Abstract
Postural orthostatic tachycardia syndrome (POTS) is a disorder epitomized by the story of the blind men and the elephant. Patients may see primary care internists or pediatricians due to fatigue, be referred to neurologists for “spells”, to cardiologists for evaluation of pre-syncope or chest pain, to gastroenterologists for nausea or dyspepsia, and even pulmonologists for dyspnea. Adoption of a more systematic approach to their evaluation and better characterization of patients has led to greater understanding of comorbidities, hypotheses prompting mechanistic investigations, and pharmacologic trials. Recent work has implicated disordered sympathetic nervous system activation in response to central (thoracic) hypovolemia. It is this pathway that leads one zero in on a putative focal point from which many of the clinical manifestations can be explained – specifically the carotid body. Despite heterogeneity in etiopathogenesis of a POTS phenotype, we propose that aberrant activation and response of the carotid body represents one potential common pathway in evolution. To understand this postulate, one must jettison isolationist or reductionist ideas of chemoreceptor and baroreceptor functions of the carotid body or sinus, respectively, and consider their interaction and interdependence both locally and centrally where some of its efferents merge. Doing so enables one to connect the dots and appreciate origins of diverse manifestations of POTS, including dyspnea for which the concept of neuro-mechanical uncoupling is wanting, thereby expanding our construct of this symptom. This perspective expounds our premise that POTS has a prominent respiratory component.
Keywords: Carotid body, Chemoreflex, Baroreflex, Dyspnea, Orthostatic
Graphical abstract
Highlights
-
•
Dyspnea affects ~⅓ patients with postural orthostatic tachycardia syndrome (POTS).
-
•
POTS is characterized by thoracic hypovolemia and compromised cephalad perfusion when upright.
-
•
Carotid body and adjacent carotid sinus mediate chemo- and baro- reflexes, respectively.
-
•
These are not independent and stimulation of either activates sympathetic discharge.
-
•
We speculate that carotid body mediates hyperventilation and dyspnea in POTS.
1. Introduction
It has been nearly 30 years since publication of results of head-up tilt testing in patients who presented with chronic orthostatic intolerance defined a unique, clinically recognizable, group with an attenuated form of dysautonomia (Schondorf and Low, 1993) – postural tachycardia syndrome (POTS). POTS is now increasingly recognized in patients with fatigue, dizziness, sleep disturbance, functional GI disorders, altered mentation (a.k.a. “brain fog”), pain, heat or cold intolerance and skin changes with acrocyanosis or livido-like discoloration in distal extremities (Kizilbash et al., 2014). A large minority also report chest discomfort or dyspnea, such that these symptoms were incorporated into the Vanderbilt Orthostatic Symptom Score (Raj et al., 2005a). Indeed, patients with POTS may present with (Popatia and Subramaniam, 2014), or be referred for (Ismail-Sayed et al., 2020), dyspnea or chest pain. The purpose of this perspective is to alert pulmonologists to POTS by summarizing extant literature and emerging data, arguing that a subset of POTS may be a respiratory disorder.
1.1. A rose by any other name
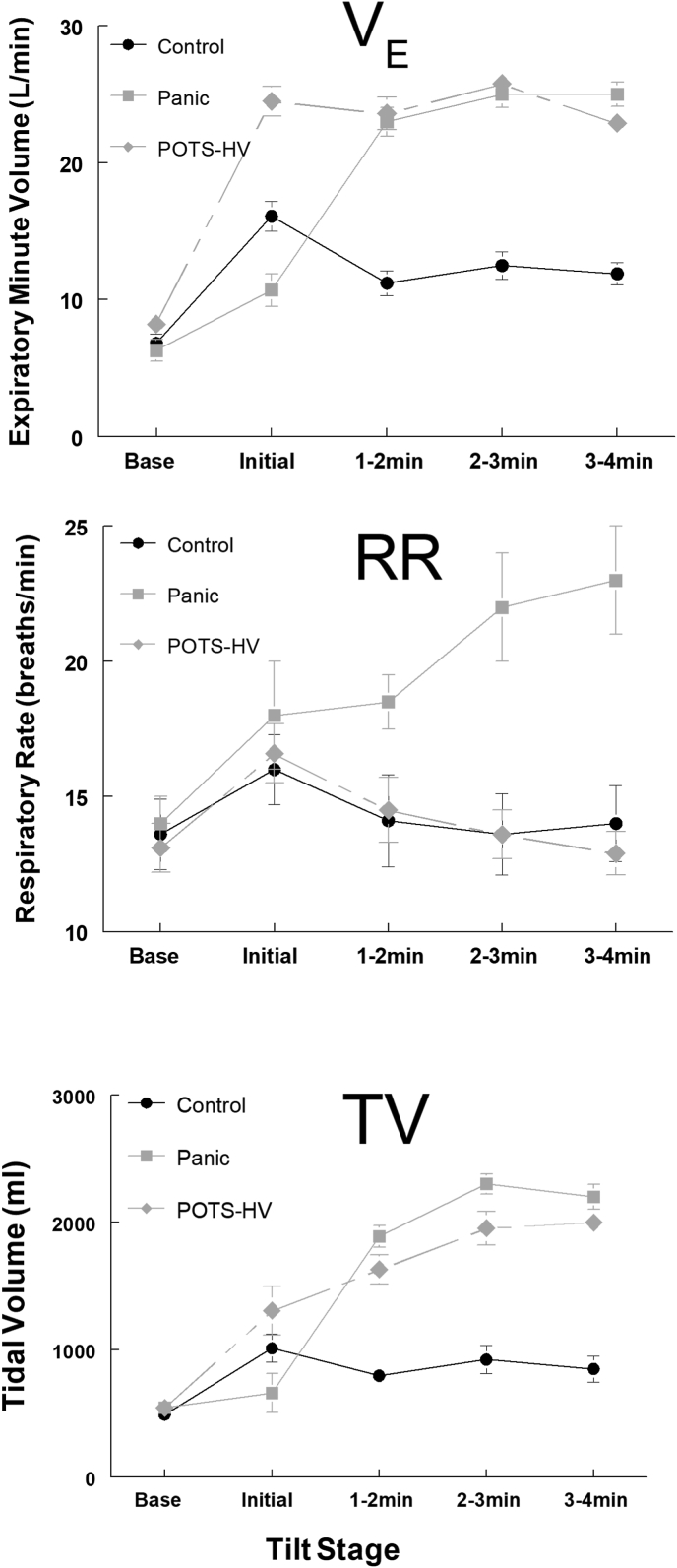
Patients presenting with dyspnea but without evidence of cardiopulmonary disease on standard tests (electro- and echo-cardiogram, pulmonary function tests, chest radiograph) are often diagnosed with hyperventilation syndrome (HVS), anxiety, or panic. Yet, we now know that breathing in panic disorder differs from breathing pattern(s) seen in patients with POTS (Stewart et al., 2018c) (Fig. 1). The Nijmegen questionnaire allegedly supports the diagnosis of HVS (van Dixhoorn and Duivenvoorden, 1985), but inspection of this tool reveals many of its questions regarding symptoms in other organ systems bear more than passing similarity to symptoms seen in POTS; the suggestion being that patients so diagnosed may in fact have POTS.
