Abstract
Objectives
To compare the clinical outcomes in terms of structure and function between the insertion of a transjugular intrahepatic portosystemic shunt (TIPS) created with the Viabahn ePTFE covered stent/bare metal stent (BMS) combination and the Fluency ePTFE covered stent/BMS combination.
Methods
A total of 101 consecutive patients who received a TIPS from February 2016 to August 2018 in our center were retrospectively analyzed. Sixty-four subjects were enrolled in the Viabahn group and 37 were enrolled in the Fluency group. The geometry characteristics of the TIPS were calculated, and the associated occurrence of shunt dysfunction, survival, overt hepatic encephalopathy, and variceal rebleeding were evaluated.
Results
The technical success rate was 100%. After the insertion of the TIPS, the rate of shunt dysfunction during the first 3 months was significantly different between the Viabahn and Fluency groups (1.6% and 13.5%, respectively; p = 0.024). Multivariate analysis indicated that the angle of portal venous inflow (α) was the only independent risk factor for shunt dysfunction (hazard ratio = 1.060, 95% confidence interval = 1.009–1.112, p = 0.020). In addition, 3 months after the TIPS insertion, the α angle distinctly increased from 20.9° ± 14.3°–26.9° ± 20.1° (p = 0.005) in the Fluency group but did not change significantly in the Viabahn group (from 21.9° ± 15.1°–22.9° ± 17.6°, p = 0.798).
Conclusions
Shunt dysfunction was related to the α angle owing to the slight effect on the α angle after the implantation of the TIPS. The Viabahn ePTFE covered stent/BMS combination was more stable in structure and promised higher short-term stent patency compared with the Fluency ePTFE covered stent/BMS combination.
Keywords: Transjugular intrahepatic portosystemic shunt, Stents, Portal venous inflow, Shunt dysfunction, Hepatic encephalopathy
Abbreviations: TIPS, transjugular intrahepatic portosystemic shunt; BMS, bare metal stent; OHE, overt hepatic encephalopathy; BCS, Budd-Chiari syndrome; ePTFE, expanded polytetrafluorethylene; FDA, Food and Drug Administration; PVT, portal vein thrombosis; MELD, model for end-stage liver disease
Introduction
Currently, transjugular intrahepatic portosystemic shunts (TIPSs) are mainly used for the treatment of complications caused by portal hypertension, especially uncontrollable or recurrent variceal hemorrhage and refractory ascites.1, 2, 3, 4 TIPSs are also used to treat gastric variceal hemorrhage, ectopic variceal bleeding, hepatic hydrothorax, hepatorenal syndrome, portal thrombosis, and Budd-Chiari syndrome.5, 6, 7 However, hepatic encephalopathy and shunt dysfunction following the insertion of a TIPS are the problematic complications of the procedure.
Increasing evidence has shown that compared with bare metal stents (BMS), expanded polytetrafluorethylene (ePTFE) covered stents can not only significantly reduce the rate of stent restenosis but are also effective in reducing rebleeding and improving survival.8,9 The use of ePTFE covered stents has completely revolutionized TIPS procedures, and the American Association for the Study of Liver Diseases (AASLD) practice guidelines recommend ePTFE as a routine TIPS material.3
The Viatorr ePTFE covered stent (WL Gore, Flagstaff AZ) was approved by the Food and Drug Administration for TIPS application in December 20, 04.10 Compared with the bare metal stent (BMS), it promises better shunt efficacy, patency rates, and survival.11,12 In China, the use of the Fluency ePTFE covered stent/BMS combination has gained great popularity as the Viatorr ePTFE covered stent was not available in the market until recently, and it is rather expensive. Several studies have confirmed the safety and feasibility of the Fluency ePTFE covered stent/BMS combination, but the stent patency appears to be lower than that of the Viatorr ePTFE covered stent.13,14
In general, the Fluency ePTFE covered stent is not entirely compatible with the soft BMS owing to its hardness. However, we consider that the Viabahn ePTFE covered stent, which has greater flexibility, might complement the BMS better.
Materials and methods
This study was approved by the ethics committee of Wuhan Union Hospital. All clinical practices and observations were conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from each patient before the study was conducted.
Patients
We conducted a retrospective study on 101 consecutive patients who underwent the TIPS procedure between February 2016 and August 2018 in our center.
The inclusion criteria were portal hypertension diagnosed by clinical symptoms and laboratory and imaging tests. Uncontrollable or recurrent variceal hemorrhage and refractory ascites were indications for TIPS implantation. The exclusion criteria were chronic heart, lung, or renal hypofunction; complications of overt hepatic encephalopathy (OHE); extensive primary or metastatic hepatic malignancy before TIPS insertion; a follow-up time <1 year; and patients with incomplete data.
According to the actual application of ePTFE covered stents, 101 subjects were recruited and classified into two groups: the Viabahn group (n = 64) and the Fluency group (n = 37). The baseline characteristics of all patients are displayed in Table 1.
Table 1.
