Abstract
This study aims to introduce the diagnosis and treatment processes of traumatic splenic bleeding and explain its emergency, medical, interventional, and surgical treatments. Furthermore, this study aims to summarize the indications and contraindications of splenic artery embolization, interventional procedures, and precautions of complications.
Keywords: Consensus, Interventional therapy, Traumatic, Splenic bleeding
1. Background
The special anatomical position and physiological structure of the spleen make it one of the most easily injured organs in the abdominal cavity. Because of the abundant blood sinuses in the spleen, the hemorrhagic shock rate is high due to traumatic splenic bleeding. Timely diagnosis and treatments can improve the survival rate of patients with traumatic splenic bleeding. Surgical treatment is the traditional treatment for traumatic splenic bleeding; however, because the spleen plays an important role in the immune mechanism of the human body, severe infection may occur after splenectomy.1 Identifying a therapeutic method that can effectively stop bleeding and preserve the normal immune function of the spleen is the focus of clinical treatment research on splenic injury.
Sclafani et al. first reported about splenic artery embolization (SAE) to treat traumatic splenic bleeding in 1981. Since then, SAE has been increasingly used to treat splenic injury and its effectiveness and safety have been clinically recognized.2,3 In recent years, the highest success rate of preserving the spleen after SAE was 97%.4 Compared with spleen-preserving surgery, interventional therapy has the following advantages: minimal invasiveness, no required general anesthesia, short operation time, and fast recovery. With the development of interventional medicine, an increasing number of doctors prefer SAE as the first treatment in traumatic splenic bleeding cases. However, for the interventional treatment of traumatic splenic bleeding, various hospitals and doctors vary widely in indications and operation techniques, and a lack of corresponding expert consensus persists domestically and internationally. The Emergency Intervention Committee of the Interventional Physicians Branch of the Chinese Medical Doctor Association and Hemorrhage Professional Committee of the Chinese Research Hospital Association has organized domestic experts to formulate an expert consensus on the interventional treatment of traumatic splenic bleeding through discussion and demonstration based on the principle of evidence-based medicine to provide a reference for the interventional treatment of traumatic splenic bleeding.
2. Diagnosis
2.1. Clinical manifestations5
The clinical manifestations of traumatic splenic injury are mainly characterized by intra-abdominal hemorrhage and peritoneal irritation signs, often closely related to the volume and speed of bleeding. After excessive and rapid hemorrhage, hypovolemic shock immediately follows, causing a critical condition. Patients with a minimal amount of bleeding and at a slow rate experience mild symptoms, with no other obvious symptoms except mild pain in the left upper abdomen that is not easily diagnosed. The amount of bleeding increases concurrently with time; once the symptoms of preshock manifest, shock eventually follows. Delayed hemorrhage can also occur-that is, if a subcapsular hematoma suddenly ruptures-leading to massive blood loss. The blood stimulates the peritoneum and causes abdominal pain, which is most obvious in the left upper abdomen at first, accompanied by tenderness, rebound pain, and abdominal muscle tension. Approximately 85% of splenic injuries that rupture clinically are true ruptures, which are more common on the upper spleen and diaphragmatic surface; sometimes, there are rib fractures in the corresponding parts. If the rupture occurs on the visceral surface, especially those adjacent to the splenic portal, there is a possibility of splenic pedicle tearing, and the amount of bleeding will be huge, shock can quickly occur, and death may result without a prompt rescue. When associated with other abdominal parenchymal organ hemorrhages or hollow organ rupture, the manifestation of bleeding, peritoneal irritation symptoms, and shock will be aggravated correspondingly or appear earlier.
2.2. Imaging examination
Assisting clinicians in the radiographic analysis of splenic injury has immense clinical significance to accurately evaluate the degree of splenic injury and make treatment decisions. The selection should follow the basic principle of “fast, comprehensive, and accurate.” The choice of imaging examination should be based on the hemodynamic condition of the patient, and CT enhancement examination is the gold standard for evaluation.6 For patients in a hemodynamically unstable condition, the implementation of diagnostic imaging examination should be limited as much as possible.
2.2.1. Multiphasic CT examination
If a patient with suspected splenic injury or rupture is in a hemodynamically stable condition or responds to volume resuscitation, consideration should be given to immediate nonenhanced and multiphasic CT examination of the whole abdomen.7 Multiphasic CT examination is performed by intravenous injection of iodine contrast agent (300–350 mg/mL, 90–120 mL) through the elbow vein using a power injector at a rate of 3–5 mL/s. The scanning delay times in the arterial, portal venous, and delayed phases are 25–35 s, 60–70 s and 3–5 min, respectively. The content of CT evaluation should include splenic parenchyma and vascular injuries.8 In addition to the spleen, other solid and hollow organs in the abdominal cavity should be evaluated at the same time.
2.2.1.1. CT grading of traumatic splenic injury
Based on the American Association for the Surgery of Trauma (AAST) splenic injury scale (1994), Marmery et al. described the CT grading system for traumatic splenic injury in 2007. This CT grading system has been widely used since then.9, 10, 11 It has been proven to be better than the AAST splenic injury scale, and some of the spleen injuries with a lower grade have been upgraded to grade Ⅳa or Ⅳb, which reduces the risk of choosing nonoperative treatment and is more helpful for clinical treatment decision-making. In 2018, the AAST updated the organ injury scale of abdominal solid organs. (see Table 1)
Table 1.
CT grading of traumatic splenic injury.12.
| Grade | CT diagnostic criteria |
|---|---|
| I | Subcapsular hematoma < 10% surface area Depth of parenchymal laceration <1 cm Capsular tear |
| II | Subcapsular hematoma = 10%–50% surface area Intraparenchymal hematoma <5 cm Depth of parenchymal laceration = 1–3 cm |
| III | Subcapsular hematoma > 50% surface area Subcapsular or intraparenchymal hematoma ≥5 cm Depth of parenchymal laceration >3 cm |
| IV | With a splenic vascular injury or active bleeding confined within the splenic capsule Parenchymal laceration involving segmental or hilar vessels producing >25% devascularization |
| V | With a splenic vascular injury with active bleeding extending beyond the spleen into the peritoneum Shattered spleen |
The results of imaging evaluation should be combined with the clinical, laboratory, and hemodynamic indexes to ensure that the clinicians choose the best treatment.
2.2.1.2. CT review and follow-up
Repeated CT scanning can be considered for patients with moderate or severe injuries according to the changes in their condition.6 Delayed complications refer to those that occur at least 48 h after splenic injury, including pseudocyst, splenic abscess, pseudoaneurysm, delayed rupture, and rebleeding. When patients have corresponding symptoms, an accurate diagnosis can be made through CT follow-up.
