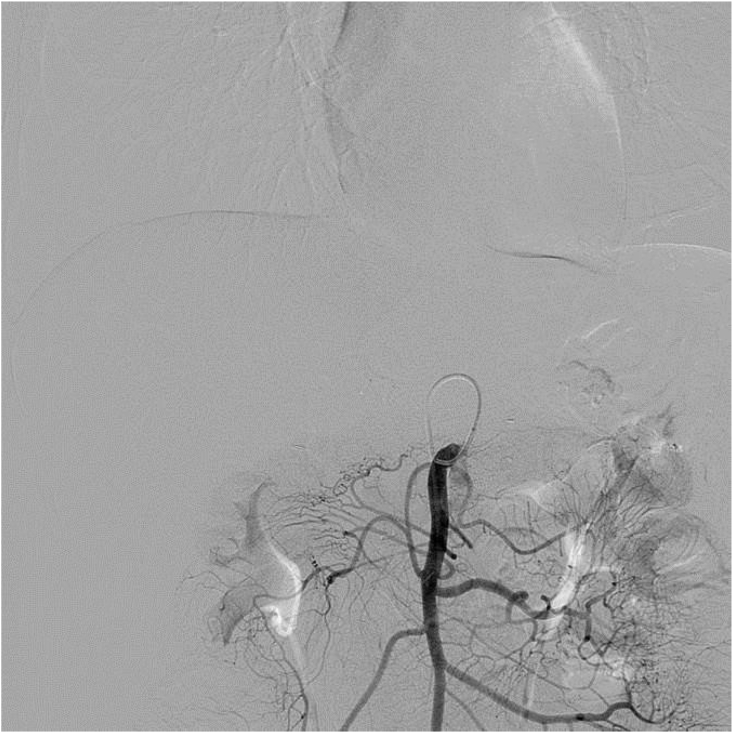
All TAI procedures were considered successful (total 85 times), withan average of 4.25 ± 1.55 procedures per patient. The treatment regimen involved 3 procedures in 10 cases, 4 procedures in 2 cases, 5 procedures in 2 cases, 7 procedures in 1 case and 8 procedures in 1 case. Five of the nine patients who did not undergo surgical resection showed a flaky, pancreatic area that showed lighter staining on superior mesenteric artery angiography (Fig. 1, Fig. 2). The remaining 15 patients showed no significant tumor staining (Fig. 3, Fig. 4).
Fig. 1.
Carcinoma staining at the head of the pancreas when angiographyat is performed at the opening of the celiac artery.
Fig. 2.
The superior mesenteric artery angiography showing carcinoma staining at the head of the pancreas.
Fig. 3.
Digital subtraction angiography (DSA) without tumor staining when angiographyat is performed at the opening of the celiac artery.
Fig. 4.
DSA without tumor staining when angiography is performed at the superior mesenteric artery.
The observed complications were mild nausea and vomiting (two cases) and mild fever (three cases), while the remaining patients had no apparent discomfort. All complications improved after symptomatic treatment.
Follow-up
All patients were followed up until December 2016 or until death, and the follow-up time ranged from 3 to 72 months (mean, 11.25 ± 15.48 months). Survival rates were 80%, 40%, 35%, 20%, 10%, and 5% in 3, 6, 12, 24, 48, and 72 months, respectively. During follow-up, four patients progressed to exhibit intrahepatic metastasis, while one patient had a complete remission without any symptoms.
Discussion
Pancreatic carcinoma is an extremely aggressive gastrointestinal tumor that progresses rapidly. The hidden anatomical position of the pancreas and the lack of specificity of clinical symptoms make early diagnosis impossible. According to the American Cancer Society, the latest statistics show that in 2019 there were 56,770 new PC cases causing up to 45,750 deaths, making PC the third leading cause of cancer mortality.1,6 Although early diagnosis is sometimes possible, surgical resection still only results in a survival rate of 10%.7,8 Unresectable PC is treated with chemotherapy, radiotherapy, or a combination of the two. Additionally, high-intensity focused ultrasound (HIFU),9,10 a noninvasive ablation modality for PC, is now accepted as another treatment option. In recent years, TAI has achieved particularly good results.11, 12, 13 We assessed the outcomes of patients diagnosed with PC and treated them with TAI at our center.