Baseline characteristics of patients included in the study.
| Variables | Viabahn (n = 64) | Fluency (n = 37) | P values |
|---|---|---|---|
| Age (years) | 51.1 ± 11.3 | 53.7 ± 10.9 | 0.268 |
| Sex (male) | 42 | 27 | 0.296 |
| Aetiology | |||
| Hepatitis B virus | 38 | 21 | 0.879 |
| Hepatitis C virus | 9 | 5 | |
| Alcohol misuse | 4 | 4 | |
| Others | 13 | 7 | |
| Comorbidity | |||
| Hypertension | 5 | 4 | 0.721 |
| Diabetes | 9 | 6 | 0.778 |
| Hepatocellular carcinoma | 3 | 3 | 0.666 |
| TIPS indications | 0.536 | ||
| Variceal hemorrhage | 56 | 34 | |
| Refractory ascites | 8 | 3 | |
| laboratory parameters | |||
| Total bilirubin (mg/dL) | 1.35 ± 0.70 | 1.32 ± 0.70 | 0.857 |
| Albumin (g/L) | 31.2 ± 6.5 | 31.5 ± 6.0 | 0.812 |
| Alanine aminotransferase (U/L) | 27.7 ± 14.2 | 36.5 ± 32.0 | 0.120 |
| Aspartate aminotransferase (U/L) | 38.9 ± 32.1 | 45.8 ± 40.8 | 0.349 |
| Creatinine (mg/dL) | 0.75 ± 0.24 | 0.73 ± 0.21 | 0.667 |
| Blood urea nitrogen (mmol/L) | 5.83 ± 3.00 | 5.78 ± 2.45 | 0.942 |
| Prothrombin time (seconds) | 16.5 ± 2.3 | 17.0 ± 2.8 | 0.365 |
| International normalized ratio | 1.36 ± 0.24 | 1.41 ± 0.30 | 0.316 |
| Hemoglobin (g/L) | 80.0 ± 24.3 | 79.2 ± 14.3 | 0.872 |
| Platelet count (109/L) | 88.4 ± 67.6 | 102.6 ± 92.5 | 0.378 |
| Serum Na (mmol/L) | 138.4 ± 4.6 | 137.9 ± 7.0 | 0.654 |
| Child-Pugh score | 7.4 ± 1.6 | 7.5 ± 1.5 | 0.600 |
| Child-Pugh class | 0.948 | ||
| A | 19 | 12 | |
| B | 40 | 22 | |
| C | 5 | 3 | |
| MELD score | 11.3 ± 3.2 | 11.1 ± 3.1 | 0.827 |
| MELD-Na score | 12.2 ± 4.5 | 12.4 ± 4.1 | 0.836 |
| Imaging evaluation | |||
| Portal vein diameter (mm) | 15.2 ± 3.5 | 15.8 ± 3.6 | 0.433 |
| Splenic vein diameter (mm) | 12.1 ± 2.5 | 12.3 ± 3.3 | 0.832 |
| Spontaneous portosystemic shunt | 12 | 3 | 0.122 |
| Pre-TIPS PP(mmHg) | 33.8 ± 6.2 | 33.9 ± 5.7 | 0.954 |
| Pre-TIPS PPG (mmHg) | 27.1 ± 5.2 | 26.1 ± 5.5 | 0.412 |
Abbreviation: MELD, model for end-stage liver disease; PVT, portal vein thrombosis; TIPS, transjugular intrahepatic portosystemic shunt; PP, portal pressure; PPG, portal pressure gradient.
TIPS procedure
A standardized TIPS technique has been described previously.15, 16, 17, 18 A pressure measurement was obtained after the catheterization of the right or middle hepatic vein was performed through the right internal jugular vein with a transjugular liver access set (RUPS-100; Cook Inc.). An intrahepatic tract was created by the puncture needle between the hepatic vein and one of the branches of the portal vein, and then the guidewire and catheter were advanced into the portal vein. At this time, portography was performed, and a pressure measurement was subsequently obtained. After the parenchymal tract was pre-balloon dilated, an 8-mm bare stent (E-Luminexx or Lifestent; Bard Inc.) combined with an ePTFE covered stent (Fluency; Bard Inc. or Viabahn; Gore Inc.) was implanted between the hepatic vein and the portal vein. The portal pressure gradient (PPG) value was then obtained; the length of the covered stent inside the portal vein was less than 1 cm, and there was no special requirement for the length inside the hepatic vein. Cyanoacrylate was utilized to embolize gastric varices via angiography.
The angle of deviation of the blood flow at the portal venous inflow (α) and the central venous outflow (β) were measured by two radiologists on portography and computed tomography (CT) images. The angles were calculated according to Fig. 1, which has been detailed previously.19
Fig. 1.
Illustration demonstrating the angle of deviation of the blood flow at the portal venous inflow (α) and central venous outflow (β).