Fig. 1.
Respiratory data are shown for 16 subjects with POTS who complained of orthostatic dyspnea; 7 patients diagnosed with recurrent complaints of upright tachycardia, shortness of breath, and hyperventilation symptoms during panic attacks triggered by orthostasis; and 7 healthy control subjects. Breathing rate contributes to the initial surge in expiratory minute volume (VE), increased tidal volume (TV) accounts for the remainder of the VE increase in POTS-HV. Reprinted from (Freeman et al., 2011).
POTS is a condition wherein chronic orthostatic intolerance is present together with inappropriate tachycardia (rise of heart rate >30 bpm in adults or >40 bpm among adolescents) within 10 min of standing or head-up tilt, in the absence of hypotension (Freeman et al., 2011). Exaggerated tachycardia is often accompanied by symptoms of cerebral hypoperfusion and autonomic over-activity, and is relieved by recumbency. The syndrome exhibits a female:male ratio ~4.5:1 and most cases occur between the ages of 15 and 25 years. Up to 50% of cases have a history of recent illness, injury, or major surgery, and 25% have a family history of similar complaints (Benarroch, 2012). It has been classified into neuropathic (Jacob et al., 2000) or hyperadrenergic (Vincent and Robertson, 2004) subtypes based on autonomic function tests and plasma norepinephrine levels; alternatively as low-flow, high-flow, or normal-flow, POTS based on measurement of leg venous pressure and calf blood flow (Stewart and Montgomery, 2004), or based on cardiac output during exercise (Pianosi et al., 2014, 2016). Its etiology is largely unknown apart from a few specific (genetic) variants but is presumably heterogeneous (Freeman et al., 2011; Benarroch, 2012). In addition to circulatory alterations, minute volume and end-tidal PCO2 (PetCO2) also change normally with orthostasis: a modest rise in ventilation and dip in PetCO2 are seen during transition from supine to upright posture (Malmberg et al., 2000; Chang et al., 2005) associated with an unpleasant sensation of breathlessness in one-quarter of patients (Gibbons and Freeman, 2005). Yet, dyspnea in this condition is not yet easily reconciled with our current paradigm of dyspnea.
1.2. Origin(s) of dyspnea in POTS
The current neurophysiologic model proposes that dyspnea results either when mismatch occurs between neural respiratory drive and ventilation achieved, sensed by length or tension changes in components of the respiratory pump (Mahler and O'Donnell, 2015; Parshall et al., 2012); or by mere conscious awareness of heightened neural respiratory drive (Zutler et al., 2012; Ingle et al., 2012). These deductions are primarily based on studies in patients with chronic obstructive lung disease or congestive heart failure in whom the concept of neuro-mechanical “uncoupling” is intuitive; but insights into dyspnea in other disease states such as cancer, palliative care, and POTS, are less explicable within this conceptual framework. There is no known or recognizable uncoupling between ventilatory drive and resultant lung stretch or thoracic displacement in POTS; but another putative cause for dyspnea was recently proposed: low cardiac filling pressures (Oldham et al., 2016). Thoracic hypovolemia is central to the circulatory pathophysiology of POTS, as many patients have lower blood or plasma volume than healthy controls, and exhibit paradoxical behavior of their renin-angiotensin system (Raj et al., 2005b). The sympathetic nervous system is one regulator of renin release and aberrant sympathetic activity (SNA) can modify the body's response to hypovolemia.
