Objective
Sprint-interval training (SIT) and intermittent fasting are effective independent methods in achieving clinical health outcomes. However, the impact of both modalities when performed concurrently is unclear. The aim of this study was to compare the effects of 6 weeks of SIT performed in the fasted versus fed state on physiological and clinical health markers in healthy adults. Methods. Thirty recreationally-active participants were equally randomised into either the fasted (FAS; 4 males, 11 females) or the fed (FED; 6 males, 9 females) group. For all exercise sessions, FAS participants had to fast ≥10 h prior to exercising while FED participants had to consume food within 3 h to exercise. All participants underwent three sessions of SIT per week for 6 weeks. Each session consists of repeated bouts of 30-s Wingate Anaerobic cycle exercise. Pre- and post-training peak oxygen uptake (VO2peak), isokinetic leg strength, insulin sensitivity, blood pressure and serum lipid levels were assessed. Results. There were no differences in baseline physiological and clinical measures between both groups (all p > 0.05). VO2peak improved by 6.0 ± 8.8% in the FAS group and 5.3 ± 10.6% in the FED group (both p < 0.05), however the difference in improvement between groups was not statistically significant (p > 0.05). A similar pattern of results was seen for knee flexion maximum voluntary contraction at 300°·s−1. SIT training in either fasted or fed state had no impact on insulin sensitivity (both p > 0.05). There was significant reduction in diastolic blood pressure (8.2 ± 4.2%) and mean arterial pressure (7.0 ± 3.2%) in the FAS group (both p < 0.05) but not FED group (both p > 0.05). Conclusion. VO2peak and leg strength improved with SIT regardless of whether participants trained in the fasted or fed state. Chronic SIT in the fasted state may potentially reduce blood pressure to a greater extent than the same chronic SIT in the fed state.
Keywords: Intermittent fasting, High intensity interval training, Blood pressure, Wingate, Isokinetic knee strength
Highlights
-
•
SIT in the fasted state leads to a significant decrease in blood pressure.
-
•
VO2peak and leg strength improves with SIT, regardless of nutrition status.
-
•
SIT, performed in fasted or fed state, does not improve insulin sensitivity, body fat percentage or lipid profile.
1. Introduction
According to the World Health Organisation, physical inactivity is the fourth leading risk factor for global mortality (World Health Organisation, 2017). The relationship between physical inactivity, weight gain and metabolic abnormalities is well established. Exercise has been shown to improve insulin sensitivity (Goodyear and Kahn, 1998), cardiorespiratory fitness, muscular strength and body composition (Albright et al., 2000). There are several challenges to habitual exercise, with ‘lack of time’ being a commonly cited barrier (Sallis et al., 1992). Many exercise protocols have been developed over the years to reduce the time spent on exercise and high-intensity interval training (HIIT) is an example of such an exercise protocol.
Two main forms of HIIT exist, namely ‘high-intensity training’ (HIT) and ‘sprint-interval training’ (SIT). HIT incorporates vigorous but submaximal exercise efforts performed between 80% and 100% of maximal aerobic power (VO2max) that last several minutes. SIT comprises bouts of supramaximal intensity exercise, i.e. ‘all-out’ efforts at >100% VO2max lasting <45-s (Gibala, 2020). An example of a typical SIT protocol employs the Wingate anaerobic cycle test (WAnT) which consists of 2–4 bouts of 30-s ‘all-out’ efforts performed consecutively and interspersed with 2–4 min of active or passive recovery periods (Gibala et al., 2012). SIT is a potent variation of HIIT that elicits adaptations similar to traditional exercises despite marked differences in training workload and time commitment (Gibala and Hawley, 2017).
Like exercise, the search for the most optimal dietary intervention for health benefits is ongoing, and one such intervention is intermittent fasting (IF). Periodic fasting, alternate-day fasting (ADF) and time-restricted feeding (TRF) are some examples of IF protocols. Periodic fasting consists of fasting for only 1 or 2 days and consuming food ad libitum for the rest of the week. ADF involves a day of fasting alternated with a day of ad libitum or unrestricted eating (Mattson et al., 2017; Anton et al., 2018), while TRF specifies window periods limiting the number of hours for eating and fasting per day. IF is popular because it has been shown to reduce weight, fat mass and blood pressure (BP), improves dyslipidaemia, lowers insulin resistance, and confers greater health benefits compared to calorie restriction (de Cabo and Mattson, 2019). There are two possible underlying mechanisms via which IF confer health benefits – “flipping of metabolic switch” from utilisation of glucose to ketones and free fatty acids (FFA) as the body's primary energy source (Anton et al., 2018); and increased availability of nitric oxide (NO) (Ergul, 2011). Endothelial dysfunction occurs when there is inadequate availability of NO. NO is synthesized by endothelial nitric oxide synthase (eNOS) and NO availability decreases in the presence of increased oxidative stress, increased inflammation, high fat diet, increased circulating FFA levels and decreased eNOS levels. During IF, the body increases the uptake of FFA via metabolic switching and upregulates pathways for the production of NO (Ghosh et al., 2017). Increased availability of NO reduces impairment to endothelium and overall vascular tone, and therefore reduces risk of development of chronic diseases such as hypertension and diabetes mellitus.
Given the independent benefits of exercise and IF, the combined effects of exercise and fasting to amplify physiological and clinical outcomes seems plausible. Effects of IF on endurance training is well understood, with consistent positive results seen to VO2max13, insulin sensitivity (Edinburgh et al., 2020), lipid levels and body composition (Bhutani et al., 2013). At the cellular level, increased Sirtuin 1 (SIRT1) protein expression improves insulin sensitivity (Sun et al., 2007), especially under insulin-resistant conditions, while phosphorylated AMP-activated protein kinase (AMPK) inhibits synthesis of fatty acids, cholesterol, triglycerides and stimulates glucose uptake in skeletal muscle (Jeon, 2016). Fasted WAnT exercise induces increased SIRT1 and AMPK expression, suggesting that fasted conditions may optimize metabolic signalling adaptations associated with SIT (Aird et al., 2018). Research into chronic SIT in the fasted state is however limited with mixed results reported. Overweight or obese women performing interval training for 6 weeks (3 d wk−1, 10 × 60-s of cycling bouts, at 90% of HRmax) in the fasted versus fed state showed no significant improvements to peak oxygen uptake (VO2peak), body composition and mitochondrial capacity between the fasted and fed groups (Gillen et al., 2013). A study comprising male cyclists completing SIT for 4 weeks (3 d wk−1, 4–7 x 30-s incremental sprint bouts) in fasted or fed state demonstrated no significant difference between groups in improvement to VO2peak, even though exercise time to exhaustion was significantly longer (i.e., better performance) in the fasted group (Terada et al., 2019). However, another cross-over randomised controlled trial with interval training (15 running bouts, 3 min at 40% VO2peak followed by 1 min at 100% VO2peak) performed in the fasted state demonstrated attenuation of interstitial glycaemic parameters in diabetic subjects (Terada et al., 2016).
To our knowledge, while previous studies have shown effects in diseased population, no study has explored the effects of chronic SIT in the fasted state on physiological and clinical markers in healthy individuals. Therefore, we embarked on a 6-week intervention study to observe and compare physiological adaptations to chronic SIT performed in the fasted against non-fasted or fed state in healthy adults. Our hypothesis was that SIT, when performed in the fasted state, will amplify improvements to the individual's physiological and clinical outcomes, relative to the same SIT performed in the non-fasted state, among a group of healthy adults.