2.2.2. Ultrasonography
Two-dimensional ultrasound and color Doppler flow imaging (CDFI) can be used to quickly diagnose splenic trauma and judge the severity of injury. It can also be used to indirectly diagnose the existence of active bleeding and its speed by observing whether the number of ascites increased and how fast it increased.13
2.2.2.1. Examination technology
In general, a convex array probe with a frequency of 3.5–5 MHz is placed in the 7th–11th rib space between the left axillary front line and posterior axillary line. It is used to scan the left costal area and left abdomen in multiple directions to observe the integrity of the spleen envelope, whether the spleen is swollen, whether the echo in the spleen substance is uniform, or whether there is effusion or weak echo, low echo, mixed echo, or other changes under and around the spleen envelope. At the same time, the operator should note whether there is effusion in the abdominal cavity and the depth and echoes of effusion. Whether there is a blood flow signal in the abnormal echo area under the envelope or in the parenchyma of spleen is also observed by CDFI.14,15
2.2.2.2. Ultrasonographic manifestations of splenic trauma and diagnostic basis
-
(1)
Subcapsular rupture: in mild cases, there is no obvious enlargement of the spleen, the shape may still be regular, the capsule may be complete, and there are spot-like hypoechoic areas in the splenic parenchyma, with different echo intensities and unclear boundaries. In severe cases, the spleen is swollen and deformed, abnormal in appearance; the capsule is still intact, while a fusiform or irregular non- or hypoechoic mass can be seen under the capsule, causing the spleen to be compressed and the surface of capsule to be uneven. There are several nonechoic areas of different sizes in the splenic parenchyma, most of which are crescent shaped, and there may be scattered small weak light spots floating inside. These nonechoic areas do not change with respiratory movement and body position. Point-shape blood signals can be seen around and inside the spleen by CDFI. When there is clot formation in the hematoma, the echo can be seen to be enhanced and further enhanced when accompanied by mechanization.16,17
-
(2)
Hematoma in the splenic parenchyma (central rupture): the volume of the spleen increases; the capsule is still intact; the parenchyma of the spleen is scattered in the areas of no, low, or mixed echo; the boundary is unclear; and the internal echo is uneven. If there are no other organ injuries, there is no obvious free fluid in the abdominal cavity. A small amount of point-shaped blood signals or no flow signal can be seen in the abnormal echo area by CDFI.18
-
(3)
Rupture of the splenic parenchyma (true rupture of spleen): the shape is abnormal, the envelope is irregular or the continuity is interrupted, the area with irregular medium and low or no echo can be seen in the splenic parenchyma, and the irregular mixed echo or slightly strong echo can be accompanied by clot and mechanization. Fluid around the spleen and free fluid in the abdominal cavity are noted. CDFI does not show a blood flow signal in the splenic rupture area.19
2.2.2.3. New ultrasonic technique of splenic trauma diagnosis
Contrast-enhanced ultrasound (CEUS) is a new technology developed in recent years. CEUS can significantly improve the diagnostic accuracy of the location and scope of splenic trauma by conventional ultrasound20 and timely detect a small-scale injury in the early stage to avoid delayed rupture to a large extent.21 The CEUS manifestations of splenic rupture are as follows: absence of subcapsular or parenchymal hematoma and no contrast medium particles entered. The laceration is characterized by irregular linear or cord-like nonechoic areas in the splenic parenchyma and no contrast medium particles entered. The contusion and laceration are manifested in the splenic parenchyma with an unequal echo intensity and irregular nonechoic area in the process of CEUS. Active bleeding is characterized by the accumulation of contrast agent microbubbles in the fissures or overflow of organs.22 In addition, CEUS can directly and accurately diagnose the presence and speed of active bleeding: when the contrast agent microbubbles do not accumulate in the injured area or spill out of the organs, it indicates no active bleeding; by contrast, when the contrast agent microbubbles rapidly accumulate in the injured area or spill out of the organs like fountains, it indicates active bleeding at a fast speed.23
2.3. Laboratory examination
To determine whether the bleeding is under control, routine laboratory examination and blood tests of patients with traumatic splenic bleeding should be monitored dynamically, including hemoglobin, hematocrit, and other indicators.24 Because of the hemodilution and decreased consumption of coagulation factors, the coagulation function of these patients should also be monitored to prevent coagulation disorder by infusing red blood cells, platelets, and coagulation factors in time.25,26
3. Treatment
3.1. Emergency treatment
The principle of emergency treatment for traumatic splenic injury is “save life first, protect spleen second.” Emergency treatment should follow the Advanced Trauma Life Support strategy and technology principally,27 especially for patients with abdominal trauma and shock. For patients with severe shock, two or even three to four infusion channels should be established to perform rapid blood transfusion and fluid replacement. For patients with uncontrolled hemorrhagic shock (with active hemorrhage), blood should be drawn quickly to prepare for the operation in the emergency room. It is recommended to adopt an allowable low-pressure resuscitation strategy in the early stage and the target blood pressure of resuscitation be controlled at 80–90 mmHg (the average arterial pressure is 50–60 mmHg). However, the time of low-pressure resuscitation should not exceed 120 min. If the allowable low-pressure resuscitation time is long, low-temperature (local) auxiliary measures can be used for a short time to reduce the body’s metabolism and protect vital organ functions.28,29 To maintain better hemodynamic parameters, vasoconstrictor drugs such as norepinephrine can be used in small doses30 to extend the golden treatment time window and gain time for definitive treatment.31,32 When the bleeding is controlled and cardiopulmonary function is tolerable, definitive resuscitation is performed to restore the body’s effective blood volume in circulation and stabilize hemodynamics. It should be quickly checked and determined whether active bleeding and shock exacerbation persist; if necessary, the patient should be sent to the operating room or interventional combined operating room for further treatment at the same time of rescue.31,32
To avoid the occurrence of serious complications and reduce the fatality rate, a dynamic evaluation should be performed based on the injury mechanism, posttraumatic clinical manifestations, and auxiliary examinations. Physical examination remains the basis of abdominal trauma assessment. For the subjective peritoneal irritation signs, it is necessary to follow the principle of “multiple inspections by multiple people” to enhance the objectivity.27 Routine emergency treatment includes vital sign, blood oxygen saturation, and electrocardiogram (ECG) monitoring, observation of drainage after nasogastric tube and urinary catheter placement, and focused evaluation of trauma ultrasound (FAST) and diagnostic abdominal puncture. Traumatic FAST ultrasound monitoring is the most effective method to check and monitor whether the abdominal organs are damaged and bleeding; it is simple and fast to operate and can be carried out by emergency and other clinicians after special ultrasound training. Trauma patients can also choose if it is conditioned.33
3.2. Medical treatment
Splenic injuries are mainly substance tears; most of them are between segments perpendicular to the spleen axis. Splenic hilar vascular injuries are rare; most of them are not connected to the intersegmental blood vessels so that bleeding can be stopped spontaneously in a short time. The aforementioned characteristics are the possible basis for the conservative treatment of splenic injury, and the clinical success of many nonsurgical treatments of splenic injury also provides confirmation. Therefore, if possible, the spleen or spleen tissue should be preserved as much as possible.
Grasping the indications strictly is the key to the success of the conservative treatment of splenic injury. However, currently, no uniform standard exists for conservative treatment indications. We believe that the principle of individualized treatment should be followed34: (1) the patient’s hemodynamics is stable, and there is no hemorrhagic shock, or the patient’s hemodynamics can be kept stable with less volume recovery; (2) CT scan of the abdomen suggests AAST grade I or II splenic injury and some grade III splenic injury; (3) other abdominal visceral organ damage is excluded, especially the rupture and perforation of hollow organs or other abdominal organ damage without emergency surgery; (4) the transfusion volume associated with splenic injury is less than 2 U; and (5) there are medical conditions for continuous monitoring and timely transfer to surgery. Conservative treatment is not advocated for patients older than 55 years and with coagulation dysfunction, severe infections, and vital organ failure in principle.