This observation implies cardiovascular volume receptors can influence ventilation, a scenario exemplified in the extreme by deep, sighing breathing during hemorrhagic shock. Clinical implications of cardiorespiratory interaction have recently sparked considerable interest as a biomarker. Inherent variability of bodily rhythms is now recognized to be a sign of health rather than physiologic noise, ensuring various organ systems function within an optimal window to find the “sweet spot” between order and entropy (chaos). Chief among these rhythms is respiratory sinus arrhythmia (RSA) (Elstad et al., 2018). RSA is most pronounced in the young and decreases with age; increases with physical conditioning; and is exaggerated with deep, slow breathing. RSA is also linked to changes in tidal volume and respiratory frequency. This coupling is caused by a cardiac-induced onset of inspiration through an unknown afferent pathway, an observation supported by mathematical modelling, and interestingly fits with the hypothesis that arterial baroreceptors – as in the carotid sinus – may operate in the genesis of cardiorespiratory coupling. Indeed, there is some evidence that RSA stabilizes mean arterial BP in a manner dependent on body posture acting via either baroreceptors or ventilatory modulation of SNA (reviewed in ref. 24). Returning to breathing in POTS, we have shown that respiratory response to pain or panic is a combination of hyperpnea and tachypnea consistent with the literature (Stewart et al., 2018c, 2018a). In contrast, minute volume increased in POTS patients who exhibited postural hyperpnea during tilt-table test by an increase in tidal volume with either no change or a small decrease in respiratory rate. Note there is respiratory modulation of SNA, with maximum discharge near end-expiration and its minimum close to end-inspiration. We speculated that a breathing pattern of active expiration at rest during orthostasis not only enhanced SNA but also caused a reduction in end-expiratory lung volume, lengthening the diaphragm and augmenting tidal volume. As such, hyperpnea in POTS may represent attempts to re-discover their “sweet spot” for cardio-respiratory interaction existing prior to onset of their malady (Bokov et al., 1985).
Whereas this compensation may be common to many or all patients with POTS, dyspnea is reported by ~25% (Stewart et al., 2018c, 2018a). Recent emphasis on a multi-dimensional approach differentiates affective and cognitive responses to perceiving dyspnea, from the sensation of breathlessness (Herigstad et al., 2011; De Peuter et al., 2004). These presumably result from corollary discharges to limbic or para-limbic brain structures such as the amygdala and cingulate gyrus which convey interoceptive information, defined as the sense of the body's physiological state. Aberrations of interoception may be a mechanism whereby simply feeling the need to breathe more, generates dyspnea irrespective of presence or absence of cardiopulmonary pathology (Bokov et al., 1985), and may contribute to dyspnea among terminally-ill (Kamal et al., 2012). Moreover, dyspnea is a symptom with notorious inter-individual variability: patients may exhibit low disease severity (or none) but report high levels of dyspnea or vice versa, implying that perception of dyspnea is loosely related to stimulus input but modifiable by cognitive and affective factors (De Peuter et al., 2004). While POTS patients are often perceived to be anxious, studies suggest it is driven mainly by overlap of orthostatic symptoms with common anxiety symptoms. Patients suffering from POTS may be more anxious than healthy subjects, but they are significantly less anxious than patients with panic disorder. However, their anxiety symptoms may be fundamentally distinct as studies attribute angst to somatic hypervigilance, meaning that they tend to report relatively mild, routine sensory information as more distressing or intense than normal (Benarroch, 2012) – perhaps a manifestation of augmented interoception. There is more consistent evidence of mild to moderate depression in POTS. Whereas several studies found a positive correlation between self-rated orthostatic symptom burden and depth of depression, none found any correlation between depression symptoms and physiological parameters (Raj et al., 2018). Thus, it may simply be a case of somatic hypervigilance leading to greater conscious awareness of heightened neural respiratory drive (Zutler et al., 2012; Ingle et al., 2012), but again, from whence does it arise?
1.3. Carotid body – a unifying hypothesis?
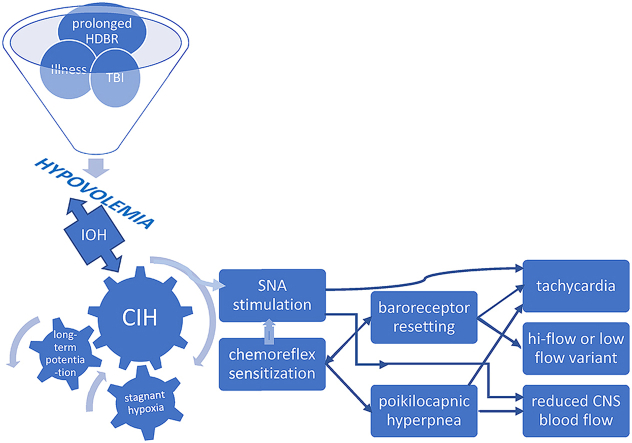
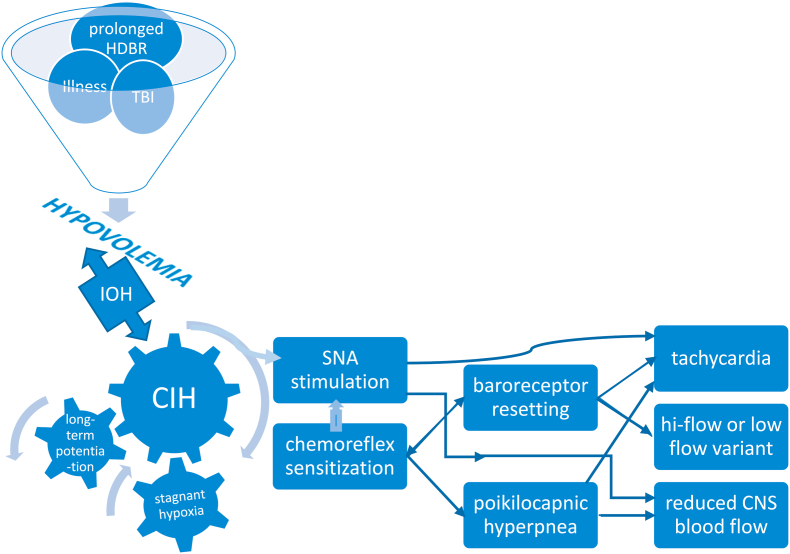
