Abstract
The in-situ post-embolization fluorescence-guided hepatectomy for hepatocellular carcinoma (HCC) requires precise embolic formulation that meets both preoperative and intraoperative needs of hepatobiliary surgeons. In this Editorial, we highlight the development of Superstable Homogeneous Iodinated Formulation Technology (SHIFT) for locoregional HCC treatment. It is believed that such an intelligent solution could resolve unmet formulation needs and make a major stride to bridge the preoperative gap of precision hepatectomy.
Keywords: Hepatectomy, Embolization, Fluorescence, SHIFT, HCC, ICG
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer in men worldwide, and the second leading cause of death from cancer in China.1,2 Among the curative treatments, including radiofrequency ablation, liver transplantation, and hepatic resection, hepatic resection remains the most viable option for resectable HCC. However, in advanced-stage HCC, especially for patients with large or multiple tumors, the risk of intraoperative bleeding and postoperative liver failure often precludes hepatic resection.3 These circumstances provide the rationale for the application of hepatic embolization, an interventional radiology catheter injection of the embolic agent into the portal vein or hepatic artery to block the blood supply of tumors in the liver. Portal vein embolization (PVE) is an effective procedure to facilitate major hepatic resection, while transcatheter arterial embolization (TAE) and chemoembolization (TACE) are not only used to treat unresectable HCCs4 but are also widely applied as a preoperative procedure to shrink the tumor size to satisfy the surgical criteria for patients with initially unresectable HCCs.5
The gap between Lipodol-based TAE and fluorescence-guided hepatectomy
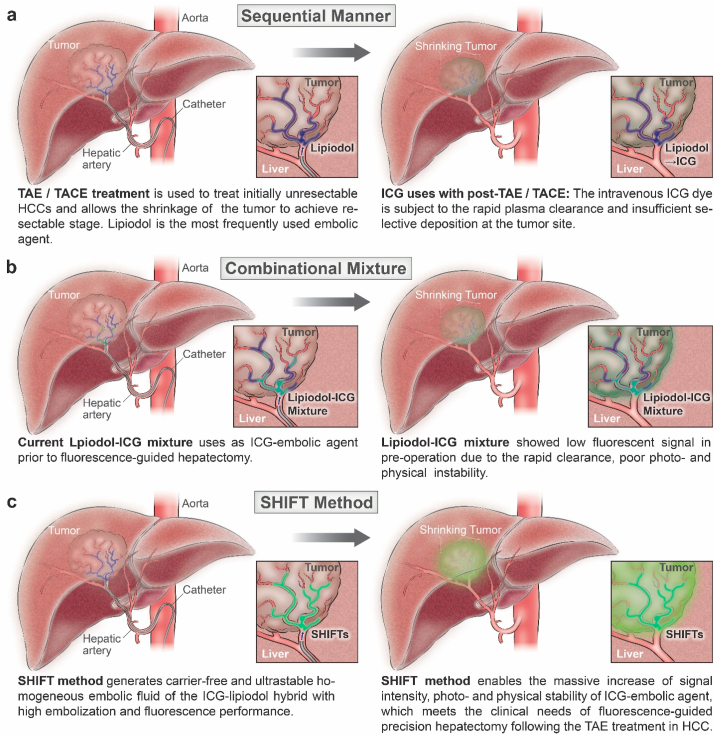
In the current clinical practice of hepatic resection, near-infrared indocyanine green (ICG) has been intensively applied to guide precision hepatectomy.6, 7, 8 This could assist in reduced intraoperative blood loss, a lower incidence of postoperative complications, a decline in the recurrence rate, and an improved survival prognosis6,.9 However, the broad clinical usage of ICG-guided hepatectomy is limited due to its instability and rapid clearance through hepatic blood flow after the intravenous injection of ICG.10,11 Moreover, a potential concern is that the intravenous dye may have limited accessibility to the tumor sites due to vessel occlusion after the embolization (Fig. 1a). To this end, the leading yet suboptimal solution is to intra-arterially perfuse a fluorescent iodized emulsion prepared by mixing lipiodol with ICG. This allows the co-deposition of lipiodol and ICG, but the existing emulsification methods are hampered by the stratification and precipitation of ICG in vivo due to the variable physical stability of the emulsion, which often results in undesired hepatic clearance of ICG in advance of hepatectomy (Fig. 1b). Given these difficulties, a robust fluorescent iodized formulation is in a pressing need for successful fluorescence-guided hepatectomy and its broad applications.
