Abstract
Purpose
A series of clinical studies have established the safety and efficacy of transcatheter arterial chemoembolization (TACE) with gelatin sponge microparticles (GSMs) in treating hepatocellular carcinoma (HCC). HCC can lead to obvious necrosis inside tumors, especially larger ones, although it is unclear whether such necrotic tumor tissue can induce favorable immune reactions against the tumor. Myeloid-derived suppressor cells (MDSCs) have immunosuppressive functions and are currently considered a very important cell type affecting tumor immunity. This study observed changes in MDSC frequency in peripheral blood before and after GSM–TACE to evaluate the effect on the immune function of HCC patients.
Methods
Eight patients diagnosed with HCC underwent GSM–TACE treatment in the Hepatobiliary Interventional Department of Beijing Tsinghua Chang Gung Hospital, Beijing, China; we followed up with the patients over a period of 30 days post-surgery. We used flow cytometry (FCM) to quantify the frequency of MDSCs in peripheral blood before TACE, 10 days after surgery and 30 days after surgery.
Results
MDSC frequency after GSM–TACE had a significant downward trend. Pre-TACE, it was 30.73% ± 11.93%, decreasing to 18.60% ± 11.37% at 10 days after operation. This decrease was not statistically significant (P > 0.05). MDSC frequency was even lower 30 days after TACE (7.63% ± 7.32%) than at 10 days after TACE (P < 0.05), and there was a significant difference compared with pre-TACE (P < 0.001). We evaluated tumor response at 30 days after GSM–TACE according to the Modified Response Evaluation Criteria in Solid Tumors (mRECIST), and all eight patients showed partial response (PR).
Conclusion
Our results confirmed that GSM–TACE was beneficial for improving anti-tumor immunity in the treatment of HCC.
Keywords: Gelatin sponge microparticles–transcatheter arterial chemoembolization (GSMs-TACE), Hepatocellular carcinoma, Myeloid-derived suppressor cells (MDSCs), Immunology
1. Introduction
Hepatocellular carcinoma (HCC) is the sixth most prevalent cancer and the third most common cause of cancer-related deaths worldwide,1 especially in Asian countries.2 Moreover, patients most patients with HCC are already in the advanced stage of the disease when they are clinically diagnosed. Therefore, transcatheter arterial chemoembolization (TACE) has become an important method for the treatment of hcc.3 Regulation of tumor-associated host immune responses following cancer treatment has been reported in recent years. For example, studies have found that treating HCC with radiofrequency ablation (RFA) or TACE increases the frequency of tumor-associated antigen (TAA)–specific T cells, likely because tumor cell necrosis causes their antigens to be exposed.4,5 There are also reports of decreased MDSC frequency in the peripheral blood of patients with HCC after hepatic arterial infusion chemotherapy (HAIC) or RFA.6 However, the current impact of TACE on MDSCs in the peripheral blood of patients with liver cancer has not been reported.
As embolic agents, gelatin sponge microparticles (GSMs) are absorptive, pose fewer complications, and cause lighter liver damage.7 Our previous study confirmed the beneficial effect of GSM–TACE on overall survival (OS) rates and tumor response in HCC in Barcelona Clinic Liver Cancer (BCLC) Stages B and C.8,9 In using GSM–TACE to treat HCC in clinical practice, we have found some special clinical phenomena, such as the disappearance of metastatic lymph nodes and pulmonary metastatic nodules after tumor necrosis, suggesting that necrosis of primary tumors may have induced anti-tumor immune response. Therefore, we designed this study to observe changes in the frequency of MDSCs in peripheral blood before and after GSM–TACE to test our hypothesis.