Patients with traumatic splenic injury who meet the aforementioned indications can all receive conservative treatment. However, during this treatment, changes in their hemodynamics and other indicators should be closely monitored, and their monitoring efforts should be even greater than those of surgical patients. The specific conservative treatment methods and efficacy evaluation are as follows: (1) absolute bed rest for more than 1 week and close monitoring of the patient’s vital signs; (2) early fasting, gastrointestinal decompression if necessary, broad-spectrum antibiotics to prevent infection, and other symptomatic support treatments; (3) avoiding the increase in the abdominal pressure, monitoring of abdominal conditions during conservative treatment, dynamic review of blood routine tests, and bedside ultrasound and abdominal CT; (4) liquid diet when the condition is stable for approximately 1 week and if the condition is improved by further treatment, proper out-of-bed activity according to the injury situation after 2 weeks but not strenuous activities within 3 months; and (5) stable hemodynamic, laboratory, and imaging examinations of patients with conservative treatment indicating that the conservative treatment is likely to succeed.
If one of the following conditions is found during the conservative treatment of traumatic splenic injury in time, the patient should be sent for emergency intervention or transfer surgery35: (1) abdominal pain and local peritoneal irritation signs are aggravated persistently; (2) the transfusion volume is > 4 U within 24 h, and the hemodynamics are still unstable; (3) the hematocrit level continues to decline and cannot be quickly corrected by blood transfusion; and (4) other abdominal internal organ damage cannot be excluded by observation. The failure of conservative treatment usually occurs within 96 h, but it is not uncommon to appear within 6–20 days, and the cause of failure may be delayed bleeding and secondary infection.
3.3. Interventional treatment
Interventional treatment for traumatic splenic bleeding includes SAE and nonvascular interventional therapy under image guidance. The main therapy is SAE.
3.3.1. Indications
The indications are as follows: (1) hemodynamically stable and low-grade (AAST-Ⅰ, AAST-Ⅱ) splenic injury15,36, 37, 38, 39, 40, 41 with active splenic bleeding or failure of conservative treatment6,41,42; (2) hemodynamically stable and high-grade (AAST-Ⅲ, AAST-Ⅳ, AAST-Ⅴ) splenic injury6,36,40,41,43, 44, 45, 46, 47, 48; (3) hemodynamically unstable and grade IV47 and some grade V splenic injury48 or SAE or surgical treatment of splenic injury with unstable blood flow according to the risk of surgical intervention, proficiency of interventional technology application, convenience of the equipment, and collaboration of the diagnosis and treatment teams45; (4) splenic injury and hemorrhage in the abdominal cavity (true rupture of the spleen)41,44,46; (5) contrast agent overflow, concentration, pseudoaneurysm, and splenic arteriovenous fistula diagnosed by imaging examination41,44,46; and, (6) for patients with splenic injury who have no surgical treatment conditions or no surgical indications, even if the hemodynamics are unstable, SAE can be implemented simultaneously with active anti-shock therapy.45
3.3.2. Contraindications
There are no absolute contraindications.49 The relative contraindications are as follows: (1) contrast agent allergy50; (2) severe infection or intractable infection49; (3) routine contraindications for endovascular interventions such as severe cardiopulmonary insufficiency and liver or kidney dysfunction50; (4) CT or other imaging examinations reveal splenic fragmentation and splenic hilar vascular rupture (grade V splenic injury); (5) unresponsive with volume recovery41,50 or hemorrhagic shock or critically ill after injury37,49; (6) splenic injury with a primary disease45,50; (7) battle-induced traumatic splenic injury45; (8) cavity organ damage, gastrointestinal perforation,37 or open injury50; (9) severe trauma in another cavity organ damage, such as chest trauma and craniocerebral injury49,50; and (10) other substantial organ injuries, such as liver and kidney ruptures,47 but those that can be treated with the same surgery should not be regarded as a contraindication.
3.3.3. Preoperative preparation
3.3.3.1. Examination and treatment preparation
-
(1)
Routine preoperative preparation: the patient’s basic vital signs, such as blood pressure, heart rate, respiration, body temperature, and pulse rate, should be closely monitored and recorded, and the patient should be kept in a hemodynamically stable condition. The preparation also includes venipuncture and infusion, antibiotic skin test, preparation for blood transfusion, skin preparation, dressing, gastric tube placement, catheterization, abrosia, ECG monitoring, and oxygen inhalation.
-
(2)
Laboratory examination: laboratory examinations, such as liver and kidney functions, blood routine, and coagulation function, should be improved; whether patients have surgical treatment indications should be clarified; and abnormal indicators that may cause serious surgical complications should be promptly corrected.
-
(3)
Imaging examination: the gold standard for diagnosing traumatic splenic bleeding is enhanced CT examination,12 which can clarify the signs, extent, location, presence and absence of active bleeding, and presence of other organ injuries of traumatic splenic injury. According to the AAST grade, it is important to determine whether to choose SAE and which embolization method to use (proximal embolization, distal embolization, proximal + distal embolization). Ultrasound can be used to observe the blood flow information of splenic blood vessels.
-
(4)
Intestinal preparation: for nonemergency surgery, laxatives are used to clean the intestines 1–3 days before surgery to reduce the presence of intestinal flora, which can effectively reduce the chance of postoperative infection.
-
(5)
Preventive use of antibiotics: the spleen is the largest immune organ of the human body, and it is easy to cause stubborn infection after embolization. Because the main infection bacteria are capsular bacteria, such as pneumococci, Neisseria gonorrhoeae, and Haemophilus influenzae, or Gram-negative bacteria, such as Escherichia coli and pseudomonas,51 infection can be prevented before surgery when phenoxymethyl penicillin, amoxicillin, and erythromycin are used.
3.3.3.2. Informed consent
Patients and their families should be fully informed on the necessity of surgery, surgical methods, risks of surgery, and possible postoperative complications. Moreover, the patient or their immediate family or an authorized person should sign the informed consent.
3.3.3.3. Equipment preparation
The digital subtraction angiography (DSA) machine, high-pressure syringe, splenic artery catheter, guide wire, puncture needle, contrast agent, PVA, and gelatin sponge or coil, as well as other embolization materials, should be prepared. If proximal embolization is required, a balloon catheter or vascular plug should also be prepared.
3.3.4. Interventional operation management
3.3.4.1. Anesthesia methods
Interventional embolization for traumatic splenic injury is a minimally invasive operation that has mild irritation and can be performed under local anesthesia in adults. From the perspective of a patient’s comfort, analgesic and sedative drugs can be administered.52 Contraindications for local anesthesia + sedation53 include the following: (1) patients who refuse sedation/general anesthesia, (2) those with an American Society of Anesthesiologists score of V, (3) those in a hemodynamically unstable condition or with respiratory diseases accompanied by uncontrolled symptoms, and (4) those with a history of sedative drug allergy and other serious anesthesia risks. For children and patients with hemodynamic instability/respiratory distress, a laryngeal mask or endotracheal intubation can be used for general anesthesia.