2. Methods
2.1. Participants
A minimum sample size of eight participants in each group was calculated to sufficiently detect an estimated VO2peak difference of >2.5 ml kg−1·min−1 with statistical power of 0.80 using an analysis of variance test (ANOVA two-tailed, α = 0.05) and effect size of 0.6. A moderate effect size of 0.6 was chosen as the smallest worthwhile change since participants recruited are healthy recreationally-active individuals. Recreationally-active was defined to be participating <2 d wk−1 in aerobic activity for a total of ≤80-min at moderate intensity (Bolgar et al., 2010). Participants were recruited via workplace posters and emails. All participants completed the Physical Activity Readiness Questionnaire (Adams, 1999) and were screened medically by a medical professional. That was to ensure that participants were free from metabolic, cardiovascular or pulmonary diseases (such as diabetes mellitus, hypertension, myocardial infarction, peripheral vascular disease, stroke, chronic obstructive pulmonary disease, asthma, etc), and had no pre-existing musculoskeletal conditions that would limit their physical function. The experimental procedures and potential risks were fully explained to all participants prior to obtaining their written-informed consent. The study was approved by the institutional review board and conformed to the Declaration of Helsinki. The study enrolled 40 participants, and 30 participants were eventually randomised equally into two groups and completed the 6-week training study. In total, 10 participants withdrew from the study because of change in work commitments (6 participants), illness (3 participants) and back injury that was not related to the study (1 participant).
2.2. Exercise-training intervention
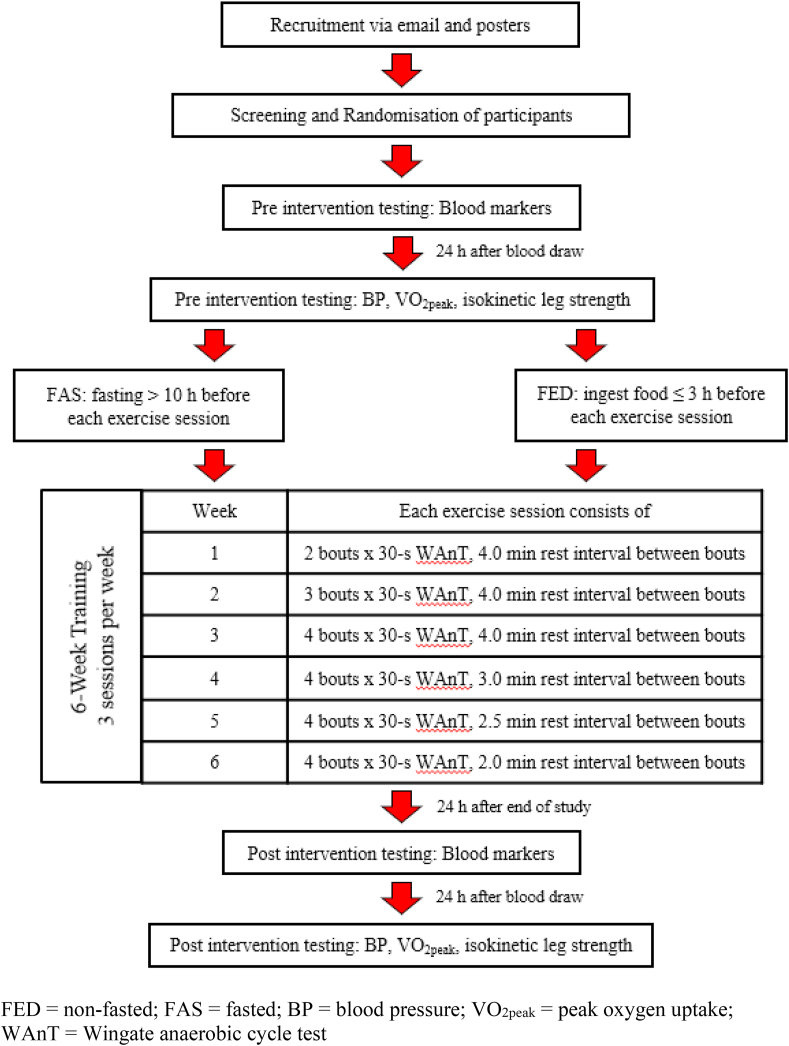
Upon enrolment, all participants were randomised via computer algorithm to either the fasted (FAS) or fed (FED) group. All training sessions took place between 08:00 to 10:00. Participants in the FED group were reminded to ingest their breakfast within 3 h prior to every exercise session. Participants in the FAS group had to go without food for at least ∼10 h (i.e., no breakfast) before the commencement of every exercise session but were allowed to ingest plain water ad libitum during the fasting hours. All participants were allowed to ingest plain water ad libitum during training. Training took place on alternate days for 3 days per week for a total intervention period of 6 weeks. Each training session consists of two to four WAnT bouts, with progressively reduced duration of recovery between bouts over the training weeks (see Fig. 1 for detailed training programme). All training sessions were conducted on a Wattbike Pro (Wattbike Ltd, Nottingham, UK) cycle ergometer, with air resistance set at level 3 and magnetic resistance set at level 1 for all participants. Participants’ peak power output, mean power output and ratings of perceived exertion (RPE) for each exercise session were recorded. RPE was measured using modified Borg perceived exertion category-ratio scale with a scale from 0 to 10, where 0 refers to the training session being very easy while 10 is very, very hard (Zhang et al., 2020). All exercise sessions were supervised by the primary investigator and performed in a laboratory setting with ambient temperature and relative humidity ranging between 20 and 22 °C and 55–65%, respectively.
Fig. 1.
Timeline of study.
2.3. Pre- and post-experimental procedures
Participants arrived in the laboratory in the morning (between 08:00 to 10:00) after at least 8 h of overnight fasting. They were advised to consume 500 ml of plain water in the morning of the test and were instructed to abstain from strenuous exercise 48 h before the test (Oberlin et al., 2014) and avoid alcohol and smoking the evening before the test. Baseline measurements for all participants consisted of basic anthropometric measures, resting BP, resting heart rate (HR), body fat percentage, VO2peak, maximal voluntary contraction (MVC), blood lipid levels, fasting blood glucose and fasting insulin levels. The same parameters were obtained post-training to determine the efficacy of the training intervention. Post-training blood markers were drawn 24 h after the individual's final training session and after an overnight fast of at least 8 h. The rest of the other measurements were repeated 24 h after the last blood draw (see Fig. 1).
2.4. Body composition, resting heart rate and resting blood pressure
Stature was measured in centimetres (cm) without shoes. Body mass and body fat percentage were determined using bioimpedance technique (Inbody770, Chungcheong, Korea) following the manufacturer's standard procedures. Resting BP and HR were assessed in a seated position, with the individual relaxed upright on a chair with a back support and arm positioned at chest level for a minimum of 3 min before any measurement was taken. All BP and HR measures were measured using an automatic digital BP/HR machine (Microlife BP 3AR1-3 P, Microlife AG, Switzerland). Two measurements of BP and HR were taken with a rest of 2 min between measures, and these were averaged to ensure the two readings were within 5% of each other. A third reading was taken if the two readings exceeded the 5% range, with the outlying measurement excluded.