Postoperative management
All patients were required to stay in hospital for several days after the procedure, with strict vital sign monitoring. The patients received symptomatic treatments, such as analgesia and antibiotic prophylaxis, as well as routine treatments to improve liver function. Anticoagulation was not routinely recommended, except in patients with portal vein thrombosis (PVT).20
Follow up
After TIPS implantation, laboratory tests (including blood tests, liver and kidney function, and coagulation function) and imaging examinations (such as ultrasound, CT, or MRI) were performed at 1, 3, 6, and 12 months in the first year and once a year thereafter. Phone calls were made regularly to keep up with the prognosis and complications of the patients, and detailed records were maintained.21
During the follow up, if a maximum shunt flow velocity of ≤50 cm/s or ≥250 cm/s or an absence of blood signal was found by ultrasonography or clinical symptoms (such as rebleeding or ascites) relapsed, then shunt dysfunction was suspected.2,22 Transjugular-route portal venography was subsequently performed on these patients, and shunt dysfunction was confirmed by a shunt stenosis of >50%; stent revision (recanalization, balloon dilation, or creation of a parallel shunt) was needed once diagnosis was confirmed.3,4 OHE was evaluated and graded based on the West Haven criteria.23
Statistical analyses
Continuous variables are expressed as mean ± standard deviation and were compared using the independent sample t-test or paired t-test. Categorical variables are expressed as frequencies and were compared using the Fisher’s exact test or the chi-squared test. Actuarial probabilities were calculated with Kaplan–Meier curves and were compared by log-rank tests. Independent predictors were identified using the Cox regression model. Differences were considered significant at p < 0.05. Statistical analyses were performed with IBM SPSS statistics version 22.0 (IBM, Inc., Chicago, IL, USA). Sample size calculations were performed using the Power and Sample Size Calculation software (version 2.1.31, Vanderbilt University, Tennessee, USA).
Results
Immediate condition following TIPS implantation
The TIPS procedure was successfully completed in all patients, and the technical success rate was 100%. After TIPS creation, the PPG of all patients reduced to at least 40%, and in 92 (91%) patients, the PPG even decreased to below 12 mmHg. Specifically, the average PPG in the Viabahn group fell from 27.1 ± 5.2 mmHg to 9.6 ± 2.4 mmHg, while in the Fluency group, it fell from 26.1 ± 5.5 mmHg to 10.2 ± 2.6 mmHg. No life-threatening complications, such as hemoperitoneum or liver or renal failure, were discovered in any of the patients during the perioperative period of the TIPS procedure.24,25
TIPS geometry
The α and β angles of both groups at the time of and 3 months after TIPS implantation are displayed in Table 2. There was no statistical difference in the baseline values between the two groups. However, 3 months after the TIPS insertion, the α angle in the Fluency group significantly increased from 20.9° ± 14.3°–26.9° ± 20.1° (p = 0.005) compared with an inconspicuous change from 21.9° ± 15.1°–22.9° ± 17.6° (p = 0.798) in the Viabahn group. With respect to the β angle, there were no significant changes in the Fluency group (12.0 ± 8.2° vs. 12.1° ± 9.9°, p = 0.956) or Viabahn group (7.8° ± 9.5° vs. 8.2° ± 9.0°, p = 0.709) between immediately and 3 months post-TIPS. Typical cases are shown in Fig. 2.
Table 2.
The angle of deviation of the blood flow.
| Variables | The angle of deviation of the blood flow |
P value | |
|---|---|---|---|
| Immediately post-TIPS | 3 months post-TIPS | ||
| Viabahn group (α angle, °) | 22.5 ± 12.1 | 22.8 ± 14.2 | 0.798 |
| Fluency group (α angle, °) | 23.0 ± 15.3 | 28.2 ± 19.5 | 0.005 |
| P value | 0.888 | 0.186 | |
| Viabahn group (β angle, °) | 22.0 ± 10.8 | 20.7 ± 11.1 | 0.317 |
| Fluency group (β angle, °) | 22.3 ± 12.8 | 24.1 ± 12.9 | 0.337 |
| P value | 0.925 | 0.257 | |
Fig. 2.
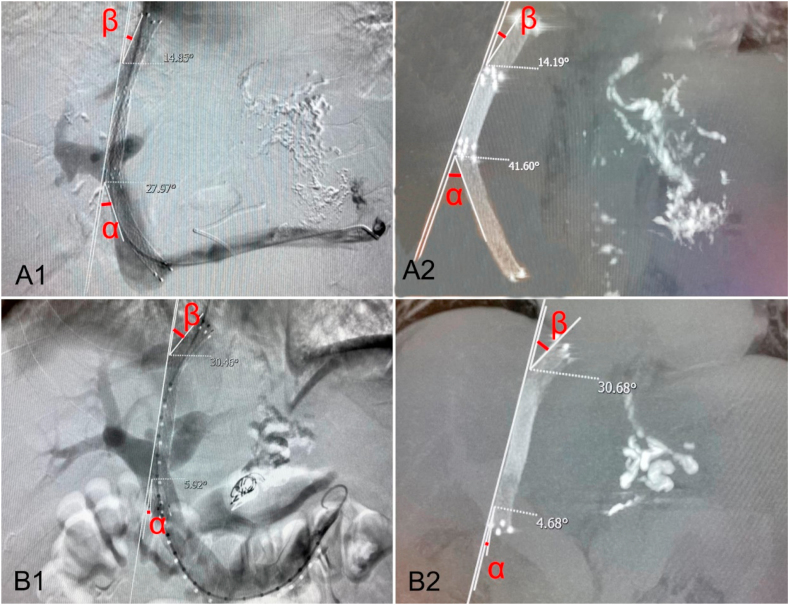
A. A 73-year-old male with variceal hemorrhage underwent TIPS created with the Fluency ePTFE covered stent/BMS combination. When TIPS insertion (A1), the α and β angle were respectively 28° and 15°; and 3 months later (A2), the α angle increased to 42° but β angle remained unchanged. B. A 38-year-old male with variceal hemorrhage received TIPS created with the Viabahn ePTFE covered stent/BMS combination. When TIPS insertion (B1), the α and β angle were respectively 6° and 30°; and 3 months later (B2), the α and β angle were respectively 5° and 31°, basically unchanged.