2. Materials and methods
2.1. Standardized GSM–TACE
Eight subjects with clinically diagnosed HCC were examined in the present study (Table 1). HCC was diagnosed based on underlying chronic liver disease, radiological findings, and elevations in tumor markers. Regarding tumor stage, the following patients were included: (1) severe vascular invasion (i.e., vascular invasion in the main trunk to the secondary branches of the portal vein; or invasion in the right, middle, or left hepatic vein) and (2) multiple intrahepatic lesions (i.e., ≥3 nodules in the left and/or right lobes as confirmed by radiology). After diagnosis, all patients were treated with GSM–TACE in the Hepatobiliary Interventional Department of Beijing Tsinghua Chang Gung Hospital, Beijing, China. All patients in this study were first-line patients. In the TACE procedure, the patient's right femoral artery was punctured. Angiography of the celiac and hepatic arteries was routinely performed with a 5F-RH hepatic duct. Angiography was performed on the inferior phrenic, superior mesenteric, left gastric, intrathoracic, and intercostal arteries according to tumor site, size, and staining integrity in order to ensure complete tumor staining. We treated patients with the chemotherapeutic drug lobaplatin (Hainan Chang'an International Pharmaceutical Co., Ltd., Haikou, China) at a dosage range of 10–30 mg based on tumor size (tumor diameter <5 cm, 10 mg; 5–10 cm, 20 mg; ≥10 cm, 30 mg),10,11 and we diluted the drug with water for injection (WFI) to 30 mL, and mixed it with GSMs 350–560 μm in diameter (Hangzhou Ailikang Pharmaceutical Technology Co., Ltd., Hangzhou, China; specification: 100 mg; product batch number: 080722). Under digital-subtraction angiography (DSA), we slowly injected the mixture of GSMs and drugs into the tumor-supplying artery. Embolization stop criteria were that tumor staining disappeared completely and blood flow in the regional artery was stagnant. Before TACE, 10 days after TACE, and 30 days after TACE, we took 4 mL peripheral venous blood to analyze changes in MDSC by flow cytometry (FCM). Response rate after GSM–TACE was determined by performing enhanced computed tomography (CT) or enhanced magnetic resonance imaging (MRI) 30 days after TACE according to the Modified Response Evaluation Criteria in Solid Tumors (mRECIST). Subjects were classified as showing complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD).12
Table 1.
Clinical characteristics of the eight patients.
| Case # | Age | Sex (M/F) | BCLC stage | Liver function (Child-pugh A/B/C) | HBsAg (positive/negative) | Diameter of main tumor (mm) |
|---|---|---|---|---|---|---|
| 1 | 66 | F | C | B | Positive | 124 |
| 2 | 50 | M | C | A | Positive | 32 |
| 3 | 55 | F | C | A | Positive | 71 |
| 4 | 71 | M | B | A | Positive | 97 |
| 5 | 47 | M | B | A | Positive | 16 |
| 6 | 63 | M | C | A | Positive | 45 |
| 7 | 44 | M | B | A | Positive | 105 |
| 8 | 49 | F | C | A | Negative | 106 |
ECOG PS: Eastern Cooperative Oncology Group Performance Score. BCLC: Barcelona Clinic Liver Cancer. Hepatitis B virus surface antigen.
All patients in this study provided written informed consent to participate in the study.
2.2. MDSC detection and analysis
2.2.1. Cell isolation and FCM analysis
We collected 4 mL of fasting peripheral venous blood from all HCC patients before GSM–TACE, 10 days after surgery, and 30 days after surgery using heparin anticoagulation tubes. We then added 100 μL whole blood to a dry blank tube, lysed it with 500 μL OptiLyse C Lysing Solution (Beckman Coulter Diagnostics, Brea, California, US), vortexed the solution, and incubated the lysed blood at room temperature for 15 min to assure lysis was complete. Then we added 2 mL phosphate-buffered saline (PBS), and we vortexed and centrifuged the mixture at 300 g for 5 min. After centrifugation, we aspirated the supernatant, resuspended the cell pellet in 500 μL PBS, added 5 μL each of CD14, CD11b, and HLA-DR antibodies, and mixed the solution low speed for 5 s. Finally, we analyzed the samples on a flow cytometer.