The efficacy of chemotherapy drugs depends on the drug concentration in the tumor and the contact time of the drugs within the carcinoma cells. The fibrous capsule surfacing PC challenges the penetration of chemotherapeutics. Additionally, PC often expresses high levels of multidrug resistance, resulting in rapid clearance of chemotherapeutic drugs from the tumor cells. Therefore, systemic chemotherapy does not result in a better prognosis. The principle of TAI is based on a local injection of high concentrations of drugs directly into the tumor tissue through the pancreatic artery or the parent artery segment. It increases the concentration and action time of the drugs in the tumor microenvironment to deliver toxicity to the tumor tissue and to overcome therapy resistance resulting in apoptosis and necrosis of PC cells and improving the therapeutic effect.11,14 This high local concentration of drugs affects only the pancreas with reduced side effects in all other organs.15 Thus, relative to intravenous chemotherapy, direct infusion of drugs intra-arterially leads to secondary perfusion through the portal vein and effectively kills tumor cells in the portal vein system. This either reduces or delays the occurrence of liver metastasis and improves the survival time of patients.16,17 We presume that in surgically resected patients with PC, postoperative intra-arterial infusion chemotherapy can destroy micrometastases that existed before surgery, thereby preventing the development of local recurrences and liver metastasis. In this study, patients treated with TAI had only mild fever (3/20) and nausea and vomiting (2/20) as side effects. Compared with the side effects of systemic chemotherapy and radiotherapy, these are almost negligible. During follow-up, also only four patients developed liver metastases (4/20). In fact, one patient who had undergone surgical resection showed complete remission at > 72 months of follow-up (Figs. 3 and 4).
Gemcitabine (GEM) is the first-line chemotherapeutic agent in PC, with an efficacy rate of 5%–15%. Clinical trials indicated that TAI with GEM improved the response and resectability, and it was also well tolerated.18 Some argue that GEM in combination with other anti-carcinoma drugs does not improve survival,19,20 while others assume that GEM in combination with other anti-carcinoma drugs, or even combined with other treatments can improve the median survival time.21, 22, 23, 24 From our experience, we favor combination therapy in safe doses. Currently, other anti-neoplastic agents such as 5-FU, oxaliplatin, and mitomycin are also being used. In this study, we used GEM combined with 5-FU, at doses of 500 mg/m2 with good results. The average survival time of patients was 11.25 ± 15.48 months, and the one-year and five-year survival rates were 35% and 5%, respectively.
Previous studies have shown that when PC is treated by TAI, arterial angiography of the hepatic artery, duodenal or gastric artery, and superior mesenteric artery could be used, with each artery injected with half the infusion dose of the chemotherapy drugs.25,26 The blood supply to the head and tail of the pancreas is different.27 The pancreatic head is mostly supplied from the hepatic artery and the superior mesenteric artery or a branch thereof. In contrast, the body and tail of the pancreas are mostly supplied by the pancreatic splenic artery from the dorsal artery, pancreatic artery, transverse pancreatic artery, and pancreatic tail artery. The head of the pancreas and pancreatic anastomosis are often connected; the superior mesenteric artery often branches into the dorsal pancreatic artery and the transverse pancreatic artery.28,29 Thus, it is not enough to provide artery infusion chemotherapy only to the hepatic artery or gastroduodenal artery and the superior mesenteric artery because it will result in some part of the pancreas not receiving the chemotherapeutic agents. We argue that artery infusion chemotherapy at the opening of the celiac artery and the superior mesenteric artery can completely cover the pancreas and improve the efficacy of local chemotherapy.
Although the value of regional chemotherapy has been demonstrated, the expansion of its clinical use is constrained by some drawbacks. TAI is more challenging for patients than systemic chemotherapy and radiotherapy as it is an invasive procedure, and patients must be hospitalized. However, TAI for PC provides advantages as it is a targeted therapy, relieves cancer-related pain, causes fewer systemic adverse reactions, reduces normal tissue damage, and limits local recurrence.
Our study has some limitations. The sample size was small, observation indicators were not comprehensive, and further studies are needed to confirm our results in larger cohorts.
Conclusion
Our data indicate that artery infusion chemotherapy with GEM and 5 FU at the opening of the celiac artery and the superior mesenteric artery can completely cover the pancreas and may be a therapeutic option in the treatment of PC.