Shunt dysfunction, survival, OHE, and variceal rebleeding after TIPS implantation
The median follow-up time of the Viabahn group was 19.5 (range: 12–30) months and that of the Fluency group was 30 (range: 14–44) months. The shunt dysfunction, survival, OHE, and variceal rebleeding of all patients are shown in Table 3, and the Kaplan–Meier curves of the follow-up are shown in Fig. 3.
Table 3.
Results of treatment and complications, including shunt dysfunction, survival, variceal rebleeding and overt hepatic encephalopathy during the follow-up.
| Variables | Viabahn (n = 64) | Fluency (n = 37) | P values |
|---|---|---|---|
| Shunt Dysfunction | |||
| 0–3 months | 1 (1.6%) | 5 (13.5%) | 0.024 |
| 0–6 months | 5 (7.8%) | 5 (13.5%) | 0.491 |
| 0–12 months | 8 (12.5%) | 6 (16.2%) | 0.541 |
| Death | |||
| 0–3 months | 2 (3.1%) | 3 (8.1%) | 0.453 |
| 0–6 months | 4 (6.3%) | 4 (10.8%) | 0.460 |
| 0–12 months | 6 (9.4%) | 4 (10.8%) | 1.000 |
| Variceal Rebleeding | |||
| 0–3 months | 1 (1.6%) | 2 (5.4%) | 0.552 |
| 0–6 months | 3 (4.7%) | 2 (5.4%) | 1.000 |
| 0–12 months | 7 (10.9%) | 4 (10.8%) | 1.000 |
| Overt Hepatic Encephalopathy | |||
| 0–3 months | 11 (17.2%) | 8 (21.6%) | 0.605 |
| 0–6 months | 13 (20.3%) | 9 (24.3%) | 0.863 |
| 0–12 months | 13 (20.3%) | 10 (27.0%) | 0.468 |
Fig. 3.
Kaplan-Meier curve of post-TIPS cumulative shunt dysfunction (A), survival (B), OHE (C) and variceal rebleeding (D), the log-rank p values of which were respectively 0.551, 0.979, 0.454 and 0.948.
Shunt dysfunction
In the year following TIPS insertion, shunt dysfunction occurred in 14 patients, including 8 (12.5%) in the Viabahn group and 6 (16.2%) in the Fluency group (p = 0.541), which indicated no statistical difference. However, after 3 months, a significant difference was found in shunt dysfunction in one patient (1.6%) in the Viabahn group and five (13.5%) patients in the Fluency group (p = 0.024) (Table 3). A similar pattern was observed in the Kaplan–Meier analysis (Fig. 3). In terms of shunt dysfunction during follow-up, the univariate analysis showed that higher model for end-stage liver disease (MELD) scores, lower international normalized ratios, and larger α angles were related. Multivariate analysis showed that only the α angle (hazard ratio [HR] = 1.060, 95% confidence interval [CI] = 1.009–1.112, p = 0.020) was an independent predictor of shunt dysfunction (Table 4).
Table 4.
Univariate and multivariate analysis of factors associated with post-TIPS shunt dysfunction.
| Variables | Shunt dysfunction |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| Yes (n = 14) | No (n = 87) | P values | HR | 95% CI | P values | |
| Male | 12 (85.7%) | 57 (65.5%) | 0.215 | – | – | – |
| Age (y) | 51.6 ± 9.5 | 52.1 ± 11.5 | 0.859 | – | – | – |
| INR | 1.31 ± 0.10 | 1.39 ± 0.28 | 0.056 | – | – | – |
| MELD score | 10.1 ± 1.7 | 11.4 ± 3.3 | 0.041 | – | – | – |
| α angle (°) | 33.6 ± 16.8 | 21.6 ± 14.9 | 0.007 | 1.060 | 1.009–1.112 | 0.020 |
Survival, OHE, and variceal rebleeding
During the 1-year follow-up, six patients died in the Viabahn group: three due to liver failure (2 weeks, 2 months, and 5 months after the TIPS procedure) and three due to variceal rebleeding (4 months, 9 months, and 12 months after the TIPS procedure). Four patients died in the Fluency group: two due to liver failure (2 weeks and 1 month after the TIPS procedure), one due to variceal rebleeding (2 months after the TIPS procedure), and one due to hepatic encephalopathy (4 months after the TIPS procedure). The survival rate at 1 year was 90.6% in the Viabahn group and 89.2% in the Fluency group (p = 1.000).