2.2.2 Antibodies and laboratory equipment: To determine MDSC frequency, we performed multicolor fluorescence-activated cell sorting analysis using Beckman Coulter CytExpert software version 1.1. We also used a DxFLEX Flow Cytometer (Beckman Coulter), a Labofuge 400R Centrifuge (Thermo Fisher Scientific, Waltham, Massachusetts, US), a LP Vortex Mixer(Thermo Fisher Waltham, Massachusetts, US), and the following anti-human monoclonal antibodies: CD11b-APC-Alexa Fluor 750, CD14-PC7, anti–HLA-DR-ECD, OptiLyse C solution, and PBS (all Beckman Coulter).
2.2.2. Detection of MDSCs
Human MDSCs are generally defined as inhibitory cells with CD11b+/CD33+/HLA-DR low/−. CD14, like CD11b and CD33, is a common MDSC surface marker.13,14 However, recent studies have found that while there is no clear definition of total MDSCs,15 they can be classified as CD11b+/CD14−/CD15+ or CD66+ (polymorphonuclear MDSCs [PNM-MDSCs), CD11b+/CD14+/HLA-DR low/− (monocytic MDSCs [M-MDSCs]), or Lin−(CD3/14/15/19/56)/HLA-DR−/CD33+ (early-stage MDSCs [e-MDSCs]).16, 17, 18 In the present study, MDSCs identified in the blood of patients with HCC were mainly of the CD11b+/CD14+/HLA-DR low/− M-MDSC phenotype.
2.3. Statistical analysis
We performed statistical analysis with Statistical Product and Service Solutions software version 11.0 (SPSS, Inc., Chicago, Illinois, US). Results are expressed as the mean ± standard deviation (SD). We used the t-test to compare changes in MDSC frequency. Count data is expressed as percentages (%). P < 0.05 was considered to indicate statistical significance in all analyses.
3. Results
3.1. Patient profiles
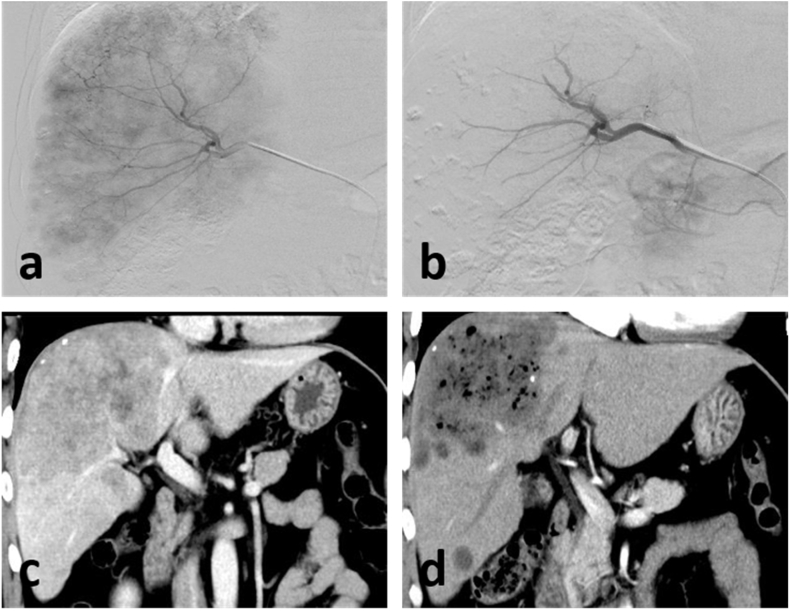
In all cases, HCC was diagnosed based on potential chronic liver disease, typical radiological findings, and elevated tumor markers. In terms of tumor diameter, there were 3 patients with tumor diameter ≥10 cm, and 5 patients with tumor diameter <10 cm. In terms of BCLC staging, 3 patients were classified as stage B, and the remaining patients were all C stage, at the same time, all patients are multiple intrahepatic. Seven patients were classified as Child–Pugh Class A, and the remaining patient was classified as Class B. All patients showed PR by 30 days after GSM–TACE, and one patient underwent tumor resection 30 days after GSM–TACE. An enhanced-CT revealed that the patient had a large lesion in the liver (Fig. 1c), and intra-operative angiography showed abundant tumor blood supply (Fig. 1a). During the TACE process, tumor-feeding arteries were blocked by the GSM–lobaplatin mixture (Fig. 1b). Thirty days later, a follow-up enhanced-CT scan showed a large amount of tumor necrosis with no significant enhancement (Fig. 1d).