2.5. Peak aerobic power
All participants performed a progressive exercise ramp test on a cycle ergometer (Lode BV, Excalibur Sport, Groningen, The Netherlands) to determine their VO2peak. Participants completed a standardized 3-min warm-up at 40 W (for females) or 60 W (for males). During the test, cycle resistance was increased by 5 W every 30 s until volitional exhaustion. Volitional exhaustion is defined as the physical limit beyond which a participant was no longer able to continue the prescribed pedal rate, i.e. a minimum of 60 revolutions per minute (rpm) for women and 65 rpm for men, continuously for 5-s duration. Subsequently, after a passive rest of ∼20 min, a verification of the initial VO2peak value (Ho et al., 2018) was carried out. In the verification test, the individual cycled for 60-s duration each at 80%, 90%, 100% and 110% of the highest power attained in the initial test, and this was followed with cycling at 120% to self-volitional exhaustion. Expired respiratory gases were measured throughout the initial and verification tests using a calibrated metabolic cart (TrueOne 2400 MMS, Parvomedics, East Sandy, Utah, USA), and VO2peak was recorded as the highest value over a 30-s period during the initial and verification tests. The coefficient of variation for VO2peak based on the above procedures in our laboratory is 2% (unpublished data).
2.6. Leg strength
MVC serves as the criterion measure of leg strength and was assessed via the maximal knee extension and flexion of the dominant leg on an isokinetic dynamometer (Biodex System 3 PRO dynamometer, Biodex Medical Systems, NY, USA). The participant's torso and thighs were securely strapped to the chair with the arms folded across the chest. The dynamometer lever arm was secured ∼2 cm above the ankle malleolus of the dominant leg and the participant's knee joint was adjusted to align to the centre of axis of rotation of the dynamometer lever arm. The limits for the range of motion of the tested leg was set accordingly for the knee extension/flexion movement. After a familiarisation set of three repetitions (reps) at ∼50–60% perceived effort, participant was instructed to forcefully extend and flex his lower leg as fast and as hard as possible, at speeds of 30°·s−1 and 300°·s−1. The participant performed five continuous knee extension-flexion reps at 30°·s−1, followed by five reps at 300°·s−1, interspersed with ∼30-s of passive rest between each tested speed. The dynamometer was calibrated prior to each participant's testing session and was corrected for gravity. MVC was defined as the highest peak torque (in Newton-meters, N·m) of the five reps at each speed.
2.7. Blood measures
Following at least 8 h of overnight fasting, a 5 ml venous blood sample was obtained from each participant using the Becton Dickinson (BD) vacutainer blood collection system and dispensed directly into a BD vacutainer serum separator tube (BD 367986). The blood tube sample was left to clot and refrigerate at 4 °C before transportation to an accredited commercial biochemistry laboratory (Innoquest Laboratories, Singapore) within 4 h of the blood draw. The blood samples were transported using a cooler pack that maintained temperature between 8 and 20 °C. Upon reaching the laboratory, each blood sample was centrifuged at 3500 rpm for 10 min to separate the serum plasma from red blood cells. As the turnover period from arrival of blood sample to the laboratory and time to blood sample analysis was short, the serum plasma was stored at room temperature (between 20 and 25 °C) until the samples were ready for analysis. Serum triglyceride was measured using GPO Trinder (without serum blank) assay while serum glucose, total cholesterol and high-density lipoprotein (HDL) were measured using hexokinase method, enzymatic method and elimination/catalase method respectively. All samples were later analysed by Siemens Advia (1800) Clinical Chemistry System analyser (Siemens Healthcare Diagnostics Inc., USA). The low-density lipoprotein (LDL) was calculated based on total cholesterol and HDL values and was not directly analysed from the analyser. Serum insulin was measured using Elecsys Insulin assay (an insulin electrochemiluminescence immunoassay) and analysed with Roche Cobas e601 analyser (Roche Diagnostics, Germany). The intra-assay coefficients of variation of these markers are within 3% (Innoquest Laboratories, Singapore).
2.8. Insulin resistance marker
An indirect index, the Homeostatic model assessment (HOMA), was used to estimate insulin resistance (Gutch et al., 2015) as it is a valid, reliable and simple indirect method for detection of insulin sensitivity. HOMA index (Matthews et al., 1985) uses the formula previously described: Insulin (Um−1) x [Glucose (mmol·l−1)/22.5].
2.9. Statistical analysis
Data collected were summarized as means and standard deviation (SD). Independent t-tests were performed to compare baseline characteristics and training load of each week between FED and FAS groups. Mann-Whitney U test was performed to compare RPE at each week between the two groups. Mixed linear model was performed to examine the differences between FED and FAS groups, taking into account ‘time’ and ‘group x time’ interactions for all the outcomes. Bonferroni correction was applied to account for multiple testing. Statistical significance was set at P < 0.05. All statistical analyses were performed using SPSS version 20.0 (IBM Corp., Armonk, NY, USA).
3. Results
The weekly mean duration of participants' last meal to commencement of their first bout of WAnT was ∼12.0 h for FAS group and ∼1.8 h for the FED group (Table 1). At least 99% of FAS training sessions and 93% of FED training sessions met targeted time duration of participants’ last meal to the first bout of WAnT. There were no significant differences in WAnT performances (i.e., peak power and average power) and RPE for both FAS and FED groups during the training sessions (Table 2).
Table 1.
Mean duration between the last meal consumed to commencement of exercise for the weekly training sessions in the fed (FED, N = 15) and fasted (FAS, N = 15) groups.
| Training Week | FED group (h) | FAS group (h) |
|---|---|---|
| 1 | 1.9 ± 0.9 | 12.0 ± 1.9 |
| 2 | 1.8 ± 1.0 | 12.4 ± 2.5 |
| 3 | 1.8 ± 1.0 | 12.1 ± 1.5 |
| 4 | 1.8 ± 1.1 | 11.9 ± 1.8 |
| 5 | 1.7 ± 0.9 | 11.8 ± 1.4 |
| 6 | 1.9 ± 1.2 | 12.0 ± 1.5 |
FED = non-fasted; FAS = fasted.
*values presented in mean ± SD.
Table 2.
Weekly exercise peak power output, average power output and RPE of individuals in the fed (FED, N = 15) and fasted (FAS, N = 15) groups.
| Training Week | Peak Power (W) |
Average Power (W) |
RPE |
|||
|---|---|---|---|---|---|---|
| FED | FAS | FED | FAS | FED | FAS | |
| 1 | 555 ± 249 | 554 ± 171 | 317 ± 108 | 330 ± 79 | 9.58 ± 0.98 | 9.83 ± 0.22 |
| 2 | 537 ± 213 | 546 ± 156 | 307 ± 90 | 320 ± 74 | 9.99 ± 0.03 | 9.96 ± 0.07 |
| 3 | 526 ± 207 | 541 ± 162 | 307 ± 85 | 315 ± 70 | 9.98 ± 0.03 | 9.98 ± 0.04 |
| 4 | 519 ± 204 | 528 ± 156 | 305 ± 85 | 306 ± 69 | 9.99 ± 0.02 | 9.99 ± 0.02 |
| 5 | 512 ± 196 | 541 ± 175 | 305 ± 85 | 310 ± 73 | 9.99 ± 0.02 | 9.99 ± 0.02 |
| 6 | 506 ± 186 | 518 ± 167 | 303 ± 81 | 298 ± 69 | 10 ± 0 | 10 ± 0 |
FED = non-fasted; FAS = fasted; RPE = rating of perceived exertion.