After TIPS insertion, OHE within 1 year occurred in 13 patients (20.3%) in the Viabahn group and 10 patients (27.0%) in the Fluency group (p = 1.000). OHE mainly developed during the first 3 months in both groups. More specifically, OHE within 3 months of TIPS insertion occurred in 11 of 13 patients (84.6%) in the Viabahn group and 8 of 10 patients (80.0%) in the Fluency group.
During the follow-up period, six (16.2%) and nine (14.1%) patients suffered from variceal rebleeding in the Fluency and Viabahn groups, respectively (p = 0.778). Excluding patients with refractory ascites, there were five (14.7%) and seven (12.5%) cases of variceal rebleeding in the Fluency and Viabahn groups, respectively (p = 1.000); this difference was still not statistically significant.
Liver and kidney function
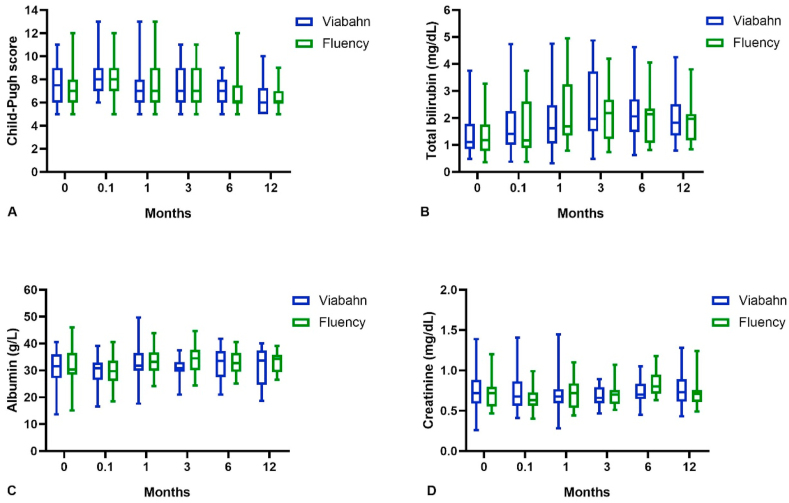
During the follow-up period, the changing trends of the Child–Pugh score, total bilirubin, albumin, and serum creatinine in all patients are depicted in Fig. 4. There was no statistical difference between the indexes at the baseline level or those at 0.1, 1, 3, 6, and 12 months after TIPS implantation between the two groups. On the whole, the liver and kidney function indices were slightly worse a short time after TIPS insertion, but they slowly returned to baseline levels within a month. Over time, the Child–Pugh score showed a progressively downward trend, which fell from 7.5 ± 1.5 years before the TIPS procedure to 6.6 ± 1.4 years after (p = 0.002).
Fig. 4.
Shown in the box plots were the changing trends of liver and kidney function indices in two groups of patients. Before TIPS implantation and 0.1, 1, 3, 6, 12 months later, the average Child-Pugh score in Viabahn group and Fluency group were respectively 7.4 ± 1.6 vs. 7.6 ± 1.5 (p = 0.526), 8.1 ± 1.8 vs. 8.4 ± 1.9 (p = 0.383), 7.5 ± 2.2 vs. 7.5 ± 2.2 (p = 0.925), 7.4 ± 1.8 vs. 7.8 ± 1.9 (p = 0.556), 6.7 ± 1.9 vs. 7.0 ± 1.9 (p = 0.586), 6.7 ± 1.4 vs. 6.4 ± 1.4 (p = 0.562), which indicated no significant difference. Similarly, these two groups did not differ from each other concerning total bilirubin, albumin and serum creatinine both before and after TIPS insertion (p > 0.05). On the whole, the Child-Pugh score, total bilirubin and serum creatinine of all patients increased at first and then decreased, while albumin presented an opposite trend.
Discussion
The development of shunt dysfunction is mainly attributed to pseudointimal hyperplasia and thrombosis.26,27 When the BMS was widely utilized, shunt dysfunction commonly occurred as a result of bile leakage and extended liver parenchyma growth into the shunt via the mesh of bare stents. However, the incidence of shunt dysfunction has been effectively reduced in the recent years owing to the widespread use of ePTFE covered stents.28
The Viatorr ePTFE covered stent, made specifically for the TIPS procedure, mainly consists of a 5–9 cm long PTFE-covered intrahepatic segment and a 2 cm long uncovered portal segment. However, the Fluency and Viabahn ePTFE covered stents that are commonly used in China are completely covered by PTFE inside out, with no bare portal component.29 Therefore, the placement of Fluency ePTFE covered stents is always performed in association with a longer BMS owing to the lack of the portal component.30,31 To date, several studies have confirmed the feasibility of the Fluency ePTFE covered stent/BMS combination.32, 33, 34
In the era of covered stents, shunt dysfunction mostly results from the stenosis of the stent ends near the portal vein and the hepatic vein, which correlates with the position of stent placement and the diameter and flexibility of the stents.35,36 Some studies have verified that the larger the α angle, the more likely it will be for shunt dysfunction to occur,25 which is similar to our conclusion. In this study, we found that the α angle (HR = 1.060, 95% CI = 1.009–1.112, p = 0.020) was the only predictor of shunt dysfunction.