Fig. 1.
(c) Pre-operative enhanced-CT image. (a–b) Intra-operative DSA image. (d) Enhanced-CT image 30 days after GSM–TACE.
3.2. Changes in MDSC
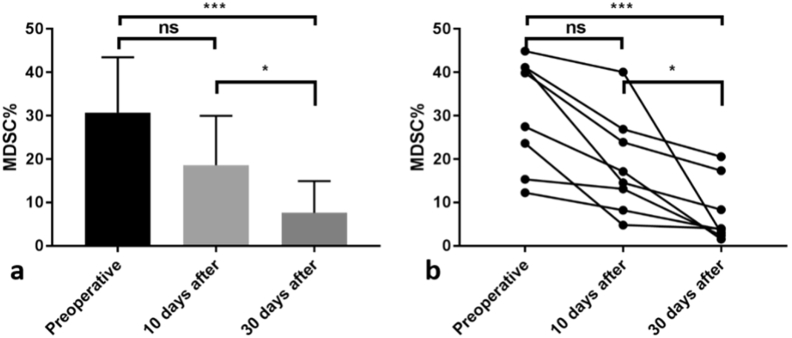
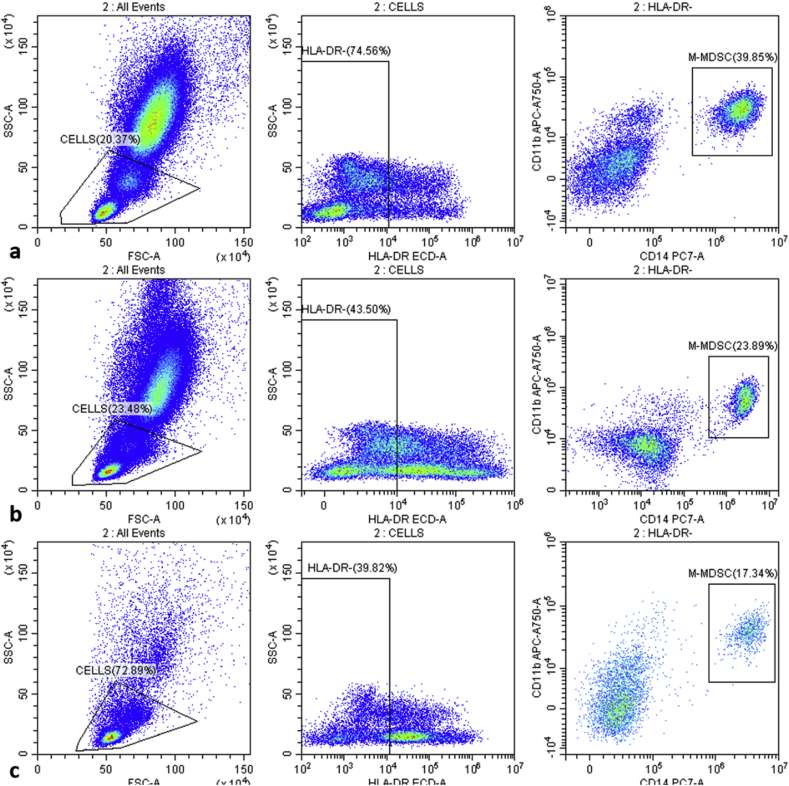
MDSC frequency after GSM–TACE had a significant downward trend (Fig. 2). Pre-TACE, the frequency was 30.73% ± 11.93%, and it decreased to 18.60% ± 11.37% 10 days post-surgery. This decrease was not statistically significant (P > 0.05). MDSC frequency 30 days after TACE (7.63% ± 7.32%) was lower than at 10 days after TACE (P < 0.05), and there was a significant difference compared with pre-TACE. (P < 0.001). Fig. 3 is a FCM diagram of a patient before TACE, 10 days after TACE, and 30 days after TACE. Fig. 3 illustrates that MDSC frequency in this patient before TACE was 39.85% (Fig. 3a), decreasing to 23.89% 10 days after GSM–TACE (Fig. 3b). In the month after surgery, the frequency continued to drop to 17.34% (Fig. 3b). In all patients, serum AFP and protein-II induced by a vitamin K absence (PIVKA-II) levels were decreased after TACE. The changes in tumor markers were almost similar to changes in MDSC frequency (Table 2).