* values presented in mean ± SD.
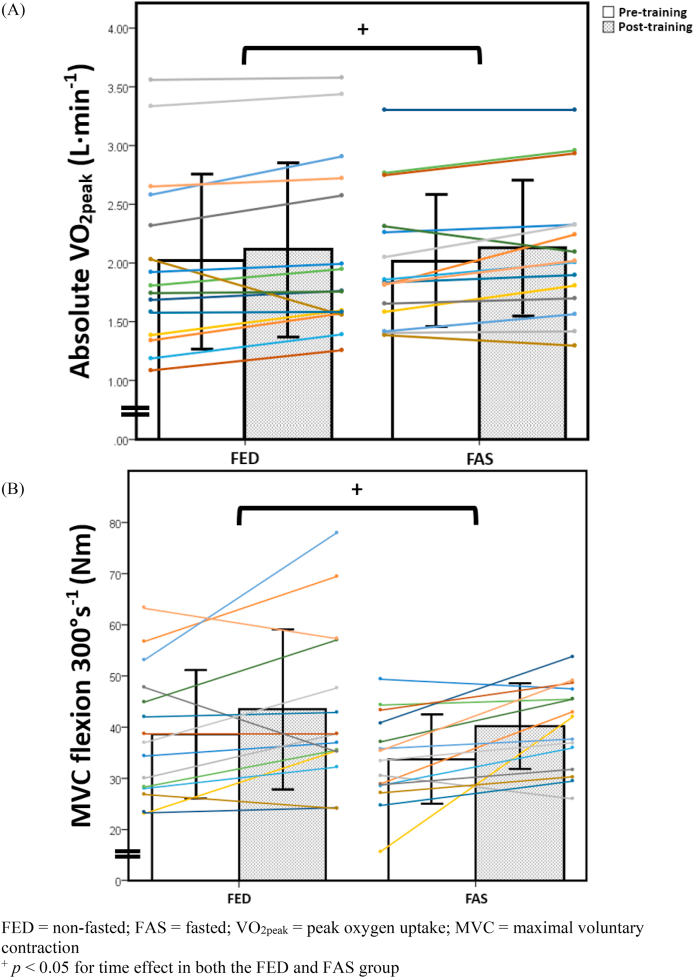
Table 3 shows both pre- and post-training results of the physiological and clinical measures between the FAS and FED groups. There were no significant differences between the FAS and FED groups in their baseline characteristics and all pre-training measures. There were statistically significant improvements in VO2peak and knee flexion MVC at 300°·s−1 in both FAS and FED groups (Table 3 and Fig. 2). More importantly, however, there were no significant differences in the magnitude of improvements in these two measures between the two groups (both p > 0.05). There was a statistically significant effect in time and interaction effects for HDL levels (p < 0.05) but not for the other measures of the lipid profile amongst FAS and FED groups (p > 0.05). There was no change to insulin sensitivity and body fat percentage between FAS and FED groups from pre-to post-training timepoint (p > 0.05).
Table 3.
Physiological and clinical measures at pre- and post-exercise intervention training in the fed (FED) and fasted (FAS) groups.
| Variables | FED group (N = 6 M + 9 F) |
FAS group (N = 4 M + 11 F) |
Time effect |
Interaction effects |
||
|---|---|---|---|---|---|---|
| Pre- | Post- | Pre- | Post- | p value | p value | |
| Age (y) | 39 ± 13 | – | 36 ± 8 | – | – | – |
| Stature (cm) | 164 ± 9 | – | 162 ± 8 | – | – | – |
| Body mass (kg) | 63.2 ± 13.9 | 63.6 ± 13.3 | 64.0 ± 11 | 63.9 ± 10.8 | 0.90 | 0.50 |
| Body mass index (kg·m−2) | 23.3 ± 3.8 | 23.5 ± 3.7 | 24.2 ± 3.4 | 24.1 ± 3.2 | 0.87 | 0.46 |
| Body fat (%) | 27.2 ± 8.0 | 27.9 ± 7.7 | 30.2 ± 5.7 | 30.3 ± 6.3 | 0.67 | 0.33 |
| Systolic BP (mmHg) | 117 ± 10 | 120 ± 15 | 120 ± 13 | 115 ± 11 | 0.10 | 0.06 |
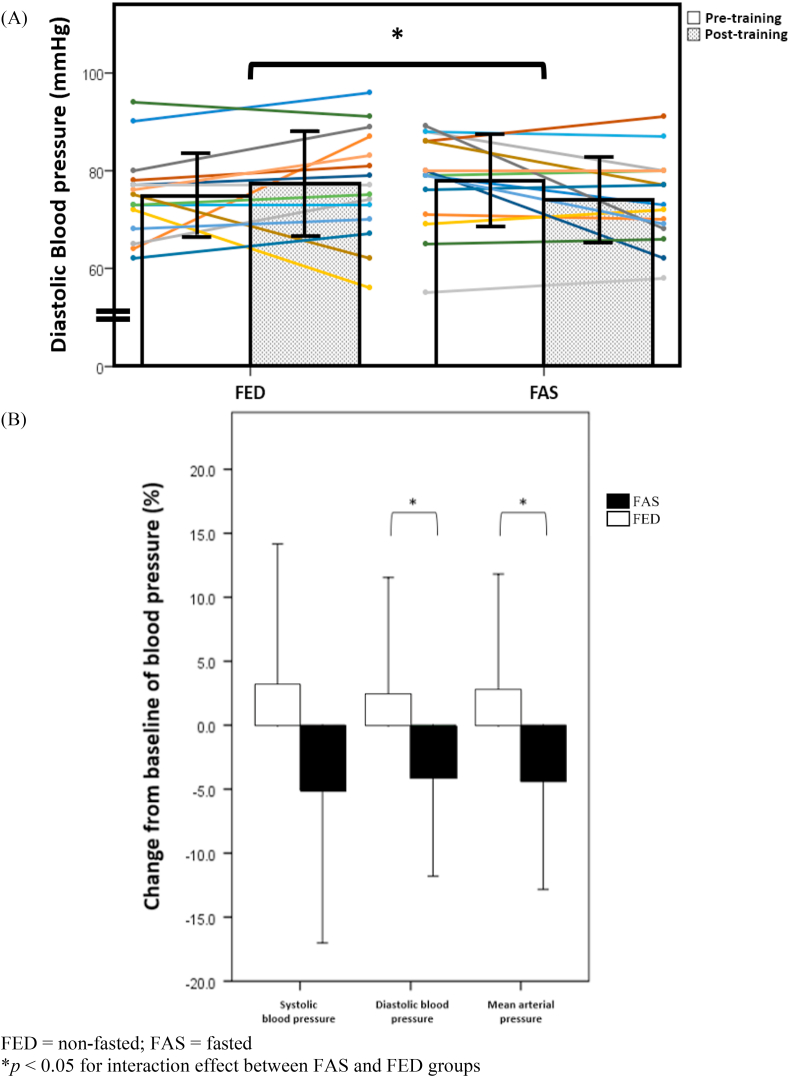
| Diastolic BP (mmHg) | 75 ± 9 | 77 ± 11 | 78 ± 10 | 74 ± 9 | 0.08 | 0.049 |
| Mean arterial pressure (mmHg) | 89 ± 9 | 91 ± 12 | 92 ± 10 | 88 ± 8 | 0.07 | 0.04 |
| Absolute VO2peak (L·min−1) | 2.02 ± 0.73 | 2.11 ± 0.73 | 2.02 ± 0.55 | 2.13 ± 0.56 | 0.02 | 0.81 |
| Relative VO2peak (ml·kg−1·min−1) | 31.8 ± 9.3 | 33.4 ± 9.5 | 31.9 ± 8.5 | 33.6 ± 8.2 | 0.03 | 0.80 |
| Fasting glucose (mg·dL−1) | 87 ± 11 | 84 ± 7 | 80 ± 6 | 80 ± 7 | 0.83 | 0.27 |
| Fasting insulin (mU·L−1) | 9.5 ± 14.4 | 6.6 ± 3.0 | 7.7 ± 5.7 | 6.9 ± 3.9 | 0.75 | 0.55 |
| HOMA | 2.3 ± 4.0 | 1.4 ± 0.7 | 1.6 ± 1.3 | 1.4 ± 0.9 | 0.78 | 0.48 |
| Total cholesterol (mg·dL−1) | 196 ± 46 | 189 ± 42 | 209 ± 50 | 204 ± 42 | 0.36 | 0.70 |
| High density lipoprotein (mg·dL−1) | 65 ± 17 | 65 ± 16 | 67 ± 20 | 58 ± 17 | <0.001 | 0.01 |
| Low density lipoprotein (mg·dL−1) | 113 ± 34 | 108 ± 38 | 124 ± 37 | 127 ± 35 | 0.50 | 0.24 |
| Triglyceride (mg·dL−1) | 97 ± 84 | 81 ± 45 | 91 ± 51 | 100 ± 54 | 0.42 | 0.13 |
| MVC extension 30°·s−1 (Nm) | 128 ± 37 | 137 ± 49 | 151 ± 40 | 147 ± 23 | 0.58 | 0.15 |
| MVC flexion 30°·s−1 (Nm) | 67 ± 26 | 70 ± 26 | 67 ± 16 | 68 ± 12 | 0.87 | 0.71 |
| MVC extension 300°·s−1 (Nm) | 66 ± 18 | 74 ± 20 | 68 ± 23 | 74 ± 23 | 0.09 | 0.72 |
| MVC flexion 300°·s−1 (Nm) | 39 ± 13 | 44 ± 16 | 34 ± 9 | 40 ± 8 | 0.01 | 0.62 |
FED = non-fasted; FAS = fasted; M = male; F = female; Pre- = pre-exercise training; Post- = post-exercise training; BP = blood pressure; VO2peak = peak oxygen uptake; MVC = maximal voluntary contraction; HOMA = Homeostatic model assessment.
*there were no significant differences in baseline characteristics or pre-training values between FED and FAS groups (p > 0.05 for all variables).
* *values presented in mean ± SD.
Fig. 2.
Absolute VO2peak (A) and MVC flexion at 300°·s−1 (B) in both fed (FED, N = 15) and fasted (FAS, N = 15) groups pre- and post-training.
A novel finding in this study is the statistically significant reduction in several measures of BP. The diastolic BP (p = 0.049) and mean arterial pressure (or MAP) (p = 0.038) were significantly lower in FAS for interaction effects, and close to being statistically significant for time effects (p = 0.07 and p = 0.08, respectively) (Table 3 and Fig. 3A). Fig. 3B shows the magnitude of change in systolic BP (FAS: −5.1 ± 12.0% vs. FED: 3.1 ± 11.0%), diastolic BP (FAS: −4.0 ± 7.8% vs. FED: 2.4 ± 9.1%) and MAP (FAS: −4.4 ± 8.5% vs. FED: 2.6 ± 9.2%) of FAS and FED groups when compared to their baseline BP measures.