As a result of the hard texture of the Fluency ePTFE covered stent, longitudinal deformation occurs in the early stage as the stent rebounces after placement, which leads to an increase in the α angle, and may even cause twists in the softer BMC due to the excessive tension.37 Viabahn ePTFE covered stents are softer than Fluency ePTFE covered stents, and the tension towards the bare stents outside is rather low, so longitudinal deformation is less likely to occur. This explains why the α angle inflow increased after TIPS implantation in the Fluency group but not in the Viabahn group. Furthermore, 3 months following TIPS insertion, the α angle distinctly increased from 20.9° ± 14.3°–26.9° ± 20.1° (p = 0.005) in the Fluency group but was not significantly changed in the Viabahn group (from 21.9° ± 15.1°–22.9° ± 17.6°, p = 0.798).
Therefore, compared with Fluency ePTFE covered stents, Viabahn ePTFE covered stents are more suitable for use in combination with bare stents, and shunt dysfunction is less likely to occur. In line with this, we found that the incidence of shunt dysfunction within 3 months was far less in the Viabahn group than in the Fluency group (1.6% vs. 11.5%, p = 0.024). This, in turn, suggests that the Viabahn ePTFE covered stent/BMS combination can be used instead of a Viatorr ePTFE covered stent, which is difficult to obtain in China.
Other studies have indicated that the Fluency ePTFE covered stent/BMS combination did not differ from the Viatorr stent in terms of survival, OHE, and variceal rebleeding.32, 33, 34 Furthermore, we found no significant difference between the different types of ePTFE covered stents in our study. The Child–Pugh score and some related laboratory indices of both groups were slightly worse after 3 days following TIPS placement but gradually restored and returned to baseline levels within a month. In 3, 6, and 12 months, we found that the Child–Pugh score fell below the baseline level and showed a downward trend over time, where an increasing number of patients progressed from Child–Pugh B and C to Child–Pugh A. This phenomenon was similar to that reported in other studies,38 which suggests that the Viabahn ePTFE covered stent/BMS combination could not only ensure the safety but also help to restore the poor liver and kidney functions of cirrhosis patients after TIPS implantation.39
Although the Viabahn ePTFE covered stent/BMS combination may be the best approach, it must be pointed out that the Viabahn ePTFE covered stent is only produced in 2.5 cm, 5 cm, and 10 cm sizes. Among these three standards, it seems that only a 5 cm long stent is suitable for establishing a passway between the hepatic vein and the portal vein, which leaves only the choice of 4 or 6 cm long other stents, such as a Fluency ePTFE covered stent, in certain cases. In TIPS insertion, a Fluency ePTFE covered stent should be placed such that it stays in a vertical position as much as possible, and a 4-cm rather than a 6-cm stent is preferred in order to prevent the influence of longitudinal deformation. Another limitation is that this was a non-randomized retrospective study, which may have led to selection bias.
Conclusion
The occurrence of shunt dysfunction was related to the α angle because of the slighter effect after TIPS implantation. The Viabahn ePTFE covered stent/BMS combination was more stable in terms of structure and promised a higher short-term stent patency compared with the Fluency ePTFE covered stent/BMS combination.
Ethics approval and consent to participate
This study was approved by the ethics committee of Wuhan Union Hospital. All clinical practices and observations were conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from each patient before the study was conducted.
Consent for publication
Written informed consent was obtained from patients for publication of these case reports and any accompanying images.
Availability of data and materials
Data sharing is not applicable to this manuscript as no datasets were generated during the study.
Declaration of interests
The authors declare that they have no conflicts of interests to this work. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81873917).
Authors’ contributions
BX conceived and designed the study. JL, JMeng and CZ collected the data. JL, CY, JMa and MC summarised all data. JL and JMeng drafted the manuscript. BX revised the final manuscript. All authors have read and approved the final manuscript.
Acknowledgements
We are grateful to the patients who participated in the study, and to the physicians and nurses who assisted.
Contributor Information
Jiacheng Liu, Email: jiacheng6jc@163.com.
Jie Meng, Email: 15927256343@163.com.
Chen Zhou, Email: zhouchenjr@126.com.
Qin Shi, Email: shiq1010@126.com.
Chongtu Yang, Email: Henrys1011@163.com.
Jinqiang Ma, Email: 18942931650@163.com.
Manman Chen, Email: 810480222@qq.com.
Bin Xiong, Email: herr_xiong@126.com.