While an array of triggers incite or initiate downstream consequences such as prolonged bed rest or an autoimmune neuropathy that result in the POTS phenotype (in a manner analogous to the swiss cheese model of accident causation), we submit that chronic intermittent hypoxia (CIH) experienced by the carotid body constitutes an integral part of at least one pathway to dysautonomia. The carotid body (CB) is the primary peripheral chemoreceptor, responding primarily to hypoxia, and to a lesser extent hypercapnia, acidosis, and hypoperfusion (Iturriaga, 2018; Dempsey and Smith, 2014), the latter being of particular interest. It is the most perfused organ per gram weight in the body. CB activation by hypercapnia or hypoxia causes circulatory changes in addition to expected hyperpnea: blood pressure and heart rate rise due to sympathetic efferent neuronal activation of vascular beds (Marshall, 1994). The adjacent carotid sinus contains mechanoreceptors that sense acute blood pressure changes, giving rise to the afferent arm of the baroreflex that increases heart rate, vascular SNA, and peripheral vasoconstriction in response to decreased venous return upon assumption of upright posture. Interactions between chemoreflex and baroreflex are recognized (Prabhakar et al., 2005) spawning the notion of a ventilatory baroreflex (Stewart et al., 2011), but current evidence indicates that interaction between baroreflex and chemoreflex occurs in the brainstem. The CB chemoreflex has the unique ability to activate sympathetic traffic to peripheral blood vessels and increase cardiac vagal activity simultaneously and said chemoreflex and baroreflex are inseparably linked in their control of SNA. The CB is not only a multi-modal sensor but sends nonspecific tonic input to the medullary, respiratory pattern-generating, neurons through multiple CNS pathways. Moreover, it is a key intercessor of vascular SNA via medullary tracts independent of the brainstem respiratory network (Dempsey and Smith, 2014). Pathways involved in chemo- and baro-receptor coupling are unclear but baro-mediated respiratory effects have been recorded in medullary rhythm-generating neurons, which sit very near barosensitive sympathetic premotor neurons regulating vasomotor tone (McMullan and Pilowsky, 2010).
Recent research underscores the importance of CB involvement in common disorders where aberrant sympathetic nervous system activation plays a key role (Iturriaga, 2018; Paton et al., 2013). The overarching stimulus in these conditions is CIH. This phenomenon is most evident in heart failure whereupon low cardiac output and reduced carotid chemoreceptor blood flow results in stagnant hypoxia of the carotid body (Ding et al., 2011). Central hypovolemia is a cardinal feature of POTS: evidence supports excessive reduction of central blood volume in typical POTS patients when upright and we demonstrated that central blood volume is reduced in POTS (Stewart et al., 2018a). This predisposes patients to hypoperfusion of tissues cephalad to the heart, causing stagnant hypoxia from reduced carotid blood flow – a key step causing SNA activation (Marshall, 1994; Prabhakar et al., 2005) in the pathophysiologic cascade in POTS. Indeed, initial orthostatic hypotension is more frequent in POTS than in controls, affecting 50% of POTS patients, with a lower minimum blood pressure (Stewart et al., 2020). Thereafter, hypocapnia and reduced cardiac output in POTS maintains low cerebral blood flow and low carotid blood flow even though BP has normalized by vasoconstriction. Individuals do not spend all their time in the upright position, and patients often learn to assume a horizontal posture to relieve symptoms, setting the stage for intermittent carotid body and sinus hypoperfusion. Symptom chronicity then develops from repeated, continual sympathetic stimulation via prolonged CB excitation due to CIH. (Fig. 2). Hyperventilation following brief hypoxic episodes interspersed with normoxia results in increased SNA that is sustained over an hour or more – so-called long-term facilitation (Mitchell et al., 1985). There are several mechanisms which perpetuate this sympathetic activation following cessation of CB stimulation (Iturriaga, 2018). Increased CB chemoreceptor sensitivity and central processing of the carotid body afferent inputs may both contribute to enhanced SNA (Prabhakar et al., 2005). Enhanced CB discharge further augments SNA, which, along with reduction in baroreflex efficiency, impairs control of vasomotor tone (Iturriaga, 2018). Repeated exposure to brief hypoxic episodes increases respiratory-sympathetic coupling (Prabhakar et al., 2015), setting up a vicious cycle. Moreover, central adaptive responses also occur with CIH, e.g. persistently high, tonic hyperactivity of medullary neurons (Guyenet et al., 2018).
Fig. 2.
Putative mechanisms leading to development of POTS phenotype. HDBR head down bed rest; TBI traumatic brain injury; IOH initial orthostatic hypotension; CIH chronic intermittent hypoxia; SNA sympathetic nerve activity; CNS central nervous system.