Fig. 3.
Diastolic blood pressure in both fed (FED, N = 15) and fasted (FAS, N = 15) groups pre- and post-training (A) and change in systolic blood pressure, diastolic blood pressure and mean arterial pressure in the fed (FED, N = 15) and the fasted (FAS, N = 15) groups post-training (B).
4. Discussion
The aim of this study was to compare the effects of 6 weeks of SIT performed in the fasted versus fed state on physiological and clinical health markers in healthy adults; and our hypothesis was SIT in the fasted state will lead to greater magnitude of change relative to similar exercise in the fed state. The main findings of the study were VO2peak and knee flexion MVC at 300°·s−1 significantly improved over 6 weeks and there was no difference in magnitude of changes between groups. Diastolic BP and MAP improved in the FAS group but not the FED group. There was no impact on other physiological and clinical markers when FAS was compared to FED group. These findings partially supported our above hypothesis.
The lack of significant change in the VO2peak gains between FAS and FED groups after six weeks of SIT was similar to that observed in another study. Terada et al. showed that gains in VO2peak achieved in young male cyclists performing 3 sessions per week of 4–7 bouts of 30-s all-out cycling for 4 weeks with overnight fasting (fasting duration not reported) did not differ significantly from those in the non-fasted group (Terada et al., 2019). The authors postulated that acute training quality and/or quantity in the fasted state could be lower relative to the fed state throughout the training period, leading to less-than-optimal training-induced adaptations. However, this is unlikely the case in the present study as there were no significant differences in the peak power output, average power output and RPE during the WAnT bouts between the FAS and FED groups across six weeks of SIT (Table 2). In contrast, Stannard et al. demonstrated significantly greater magnitude of improvement of 9.7% in VO2peak amongst healthy participants who underwent 4 weeks of endurance exercise (5 d wk−1, 25–100 min incrementally at 65% VO2peak) in the overnight fasted state (fasting duration not reported) relative to the 2.5% improvement in VO2peak in the fed group (Stannard et al., 2010). Fasting lowers circulating insulin levels and shifts the body to utilise plasma glycerol and FFA as the main fuel source due to increased activation of adipocyte lipolysis (Anton et al., 2018; Zouhal et al., 2020). However, when compared to continuous endurance-based exercises, the body's preferred energy source during SIT type of exercise is creatine phosphate and anaerobic glycolysis of muscle glycogen, rather than FFA oxidation (Berg et al., 2002). Hence, the mismatch in fuel source for SIT could have accounted for the absence of greater magnitude of VO2peak improvement in the FAS relative to FED groups in this study, when compared to the endurance exercise used in the study by Stannard and colleagues.
IF may potentially negate muscle strength gains from exercise through reduction in total calorie intake and affecting the overall availability of carbohydrates in the working muscle tissues. This could consequently impair physical exertion and performance, especially for high intensity exercise such as SIT. The present study however showed that both the FAS and FED participants had significant improvement in knee flexion MVC at 300°·s−1, albeit there was no significant change in magnitude of improvement between the FED and FAS groups. The above improvement is likely secondary to neural adaptation to WAnT training, based on the relatively untrained participants utilized in this study (Folland and Williams, 2007). Our finding is supported by a study that showed young recreationally active males who performed resistance training (6 upper body workouts, 5 lower body work outs, 3 days∙wk−1, 4 sets of 8–12 reps per workout) under time restricted feeding (calorie consumption within 4 h for 4 days∙wk−1) versus normal diet showed increased in muscular strength (1-RM hip sled and bench press strength) in both groups over 8 weeks, but no interaction effects were present (Tinsley et al., 2017).