Fig. 2.
(a) A statistically significant distribution. (b) Decrease in MDSC frequency at different time periods. *P < 0.05. **P < 0.01. ***P < 0.001. ns: No significant difference.
Fig. 3.
MDSC changes in one patient. (a) Pre-surgery, MDSC frequency was 39.85%. (b) MDSC frequency decreased to 23.89% by 10 days after TACE. (c) MDSC frequency continued to decrease to 17.34% by 30 days after TACE.
Table 2.
Changes in tumor markers and MDSC frequencies in patients before and after TACE.
| AFP (ng/ml) |
PIVKA-II (mAU/m) |
MDSC (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Case # | Pre-op. | 10 days after | 30 days after | Pre-op. | 10 days after | 30 days after | Pre-op. | 10 days after | 30 days after |
| 1 | 298.99 | 221.37 | 304.26 | 1305.27 | 14.06 | 20.82 | 44.90% | 40.08% | 3.01% |
| 2 | 20.2 | 12.09 | 5.95 | 10,556.75 | 3616.3 | 848.52 | 15.36% | 13.13% | 2.41% |
| 3 | 47.86 | 15.72 | 10.63 | 610.23 | 114.56 | 14.49 | 27.48% | 17.15% | 1.58% |
| 4 | 7.11 | 4.21 | 2.11 | 1260.48 | 298.01 | 78.53 | 23.63% | 4.82% | 4.02% |
| 5 | 986.61 | 185.82 | 168.5 | >30,000.00 | 7185.8 | 4278.23 | 12.29% | 8.22% | 3.75% |
| 6 | 436.67 | 394.38 | 333.58 | 14,841.44 | 14,048.34 | 11,358.09 | 39.85% | 23.89% | 17.34% |
| 7 | 1822.74 | 857.71 | 255.3 | 799.94 | 191.89 | 107.22 | 41.16% | 14.56% | 8.35% |
| 8 | 1094.84 | 979.7 | 791.06 | 35.22 | 11.56 | 5.78 | 41.15% | 26.91% | 20.57% |
4. Discussion
TACE has become one of the main treatment approaches for advanced liver cancer. The consensus guidelines developed by experts in the United States, Asia-Pacific, and Europe recommend that TACE be used as the first-line treatment for patients with advanced liver cancer who have (a) large or multiple tumors that cannot be surgically removed, and (b) vascular infiltration or (c) extrahepatic dissemination. These guidelines have significantly improved the survival rate of liver cancer patients.19, 20, 21 BCLC Stage B patients are recommended for TACE treatment.22 Previous studies by our team indicate that the clinical application of GSM–TACE can improve patients' immune status' by destroying tumors, exposing tumor antigens, and ultimately improving the long-term efficacy of TACE. It can also reduce the frequency of regulatory T cells (Tregs) in peripheral blood, confirming that the method can positively regulate the body's anti-tumor immune response.23,24