1.4. What is the evidence
We have thus far conceptualized a framework for studying mechanisms responsible for POTS phenotype, but what is the evidence that CB is altered in patients with POTS? It has nothing to do with sleep apnea, likely the first thought when pulmonologists think CIH. Studies have demonstrated that patients with POTS report more sleep complaints than controls, mainly sleep initiation and sleep maintenance, with mildly delayed sleep-wake phase cycle; but they have greater subjective symptoms (resembling fatigue more than sleepiness) than objective findings on polysomnography; nor do they show any consistent polysomnographic abnormalities that explain severity of their symptoms (Miglis and Barwick, 2018). The fact is that changes or differences in both cardiovascular and respiratory CB-mediated reflexes have been demonstrated in patients with POTS. Cardiac baroreflex sensitivity (i.e., the slope or gain of cardiac baroreflex curve) was reduced in POTS patients compared with healthy control subjects, and available data suggests that cardiac baroreflex sensitivity may range from normal to decreased in the POTS population as a whole (Muenter Swift et al., 2005). It is unclear whether this represents a cause or effect of POTS. Stewart and co-workers showed that peripheral chemoresponsiveness to hypoxia is heightened in patients with POTS compared with control subjects while supine and was accentuated by orthostasis, i.e. baroreflex unloading during head-up tilt potentiated their ventilatory response to hypoxia (Taneja et al., 2011). They concluded that baroreflex unloading during head-up tilt stimulates peripheral O2-dependent chemoreflexes causing hyperventilation that is unopposed by the normal, restraining, effect of hypocapnia in POTS patients. The latter phenomenon raises the possibility of abnormal central integration of respiratory and cardiovascular afferents in the ventrolateral medulla. Respiratory chemoreflexes are abnormal in POTS patients, and function is further perturbed by baroreflex unloading resulting in hyperventilation. We recently posited that hyperventilation in POTS patients signifies a forme fruste of CIH (Stewart et al., 2018a). Alkalosis from hypocapnic hyperventilation may cause or exacerbate typical symptoms of POTS: hypocapnic cerebral vasoconstriction can cause lightheadedness; coronary vasoconstriction may contribute to chest pain, and alkalemia due to hypocapnia can provoke paresthesiae. The Bohr effect aggravates ischemic effects of cerebral and coronary vasoconstriction because leftward shift of the oxyhemoglobin dissociation curve reduces O2 unloading in tissues (Laffey et al., 2002). We suspect that many or most patients with a POTS phenotype (not only POTS patients who hyperventilate) have abnormal function of the ventilatory baroreflex as a result of the common sine qua non of central hypovolemia. This accounts for activation of SNA that could account for low-flow variant by mediating intense vasoconstriction (Pianosi et al., 2016); and high-flow variant as it drives a hyperkinetic circulatory response to exercise (Pianosi et al., 2014). Whereas the former situation is easily grasped as SNA is often equated with vasoconstriction, the latter scenario is more difficult to conceptualize. Contracting skeletal muscle can overcome sympathetic vasoconstrictor activity (functional sympatholysis) that normally facilitates matching perfusion to metabolic demand during exercise (Mortensen et al., 2012). NO and ATP have been proposed as important inhibitors of norepinephrine-mediated vasoconstriction and presently an inhibitory effect of ATP on norepinephrine signaling via purinergic (P2) receptors appears most likely (see below) (Saltin and Mortensen, 2012). Excessive SNA coupled with impaired functional sympatholysis could have deleterious consequences for cardiovascular regulation during exercise if sympatholysis occurred in non-essential vascular (e.g. splanchnic) beds (Thomas, 2015). Faced with inappropriately (for exercise) low systemic blood pressure, the heart responds by raising cardiac output above prevailing metabolic demand to defend perfusion pressure (Holmgren et al., 1957).
1.5. Current and future directions
This carotid (body and sinus) hypothesis was recently tested experimentally by applying a neck collar to create pressure around the carotid body and reduce carotid artery cross-sectional area (including carotid sinus) in a proof-of concept study. Such application reduced orthostatic symptoms and resting ventilation at baseline and during head-up tilt in patients with POTS (Nardone et al., 1985) accompanied by improvement in the chest discomfort component of the Vanderbilt POTS Symptom Score. Replication of this result with hyperoxia to silence CB chemoreceptor reflexes would further support the contention that CB sensitization to stagnant hypoxia contributes to pathogenesis of POTS. Should this substantiate our contention, the next step should be clinical therapeutic trials aimed at modulating CB function (Paton et al., 2013). New pharmacologic strategies must be sought given failure of current vasoactive drugs to reliably ameliorate symptoms. Chronic supplementation of inorganic nitrate boosts circulating NO bioavailability and attenuates carotid chemoreflex sensitivity (Bock et al., 2018). ATP acting via P2X3 receptors leads to CB hyperactivity and hyperreflexia, suggesting modulation of P2X3 receptors as another potential pharmacologic strategy (Pijacka et al., 2016).
Increasing awareness of this condition is a prerequisite before therapeutic trials can begin. Patients with POTS must be identified and characterized because non-pharmacologic therapies are readily available. These are summarized in our recent consensus statement (Stewart et al., 2018b), but fluid repletion and salt loading are the first steps. Outcomes and prognosis are very favorable (Kizilbash et al., 2014) but these cannot be realized unless patients are identified and channeled appropriately.