The present study found that FAS participants, when compared to FED participants, showed statistically significant decreases in diastolic BP and MAP values after 6 weeks of SIT. This is a novel and possibly an important finding from the study. A previously published large scale meta-analysis found that fasting and energy restriction diets produce a weighted mean difference in DBP of −1.32 mmHg (95% CI -1.81, −0.84) (Kord-Varkaneh et al., 2020) whereas our present study showed a greater mean difference in DBP of −6.4 mmHg (95% CI -12.76, −0.04). This suggests that exercise seems to amplify the effects of fasting on DBP. The BP lowering effect of SIT results from intensity-dependent increases to blood flow velocity due to increased levels of NO (Batacan et al., 2017). SIT increases availability and production of NO by increasing eNOS activity (Fallahi et al., 2015; Gibala, 2009) and reducing endothelin-1 levels (Ciolac, 2012) concurrently. These mechanisms improve endothelial function and arterial wall stiffness, reduce peripheral vascular resistance and improve overall NO-dependent vascular vasodilation (Costa et al., 2018). In previous literature, Ramadan fasting, which is a form of IF, was thought to reduce blood pressure through reduction in body weight (Dewanti et al., 2006). However, our present study did not show any significant loss of body weight in the FAS nor the FED participants. Physiologically, IF has been shown to reduce BP via decreasing sympathetic and increasing parasympathetic activities (by increasing brain derived neurotrophic factor to decrease catecholamine levels) (Malinowski et al., 2019; Dong et al., 2020) and affects natriuresis (via elevation of cardiac natriuretic peptides), renin-angiotensin-aldosterone and endothelial (via adiponectin-mediated endothelial nitrogen monoxide production) pathways (Grundler et al., 2020). Furthermore, fasting indirectly promotes the vasodilatory effect of insulin by reducing insulin resistance (Grundler et al., 2020) and increasing FFA availability for uptake by liver and muscles as substrate for aerobic oxidation (Zouhal et al., 2020). This increased uptake of FFA by the body via metabolic switching is of particular importance as decreased circulating plasma FFA may promote better BP measurements through reduced oxidative stress to endothelial cells and upregulation of pathways (e.g. tyrosine phosphorylation IRS-1/2, PI3K/Akt and AMPK/PI3K/Akt/eNOS) to increase production of NO (Ghosh et al., 2017). Therefore, the two different mechanisms elicited by fasting and SIT exercise could have combined to amplify the positive effects on BP observed in the present study.
The present study did not show any improvement in insulin sensitivity in either the FAS or FED groups (see Table 3). Several confounders, such as the duration of the exercise, population type (healthy non-overweight participants with absence of diabetes) and duration of fast, could have accounted for the absence of results for insulin sensitivity in the present study. Edinburgh et al. showed that 6 weeks of endurance exercise (3 sessions∙wk−1, 30-50-min of cycling, at 50–55% peak power output) after overnight fast improved insulin sensitivity in overweight subjects (Edinburgh et al., 2020). From the above study, endurance training with higher training volume seems to improve insulin sensitivity more significantly than SIT (Houmard et al., 2004). Besides, participants in above study were overweight. During SIT, glucose production rises seven-to eightfold and glucose utilisation rises three-to fourfold; therefore, glycemia increases and plasma insulin decreases minimally (Marliss and Vranic, 2002). The exercise induced hyperglycaemia seems to preferentially increase and activate GLUT 4 receptors in peripheral skeletal muscles, and therefore improves insulin resistance, more significantly in overweight and insulin resistant population than in healthy adult population (Röhling et al., 2016). However, for the exercising non-obese participants who are normoglycemic, the hyperglycaemia is matched instead by secreting more insulin to maintain blood sugar levels. Thus, this homeostatic regulation may “worsen” insulin sensitivity as there is relative increase in serum insulin levels instead. This point is illustrated by a study that showed non-obese participants who performed SIT had an increase in insulin level but glucose level did not significantly change from baseline, while obese participants who performed SIT had decrease in glucose level but showed no change to insulin level (Colpitts et al., 2021). The duration of fast could play a role in augmenting insulin sensitivity. Several IF studies (Sutton et al., 2018; Halberg et al., 2005) found that subjects who underwent minimum fasting duration of 18–20 h had greater insulin sensitivity when compared to overnight fasting of 8–12 h. Therefore, the present study is different from previous studies as SIT was performed instead of endurance training, minimum overnight fasting duration of ∼10 h was employed and only non-overweight, healthy participants were enrolled.
The present study did not show any difference in time or interaction effects for body fat percentage or lipid except for HDL, which decreased at post-training in the FAS group. This finding differs from previous studies. Gillian et al. showed that overweight females who underwent 6-week HIIT with overnight fasting (fasting duration not reported) achieved lower body fat percentages and body fat mass (Gillen et al., 2013). Likewise, Bhutani et al. showed that obese adults who underwent ADF (duration of fast 24 h) with 12 weeks of moderate intensity exercise (3 sessions∙wk-1, 25 min at 60% HRmax to 40 min at 75% HRmax incrementally) had decreased LDL and increased HDL levels (Bhutani et al., 2013). It is likely that lipid lowering effects post training could be more pronounced in obese individuals, as compared to individuals in the present study whose mean BMI lie in the healthy range. Endurance training, rather than SIT, in the fasted state also appears more effective in decreasing insulin, increasing catecholamines (Kirkendall et al., 2008) and upregulating the genes involved in fatty acid transport (Fakhrzadeh et al., 2003), thereby enhancing the rate of fat oxidation and reduction in body fat content (Chaouachi et al., 2009). In addition, Nybo et al. found that men who did interval training (12 wk, 3 sessions∙week−1, 5 x 2-min interval running, at >95% HRmax) when compared to prolonged running (12 wk, 3 sessions∙week−1, 1 h continuous running, at 80% HRmax) had poorer lipid profiles (Nybo et al., 2010), suggesting that training volume could be more important than training intensity to elicit more favourable lipid profiles. This could explain why the low volume, albeit supramaximal intensity exercise intervention in the present study did not yield meaningful lipid profile outcomes despite a fasting period of between 10 and 12 h.
The present study showed an absence in most physiological and clinical markers in interaction effects between fasting and SIT. The current concept of SIT is to deplete the skeletal muscle of phosphagen system as quickly as possible during intense exercise to optimize skeletal muscle uptake of glucose (to reduce free glucose molecules in the vascular system), improve skeletal muscle mitochondrial number and capacity and to enhance fat metabolism when performed chronically. The current assumption is fasting may amplify the effects of SIT by depleting the muscle glycogen stores further to enhance glucose uptake by skeletal muscles and/or switching the body's use of lipids to a greater extent relative to the same SIT exercise in the fed state. However, it is also plausible that there lies a saturation effect whereby fasting and training performed on the same day may instead lead to a decreased response to fasting on the day of SIT. Future studies may test this alternate hypothesis of performing fasting and training on separate or alternate days, where fasting and training separately may complement the effects of each modality.