Fig. 1.
A schematic diagram showing the differences between conventional procedures and the SHIFT method for the fluorescence-guided hepatectomy following the TAE/TACE treatment. a) The direct use of ICG on post-TAE/TACE treatment as a sequential manner. b) The use of conventional lipiodol-ICG mixture for TAE/TACE treatment followed by fluorescence-guided hepatectomy. c) The SHIFT method is introduced to counter the existing issues of conventional ICG-lipiodol formulation and bridge the preoperative gap of fluorescence-guided hepatectomy of HCC.
Key technological advances of SHIFT
Recently, Chen et al. reported an instant, clinically applicable formulation technology, referred to as superstable homogeneous iodinated formulation technology (SHIFT)12, which can generate an ultrastable homogeneous embolic fluid of the ICG-lipiodol hybrid via the supercritical fluid (SCF) homogenization process. The authors used supercritical carbon dioxide (CO2) as a dissolution medium in the ICG-lipiodol mixing interaction. Unlike conventional liquid solvents, supercritical CO2 is a fluid state of CO2, where it exhibits fine-tunable solvation strength with the modified temperature and pressure13. In addition, CO2 is nontoxic and leaves minimal residue in products relative to organic solvents.13 Notably, with its environmentally benign nature and highly commercialized availability, supercritical CO2 has been applied in both food and pharmaceutical industries.
This robust SHIFT method can address the issues associated with conventional ICG-lipiodol formulation and bridge the preoperative gap of fluorescence-guided hepatectomy of HCC (Fig. 1c). By controlling and reforming the intermolecular interaction of ICG via SHIFT, the authors demonstrated a drastic improvement in the fluorescence intensity, photostability, and physical stability of ICG-embolic agents, which may fulfill the clinical needs of ICG-guided precision hepatectomy following TAE/TACE treatment in HCC.
From a clinical point of view, desirable viscosity is essential for the lipiodol embolic effect and high-sensitivity CT imaging.14 Thus, the effects of SHIFT on the viscosity of ICG-lipiodol agents were evaluated. As expected, the SHIFT agent is virtually identical to that of the free lipiodol agent12. The equivalent embolic effect and CT value between the SHIFT and lipiodol groups were further confirmed in the rabbit VX2 tumor model with the assistance of CT and MRI. To verify the intraoperative navigation performance of SHIFTs, it was compared to the conventional lipiodol-ICG mixture in fluorescence-guided hepatectomy.12 The SHIFT agent demonstrated a boosted fluorescence signal-to-noise ratio with long-lasting signal intensity (>5 h) under the holographic navigation system12. During resection, the tumor site was clearly located with tumor boundaries identified by the SHIFT agent, whereas the lipiodol-ICG mixture showed insufficient fluorescent signals pre- and postoperatively due to rapid plasma clearance and unstable fluorescence. In addition, the benefit of the SHIFT agent compared with either mono-agent (ICG) or combinational mixture (ICG-lipiodol) was also exhibited in both promoted photothermal conversion efficiency and enhanced photoacoustic imaging. Based on these promising results, the SHIFT agent may be rapidly moved to clinical development with further evaluation in large animals to confirm its safety and benefits.
Conclusion and implications
The SHIFT strategy is a green physical method for multiagent formulation. It enables in situ post-TAE, ICG-guided hepatectomy for HCC, with high embolization and prolonged fluorescence performance. This study makes a major stride to bridge the preoperative gap of fluorescence-guided hepatectomy. From a future perspective, new developments in TAE/TACE formulations and novel theranostics (phototherapeutics, chemotherapeutics, and radiotherapeutics et al.) using SHIFT hold great potential in precision surgery, interventional therapy, and drug delivery, leading to better therapeutic outcomes and survival for patients with HCC.