CRediT authorship contribution statement
Julian M. Stewart: Conceptualization, Investigation, Writing - review & editing, Supervision. Paolo T. Pianosi: Writing - original draft, creation and/or presentation of the published work, Writing - review & editing, Visualization, The corresponding author (PP) is responsible for ensuring that the descriptions are accurate and agreed by all authors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Benarroch E.E. Postural tachycardia syndrome: a heterogeneous and multifactorial disorder. Mayo Clin. Proc. 2012;87(12):1214–1225. doi: 10.1016/j.mayocp.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock J.M., Ueda K., Schneider A.C., Hughes W.E., Limberg J.K., Bryan N.S., Casey D.P. Inorganic nitrate supplementation attenuates peripheral chemoreflex sensitivity but does not improve cardiovagal baroreflex sensitivity in older adults. Am. J. Physiol. Heart Circ. Physiol. 2018;314(1):H45–H51. doi: 10.1152/ajpheart.00389.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokov P., Fiamma M.N., Chevalier-Bidaud B., Chenivesse C., Straus C., Similowski T., Delclaux C. Increased ventilatory variability and complexity in patients with hyperventilation disorder. J. Appl. Physiol. 1985;120(10):1165–1172. doi: 10.1152/japplphysiol.00859.2015. [DOI] [PubMed] [Google Scholar]
- Chang A.T., Boots R.J., Brown M.G., Paratz J.D., Hodges P.W. Ventilatory changes following head-up tilt and standing in healthy subjects. Eur. J. Appl. Physiol. 2005;95(5–6):409–417. doi: 10.1007/s00421-005-0019-2. [DOI] [PubMed] [Google Scholar]
- De Peuter S., Van Diest I., Lemaigre V., Verleden G., Demedts M., Van den Bergh O. Dyspnea: the role of psychological processes. Clin. Psychol. Rev. 2004;24(5):557–581. doi: 10.1016/j.cpr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Dempsey J.A., Smith C.A. Pathophysiology of human ventilatory control. Eur. Respir. J. 2014;44(2):495–512. doi: 10.1183/09031936.00048514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Li Y.L., Schultz H.D. Role of blood flow in carotid body chemoreflex function in heart failure. J. Physiol. 2011;589(Pt 1):245–258. doi: 10.1113/jphysiol.2010.200584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstad M., O'Callaghan E.L., Smith A.J., Ben-Tal A., Ramchandra R. Cardiorespiratory interactions in humans and animals: rhythms for life. Am. J. Physiol. Heart Circ. Physiol. 2018;315(1):H6–H17. doi: 10.1152/ajpheart.00701.2017. [DOI] [PubMed] [Google Scholar]
- Freeman R., Wieling W., Axelrod F.B., Benditt D.G., Benarroch E., Biaggioni I., Cheshire W.P., Chelimsky T., Cortelli P., Gibbons C.H., Goldstein D.S. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin. Auton. Res. 2011;21(2):69–72. doi: 10.1007/s10286-011-0119-5. [DOI] [PubMed] [Google Scholar]
- Gibbons C.H., Freeman R. Orthostatic dyspnea: a neglected symptom of orthostatic hypotension. Clin. Auton. Res. 2005;15(1):40–44. doi: 10.1007/s10286-005-0227-1. [DOI] [PubMed] [Google Scholar]
- Guyenet P.G., Bayliss D.A., Stornetta R.L., Kanbar R., Shi Y., Holloway B.B., Souza G., Basting T.M., Abbott S.B.G., Wenker I.C. Interdependent feedback regulation of breathing by the carotid bodies and the retrotrapezoid nucleus. J. Physiol. 2018;596(15):3029–3042. doi: 10.1113/JP274357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herigstad M., Hayen A., Wiech K., Pattinson K.T. Dyspnoea and the brain. Respir. Med. 2011;105(6):809–817. doi: 10.1016/j.rmed.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Holmgren A., Jonsson B., Levander M., Linderholm H., Sjostrand T., Strom G. Low physical working capacity in suspected heart cases due to inadequate adjustment of peripheral blood flow (vasoregulatory asthenia) Acta Med. Scand. 1957;158(6):413–436. doi: 10.1111/j.0954-6820.1957.tb15509.x. [DOI] [PubMed] [Google Scholar]
- Ingle L., Cleland J.G., Clark A.L. Body mass index is related to the perception of exertional breathlessness in patients presenting with dyspnoea of unknown origin. Int. J. Cardiol. 2012;157(2):300–303. doi: 10.1016/j.ijcard.2012.03.113. [DOI] [PubMed] [Google Scholar]
- Ismail-Sayed I.F.J., Chrzanowski S., Young K.R., Hansen-Flaschen J.H., Van Scoy L.J. American Thoracic Society; 2020. Unexplained chronic dyspnea on exertion with acrocyanosis; p. A3225. [Google Scholar]
- Iturriaga R. Translating carotid body function into clinical medicine. J. Physiol. 2018;596(15):3067–3077. doi: 10.1113/JP275335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob G., Costa F., Shannon J.R., Robertson R.M., Wathen M., Stein M., Biaggioni I., Ertl A., Black B., Robertson D. The neuropathic postural tachycardia syndrome. N. Engl. J. Med. 2000;343(14):1008–1014. doi: 10.1056/NEJM200010053431404. [DOI] [PubMed] [Google Scholar]