3.3.4.2. Puncture and angiography
The right or left femoral artery is punctured, and an arterial sheath is inserted. The angiography catheter is selectively inserted into the middle and distal parts of the splenic artery for DSA examination. It is recommended to use a low-flow-rate, low-dose, and low-pressure injection of contrast agent under the premise of clear bleeding lesions to prevent fresh blood clots from falling off that may induce or aggravate intraoperative bleeding.54, 55, 56 If low-flow-rate and low-dose contrast agent angiography cannot show the bleeding lesion, the dose can be increased appropriately. The recommended injection rate is 3–5 mL/s, and the total amount is 15–25 mL. The main signs of DSA in traumatic splenic bleeding include54,56 the following: (1) changes in blood vessel orientation: the splenic artery branch is displaced by compression or surrounded by bleeding signs, the peripheral blood vessels are separated, and there is a nonvascular area (contrast agent filling defect) in the splenic parenchyma; (2) splenic parenchymal contusion: local irregular small pieces or spot and mass shadows diffused in the parenchymal stage are noted; sometimes, only small blood vessels are seen with rough edges and continue to the venous stage. (3) hematoma: the central and subcapsular types of hematomas are noted, the arterial phase shows a blood vessel shift, the venous phase shows different forms of the translucent area, vignetting occurs, and the subcapsular type peripheral blood vessels cannot reach the periphery of the spleen. (4) blood ejection sign: contrast agent gushes out from damaged and ruptured blood vessels, which is more common in patients with massive hemorrhage and a dangerous condition; (5) traumatic pseudoaneurysm: a round or quasi-circular capsule convex shadow is seen next to the branch of the splenic artery, with smooth edges. If there is thrombosis in the mass, it shows that the mass is incomplete; (6) interruption of the arterial branch: the branch of the splenic artery is interrupted, and the distal end of the blood vessel may or may not overflow; (7) arteriovenous fistula: the splenic vein is developed early in the arterial phase, and the branches of the splenic artery are insufficiently exposed or not exposed because of the effect of “stealing blood”; (8) rupture of organs: the splenic injury is often associated with other organ injuries, such as the liver, kidney, pancreas, stomach, and intestine; thus, their diagnosis and treatment should also be checked. After SAE, DSA should be performed on the hepatic, renal, and superior and inferior mesenteric arteries according to the condition and preoperative imaging examination.
3.3.4.3. Vascular embolization methods
Principle: according to the AAST classification and hemodynamic stability of the patients, appropriate treatment methods are selected, including conservative treatment, distal SAE, proximal SAE, distal SAE + proximal SAE, and surgery. The aforementioned treatments are all based on a simple splenic injury and do not involve multiorgan injury. (1) If the AAST grade is I or II with no active splenic bleeding, pseudoaneurysm, or traumatic arteriovenous fistula, and the hemodynamics are stable, conservative treatment is feasible. (2) If the AAST grade is I or II, accompanied by active splenic hemorrhage, pseudoaneurysm, or traumatic arteriovenous fistula, distal SAE is feasible. (3) If the AAST grade is III or above, without splenic active bleeding, pseudoaneurysm, or traumatic arteriovenous fistula, proximal SAE is feasible. (4) If the AAST grade is III or above, accompanied by active splenic bleeding, pseudoaneurysm, or traumatic arteriovenous fistula, distal SAE + proximal embolization is feasible. (5) Patients with unstable hemodynamics or those with delayed splenic rupture should undergo emergency surgery or surgical splenectomy/partial splenectomy.
Proximal embolization: the precise position of the proximal embolization of the splenic artery should be between the dorsal pancreatic and great pancreatic arteries, and coil embolization is routinely used (including controlled coil embolization). Because the blood flow of the splenic artery is faster and the flow is larger, the coil is displaced along the blood flow, resulting in ectopic embolization. The following two methods are recommended for proximal SAE: vascular embolization and balloon-assisted coil spleen embolization of the proximal artery.
Distal embolization: the catheter is superselectively inserted into the responsible blood vessel (the microcatheter is applicable if necessary), and then the microcoil, gelatin sponge, PVA particles or microspheres are selected according to the bleeding site. The embolization particles should be mixed with an appropriate amount of contrast agent and slowly injected under fluoroscopy. Embolization particles with a diameter of 700–1000 μm are recommended for rupture below the splenic artery, and those with a diameter greater than 1000 μm and/or microcoil are recommended for rupture of the splenic artery.46,54,55,57,58 If the blood flow of the branch of the embolized artery is slow, splenic artery DSA is performed again to observe the hemostatic effect. Bleeding patients are still embolized again until the signs of bleeding disappear. Next, intubation to the common hepatic, renal, and superior and inferior mesenteric arteries for DSA is performed, other organ damage is observed, and the corresponding processing is conducted.54,55
3.3.4.4. Abdominal drainage and autologous blood transfusion
For drainage after embolization, CT- or ultrasound-guided puncture should be performed. The location where the hemorrhage has accumulated is determined. After local anesthesia, a guide wire is inserted into the abdomen or pelvic cavity through a needle, and the porous drainage tube is inbounded along the guide wire to drain the peritoneal hemorrhage.54 Patients with simple traumatic splenic injury (not associated with other abdominal cavity organ damage) can salvage blood by puncturing the drainage tube.59 The drainage tube is connected to the cell salvage device to collect, hemagglutinate, wash, and filter the blood and then transfuse it back into the patient.60 Qualified equipment is essential for cell salvage, and the autologous blood should meet certain quality standards. A separate sterile suction tube is required for cell salvage during application, and a leukocyte or micropolymer filter is needed during reinfusion.61,62 The contraindications for autologous transfusion are as follows63: (1) the blood flows out of the blood vessel for more than 6 h; (2) the blood is suspected to contain cancer cells; (3) the blood is suspected to be contaminated with bacteria and feces; and (4) the blood is severely hemolyzed. (5) In emergencies, when used in combination with a leukocyte filter, the indications can be appropriately broadened.
3.3.5. Postoperative treatment
3.3.5.1. Postoperative routine management
After SAE, anti-infection, abrosia, and fluid replacement are routinely required. Abdominal pain can be treated using a painkiller when other causes of abdominal pain are excluded. Low-molecular-weight heparin can be used to prevent portal vein thrombosis without anticoagulation contraindications. Severe patients require rigorous ECG monitoring and circulatory support treatment. If the condition worsens, they should be reintervened or surgically treated in time.
3.3.5.2. Management of complications
Due to the location of the splenic artery and its vascular conditions and the choice of embolization method, a series of complications may occur after surgery. The complications after SEA need to be identified and treated in time. If they are not handled properly, serious consequences and even death may occur.