Ethical approval
The study was approved by the ethics committee of The First Affiliated Hospital, Zhejiang University School of Medicine. All clinical practices and observations were conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from each patient before the study was conducted.
Funding
The present work was funded by Zhejiang Provincial Natural Science Foundation of China (Grant No. LZ18H180001), National Natural Science Foundation of China (Grant No. 81971713 and 81,371,658), National S&T Major Project of China (NO.2018ZX10301201), Grant from Health Commission of Zhejiang Province (JBZX-202004), Research Unit of Collaborative Diagnosis and Treatment For Hepatobiliary and Pancreatic Cancer, Chinese Academy of Medical Sciences (2019RU019), The Key Research Development Program of Zhejiang province (Grant No.2018C03018), Key Science and Technology Program of Zhejiang province (No.WKJ-ZJ-1923) and National Key R&D Program of China (No.2017YFC0114102).
Patient consent
Written informed consent was obtained from patients for publication of these case reports and any accompanying images.
Declaration of competing interest
The authors declare that they have no conflicts of interests to this work. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics. CA A Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. 2019. [DOI] [PubMed] [Google Scholar]
- 2.Mettu N.B., Abbruzzese J.L. Clinical insights into the biology and treatment of pancreatic carcinoma. J OncolPract. 2016;12:17–23. doi: 10.1200/JOP.2015.009092. [DOI] [PubMed] [Google Scholar]
- 3.Schnelldorfer T., Sarr M.G., Nagorney D.M. Experience with 208 resections for intraductal papillary mucinous neoplasm of the pancreas. Arch Surg. 2008;143:639–646. doi: 10.1001/archsurg.143.7.639. [DOI] [PubMed] [Google Scholar]
- 4.Merrell K.W., Haddock M.G., Quevedo J.F. Predictors of locoregional failure and impact on overall survival in patients with resected exocrine pancreatic carcinoma. Int J Radiat Oncol Biol Phys. 2016;94:561–570. doi: 10.1016/j.ijrobp.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Jang J.Y., Han Y., Lee H. Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer: a prospective, randomized, open-label, multicenter phase 2/3 trial. Ann Surg. 2018;268:215–222. doi: 10.1097/SLA.0000000000002705. [DOI] [PubMed] [Google Scholar]
- 6.Rahib L., Smith B.D., Aizenberg R. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Can Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 7.Spadi R., Brusa F., Ponzetti A. Current therapeutic strategies for advanced pancreatic carcinoma: a review for clinicians. World J Clin Oncol. 2016;7:27–43. doi: 10.5306/wjco.v7.i1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiltberger G., Bucher J.N., KrenzienF Extended resection in pancreatic metastases: feasibility, frequency, and long-term outcome: a retrospective analysis. BMC Surg. 2015;15:126. doi: 10.1186/s12893-015-0114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Copelan A., Hartman J., Chehab M. High-intensity focused ultrasound: current status for image-guided therapy. Semin Intervent Radiol. 2015;32:398–415. doi: 10.1055/s-0035-1564793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Feng. High intensity focused ultrasound: a noninvasive therapy for locally advanced pancreatic cancer. World J Gastroenterol. 2014;20:16480–16488. doi: 10.3748/wjg.v20.i44.16480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Committee of Interventional Medicine Cancer Foundation of China; Committee of Interventional Medicine and Bioengineering, Chinese Society of Interventional Physicians. Clinical practice guidelines for the interventional treatment of advanced pancreatic carcinoma (on trial) (3rd edition) J Clin Hepatol. 2019;35:744–754. [Google Scholar]
- 12.Hashimoto A., Tanaka T., ShoM Adjuvant hepatic arterial infusion chemotherapy after resection for pancreatic cancer using coaxial catheter-port system compared with conventional system. Cardiovasc Intervent Radiol. 2016;39:831–839. doi: 10.1007/s00270-016-1292-7. [DOI] [PubMed] [Google Scholar]
