Abstract
A case report of eight year complete remission post bronchial artery infusion (BAI) chemotherapy (Pimetrazine and cisplatin) for unresectable lung adenocarcinoma.
Keywords: Bronchial artery infusion, Bronchial lung cancer, Regional chemotherapy
Lung cancer is one of the most common forms of malignant tumors,1 with high morbidity and mortality.2 In recent years, bronchial artery infusion chemotherapy has been used to treat lung cancer. Here, we report a case of complete remission at eight years following bronchial artery infusion (BAI) chemotherapy in a patient with unresectable lung adenocarcinoma.
Case report
A 49-year-old woman was hospitalized due to a history of coughing that had persisted for more than a month, with progressive edema in the right upper limb, head, and face. She had also been experiencing increased breathing difficulties for a week. Her Eastern Cooperative Oncology Group (ECOG) performance status was 4. On physical examination, the vital signs were: blood pressure (BP), 121/83; pulse, 80/min; and respiratory rate (RR), 20/min. The patient had shortness of breath in the semi-recumbent position. There was edema of the head, face, and right upper limb. Cervical lymphadenopathy was observed. There were only two abnormal biochemical results: carcinoembryonic antigen (CEA) 87 ng/ml and Squamous Cell Carcinoma (SCC) Antigen 65 ng/ml. Testing results for the expression of EGFR, ALT, RAS, and other genes in this patient were negative.
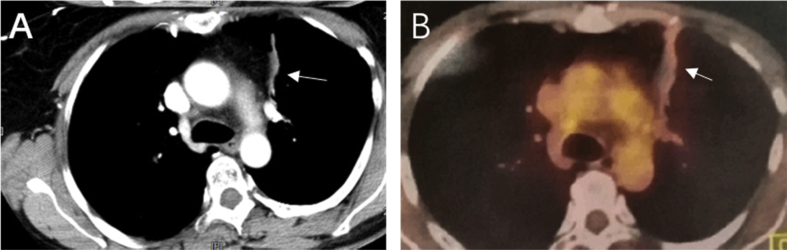
Computed tomography (CT) examination indicated masses in the left upper lung, right upper lung, and the mediastinum with tracheal compression. The right frontal chest wall vein was dilated (Fig. 1 A, B). A biopsy of the upper left lung mass was reported as a poorly differentiated adenocarcinoma. Pathological staging indicated lung adenocarcinoma (Fig. 2), cT4NxM1 (right upper lung with mediastinal lymph node metastasis). The patient was assessed as having unresectable lung cancer. The patient refused systemic chemotherapy. Instead, the patient provided informed consent for bronchial artery infusion chemotherapy.
Fig. 1.
Enhanced CT scan of chest at first visit: (A) soft tissue mass in the left upper lung (→) and enlarged lymph nodes in mediastinum (∗) and (B) right upper lung and mediastinal tumor metastasis (∗), superior vena cava compressed by tumors and right chest wall vein dilatation (→).
Fig. 2.
(A) CT-guided lung puncture biopsy (B) 40-fold pathological image (C) 100-fold pathological image (D) 200-fold pathological image.
A superior vena cava stent was implanted to relieve the upper vena cava stenosis with resultant regional edema (Fig. 3). The edema subsided postoperatively.
Fig. 3.

X-ray chest radiograph post superior vena cava stent implantation.
BAI was performed under local anesthesia (Fig. 4 A, B). The right femoral artery was punctured using the Seldinger technique and trans-aortic selective arterial cannulation of the left and right bronchial arteries was performed. The spinal artery was excluded using DSA. Pimetrazine 1.0 g and cisplatin 60 mg were diluted in 50 ml saline and infused over 15 min.
Fig. 4.
Digital subtraction angiography (DSA): (A) right bronchial artery (→) supplying the tumor in right lung, (B) left bronchial artery (→) supplying the tumor in the left lung.
BAI was repeated every 3 weeks for a total of 6 cycles. Enhanced chest CT scans were performed every 2-monthly and As mentioned above, please check whether this abbreviation can be used without explanation. Otherwise, please write this as “positron emission tomography CT (PETCT)” was performed after 6 months. The patient did not receive any specific antitumor therapy. The lung masses and lymphadenopathy decreased over several months. A residual left upper lung scar remained (Fig. 5). Tumor markers returned to normal, and the patient’s performance status evaluation score recovered to normal (EOCG 0).
Fig. 5.