3.3.5.2.1. Postembolization syndrome
The most common complication after SAE is postembolization syndrome, which has a high incidence and can be manifested as abdominal pain, bloating, nausea, vomiting, and intermittent fever.64 The causes for consideration are as follows: ischemic and necrotic spleen tissue after SAE; swelling of the spleen; a tense capsule; the release of prostaglandin E2, interleukin, and other inflammatory mediators and endogenous pyrogens; and paroxysmal spasm of the diaphragm caused by the diaphragmatic or phrenic nerves.65 Abdominal pain is mostly located in the left upper abdomen, and the size of the embolized area is directly related to the degree of pain. If the embolization area is less than 40%, the pain is lighter. By contrast, if the embolization area is more than 80%, the pain is more serious. It is recommended to control the embolization area to be less than 70%, preferably within 50%.66 Generally, mild abdominal pain can be treated symptomatically. If there is severe abdominal pain, bucinnazine hydrochloride and morphine can be administered to relieve pain, and an anesthesia analgesia pump can also be provided. Patients with abdominal distension, nausea, and vomiting can be treated with abrosia and rehydration. Fever generally occurs approximately 3 days after embolization, mostly due to spleen tissue necrosis and hematoma absorption, and physical hypothermia can be taken. Patients with splenic injury and hemorrhage should be monitored for splenic abscess and intraperitoneal infection. Antibiotics can be used to prevent infection.
3.3.5.2.2. Severe infection
Severe infection is a serious complication, which includes peritonitis and splenic abscess. It is related to the large area of splenic embolism and impaired immune function. Poor aseptic treatment during the operation and translocation infection of intestinal anaerobic bacteria may also be the causes of infection. Some experts use the method of pushing antibiotics into the splenic artery during embolization to prevent postoperative splenic abscesses.67 Postoperative antibiotic treatment is also necessary. In the case of serious infection, puncture or drainage can be performed if necessary, and some patients require surgical treatment.68,69
3.3.5.2.3. Pulmonary complications
Pulmonary complications after SAE include pneumonia, atelectasis, and pleural effusion. These complications usually appear on the left side and is related to the anatomical position of the spleen. In particular, after embolization of the upper pole of the spleen, due to increased pain in the left upper abdomen, restricted breathing, pleural inflammation, and insufficient lymphatic drainage, the exudate increases, causing lung infection, atelectasis, and pleural effusion. Pneumonia and mild to moderate pleural effusion can be absorbed after effective antibiotics and analgesic treatment. Thoracentesis and drainage treatment are needed with massive pleural effusion. Embolization of the middle and lower poles of the splenic artery in SAE can reduce the incidence of these complications.68,69
3.3.5.2.4. Ectopic embolism
The unexpected embolization of the pancreatic arteries and use of contrast agents may lead to pancreatitis68 and even rare intestinal blood flow disorders, acute intestinal obstruction, and acute peritonitis. These conditions can usually be alleviated by conservative treatments such as abrosia, anti-infection, and fluid replacement. However, for the sudden increase in abdominal pain, accompanied by symptoms of total abdominal and rebound tenderness, attention should be given to the occurrence of tissue and organ necrosis, suppurative pancreatitis, and intestinal perforation caused by ectopic embolism, which require timely surgical treatment.
3.3.5.2.5. Portal vein thrombosis
After SAE, the portal vein blood flow decreases, and the platelet count rapidly increases, especially if the embolization area is too large, leading to the high coagulation state of the portal vein blood and then portal vein thrombosis.68,69 If there are no active bleeding and no anticoagulation contraindications, low-molecular-weight heparin or oral anticoagulation drugs can be administered for anticoagulation.
3.3.5.2.6. Bleeding and splenic rupture
Active bleeding may still occur after SAE. First, whether there are other organ injuries must be determined. Second, splenic vein tear bleeding caused by splenic injury is also a possible reason. In some patients, trauma or increased activity can cause the spleen to rupture and bleed again. In these cases, timely surgical treatment should be considered.68,69
3.3.6. Therapeutic effect evaluation
The results and outcomes of interventional therapy for traumatic splenic bleeding include the following. (1) Cure: after treatment, the symptoms and signs disappear, bleeding stops, and no complications occur. (2) Improvement: after treatment, the general condition improves, bleeding stops, and complications occur but are cured after symptomatic treatment. (3) Unhealed: after interventional surgery, the spleen is still bleeding, and hematoma secondary to infection, subphrenic abscess and other complications need to be treated by second-stage surgery. Studies have shown that the success rate of SAE in treating traumatic splenic injury is more than 90%.48
3.4. Nonvascular interventional therapy
Nonvascular intervention therapy includes ultrasound- and CT-guided interventional therapies, mainly the former therapy. Ultrasound-guided microwave ablation hemostasis70 and radiofrequency ablation hemostasis71 have been applied for the hemostasis of traumatic splenic bleeding. In addition, ultrasound combined with microbubble cavitation treatment technology is expected to become a new noninvasive treatment of traumatic splenic bleeding in the future.72
3.5. Surgical treatment
Surgical operation is one of the most effective treatment methods for splenic trauma. The key points of surgical treatment for splenic trauma include the following: rapid and comprehensive diagnosis of the traumatic condition, identification of the operative indication, rational arrangement of the order, use of the appropriate surgical method, and implementation of individual treatment. Total splenectomy is the fundamental surgical method; however, it is associated with major postoperative complications and impaired human immune function.73 Various effective spleen-preserving operations and self splenic tablet transplantation can retain a part of the spleen tissue to preserve the splenic function.74 Laparoscopic surgery is a minimally invasive method for the diagnosis and treatment of splenic trauma and is consistent with the principle of damage control surgery.75 In clinical work, we shall follow the principles of “rescuing life first, retaining spleen second” and “controlling injury”.15 However, the spleen should be removed without hesitation if necessary to avoid serious consequences caused by an increase in blood loss. Moreover, appropriate spleen-preserving surgery and interventional embolization can be chosen to treat splenic trauma according to the condition of equipment and surgeon experience if there are no serious associated injuries and the degree of splenic trauma is low.
Expert consensus steering committee
Yimin Zhu (Hunan Provincial Health Commission), Hua Xiang (Hunan Provincial People’s Hospital), Jinshu Wu (Hunan Provincial People’s Hospital), Gaojun Teng (The Affiliated Zhongda Hospital of Southeast University), Chuanzhu Lv (Hainan Medical College), Yuguo Chen (Qilu Hospital of Shandong University), Ke Xu (The First Affiliated Hospital of China Medical University), Xuezhong Yu (Peking Union Medical College Hospital), Guoqiang Zhang (China-Japan Friendship Hospital), Mao Zhang (The Second Affiliated Hospital of Zhejiang University), Huadong Zhu (Peking Union Medical College Hospital), Xiaoming Zhang (People’s Hospital of Peking University), Hong Shan (The Fifth Affiliated Hospital of Sun Yat-sen University), Yingsheng Cheng (The East Hospital of Shanghai Sixth People’s Hospital), and Yinghua Zou (The First Hospital of Peking University).