References
- 1.de Franchis R., VI Faculty Baveno. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63(3):743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 2.Dariushnia S.R., Haskal Z.J., Midia M. Quality improvement guidelines for transjugular intrahepatic portosystemic shunts. J Vasc Intervent Radiol. 2016;27(1):1–7. doi: 10.1016/j.jvir.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 3.Boyer T.D., Haskal Z.J. American association for the study of liver diseases. The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension: update 2009. Hepatology. 2010;51(1):306. doi: 10.1002/hep.23383. [DOI] [PubMed] [Google Scholar]
- 4.Boyer T.D., Haskal Z.J., American Association for the Study of Liver Diseases The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology. 2005;41(2):386–400. doi: 10.1002/hep.20559. [DOI] [PubMed] [Google Scholar]
- 5.Sankar K., Moore C.M. Transjugular intrahepatic portosystemic shunts. J Am Med Assoc. 2017;317(8) doi: 10.1001/jama.2016.20899. 880-880. [DOI] [PubMed] [Google Scholar]
- 6.Rössle M. TIPS: 25 years later. J Hepatol. 2013;59(5):1081–1093. doi: 10.1016/j.jhep.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Patidar K.R., Sydnor M., Sanyal A.J. Transjugular intrahepatic portosystemic shunt. Clin Liver Dis. 2014;18(4):853–876. doi: 10.1016/j.cld.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perarnau J.M., Le Gouge A., Nicolas C. Covered vs. uncovered stents for transjugular intrahepatic portosystemic shunt: a randomized controlled trial. J Hepatol. 2014;60(5):962–968. doi: 10.1016/j.jhep.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Wang L., Xiao Z., Yue Z. Efficacy of covered and bare stent in TIPS for cirrhotic portal hypertension: a single-center randomized trial. Sci Rep. 2016;6:21011. doi: 10.1038/srep21011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cejna M. Should stent-grafts replace bare stents for primary transjugular intrahepatic portosystemic shunts? Semin Intervent Radiol. 2005;22(4):287–299. doi: 10.1055/s-2005-925555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferral H., Gomez-Reyes E., Fimmel C.J. Post–transjugular intrahepatic portosystemic shunt follow-up and management in the VIATORR era. Tech Vasc Intervent Radiol. 2016;19(1):82–88. doi: 10.1053/j.tvir.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Sommer C.M., Gockner T.L., Stampfl U. Technical and clinical outcome of transjugular intrahepatic portosystemic stent shunt: bare metal stents (BMS) versus viatorr stent-grafts (VSG) Eur J Radiol. 2012;81(9):2273–2280. doi: 10.1016/j.ejrad.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 13.Li Y.H., Xu Z.Y., Wu H.M. Long-term shunt patency and overall survival of transjugular intrahepatic portosystemic shunt placement using covered stents with bare stents versus covered stents alone. Clin Radiol. 2018;73(6):580–587. doi: 10.1016/j.crad.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Wang C.M., Li X., Fu J. Construction of transjugular intrahepatic portosystemic shunt: bare metal stent/stent-graft combination versus single stent-graft, a prospective randomized controlled study with long-term patency and clinical analysis. Chin Med J. 2016;129(11):1261–1267. doi: 10.4103/0366-6999.182830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pereira K., Baker R., Salsamendi J. An approach to endovascular and percutaneous management of transjugular intrahepatic portosystemic shunt (TIPS) dysfunction: a pictorial essay and clinical practice algorithm. Cardiovasc Intervent Radiol. 2016;39(5):639–651. doi: 10.1007/s00270-015-1247-4. [DOI] [PubMed] [Google Scholar]
- 16.Rosado B., Kamath P.S. Transjugular intrahepatic portosystemic shunts: an update. Liver Transplant. 2003;9(3):207–217. doi: 10.1053/jlts.2003.50045. [DOI] [PubMed] [Google Scholar]
- 17.Liu J., Shi Q., Xiao S. Using transjugular intrahepatic portosystemic shunt as the first-line therapy in secondary prophylaxis of variceal hemorrhage. J Gastroenterol Hepatol. 2020;35(2):278–283. doi: 10.1111/jgh.14761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J., Zhou B., Chen D. Transjugular intrahepatic portosystemic shunt placement in patients with schistosomiasis-induced liver fibrosis. Cardiovasc Intervent Radiol. 2019;42(12):1760–1770. doi: 10.1007/s00270-019-02295-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klasen-Sansone J., Bode J., Lanzman R.S. TIPS geometry influences patency. Z Gastroenterol. 2015;53(1):28–32. doi: 10.1055/s-0034-1385430. [DOI] [PubMed] [Google Scholar]
- 20.Senzolo M., Sartori M., Rossetto V. Prospective evaluation of anticoagulation and transjugular intrahepatic portosistemic shunt for the management of portal vein thrombosis in cirrhosis. Liver Int. 2012;32(6):919–927. doi: 10.1111/j.1478-3231.2012.02785.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim S.K., Belikoff B.G., Guevara C.J. An algorithm for management after transjugular intrahepatic portosystemic shunt placement according to clinical manifestations. Dig Dis Sci. 2017;62(2):305–318. doi: 10.1007/s10620-016-4399-4. [DOI] [PubMed] [Google Scholar]