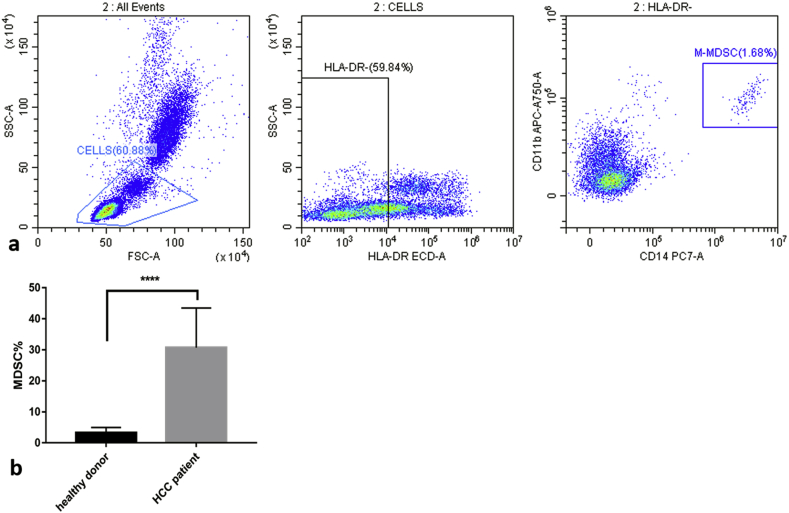
MDSCs are a type of immature cells in the bone marrow shown to play a pivotal role in immune responses, such as pro-angiogenesis, inflammation, tumor progression, and tumor cell immune escape; MDSCs are closely related to the prognosis of cancer.25 MDSCs expand under pathological conditions, such as malignant tumors, infections, or wounds, losing the ability to differentiate into mature macrophages, dendritic cells, or granulocytes.26 This expansion strongly inhibits the anti-tumor activity of T and natural-killer (NK) cells, and activates Treg amplification, which ultimately leads to tumor progression.27 As a monocyte subpopulation of MDSCs, CD14−/HLA-DR low/− M-MDSCs have been reported to increase significantly in malignant tumors, including melanoma, multiple myeloma, prostate cancer, and bladder cancer.28 Also, in a recent study, Hoechst et al.29 reported a significant increase in CD14−/HLA−DR low/− M-MDSCs in HCC patients and that these M-MDSCs inhibited T cell function. In this study, we also analyzed peripheral blood from nine healthy donors and compared the results with pre-operative data from HCC patients (Table 3). We found that the frequency of healthy donors’ MDSCs was very low (Fig. 4a), 3.26% ± 1.71%, while that of pre-operative HCC patients was 30.73% ± 11.93%. The two sets of data showed significant differences (P < 0.0001) after analysis (Fig. 4b). Therefore, we believe MDSCs increased significantly in our HCC patients. However, due to an insufficient number of patients and related conditions such as lack of relevant experimental protocols and reagents, we did not conduct multivariate analysis; thus, it was not clear whether MDSCs affected the number and function of T cells in these patients.
Table 3.
Comparison of MDSCs between healthy donors and HCC patients.
| Donor | M-MDSC Frequency | Comparable HCC Patient |
|---|---|---|
| 1 | 5.93% | 44.90% |
| 2 | 0.96% | 15.36% |
| 3 | 5.13% | 27.48% |
| 4 | 1.68% | 23.63% |
| 5 | 2.34% | 12.29% |
| 6 | 4.8% | 39.85% |
| 7 | 1.94% | 41.16% |
| 8 | 3.4% | 41.15% |
| 9 | 3.12% |
Fig. 4.
(a) The frequency of M-MDSCs in peripheral blood from a healthy person is 1.68%. (b) For HCC patients, the figure is significantly higher than for healthy donors. ****P < 0.0001.