Members of the expert consensus committee (last name Pinyin sort)
Youde Cao (Hunan Provincial People’s Hospital), Wei Cheng (Hunan Provincial People’s Hospital), Jianping Gu (Nanjing First Hospital), Yuming Gu (The Affiliated Hospital of Xuzhou Medical University), Jinhe Guo (The Affiliated Zhongda Hospital of Southeast University), Guohong Han (Xijing Hospital), Xiaotong Han (Hunan Provincial People’s Hospital), Xinwei Han (The First Affiliated Hospital of Zhengzhou University), Xiaofeng He (Southern Hospital of Southern Medical University), Jiansong Ji (Lishui Central Hospital, Zhejiang Province), Bo Jiang (Hunan Provincial People’s Hospital), Hailiang Li (Henan Cancer Hospital), Maoquan Li (Shanghai Tenth People’s Hospital), Tianxiao Li (Henan Provincial People’s Hospital), Xiao Li (Cancer Hospital of Chinese Academy of Medical Sciences), Ligong Lu (Zhuhai People’s Hospital), Jitong Liu (Hunan Provincial People’s Hospital), Peng Liu (Hunan Provincial People’s Hospital), Rongguang Luo (The First Affiliated Hospital of Nanchang University), Weifu Lv (Anhui Provincial Hospital), Caifang Ni (The First Affiliated Hospital of Suzhou University), Haibin Shi (Jiangsu Provincial People’s Hospital), Jingchun Song (The Great Wall Hospital Affiliated to Nanchang University), Wei Song (Hainan Provincial People’s Hospital), Zhenju Song (Zhongshan Hospital Affiliated to Fudan University), Feng Wang (The First Affiliated Hospital of Dalian Medical University), Gang Wang (The Second Affiliated Hospital of Xi’an Jiaotong University), Maoqiang Wang (General Hospital of Chinese People’s Liberation Army), Yi Wang (The Third Affiliated Hospital of Army Military Medical University), An Wei (Hunan Provincial People’s Hospital), Zhongmin Wang (Ruijin Hospital Affiliated to Medical College of Shanghai Jiaotong University), Hao Xu (The Affiliated Hospital of Xuzhou Medical University), Shuogui Xu (The Affiliated Changhai Hospital of Naval Medical University), Zhiping Yan (Zhongshan Hospital Affiliated to Fudan University), Weizhu Yang (The Affiliated Union Hospital of Fujian Medical University), Yefa Yang (Oriental Hepatobiliary Surgery Hospital of Naval Medical University), Shuiting Zhai (Henan Provincial People’s Hospital), Jianfeng Zhang (The Second Affiliated Hospital of Guangxi Medical University), Lin Zhang (Beijing Tsinghua Chang Gung Memorial Hospital Affiliated to Tsinghua University), Yi Zhang (Hunan Provincial People’s Hospital), Zengren Zhao (The First Hospital of Hebei Medical University), Chuansheng Zheng (Union Hospital Affiliated to Tongji Medical College, Huazhong University of Science and Technology), Hongshan Zhong (The First Affiliated Hospital of China Medical University), Shi Zhou (The Affiliated Hospital of Guiyang Medical College), and Hui Zhu (The Second Xiangya Hospital of Central South University).
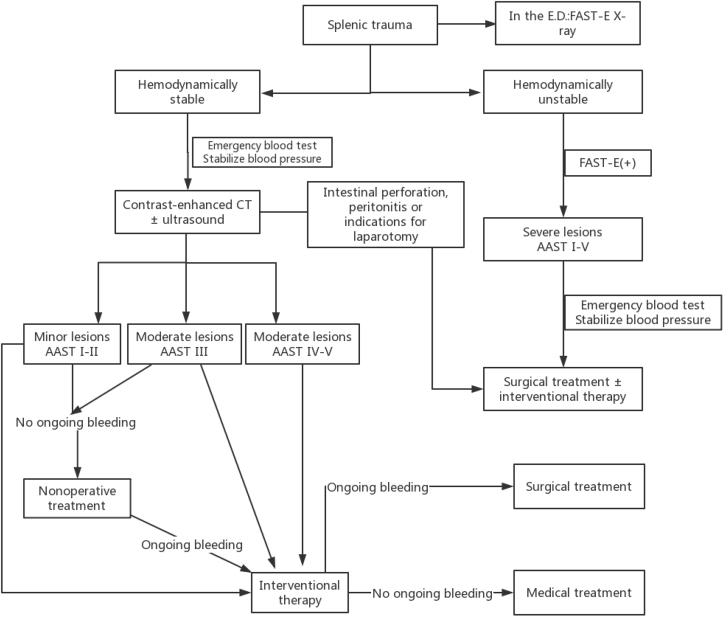
Splenic trauma management algorithm
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Huangxing Cai, Email: 120525151@qq.com.
Lingyun Zhang, Email: 58368287@qq.com.
Hua Xiang, Email: vipxiangh@163.com.
References
- 1.Rosling M., Trenker C., Neesse A. Spontaneous and traumatic splenic rupture: retrospective clinical, B-Mode and CEUS analysis in 62 patients. Ultrasound Int Open. 2018;4:E30–E34. doi: 10.1055/s-0043-125311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ekeh A.P., Khalaf S., Ilyas S. Complications arising from splenic artery embolization: a review of an 11-year experience. Am J Surg. 2013;205:250–254. doi: 10.1016/j.amjsurg.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Ming Hua, Yang Hongyun, Cao Chongqi. Significance of selective splenic artery embolization in non-surgical treatment of traumatic splenic rupture. J Nan Jing Med Univ. 2019;39:1207–1210. [Google Scholar]
- 4.Schnuriger B., Inaba K., Konstantinidis A. Outcomes of proximal versus distal splenic artery embolization after trauma: a systematic review and meta-analysis. J Trauma. 2011;70:252–260. doi: 10.1097/TA.0b013e3181f2a92e. [DOI] [PubMed] [Google Scholar]
- 5.Wang Jianping, Zhao Jizong, Chen Xiaoping. People’s Health Press; 2018. Surgery Ninth Edition; p. 323. [Google Scholar]
- 6.Coccolini F., Montori G., Catena F. Splenic Trauma: WSES classification and guidelines for adult and pediatric patients. World J Emerg Surg. 2017;12:40. doi: 10.1186/s13017-017-0151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marmery H., Shanmuganathan K., Alexander M.T. Optimization of selection for nonoperative management of blunt splenic injury: comparison of MDCT grading systems. AJR Am J Roentgenol. 2007;189:1421–1427. doi: 10.2214/AJR.07.2152. [DOI] [PubMed] [Google Scholar]
- 8.Shi H., Tech W.C., Chin F.W.K. CT of blunt splenic injuries: what the trauma team wants to know from the radiologist. Clin Radiol. 2019;74:903–911. doi: 10.1016/j.crad.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Adibi A., Ferasat F., Baradaran Mahdavi M.M. Assessment of blunt splenic trauma: which imaging scoring system is superior? J Res Med Sci. 2018;23:29. doi: 10.4103/jrms.JRMS_875_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saksobhavivat N., Shanmuganathan K., Chen H.H. Blunt splenic injury: use of a multidetector CT-based splenic injury grading system and clinical parameters for triage of patients at admission. Radiology. 2015;274:702–711. doi: 10.1148/radiol.14141060. [DOI] [PubMed] [Google Scholar]
- 11.Boscak A., Shanmuganathan K. Splenic trauma: what is new? Radiol Clin North Am. 2012;50:105–122. doi: 10.1016/j.rcl.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Kozar R.A., Crandall M., Shanmuganathan K. Organ injury scaling 2018 update: spleen, liver, and kidney. J Trauma Acute Care Surg. 2018;85:1119–1122. doi: 10.1097/TA.0000000000002058. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Xiancun, Wang Ming, Ou Guangchao. Diagnostic value of contrast-enhanced ultrasonography in abdominal parenchymatous organ injury. Chin J Gen Pract. 2014;12:434–436. [Google Scholar]
- 14.Lin Peiren. Diagnosis and research of emergency ultrasound in blunt abdominal trauma with visceral rupture and hemorrhage. J Mod Clin Med. 2019;45:364–365. [Google Scholar]
- 15.Wang Qian, Jiang Hongchi, Li Zongfang. Group of splenic function and spleen surgery, Chinese society of surgery, Chinese medical association. Expert consensus on treatment methods for spleen injury (2014) J Clin Hepatol. 2015;31:1002–1003. [Google Scholar]