Images after treatment with BAI for 6 cycles. The CT (A) and PET-CT (B) scans showed the left lung with fibrous scar (→) and resolving lesions, and the right upper lung and mediastinum with resolving lesions.
The patient has survived for more than 8 years. She has been reviewed every 6–12 months. At her most recent follow up, the patient’s performance status was reported as EOCG grade 0 and her chest CT indicated that the left upper lung scar was stable (Fig. 6) The radiologic assessment of the response (RECIST1.13 standard) was considered complete.
Fig. 6.
Chest enhanced CT at 20 month (A), 35month (B), 52 month (C) and 64 month (D) post treatment. Scans show only right upper lung scar.
Discussion
BAI chemotherapy has been used to treat lung tumors for more than 50 years.4 A growing body of literature supports BAI for patients with advanced lung cancer who cannot be surgically operated on and who cannot tolerate systemic chemotherapy or radiotherapy.5,6 A study by Fu et al.7 have shown that the response rate to BAI treatment in patients with advanced NSCLC was 32.5%, and the disease control rate was 92.5%. The average overall survival was 13 months ± 2.1 months. Nevazaki et al.8 have shown that the total remission rate of unresectable lung cancer can reach higher than 50% with BAI. Therefore, BAI is an effective treatment option for unresectable NSCLC.
The source of tumor blood supply is very important for tumor growth. It is also the anatomical basis of regional treatment through blood vessels. There has been a debate about the source of blood supply for lung cancer because the lungs have double circulation: the lung circulation responsible for gas exchange, and the bronchial artery circulation which is responsible for the nutrition of the lungs and bronchus. A study have shown that among all arterial infusion chemotherapy for lung cancer, the bronchial artery infusion chemotherapy is the best therapeutic approach.9 Although the lung circulation can meet the metabolic needs of tumors, the essential growth of the tumor requires nutrient supplementation from the highly proliferative bronchial circulation.10 A study that examined the blood supply of lung cancer in 59 patients by multi-slice spiral CT angiography suggested that the bronchial artery supplied blood to the tumor in approximately 79% of lung cancer cases.11
BAI chemotherapy has the advantages of being minimally invasive with reduced side effects compared to systemic chemotherapy. Multiple courses of chemotherapy can be administered in a repeatable fashion. The direct injection of anticancer drugs into local tumors reduces the required drug dose by one half compared to systemic chemotherapy.12,13 Moreover, the antitumor effect in local tumors is 2–6 times better than that of intravenous chemotherapy using the same drug dosage.14 BAI reduces the side effects of chemotherapy while improving the efficiency of tumor treatment and the patient’s quality of life.15 The effect of regional tumor treatment is influenced by the blood supply available to the target artery. In this case, the blood supply from the bronchial artery was abundant in the tumor area, and there was complete remission following BAI chemotherapy.
BAI can lead to serious adverse reactions, such as spinal cord paralysis, bronchial ulcers, esophageal ulcers, hemoptysis, pulmonary toxicity, and kidney damage.16,17 However, arterial puncture cannulation has a low complication rate. Alternatively, systemic venous chemotherapy has side effects including bone marrow inhibition, neurotoxicity, liver and kidney dysfunctions, and gastrointestinal reactions.18 With improvements in DSA equipment and ultra-selective arterial cannulation technology, the rate of BAI-related complications has become more acceptable. Unlike systemic outpatient chemotherapy, patients require repeated hospitalization during BAI treatment, which increases the costs. These limitations hinder the widespread use of BAI as a standard clinical treatment for lung cancer.
This unusual case suggests a significant adaptive immune response following treatment. To further determine the clinical efficacy of BAI for lung cancer, prospective randomized trials are warranted.
Authors’ contributions
Kunlin Huang wrote the manuscript. Yujin Liu edited and proofread this manuscript and revised the manuscript. All authors read and approved the final manuscript.
Ethical approval
The study was approved by the ethics committee of Yueyang Hospital of Integrated Traditional Chinese and Western Medicine affiliated to Shanghai University of Traditional Chinese Medicine. All clinical practices and observations were conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from each patient before the study was conducted.
Patient consent
Written informed consent was obtained from patients for publication of these case reports and any accompanying images.
Declaration of competing interest
The authors declare that they have no conflicts of interests to this work. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Acknowledgments
None.
Contributor Information
Kunlin Huang, Email: kunlinh@163.com.
Yujin Liu, Email: liuyujin@shyueyanghospital.com.