In addition, studies have found that the use of certain chemotherapeutic drugs can reduce MDSC frequency and produce significant anti-tumor effects.27, 30 Examples include the use of oxaliplatin in the treatment of colon cancer and the use of gemcitabine for perfusion therapy in patients with pancreatic cancer. Mizukoshi E et al.6 treated HCC patients with a HAIC regimen of 5-fluorouracil combined with cisplatin and found that the frequency of MDSCs and Tregs in the patients' peripheral blood decreased after surgery. In this study, we examined changes in MDSC frequency in peripheral blood from HCC patients before and after GSM–TACE treatment and found that frequency decreased as time after surgery increased. This indicated that GSM–TACE treatment could improve the tumor immune microenvironment by reducing MDSC frequency. However, this phenomenon may be due to tumor necrosis caused by embolization, tumor burden reduction or apoptosis, or chemotherapeutic drugs, resulting in decreased MDSC levels in the tumor microenvironment. It is possible that the effect is due to synergy, but we don't know the specific cause or mechanism.
In conclusion, this study showed a significant reduction in MDSC frequency after GSM–TACE, indicating that this method had a positive regulatory effect on the body's anti-tumor immune function. These results provide preliminary evidence that GSM–TACE combined with other methods, especially immunotherapy, may be helpful for improving the efficacy of HCC treatment.
Funding
Supported by a grant from National Natural Science Foundation of China(grant number 81571783) and National Major Project for Infectious Diseases of China(2017ZX100203205005).
References
- 1.Lencioni R., Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology. 2012;262(1):43–58. doi: 10.1148/radiol.11110144. [DOI] [PubMed] [Google Scholar]
- 2.Llovet J.M., Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48(supp-S1):S20–S37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 3.Ayaru L., Pereira S.P., Alisa A. Unmasking of alpha-fetoprotein-specific CD4(+) T cell responses in hepatocellular carcinoma patients undergoing embolization. J Immunol. 2007;178(3):1914–1922. doi: 10.4049/jimmunol.178.3.1914. [DOI] [PubMed] [Google Scholar]
- 4.Mizukoshi E., Yamashita T., Arai K. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2013;57(4):1448–1457. doi: 10.1002/hep.26153. [DOI] [PubMed] [Google Scholar]
- 5.Mizukoshi E., Yamashita T., Arai K. Myeloid-derived suppressor cells correlate with patient outcomes in hepatic arterial infusion chemotherapy for hepatocellular carcinoma. Cancer Immunol Immunother. 2016;65(6):715–725. doi: 10.1007/s00262-016-1837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin A.O., Yue-Wei Z. Gelatin sponge particle used as an embolic agent in transcatheter arterial chemoembolization treatment for primary hepatocellular carcinoma: its current situation in research. J Interv Radiol. 2011;18(3):229–234. [Google Scholar]
- 7.Kamran A.U., Liu Y., Li F.E. Transcatheter arterial chemoembolization with gelatin sponge microparticles treated for BCLC stage B hepatocellular carcinoma: a single center retrospective study. Medicine. 2015;94(52):e2154. doi: 10.1097/MD.0000000000002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou J., Liu Y., Ren Z. Transarterial chemoembolization with gelatin sponge microparticles for barcelona clinic liver cancer Stage C and large hepatocellular carcinoma: initial clinical experience. J Cancer Res Ther. 2017;13(5):767–772. doi: 10.4103/jcrt.JCRT_297_17. [DOI] [PubMed] [Google Scholar]
- 9.Wu Q., Qin S.K., Teng F.M. Lobaplatin arrests cell cycle progression in human hepatocellular carcinoma cells. J Hematol Oncol. 2010;3(1):43. doi: 10.1186/1756-8722-3-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang N., Lv Y.Z., Xu A.H. Application of lobaplatin in trans-catheter arterial chemoembolization for primary hepatic carcinoma. Asian Pac J Cancer Prev. 2014;15(2):647–650. doi: 10.7314/apjcp.2014.15.2.647. [DOI] [PubMed] [Google Scholar]