- 13.Zheng Y.Y., Tang C.W., Xu Y.Q. Hepatic arterial infusion chemotherapy reduced hepatic metastases from pancreatic carcinoma after pancreatectomy. Hepato-Gastroenterology. 2014;61:1415–1420. [PubMed] [Google Scholar]
- 14.Whitehouse P.A., Cooper A.J., Johnson C.D. Synergistic activity of gamma-linolenic acid and cytotoxic drugs against pancreatic adenocarcinoma cell lines. Pancreatology. 2003;3:367–373. doi: 10.1159/000073651. discussion 373-4. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka T., Yamamoto K., Sho M. Pharmacokinetic evaluation of pancreatic arterial infusion chemotherapy after unification of the blood supply in an animal model. J Vasc Intervent Radiol. 2010;21:116–121. doi: 10.1016/j.jvir.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 16.Aigner K.R., Gailhofer S., Kopp S. Regional versus systemic chemotherapy for advanced pancreatic cancer: a randomized study. Hepato-Gastroenterology. 1998;45:1125–1129. [PubMed] [Google Scholar]
- 17.Nakchbandi W., Muller H., Singer M.V. Prospective study on warfarin and regional chemotherapy in patients with pancreatic carcinoma. J Gastrointestin Liver Dis. 2008;17:285–290. [PubMed] [Google Scholar]
- 18.Aigner K.R., Gailhofer S. Celiac axis infusion and microembolization for advanced stage III/IV pancreatic carcinoma–a phase II study on 265 cases. Anticarcinoma Res. 2005;25:4407–4412. [PubMed] [Google Scholar]
- 19.Colucci G., Labianca R., Di Costanzo F. Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic carcinoma: the GIP-1 study. J Clin Oncol. 2010;28:1645–1651. doi: 10.1200/JCO.2009.25.4433. [DOI] [PubMed] [Google Scholar]
- 20.Arshad A., Al-Leswas D., Stephenson J. Potential applications of fish oils rich in n-3 fatty acids in the palliative treatment of advanced pancreatic carcinoma. Br J Nutr. 2011;106:795–800. doi: 10.1017/S0007114511003060. [DOI] [PubMed] [Google Scholar]
- 21.Takamori H., Kanemitsu K., Hirota M. Perioperative intra-arterial and systemic chemotherapy for pancreatic carcinoma. Ann Surg Oncol. 2011;18:1110–1115. doi: 10.1245/s10434-010-1384-6. [DOI] [PubMed] [Google Scholar]
- 22.Oettle H., Neuhaus P., Hochhaus A. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic carcinoma: the CONKO-001 randomized trial. J Am Med Assoc. 2013;310:1473–1481. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 23.Postlewait L.M., Ethun C.G., Kooby D.A. Combination gemcitabine/cisplatin therapy and ERCC1 expression for resected pancreatic adenocarcinoma: results of a Phase II prospective trial. J Surg Oncol. 2016;114:336–341. doi: 10.1002/jso.24317. [DOI] [PubMed] [Google Scholar]
- 24.Moon do C., Lee H.S., Lee Y.I. Concomitant statin use has a favorable effect on gemcitabine-erlotinib combination chemotherapy for advanced pancreatic cancer. Yonsei Med J. 2016;57:1124–1130. doi: 10.3349/ymj.2016.57.5.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka T., Sho M., Nishiofuku H. Unresectable pancreatic cancer: arterial embolization to achieve a single blood supply for intraarterial infusion of 5-fluorouracil and full-dose IV gemcitabine. AJR Am J Roentgenol. 2012;198:1445–1452. doi: 10.2214/AJR.11.8008. [DOI] [PubMed] [Google Scholar]
- 26.Sada T., Denno R., Tanaka T. Intra-arterial infusion chemotherapy with 5-fluorouracil and cisplatin in advanced pancreatic cancer: a feasibility study. Am J Clin Oncol. 2008;31:71–78. doi: 10.1097/COC.0b013e31807a328c. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka T., Sakaguchi H., Sho M. A novel interventional radiology technique for arterial infusion chemotherapy against advanced pancreatic cancer. AJR Am J Roentgenol. 2009;92:W168–W177. doi: 10.2214/AJR.08.1392. [DOI] [PubMed] [Google Scholar]
- 28.Collins J.M., Silva A.C., Hayman L.A. Arterial anatomy of the pancreas: part 2. Axial. J Comput Assist Tomogr. 2010;34:795–798. doi: 10.1097/RCT.0b013e3181dd5bda. [DOI] [PubMed] [Google Scholar]
- 29.Balachandran A., Darden D.L., Tamm E.P. Arterial variants in pancreatic adenocarcinoma. Abdom Imag. 2008;33:214–221. doi: 10.1007/s00261-007-9235-z. [DOI] [PubMed] [Google Scholar]