- 16.Yu Hua, Han Xiaoli. Signs and value of color Doppler ultrasound in diagnosis of splenic rupture. Clin Med Res Pract. 2019;4:136–138. [Google Scholar]
- 17.Lv F., Ning Y., Zhou X. Effectiveness of contrast-enhanced ultrasound in the classification and emergency management of abdominal trauma. Eur Radiol. 2014;24:2640–2648. doi: 10.1007/s00330-014-3232-8. [DOI] [PubMed] [Google Scholar]
- 18.Riezzo I., Di Battista B., De Salvia A. Delayed splenic rupture: dating the sub-capsular hemorrhage as a useful task to evaluate causal relationships with trauma. Forensic Sci Int. 2014;234:64–71. doi: 10.1016/j.forsciint.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 19.Luo Xiaomin, Wang Dongfeng, Wei Jie. Application of focused assessment with sonography for trauma in emergency treatment of chest and abdominal injuries. J Trauma Surg. 2015;17:181–184. [Google Scholar]
- 20.Lv Faqin, Tang Jie, Luo Yukun. The contrast-enhanced ultrasound for classification treatment of trauma of abdominal parenchymal organs. Chin J Med Ultrasound (Electron Ed) 2009;6:25–30. [Google Scholar]
- 21.Liu J., Feng Y., Li A. Diagnosis and treatment of atraumatic splenic rupture: experience of 8 cases. Gastroenterol Res Pract. 2019;2019:5827694. doi: 10.1155/2019/5827694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durkin N., Deganello A., Sellars M.E. Post-traumatic liver and splenic pseudoaneurysms in children: diagnosis, management, and follow-up screening using contrast enhanced ultrasound (CEUS) J Pediatr Surg. 2016;51:289–292. doi: 10.1016/j.jpedsurg.2015.10.074. [DOI] [PubMed] [Google Scholar]
- 23.Menichini G., Sessa B., Trinci M. Accuracy of contrast-enhanced ultrasound (CEUS) in the identification and characterization of traumatic solid organ lesions in children: a retrospective comparison with baseline US and CE-MDCT. Radiol Med. 2015;120:989–1001. doi: 10.1007/s11547-015-0535-z. [DOI] [PubMed] [Google Scholar]
- 24.El-Matbouly M., Jabbour G., El-Menyar A. Blunt splenic trauma: assessment, management and outcomes. Surgeon. 2016;14:52–58. doi: 10.1016/j.surge.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Cosgriff N., Moore E.E., Sauaia A. Predicting life-threatening coagulopathy in the massively transfused trauma patient: hypothermia and acidoses revisited. J Trauma. 1997;42:857–862. doi: 10.1097/00005373-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Ferrara A., MacArthur J.D., Wright H.K. Hypothermia and acidosis worsen coagulopathy in the patient requiring massive transfusion. Am J Surg. 1990;160:515–518. doi: 10.1016/s0002-9610(05)81018-9. [DOI] [PubMed] [Google Scholar]
- 27.Special committee on trauma first aid and multiple injuries of the trauma branch of the Chinese medical association, special committee of multiple injured Physicians of the trauma surgeon branch of the Chinese medical doctor association. Expert consensus on laparoscopic diagnosis and treatment of abdominal trauma. Chin J Traumatol. 2016;32:493–496. [Google Scholar]
- 28.Butler F.K., Jr. Fluid resuscitation in tactical combat casualty care: yesterday and today. Wilderness Environ Med. 2017;28:S74–S81. doi: 10.1016/j.wem.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Hamada S.R., Gauss T., Pann J. European trauma guideline compliance assessment: the ETRAUSS study. Crit Care. 2015;19:423. doi: 10.1186/s13054-015-1092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Haren R.M., Thorson C.M., Valle E.J. Vasopressor use during emergency trauma surgery. Am Surg. 2014;80:472–478. [PubMed] [Google Scholar]
- 31.Spahn D.R., Bouillon B., Cerny V. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care. 2013;17:R76. doi: 10.1186/cc12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carrick M.M., Morrison C.A., Tapia N.M. Intraoperative hypotensive resuscitation for patients undergoing laparotomy or thoracotomy for trauma: early termination of a randomized prospective clinical trial. J Trauma Acute Care Surg. 2016;80:886–896. doi: 10.1097/TA.0000000000001044. [DOI] [PubMed] [Google Scholar]
- 33.Ghafouri H.B., Zare M., Bazrafshan A. Diagnostic accuracy of emergency-performed focused assessment with sonography for trauma (FAST) in blunt abdominal trauma. Electron Physician. 2016;8:2950–2953. doi: 10.19082/2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Yong, Shang Chao, Hu Jianping. Analysis of conservative treatment for traumatic splenic rupture. Heilongjiang Med J. 2018;31:1383–1385. [Google Scholar]
- 35.Wu Zhaohan, Qin Xinyu, Ding Qiang. forth ed. People’s Health Press; 2017. Practical Surgery; pp. 415–416. [Google Scholar]
- 36.Ou Hui, Lao Jingmao. Clinical evaluation of different surgical methods for traumatic splenic rupture. Chin Med Guidel. 2019;17:110–111. [Google Scholar]
- 37.Zheng Kun, Zheng Xiaohan, Pang Wenyong. Clinical analysis of 22 cases of traumatic splenic rupture treated by interventional therapy. Guizhou Med. 2013;37:535–536. [Google Scholar]
- 38.Wu Ruike, Chen Xinguo, Xia Li. Analysis of the efficacy of selective splenic artery embolization in the treatment of traumatic splenic rupture with shock. ZH J J Traumatic. 2019;24:472–473. [Google Scholar]
- 39.Muroya T., Ogura H., Shimizu K. Delayed formation of splenic pseudoaneurysm following nonoperative management in blunt splenic injury: multi-institutional study in Osaka. J Trauma Acute Care Surg. 2013;75:417–420. doi: 10.1097/TA.0b013e31829fda77. [DOI] [PubMed] [Google Scholar]
- 40.Stassen N.A., Bhullar I., Cheng J.D. Selective nonoperative management of blunt splenic injury: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg. 2012;73:S294–S300. doi: 10.1097/TA.0b013e3182702afc. [DOI] [PubMed] [Google Scholar]
- 41.Rowell S.E., Biffl W.L., Brasel K. Western trauma association critical decisions in trauma: management of adult blunt splenic trauma-2016 updates. J Trauma Acute Care Surg. 2017;82:787–793. doi: 10.1097/TA.0000000000001323. [DOI] [PubMed] [Google Scholar]
- 42.Corn S., Reyes J., Helmer S.D. Outcomes following blunt traumatic splenic Injury treated with conservative or operative management. Kans J Med. 2019;12:83–88. [PMC free article] [PubMed] [Google Scholar]