There are several limitations that should be considered when interpreting the results of the present study. The present study recruited healthy adults with no chronic diseases. This could have contributed to the null results seen in insulin sensitivity and blood lipids, whereas significant changes tend to be seen in cohorts such as the overweight/obese or diabetic populations when they perform SIT exercise in fasted state. The impact of sex-related differences on exercise and diet could also have affected the results of the present study as two-thirds of the participants in the present study were females. The present study also did not control for the type and amount of calorie intake, or diet prior to all the exercise sessions in the FED group. It was not possible to fully control for physical activities outside of the prescribed training program, although participants were instructed to maintain their normal routine habits. Due to invasive nature and lack of equipment, insulin resistance was not determined with the gold-standard measurement of insulin sensitivity, i.e., the hyperinsulinemia euglycemic clamp technique, which may have resulted in higher variability in our results.
5. Conclusion
The results of the present study showed that 6 weeks of SIT, either in the fed (ingestion of food ≤3 h prior to all exercise sessions) or fasted state (no food ≥10 h prior to all exercise sessions) enhanced VO2peak and leg strength in healthy, recreationally active adults. However, the magnitude of training-induced adaptations in VO2peak and leg strength were similar in both FAS and FED groups. A novel finding of this study was SIT in the fasted state led to a significant decrease in diastolic BP and MAP.
CRediT authorship contribution statement
Victor Tan: Investigation, Formal analysis, Resources, Writing – original draft, Writing – review & editing, Visualizsation, Project administration. Ivy Lim: Writing – review & editing, Supervision. Pei Ting Tan: Validation, Formal analysis, Data curation. Frankie Tan: Writing – review & editing, Supervision. Abdul Rashid Aziz: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Adams R. Revised physical activity readiness Questionnaire. Can. Fam. Physician. 1999;45:1004–1005. 992, 995. [PMC free article] [PubMed] [Google Scholar]
- Aird T.P., Davies R.W., Carson B.P. Effects of fasted vs fed-state exercise on performance and post-exercise metabolism: a systematic review and meta-analysis. Scand. J. Med. Sci. Sports. 2018;28(5):1476–1493. doi: 10.1111/sms.13054. [DOI] [PubMed] [Google Scholar]
- Albright A., Franz M., Hornsby G. Exercise and type 2 diabetes. Med. Sci. Sports Exerc. 2000;32(7):1345–1360. doi: 10.1097/00005768-200007000-00024. [DOI] [PubMed] [Google Scholar]
- Anton S.D., Moehl K., Donahoo W.T. Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity. 2018;26(2):254–268. doi: 10.1002/oby.22065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batacan R.B., Duncan M.J., Dalbo V.J., Tucker P.S., Fenning A.S. Effects of high-intensity interval training on cardiometabolic health: a systematic review and meta-analysis of intervention studies. Br. J. Sports Med. 2017;51(6):494. doi: 10.1136/bjsports-2015-095841. [DOI] [PubMed] [Google Scholar]
- Berg J.M., Tymoczko J.L., Stryer L. fifth ed. W H Freeman; New York, NY: 2002. Biochemistry. [Google Scholar]
- Bhutani S., Klempel M.C., Kroeger C.M., Trepanowski J.F., Varady K.A. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity. 2013;21(7):1370–1379. doi: 10.1002/oby.20353. [DOI] [PubMed] [Google Scholar]
- Bolgar M.R., Baker C.E., Goss F.L., Nagle E., Robertson R.J. Effect of exercise intensity on differentiated and undifferentiated ratings of perceived exertion during cycle and treadmill exercise in recreationally active and trained women. J. Sports Sci. Med. 2010;9(4):557–563. [PMC free article] [PubMed] [Google Scholar]
- Chaouachi A., Coutts A.J., Chamari K. Effect of ramadan intermittent fasting on aerobic and anaerobic performance and perception of fatigue in male elite judo athletes. J. Strength Condit Res. 2009;23(9):2702–2709. doi: 10.1519/JSC.0b013e3181bc17fc. [DOI] [PubMed] [Google Scholar]
- Ciolac E.G. High-intensity interval training and hypertension: maximizing the benefits of exercise? Am J Cardiovasc Dis. 2012;2(2):102–110. [PMC free article] [PubMed] [Google Scholar]
- Colpitts B.H., Seaman K., Eadie A.L., Brunt K.R., Bouchard D.R., Sénéchal M. Effects of sprint interval training on substrate oxidation in adults living with and without obesity: the i‐FLEX study. Phys. Rep. 2021;9(11) doi: 10.14814/phy2.14916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa E.C., Hay J.L., Kehler D.S. Effects of high-intensity interval training versus moderate-intensity continuous training on blood pressure in adults with pre- to established hypertension: a systematic review and meta-analysis of randomized trials. Sports Med. 2018;48(9):2127–2142. doi: 10.1007/s40279-018-0944-y. [DOI] [PubMed] [Google Scholar]
- de Cabo R., Mattson M.P. Effects of intermittent fasting on health, aging, and disease. N. Engl. J. Med. 2019;381(26):2541–2551. doi: 10.1056/NEJMra1905136. [DOI] [PubMed] [Google Scholar]
- Dewanti L., Watanabe C., Sulistiawati Ohtsuka R. Unexpected changes in blood pressure and hematological parameters among fasting and nonfasting workers during Ramadan in Indonesia. Eur. J. Clin. Nutr. 2006;60(7):877–881. doi: 10.1038/sj.ejcn.1602393. [DOI] [PubMed] [Google Scholar]
- Dong T.A., Sandesara P.B., Dhindsa D.S. Intermittent fasting: a heart healthy dietary pattern? Am. J. Med. 2020;133(8):901–907. doi: 10.1016/j.amjmed.2020.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinburgh R.M., Bradley H.E., Abdullah N.F. Lipid metabolism links nutrient-exercise timing to insulin sensitivity in men classified as overweight or obese. J. Clin. Endocrinol. Metab. 2020;105(3):660–676. doi: 10.1210/clinem/dgz104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergul A. Endothelin-1 and diabetic complications: focus on the vasculature. Pharmacol. Res. 2011;63(6):477–482. doi: 10.1016/j.phrs.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhrzadeh H., Lariiani B., Sanjari M., Baradar-Jalili R., Amini M.R. Effect of ramadan fasting on clinical and biochemical parameters in healthy adults. Ann. Saudi Med. 2003;23(3–4):223–226. doi: 10.5144/0256-4947.2003.223. [DOI] [PubMed] [Google Scholar]
- Fallahi A., Gaeini A., Shekarfroush S., Khoshbaten A. Cardioprotective effect of high intensity interval training and nitric oxide metabolites (NO2-, NO3-) Iran. J. Public Health. 2015;44:7. [PMC free article] [PubMed] [Google Scholar]
- Folland J.P., Williams A.G. The adaptations to strength training: morphological and neurological contributions to increased strength. Sports Med. 2007;37(2):145–168. doi: 10.2165/00007256-200737020-00004. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Gao L., Thakur A., Siu P.M., Lai C.W.K. Role of free fatty acids in endothelial dysfunction. J. Biomed. Sci. 2017;24(1):50. doi: 10.1186/s12929-017-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibala M.J. Molecular responses to high-intensity interval exercise. Appl. Physiol. Nutr. Metabol. 2009;34(3):428–432. doi: 10.1139/H09-046. [DOI] [PubMed] [Google Scholar]