- Kamal A.H., Maguire J.M., Wheeler J.L., Currow D.C., Abernethy A.P. Dyspnea review for the palliative care professional: treatment goals and therapeutic options. J. Palliat. Med. 2012;15(1):106–114. doi: 10.1089/jpm.2011.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizilbash S.J., Ahrens S.P., Bruce B.K., Chelimsky G., Driscoll S.W., Harbeck-Weber C., Lloyd R.M., Mack K.J., Nelson D.E., Ninis N., Pianosi P.T. Adolescent fatigue, pots, and recovery: a guide for clinicians. Curr. Probl. Pediatr. Adolesc. Health Care. 2014;44(5):108–133. doi: 10.1016/j.cppeds.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffey J.G., Kavanagh B.P., Hypocapnia N. Engl. J. Med. 2002;347(1):43–53. doi: 10.1056/NEJMra012457. [DOI] [PubMed] [Google Scholar]
- Mahler D.A., O'Donnell D.E. Recent advances in dyspnea. Chest. 2015;147(1):232–241. doi: 10.1378/chest.14-0800. [DOI] [PubMed] [Google Scholar]
- Malmberg L.P., Tamminen K., Sovijarvi A.R. Orthostatic increase of respiratory gas exchange in hyperventilation syndrome. Thorax. 2000;55(4):295–301. doi: 10.1136/thorax.55.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J.M. Peripheral chemoreceptors and cardiovascular regulation. Physiol. Rev. 1994;74(3):543–594. doi: 10.1152/physrev.1994.74.3.543. [DOI] [PubMed] [Google Scholar]
- McMullan S., Pilowsky P.M. The effects of baroreceptor stimulation on central respiratory drive: a review. Respir. Physiol. Neurobiol. 2010;174(1–2):37–42. doi: 10.1016/j.resp.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Miglis M.G., Barwick F. Sleep disorders in patients with postural tachycardia syndrome: a review of the literature and guide for clinicians. Auton. Neurosci. 2018;215:62–69. doi: 10.1016/j.autneu.2018.05.002. [DOI] [PubMed] [Google Scholar]
- Mitchell G.S., Baker T.L., Nanda S.A., Fuller D.D., Zabka A.G., Hodgeman B.A., Bavis R.W., Mack K.J., Olson E.B., Jr. Invited review: intermittent hypoxia and respiratory plasticity. J. Appl. Physiol. 1985;90(6):2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Mortensen S.P., Morkeberg J., Thaning P., Hellsten Y., Saltin B. Two weeks of muscle immobilization impairs functional sympatholysis but increases exercise hyperemia and the vasodilatory responsiveness to infused atp. Am. J. Physiol. Heart Circ. Physiol. 2012;302(10):H2074–H2082. doi: 10.1152/ajpheart.01204.2011. [DOI] [PubMed] [Google Scholar]
- Muenter Swift N., Charkoudian N., Dotson R.M., Suarez G.A., Low P.A. Baroreflex control of muscle sympathetic nerve activity in postural orthostatic tachycardia syndrome. Am. J. Physiol. Heart Circ. Physiol. 2005;289(3):H1226–H1233. doi: 10.1152/ajpheart.01243.2004. [DOI] [PubMed] [Google Scholar]
- Nardone M., Guzman J., Harvey P.J., Floras J.S., Edgell H. Effect of a neck compression collar on cardiorespiratory and cerebrovascular function in postural orthostatic tachycardia syndrome (pots) J. Appl. Physiol. 1985;128(4):907–913. doi: 10.1152/japplphysiol.00040.2020. [DOI] [PubMed] [Google Scholar]
- Oldham W.M., Lewis G.D., Opotowsky A.R., Waxman A.B., Systrom D.M. Unexplained exertional dyspnea caused by low ventricular filling pressures: results from clinical invasive cardiopulmonary exercise testing. Pulm. Circ. 2016;6(1):55–62. doi: 10.1086/685054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parshall M.B., Schwartzstein R.M., Adams L., Banzett R.B., Manning H.L., Bourbeau J., Calverley P.M., Gift A.G., Harver A., Lareau S.C., Mahler D.A. An official american thoracic society statement: update on the mechanisms, assessment, and management of dyspnea. Am. J. Respir. Crit. Care Med. 2012;185(4):435–452. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton J.F., Sobotka P.A., Fudim M., Engelman Z.J., Hart E.C., McBryde F.D., Abdala A.P., Marina N., Gourine A.V., Lobo M., Patel N. The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension. 2013;61(1):5–13. doi: 10.1161/HYPERTENSIONAHA.111.00064. [DOI] [PubMed] [Google Scholar]
- Pianosi P.T., Goodloe A.H., Soma D., Parker K.O., Brands C.K., Fischer P.R. High flow variant postural orthostatic tachycardia syndrome amplifies the cardiac output response to exercise in adolescents. Phys. Rep. 2014;2(8) doi: 10.14814/phy2.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pianosi P.T., Schroeder D.R., Fischer P.R. Cardiac responses to exercise distinguish postural orthostatic tachycardia syndrome variants. Phys. Rep. 2016;4(22) doi: 10.14814/phy2.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijacka W., Moraes D.J., Ratcliffe L.E., Nightingale A.K., Hart E.C., da Silva M.P., Machado B.H., McBryde F.D., Abdala A.P., Ford A.P., Paton J.F. Purinergic receptors in the carotid body as a new drug target for controlling hypertension. Nat. Med. 2016;22(10):1151–1159. doi: 10.1038/nm.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popatia R., Subramaniam M. An interesting case of dysautonomia presenting with dyspnea. Pediatr. Pulmonol. 2014;49(3):E25–E26. doi: 10.1002/ppul.22780. [DOI] [PubMed] [Google Scholar]