- 43.Miller P.R., Chang M.C., Hoth J.J. Prospective trial of angiography and embolization for all grade III to V blunt splenic injuries: nonoperative management success rate is significantly improved. J Am Coll Surg. 2014;218:644–648. doi: 10.1016/j.jamcollsurg.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 44.Salottolo K., Carrick M.M., Madaya R.M. Predictors of splenic artery embolization as an adjunct to non-operative management of stable blunt splenic injury: a multi-institutional study. Trauma Surg Acute Care Open. 2019;4 doi: 10.1136/tsaco-2019-000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang Zhongfei, Qiang Li, Wang Maoqiang. Application of vascular interventional therapy in splenic rupture and hemorrhage. Chin J Clin (Electron Ed) 2016;10:2039–2043. [Google Scholar]
- 46.Ahuja C., Farsad K., Chadha M. An overview of splenic embolization. AJR Am J Roentgenol. 2015;205:720–725. doi: 10.2214/AJR.15.14637. [DOI] [PubMed] [Google Scholar]
- 47.Lin W.C., Chen Y.F., Lin C.H. Emergent transcatheter arterial embolization in hemodynamically unstable patients with blunt splenic injury. Acad Radiol. 2008;15:201–208. doi: 10.1016/j.acra.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 48.Hagiwara A., Fukushima H., Murata A. Blunt splenic injury: usefulness of transcatheter arterial embolization in patients with a transient response to fluid resuscitation. Radiology. 2005;235:57–64. doi: 10.1148/radiol.2351031132. [DOI] [PubMed] [Google Scholar]
- 49.Han Xinwei. Zhengzhou University Press; 2019. Interventional Medicine [M] pp. 374–453. [Google Scholar]
- 50.Yang Renjie, Li Wenhua. People’s Health Press; 2016. Clinical Emergency Interventional Therapy [M] pp. 433–437. [Google Scholar]
- 51.Luu S., Spelman D., Woolley I.J. Post-splenectomy sepsis: preventative strategies, challenges, and solutions. Infect Drug Resist. 2019;12:2839–2851. doi: 10.2147/IDR.S179902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu P., Harmon D., Frizelle H. Patient comfort during regional anesthesia. J Clin Anesth. 2007;19:67–74. doi: 10.1016/j.jclinane.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 53.American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004–1017. doi: 10.1097/00000542-200204000-00031. [DOI] [PubMed] [Google Scholar]
- 54.Lv Weifu, Zhang Xuebin, Zhang Xingming. An analysis of the clinical efficacy and experience on the management of traumatic splenic rupture by partial splenic embolization. Chin J Radiol. 2003;37:1092–1096. [Google Scholar]
- 55.Long Li, Chen Yong, Shen Liping. Clinical study of partial splenic embolization for traumatic splenic rupture. Chin J Surg. 2005;43:595–596. [Google Scholar]
- 56.Yang Qiuhong, Lv Weifu, Zhao Yingming. Interventional treatment of traumatic splenic rupture. J Med Imaging. 2003;13:912–914. [Google Scholar]
- 57.Rong J.J., Liu D., Liang M. The impacts of different embolization techniques on splenic artery embolization for blunt splenic injury: a systematic review and meta-analysis. Mil Med Res. 2017;4:17. doi: 10.1186/s40779-017-0125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Zhengran, Shan Hong, Zhu Kangshun. Clinical quantitative study of therapeutic effect of partial splenic embolization (PSE) on portal vein hemodynamics. Chin J Radiol. 2002;36:913–917. [Google Scholar]
- 59.Emergency physicians branch of Chinese Medical Association Expert consensus on emergency transfusion in special cases. China Emerg Med. 2013;33:481–483. [Google Scholar]
- 60.British Committee for Standards in Haematology, Stainsby D., Maclennan S. Guidelines on the management of massive blood loss. Br J Haematol. 2006;135:634–641. doi: 10.1111/j.1365-2141.2006.06355.x. [DOI] [PubMed] [Google Scholar]
- 61.Carson J.L., Grossman B.J., Kleinman S. Red blood cell transfusion: a clinical practice guideline from the AABB∗. Ann Intern Med. 2012;157:49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 62.Ashworth A., Klein A.A. Cell salvage as part of a blood conservation strategy in anaesthesia. Br J Anaesth. 2010;105:401–416. doi: 10.1093/bja/aeq244. [DOI] [PubMed] [Google Scholar]
- 63.Clinical transfusion committee of China transfusion Association Expert consensus on clinical pathway management of autotransfusion (2019) J Clin Hematol. 2019;32:81–86. [Google Scholar]
- 64.Shah R., Mahour G.H., Ford E.G. Partial splenic embolization. An effective alternative to splenectomy for hypersplenism. Am Surg. 1990;56:774–777. [PubMed] [Google Scholar]
- 65.Watanabe Y., Todani T., Noda T. Changes in splenic volume after partial splenic embolization in children. J Pediatr Surg. 1996;31:241–244. doi: 10.1016/s0022-3468(96)90006-8. [DOI] [PubMed] [Google Scholar]
- 66.Qin Wei, Fuhua Ji, Li Yanzhuo. Application of partial splenic artery embolization in treating hypersplenism and prevention of complications. J China Clin Med Imaging. 2014;25:206–209. [Google Scholar]
- 67.Liu Dongbo, Yang Zhi, Zhang Lina. Progression treatment of traumatic splenic rupture by splenic arterial embolization. J Intervent Radiol. 2007;16:645–647. [Google Scholar]
- 68.Piffaretti G., Tozzi M., Lomazzi C. Splenic artery aneurysms: postembolization syndrome and surgical complications. Am J Surg. 2007;193:166–170. doi: 10.1016/j.amjsurg.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 69.Guan Y.S., Hu Y. Clinical application of partial splenic embolization. Sci World J. 2014;2014:961345. doi: 10.1155/2014/961345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang G., Sun Y., Yu J. Microwave coagulation therapy and drug injection to treat splenic injury. J Surg Res. 2014;186:226–233. doi: 10.1016/j.jss.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 71.Yi Li, Zhang Wei, Tian Yinsheng. The clinical application of radio frequency ablation in the operation of preserving spleen after traumatic rupture of spleen. Chin J Hepatobiliary Surg. 2013;19:67–69. [Google Scholar]
- 72.Zhong Yu, Sun Danning, Qu Wencai. Efficacy of different peak negative pressure pulse ultrasounds combined with microvesicles on splenic trauma hemostasis. Southwest Nat Defense Med. 2016;26:1422–1425. [Google Scholar]
- 73.Liu Zeliang, Zhu Jianfang, He Wei. The treatment experience of 147 cases of splenic trauma. J Hepatopancreatobiliary Surg. 2016;28:140–142. [Google Scholar]
- 74.Xu Wulin, Vahav Alemujiang, Toldi Wusman. The diagnosis and treatment experience of 18 cases of splenic trauma. World Latest Med Inf. 2018;18:32. [Google Scholar]
- 75.Jiang G.Q., Bai D.S., Chen P. Predictors of portal vein system thrombosis after laparoscopic splenectomy and azygoportal disconnection: a Retrospective Cohort Study of 75 Consecutive Patients with 3-months follow-up. Int J Surg. 2016;30:143–149. doi: 10.1016/j.ijsu.2016.04.047. [DOI] [PubMed] [Google Scholar]