- 22.Kanterman R.Y., Darcy M.D., Middleton W.D. Doppler sonography findings associated with transjugular intrahepatic portosystemic shunt malfunction. AJR Am J Roentgenol. 1997;168(2):467–472. doi: 10.2214/ajr.168.2.9016228. [DOI] [PubMed] [Google Scholar]
- 23.Vilstrup H., Amodio P., Bajaj J. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European association for the study of the liver and the American association for the study of liver diseases. J Hepatol. 2014;61(3):642–659. doi: 10.1016/j.jhep.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 24.Shah R.P., Sze D.Y. Complications during transjugular intrahepatic portosystemic shunt creation. Tech Vasc Intervent Radiol. 2016;19(1):61–73. doi: 10.1053/j.tvir.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Vizzutti F., Schepis F., Arena U. Transjugular intrahepatic portosystemic shunt complications: prevention and management. Semin Intervent Radiol. 2015;32(2):123–132. doi: 10.1055/s-0035-1549376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark T.W. Management of shunt dysfunction in the era of TIPS endografts. Tech Vasc Intervent Radiol. 2008;11(4):212–216. doi: 10.1053/j.tvir.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Owen J.W., Saad N.E., Foster G. The feasibility of using volumetric phase-contrast MR imaging (4D flow) to assess for transjugular intrahepatic portosystemic shunt dysfunction. J Vasc Intervent Radiol. 2018;29(12):1717–1724. doi: 10.1016/j.jvir.2018.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Z., Han G., Wu Q. Patency and clinical outcomes of transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stents versus bare stents: a meta-analysis. J Gastroenterol Hepatol. 2010;25(11):1718–1725. doi: 10.1111/j.1440-1746.2010.06400.x. [DOI] [PubMed] [Google Scholar]
- 29.Qi X.S., Bai M., Yang Z.P. Selection of a TIPS stent for management of portal hypertension in liver cirrhosis: an evidence-based review. World J Gastroenterol. 2014;20(21):6470. doi: 10.3748/wjg.v20.i21.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Q., Jiang J., He Y. Transjugular intrahepatic portosystemic shunt using the FLUENCY expanded polytetrafluoroethylene-covered stent. Exp Ther Med. 2013;5(1):263–266. doi: 10.3892/etm.2012.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo X., Zhao M., Wang X. Long-term patency and clinical outcome of the transjugular intrahepatic portosystemic shunt using the expanded polytetrafluoroethylene stent-graft. PLoS One. 2019;14(2) doi: 10.1371/journal.pone.0212658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saad W.E., Darwish W.M., Davies M.G. Stent-grafts for transjugular intrahepatic portosystemic shunt creation: specialized TIPS stent-graft versus generic stent-graft/bare stent combination. J Vasc Intervent Radiol. 2010;21(10):1512–1520. doi: 10.1016/j.jvir.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Wan Y.M., Li Y.H., Xu Y. Predictors of shunt dysfunction and overall survival in patients with variceal bleeding treated with transjugular portosystemic shunt creation using the fluency stent graft. Acad Radiol. 2018;25(7):925–934. doi: 10.1016/j.acra.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 34.Luo X.F., Nie L., Wang Z. Stent-grafts for the treatment of TIPS dysfunction: Fluency stent vs Wallgraft stent. World J Gastroenterol. 2013;19(30):5000. doi: 10.3748/wjg.v19.i30.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weber C.N., Nadolski G.J., White S.B. Long-term patency and clinical analysis of expanded polytetrafluoroethylene–covered transjugular intrahepatic portosystemic shunt stent grafts. J Vasc Intervent Radiol. 2015;26(9):1257–1265. doi: 10.1016/j.jvir.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Cura M., Cura A., Suri R. Causes of TIPS dysfunction. AJR Am J Roentgenol. 2008;191(6):1751. doi: 10.2214/AJR.07.3534. [DOI] [PubMed] [Google Scholar]
- 37.McGrath D.J., O’Brien B., Bruzzi M. Evaluation of cover effects on bare stent mechanical response. J Mech Behav Biomed Mater. 2016;61:567–580. doi: 10.1016/j.jmbbm.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 38.Lv Y., Li K., He C. TIPSS for variceal bleeding in patients with idiopathic non-cirrhotic portal hypertension: comparison with patients who have cirrhosis. Aliment Pharmacol Ther. 2019;49(7):926–939. doi: 10.1111/apt.15186. [DOI] [PubMed] [Google Scholar]
- 39.Busk T.M., Bendtsen F., Poulsen J.H. Transjugular intrahepatic portosystemic shunt: impact on systemic hemodynamics and renal and cardiac function in patients with cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2018;314(2):G275–G286. doi: 10.1152/ajpgi.00094.2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this manuscript as no datasets were generated during the study.