- Prabhakar N.R., Peng Y.J., Jacono F.J., Kumar G.K., Dick T.E. Cardiovascular alterations by chronic intermittent hypoxia: importance of carotid body chemoreflexes. Clin. Exp. Pharmacol. Physiol. 2005;32(5–6):447–449. doi: 10.1111/j.1440-1681.2005.04209.x. [DOI] [PubMed] [Google Scholar]
- Prabhakar N.R., Peng Y.J., Kumar G.K., Nanduri J. Peripheral chemoreception and arterial pressure responses to intermittent hypoxia. Comp. Physiol. 2015;5(2):561–577. doi: 10.1002/cphy.c140039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj S.R., Black B.K., Biaggioni I., Harris P.A., Robertson D. Acetylcholinesterase inhibition improves tachycardia in postural tachycardia syndrome. Circulation. 2005;111(21):2734–2740. doi: 10.1161/CIRCULATIONAHA.104.497594. [DOI] [PubMed] [Google Scholar]
- Raj S.R., Biaggioni I., Yamhure P.C., Black B.K., Paranjape S.Y., Byrne D.W., Robertson D. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation. 2005;111(13):1574–1582. doi: 10.1161/01.CIR.0000160356.97313.5D. [DOI] [PubMed] [Google Scholar]
- Raj V., Opie M., Arnold A.C. Cognitive and psychological issues in postural tachycardia syndrome. Auton. Neurosci. 2018;215:46–55. doi: 10.1016/j.autneu.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin B., Mortensen S.P. Inefficient functional sympatholysis is an overlooked cause of malperfusion in contracting skeletal muscle. J. Physiol. 2012;590(24):6269–6275. doi: 10.1113/jphysiol.2012.241026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schondorf R., Low P.A. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43(1):132–137. doi: 10.1212/wnl.43.1_part_1.132. [DOI] [PubMed] [Google Scholar]
- Stewart J.M., Kota A., O’Donnell-Smith M.B., Visintainer P., Terilli C., Medow M.S. The preponderance of initial orthostatic hypotension in postural tachycardia syndrome. J. Appl. Physiol. 2020;129(3):459–466. doi: 10.1152/japplphysiol.00540.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J.M., Montgomery L.D. Regional blood volume and peripheral blood flow in postural tachycardia syndrome. Am. J. Physiol. Heart Circ. Physiol. 2004;287(3):H1319–H1327. doi: 10.1152/ajpheart.00086.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J.M., Pianosi P., Shaban M.A., Terilli C., Svistunova M., Visintainer P., Medow M.S. Hemodynamic characteristics of postural hyperventilation: pots with hyperventilation versus panic versus voluntary hyperventilation. J. Appl. Physiol. 1985;125(5):1396–1403. doi: 10.1152/japplphysiol.00377.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J.M., Rivera E., Clarke D.A., Baugham I.L., Ocon A.J., Taneja I., Terilli C., Medow M.S. Ventilatory baroreflex sensitivity in humans is not modulated by chemoreflex activation. Am. J. Physiol. Heart Circ. Physiol. 2011;300(4):H1492–H1500. doi: 10.1152/ajpheart.01217.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J.M., Pianosi P., Shaban M.A., Terilli C., Svistunova M., Visintainer P., Medow M.S. Postural hyperventilation as a cause of postural tachycardia syndrome: increased systemic vascular resistance and decreased cardiac output when upright in all postural tachycardia syndrome variants. J Am Heart Assoc. 2018;7(13) doi: 10.1161/JAHA.118.008854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J.M., Boris J.R., Chelimsky G., Fischer P.R., Fortunato J.E., Grubb B.P., Heyer G.L., Jarjour I.T., Medow M.S., Numan M.T., Pianosi P.T. Pediatric disorders of orthostatic intolerance. Pediatrics. 2018;141(1) doi: 10.1542/peds.2017-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja I., Medow M.S., Clarke D.A., Ocon A.J., Stewart J.M. Baroreceptor unloading in postural tachycardia syndrome augments peripheral chemoreceptor sensitivity and decreases central chemoreceptor sensitivity. Am. J. Physiol. Heart Circ. Physiol. 2011;301(1):H173–H179. doi: 10.1152/ajpheart.01211.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G.D. Functional sympatholysis in hypertension. Auton. Neurosci. 2015;188:64–68. doi: 10.1016/j.autneu.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dixhoorn J., Duivenvoorden H.J. Efficacy of nijmegen questionnaire in recognition of the hyperventilation syndrome. J. Psychosom. Res. 1985;29(2):199–206. doi: 10.1016/0022-3999(85)90042-x. [DOI] [PubMed] [Google Scholar]
- Vincent S., Robertson D. second ed. Elsevier; 2004. Hyperadrenergic Postural Tachycardia Syndrome; pp. 344–345. Primer on the autonomic nervous system. [Google Scholar]
- Zutler M., Singer J.P., Omachi T.A., Eisner M., Iribarren C., Katz P., Blanc P.D. Relationship of obesity with respiratory symptoms and decreased functional capacity in adults without established copd. Prim. Care Respir. J. 2012;21(2):194–201. doi: 10.4104/pcrj.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]