- Gibala M.J. Physiological basis of interval training for performance enhancement. Exp. Physiol. 2020:1–4. doi: 10.1113/EP088190. [DOI] [PubMed] [Google Scholar]
- Gibala M.J., Hawley J.A. Sprinting toward fitness. Cell Metabol. 2017;25(5):988–990. doi: 10.1016/j.cmet.2017.04.030. [DOI] [PubMed] [Google Scholar]
- Gibala M.J., Little J.P., MacDonald M.J., Hawley J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012;590(5):1077–1084. doi: 10.1113/jphysiol.2011.224725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillen J.B., Percival M.E., Ludzki A., Tarnopolsky M.A., Gibala M.J. Interval training in the fed or fasted state improves body composition and muscle oxidative capacity in overweight women. Obesity. 2013;21(11):2249–2255. doi: 10.1002/oby.20379. [DOI] [PubMed] [Google Scholar]
- Goodyear L.J., Kahn B.B. Exercise, glucose transport, and insulin sensitivity. Annu. Rev. Med. 1998;49(1):235–261. doi: 10.1146/annurev.med.49.1.235. [DOI] [PubMed] [Google Scholar]
- Grundler F., Mesnage R., Michalsen A., de Toledo F.W. Blood pressure changes in 1610 subjects with and without antihypertensive medication during long‐term fasting. J Am Heart Assoc. 2020;9(23) doi: 10.1161/JAHA.120.018649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutch M., Kumar S., Mohd Razi S., Gupta K.K., Gupta A. Assessment of insulin sensitivity/resistance. Indian J Endocrinol Metab. 2015;19(1):160–164. doi: 10.4103/2230-8210.146874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberg N., Henriksen M., Söderhamn N. Effect of intermittent fasting and refeeding on insulin action in healthy men. J. Appl. Physiol. 2005;99(6):2128–2136. doi: 10.1152/japplphysiol.00683.2005. [DOI] [PubMed] [Google Scholar]
- Ho B.H., Lim I., Tian R., Tan F., Aziz A.R. Effects of a novel exercise training protocol of Wingate-based sprint bouts dispersed over a day on selected cardiometabolic health markers in sedentary females: a pilot study. BMJ Open Sport Exerc Med. 2018;4(1) doi: 10.1136/bmjsem-2018-000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houmard J.A., Tanner C.J., Slentz C.A., Duscha B.D., McCartney J.S., Kraus W.E. Effect of the volume and intensity of exercise training on insulin sensitivity. J. Appl. Physiol. 2004;96(1):101–106. doi: 10.1152/japplphysiol.00707.2003. [DOI] [PubMed] [Google Scholar]
- Jeon S.-M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016;48(7) doi: 10.1038/emm.2016.81. e245-e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkendall D.T., Leiper J.B., Bartagi Z., Dvorak J., Zerguini Y. The influence of Ramadan on physical performance measures in young Muslim footballers. J. Sports Sci. 2008;26(Suppl. 3):S15–S27. doi: 10.1080/02640410802422199. [DOI] [PubMed] [Google Scholar]
- Kord-Varkaneh H., Nazary-Vannani A., Mokhtari Z. The influence of fasting and energy restricting diets on blood pressure in humans: a systematic review and meta-analysis. High Blood Pres. Cardiovasc. Prev. 2020;27(4):271–280. doi: 10.1007/s40292-020-00391-0. [DOI] [PubMed] [Google Scholar]
- Malinowski B., Zalewska K., Węsierska A. Intermittent fasting in cardiovascular disorders-an overview. Nutrients. 2019;11(3):673. doi: 10.3390/nu11030673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marliss E.B., Vranic M. Intense exercise has unique effects on both insulin release and its roles in glucoregulation: implications for diabetes. Diabetes. 2002;51(Suppl. 1):S271–S283. doi: 10.2337/diabetes.51.2007.S271. [DOI] [PubMed] [Google Scholar]
- Matthews D.R., Hosker J.P., Rudenski A.S. Homeostasis model assessment: insulin resistance and ß-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Mattson M.P., Longo V.D., Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017;39:46–58. doi: 10.1016/j.arr.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nybo L., Sundstrup E., Jakobsen M.D. High-intensity training versus traditional exercise interventions for promoting health. Med. Sci. Sports Exerc. 2010;42(10):1951–1958. doi: 10.1249/MSS.0b013e3181d99203. [DOI] [PubMed] [Google Scholar]
- Oberlin D.J., Mikus C.R., Kearney M.L. One bout of exercise alters free-living postprandial glycemia in type 2 diabetes. Med. Sci. Sports Exerc. 2014;46(2):232–238. doi: 10.1249/MSS.0b013e3182a54d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhling M., Herder C., Stemper T., Müssig K. Influence of acute and chronic exercise on glucose uptake. J Diabetes Res. 2016;2016:1–33. doi: 10.1155/2016/2868652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallis J.F., Hovell M.F., Richard Hofstetter C. Predictors of adoption and maintenance of vigorous physical activity in men and women. Prev. Med. 1992;21(2):237–251. doi: 10.1016/0091-7435(92)90022-A. [DOI] [PubMed] [Google Scholar]
- Stannard S.R., Buckley A.J., Edge J.A., Thompson M.W. Adaptations to skeletal muscle with endurance exercise training in the acutely fed versus overnight-fasted state. J. Sci. Med. Sport. 2010;13(4):465–469. doi: 10.1016/j.jsams.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Sun C., Zhang F., Ge X. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metabol. 2007;6(4):307–319. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Sutton E.F., Beyl R., Early K.S., Cefalu W.T., Ravussin E., Peterson C.M. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metabol. 2018;27(6):1212–1221. doi: 10.1016/j.cmet.2018.04.010. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada T., Wilson B.J., Myette-Côté E. Targeting specific interstitial glycemic parameters with high-intensity interval exercise and fasted-state exercise in type 2 diabetes. Metab. Clin. Exp. 2016;65(5):599–608. doi: 10.1016/j.metabol.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Terada T., Toghi Eshghi S.R., Liubaoerjijin Y. Overnight fasting compromises exercise intensity and volume during sprint interval training but improves high-intensity aerobic endurance. J. Sports Med. Phys. Fit. 2019;59(3) doi: 10.23736/S0022-4707.18.08281-6. [DOI] [PubMed] [Google Scholar]
- Tinsley G.M., Forsse J.S., Butler N.K. Time-restricted feeding in young men performing resistance training: a randomized controlled trial. Eur. J. Sport Sci. 2017;17(2):200–207. doi: 10.1080/17461391.2016.1223173. [DOI] [PubMed] [Google Scholar]
- World Health Organisation Physical activity. May 23, 2017. https://www.who.int/dietphysicalactivity/pa/en/ Published.
- Zhang J., Schaeffer M.R., Mitchell R.A. A multidimensional assessment of dyspnoea in healthy adults during exercise. Eur. J. Appl. Physiol. 2020;120(11):2533–2545. doi: 10.1007/s00421-020-04479-2. [DOI] [PubMed] [Google Scholar]
- Zouhal H., Saeidi A., Salhi A. Exercise training and fasting: current insights. Open Access J. Sports Med. 2020;11:1–28. doi: 10.2147/OAJSM.S224919. [DOI] [PMC free article] [PubMed] [Google Scholar]