References
- 1.Huang X. Interpretation of China experts consensus on the diagnosis and treatment of brain metastases of lung cancer (2017 version) J Int Transl Med. 2017;5:45–52. [Google Scholar]
- 2.Chen W., Zheng R., Baade P.D. Cancer statistics in China, 2015. Ca Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Eisenhauer E.A., Therasse P., Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 4.Kahn P.C., Paul R.E., Rheinlander H.F. Selective bronchial arteriography and intra-arterial chemotherapy in carcinoma of the lung. J Thorac Cardiovasc Surg. 1965;50:640–645. [PubMed] [Google Scholar]
- 5.Nakanishi M., Yoshida Y., Natazuka T. Prospective study of transarterial infusion of docetaxel and cisplatin to treat non-small-cell lung cancer in patients contraindicated for standard chemotherapy. Lung Cancer. 2012;77:353–358. doi: 10.1016/j.lungcan.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Yuan Z., Li W.T., Ye X.D. Intra-arterial infusion chemotherapy for advanced non-small-cell lung cancer: preliminary experience on the safety, efficacy, and clinical outcomes. J Vasc Intervent Radiol. 2013;24:1521–1528.e4. doi: 10.1016/j.jvir.2013.05.065. [DOI] [PubMed] [Google Scholar]
- 7.Fu Y.F., Li Y., Wei N. Transcatheter arterial chemical infusion for advanced non-small-cell lung cancer: long-term outcome and predictor of survival. Radiol Med. 2016;121:605–610. doi: 10.1007/s11547-016-0629-2. [DOI] [PubMed] [Google Scholar]
- 8.Neyazaki T., Ikeda M., Seki Y. Bronchial artery infusion therapy for lung cancer. Cancer. 1969;24:912–922. doi: 10.1002/1097-0142(196911)24:5<912::aid-cncr2820240508>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 9.Nakanishi M., Demura Y., Umeda Y. Multi-arterial infusion chemotherapy for non-small cell lung carcinoma--significance of detecting feeding arteries and tumor staining. Lung Cancer. 2008;61:227–234. doi: 10.1016/j.lungcan.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Eldridge L., Moldobaeva A., Zhong Q. Bronchial artery angiogenesis drives lung tumor growth. Cancer Res. 2016;76:5962–5969. doi: 10.1158/0008-5472.CAN-16-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye X.D., Yuan Z., Ye J.D. Assessment of the feeding arteries by three-dimensional computed tomography angiography prior to multi-arterial infusion chemotherapy for lung cancer. Oncol Lett. 2013;5:363–367. doi: 10.3892/ol.2012.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe Y., Shimizu J., Murakami S. Reappraisal of bronchial arterial infusion therapy for advanced lung cancer. Jpn J Surg. 1990;20:27–35. doi: 10.1007/BF02470710. [DOI] [PubMed] [Google Scholar]
- 13.Osaki T., Oyama T., Takenoyama M. Feasibility of induction chemotherapy using bronchial arterial infusion for locally advanced non-small cell lung cancer: a pilot study. Surg Today. 2002;32:772–778. doi: 10.1007/s005950200148. [DOI] [PubMed] [Google Scholar]
- 14.Zhao G., Huang Y., Lianhua Y. Therapeutic efficacy of traditional vein chemotherapy and bronchial arterial infusion combining with CIKs on Ⅲ stage non-small cell lung cancer. Chin J Lung Cancer. 2009;12:1000–1004. doi: 10.3779/j.issn.1009-3419.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Nakanishi M., Umeda Y., Demura Y. Effective use of multi-arterial infusion chemotherapy for advanced non-small cell lung cancer patients: four clinical specified cases. Lung Cancer. 2007;55:241–247. doi: 10.1016/j.lungcan.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka O., Hashimoto S., Narimatsu Y. Can selective CT angiography reduce the incidence of severe complications during transcatheter arterial embolization or infusion chemotherapy for thoracic diseases? Diagn Interv Radiol. 2006;12:201–205. [PubMed] [Google Scholar]
- 17.Yang N., Xiong F., He Q. Achievable complete remission of advanced non-small-cell lung cancer: case report and review of the literature. World J Clin Cases. 2018;6:150–155. doi: 10.12998/wjcc.v6.i7.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gießen-Jung C., von Baumgarten L. Chemotherapie-induzierte periphere Neuropathie [Peripheral neuropathy as a side effect of chemotherapy and targeted therapy] Dtsch Med Wochenschr. 2018;113:970–978. doi: 10.1055/s-0043-120839. German. [DOI] [PubMed] [Google Scholar]