- 11.Lencioni R. New data supporting modified RECIST (mRECIST) for Hepatocellular Carcinoma. Clin Canc Res Off J Am Assoc Canc Res. 2013;19(6):1312–1314. doi: 10.1158/1078-0432.CCR-12-3796. [DOI] [PubMed] [Google Scholar]
- 12.Dong W., An G., Xie S. The clinical and prognostic significance of CD14 + HLA-DR −/low, myeloid-derived suppressor cells in hepatocellular carcinoma patients receiving radiotherapy. Tumour Biol. 2016;37(8):10427–10433. doi: 10.1007/s13277-016-4916-2. [DOI] [PubMed] [Google Scholar]
- 13.Wan S., Kuo N., Kryczek I. Myeloid cells in hepatocellular carcinoma. Hepatology. 2015;62(4):1304–1312. doi: 10.1002/hep.27867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bronte V., Brandau S., Chen S.H. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150–12159. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabrilovich D.I., Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Condamine T., Gabrilovich D.I. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32(1):19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabrilovich D.I., Ostrand-Rosenberg S., Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heimbach J., Kulik L.M., Finn R. Aasld guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2017;67(1):358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 19.Omata M., Lesmana L.A., Tateishi R. Asian pacific association for the study of the liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4(2):439–474. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han K.H., Kudo M., Ye S.L. Asian consensus workshop report: expert consensus guideline for the management of intermediate and advanced hepatocellular carcinoma in Asia. Oncology. 2015;81(Suppl.1):158–164. doi: 10.1159/000333280. [DOI] [PubMed] [Google Scholar]
- 21.Han Kichang, Kim Jin Hyoung. Transarterial chemoembolization in hepatocellular carcinoma treatment: barcelona clinic liver cancer staging system. World J Gastroenterol. 2015;21(36):10327–10335. doi: 10.3748/wjg.v21.i36.10327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song L., Ran-Ran M., Yue-Wei Z. TACE combined with dendritic cells for the treatment of hepatic cancers: its influence on patient's immune function. J Interv Radiol. 2014;23(2):181–184. [Google Scholar]
- 23.Zhi-Zhong R., Ying L., Yuan-Xun Y. Postoperative changes and its significance of Treg cells in peripheral blood after GSMs- TACE treatment for large hepatocellular carcinoma in 15 patients. J Interv Radiol. 2018;27(12):1151–1154. [Google Scholar]
- 24.Chang C.J., Yang Y.H., Chiu C.J. Targeting tumor-infiltrating Ly6G+ myeloid cells improves sorafenib efficacy in mouse orthotopic hepatocellular carcinoma. Int J Cancer. 2018;142(9):1878–1889. doi: 10.1002/ijc.31216. [DOI] [PubMed] [Google Scholar]
- 25.Jiang N., Xing Y.F., Hu B. Endoplasmic reticulum stress induced Lox-1+CD15+ polymorphonuclear myeloid-derived suppressor cells in hepatocellular carcinoma. Immunology. 2017;154(1):144–155. doi: 10.1111/imm.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber R., Fleming V., Hu X. Myeloid-derived suppressor cells hinder the anti-cancer activity of immune checkpoint inhibitors. Front Immunol. 2018;9:1310–1318. doi: 10.3389/fimmu.2018.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo B., Xie N., Wang Y. Cooperative effect of Bifidobacteria lipoteichoic acid combined with 5-fluorouracil on hepatoma-22 cells growth and apoptosis. Bull Du Canc. 2015;102(3):204–212. doi: 10.1016/j.bulcan.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Hoechst B., Ormandy L.A., Ballmaier M. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135(1):234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 29.Moehler M., Göpfert K., Outlook Lenz H.J. Immunotherapy in gastrointestinal carcinoma - innovative strategies. Oncol Res Treat. 2018;41(5):313–315. doi: 10.1159/000489047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moehler M., Göpfert K., Lenz H.J. Outlook: Immunotherapy in Gastrointestinal Carcinoma - innovative strategies. Oncol Res Treat. 2018;41(5):313. doi: 10.1159/000489047. [DOI] [PMC free article] [PubMed] [Google Scholar]