CRediT authorship contribution statement
Zhe Wang: Project administration, Writing - original draft. Junqing Wang: Project administration, Writing - original draft. Gang Liu: Conceptualization, Funding acquisition, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was in part supported by the Major State Basic Research Development Program of China (2017YFA0205201), the National Natural Science Foundation of China (81901876, 81422023 and U1505221), the Fundamental Research Funds for the Central Universities (20720190088 and 20720200019), and the Program for New Century Excellent Talents in University, China (NCET-13-0502). JW was supported by the Hundred Talents Program (75110-18841227) from Sun Yat-sen University, Guangzhou, China; and the Guangdong Basic and Applied Basic Research Foundation (2019A1515110326).
Footnotes
Comment on: Chen H, Cheng H, Dai Q et al. A superstable homogeneous lipiodol-ICG formulation for locoregional hepatocellular carcinoma treatment. J Control Release 2020; 323:635–43.
Contributor Information
Junqing Wang, Email: wangjunqing@mail.sysu.edu.cn.
Gang Liu, Email: gangliu.cmitm@xmu.edu.cn.
References
- 1.Ferlay J., Colombet M., Soerjomataram I. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Canc. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Ji M., Liu Z., Chang E.T. Mass screening for liver cancer: results from a demonstration screening project in Zhongshan City, China. Sci Rep. 2018;8:12787. doi: 10.1038/s41598-018-31119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohanty S., Rajaram R., Bilimoria K.Y. Assessment of non-surgical versus surgical therapy for localized hepatocellular carcinoma. J Surg Oncol. 2016;113:175–180. doi: 10.1002/jso.24113. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y.X., De Baere T., Idee J.M. Transcatheter embolization therapy in liver cancer: an update of clinical evidences. Chin J Canc Res. 2015;27:96–121. doi: 10.3978/j.issn.1000-9604.2015.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galle P.R., Tovoli F., Foerster F. The treatment of intermediate stage tumours beyond TACE: from surgery to systemic therapy. J Hepatol. 2017;67:173–183. doi: 10.1016/j.jhep.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Xu D., Li L., Chu C. Advances and perspectives in near-infrared fluorescent organic probes for surgical oncology. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12:e1635. doi: 10.1002/wnan.1635. [DOI] [PubMed] [Google Scholar]
- 7.Chen H., Cheng H., Wu W. The blooming intersection of transcatheter hepatic artery chemoembolization and nanomedicine. Chin Chem Lett. 2020;31:1375–1381. [Google Scholar]
- 8.Byrne W.L., DeLille A., Kuo C. Use of optical imaging to progress novel therapeutics to the clinic. J Contr Release. 2013;172:523–534. doi: 10.1016/j.jconrel.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Vahrmeijer A.L., Hutteman M., van der Vorst J.R. Image-guided cancer surgery using near-infrared fluorescence. Nat Rev Clin Oncol. 2013;10:507–518. doi: 10.1038/nrclinonc.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakka S.G. Assessment of liver perfusion and function by indocyanine green in the perioperative setting and in critically ill patients. J Clin Monit Comput. 2018;32:787–796. doi: 10.1007/s10877-017-0073-4. [DOI] [PubMed] [Google Scholar]
- 11.Mindt S., Karampinis I., John M. Stability and degradation of indocyanine green in plasma, aqueous solution and whole blood. Photochem Photobiol Sci. 2018;17:1189–1196. doi: 10.1039/c8pp00064f. [DOI] [PubMed] [Google Scholar]
- 12.Chen H., Cheng H., Dai Q. A superstable homogeneous lipiodol-ICG formulation for locoregional hepatocellular carcinoma treatment. J Contr Release. 2020;323:635–643. doi: 10.1016/j.jconrel.2020.04.021. [DOI] [PubMed] [Google Scholar]
- 13.Kankala R.K., Zhang Y.S., Wang S.B. Supercritical fluid technology: an emphasis on drug delivery and related biomedical applications. Adv Healthc Mater. 2017;6 doi: 10.1002/adhm.201700433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kora S., Urakawa H., Mitsufuji T. Warming effect on miriplatin-lipiodol suspension for potential use as a chemotherapeutic agent for transarterial chemoembolization of hepatocellular carcinoma: in vitro study. Hepatol Res. 2013;43:1100–1104. doi: 10.1111/hepr.12050. [DOI] [PubMed] [Google Scholar]