Abstract
Aim
To evaluate the diagnosis and treatment strategies for the iliac vein compression syndrome (IVCS) and the factors that affect the treatment outcome.
Methods
In total, 69 patients with IVCS were enrolled in the study. The patients underwent computed tomography (CT) venography before treatment. CT observations included assessment of the iliac venous channel sagittal diameter (IVCD) before the lower lumbar vertebra, causes of oppression, thrombus density, and embolization range. The patients with IVCS were divided into the simple IVCS (sIVCS, n = 22), lumbar degeneration-related type IVCS (dIVCS, n = 33), and IVCS of other causes (oIVCS, n = 14) including lumbar fracture, hematoma of infection, and abscess wraping around and compressing the iliac vein, groups. The treatment methods included target venous catheter-directed thrombolysis (CDT), a mechanical breaking and sucking treatment for the thrombi, followed by balloon dilatation and iliac vein stent implantation. The factors that may possibly affect the treatment outcomes included IVCS type, duration of disease, thrombus hardness, embolization length, and treatment regimen. Logistic regression was used to analyze the factors that affected the therapeutic efficacy.
Results
At the first stage, CDT was only effective in 15 cases (5 dIVCSs and 10 oIVCSs) and was ineffective in the remaining 54 cases, which required further mechanical breaking and sucking of the thrombi and intravenous balloon dilatation. In the second stage, combination of thrombi breaking and suction and balloon dilatation was preliminarily effective in 26 cases (6 sIVCSs, 16 dIVCSs and 4 oIVCSs), but during follow-up from 1 to 6 months, treatment was considered futile for 9 recurrent cases (3 sIVCSs and 6 dIVCSs). So, 28 cases of preliminary ineffective treatment and 9 relapse in the second stage were arranged to the third stage of treatment by iliac vein stent implantation. All 37 cases were treated effectively and achieved a satisfactory iliac vein patency, and were followed-up for 24 months without recurrence. Logistic regression analysis showed that IVCS type (β = 4.14; Wald test, P < 0.01), duration of illness (β = -5.33; Wald test, P = 0.02), thrombus density (β = -6.46; Wald test, P = 0.01), embolization length (β = 2.74; Wald test, P = 0.03), and treatment regimens (β = 11.92; Wald test, P = 0.01) all had a significant effect on the treatment outcomes.
Conclusion
The selection of a suitable intervention treatment regimen for different types of IVCS may aid in improving the curative effect.
Keywords: Iliac vein compression syndrome, Radiation intervention, Efficacy, Influencing factors
Introduction
Iliac vein compression syndrome (IVCS) is a severe deep vein thrombosis with a complex pathophysiology. IVCS occurs when the left iliac vein, passing from right to left through the narrow space between the right iliac artery and lower lumbar vertebra, is compressed by the right iliac artery, leading to long-term pulsative compression. Chronic thrombosis occurs in the lumen of the vein, as well as pelvic congestion and the formation of collateral circulation; this is followed by left lower extremity deep vein thrombosis, lower extremity swelling, pain, and a series of acute clinical manifestations.1 The placement of a vena cava filter and iliac vein stent, together with assisted intravascular thrombolysis is currently recognized as the first line treatment method2; however, the efficacy of treatment is variable, with results including both successful iliac vein recanalization and embolism recurrence.3 This article examines the pathways of IVCS diagnosis and treatments, as well as the factors that influence the treatment outcome. The ultimate aim of this study is to emphasize the accurate preoperative assessment of IVCS and to optimize the treatment strategy based on accurate diagnosis.
1. Materials and methods
1.1. General information
From July 2009 to June 2014, 69 patients with IVCS were enrolled; the patients included 25 males and 44 females, aged 24–86 years (median age, 53 years). The clinical manifestations were left lower limb swelling and progressively worsening pain for 1 day to 6 weeks. The inclusion criteria were as follows: clear clinical and imaging-based diagnosis of IVCS, with no limitation with respect to sex and age; clinical and imaging (ultrasound, CT, and DSA) data were complete; and treatment and results of follow-up are traceable. The exclusion criteria were as follows:lower extremity fractures, pregnancy, lower extremity venous lesions, abdominal or retroperitoneal space lesions, and IVCS caused by non-left iliac vein compression; treatment was incomplete or with a follow-up of less than 24 months.
The patients were divided into the following three types based on the cause of IVCS as determined by CT:
sIVCS (simple type IVCS), the iliac vein was compressed only by the iliac artery in front of the vertebra.
dIVCS (lumbar degeneration-related type IVCS), the iliac vein was compressed by both the iliac artery from the front and lower lumbar degenerative changes from the rear, including intervertebral disc bulge or herniation, osteophyte, lumbar spondylolisthesis and anterior longitudinal ligament ossification, which compress the iliac vein like a wedge.
oIVCS (IVCS from other causes), the iliac vein was compressed by abscess or hematoma due to lumbar tuberculosis, inflammation and fracture.
1.2. Imaging diagnosis
According to the previous studies4,5 and our clinical experience, an emergency ultrasound was performed prior to admission for the initial diagnosis and assessment of the extent of venous thrombosis; this included embolism localized in the iliac vein, extending to the femoral vein, or involving the distant popliteal vein. After hospitalization, the iliac waist CT scan and deep vein imaging were supplemented in order to determine the diagnosis, understand the anatomical location and interrelationship around the iliac vascular, evaluate the changes of lumbar degeneration and the iliac vein channel vertebral anterior to the vertebra, and to exclude other diseases in the abdominal cavity, pelvic cavity, and lumbosacral.
1.3. Computed tomography (CT) observation index
The left iliac vein channel (IVC) is the space through which the left iliac vein advances from the right to the left between the right iliac artery and lower lumbar vertebra.
Iliac vein channel diameter (IVCD) (Fig. 1A): Measured on the enhanced cross-sectional CT images of the left iliac vein from the right to the left side. The minimum sagittal diameter between the rear edge of the contrasted iliac artery to the leading edge of the anterior longitudinal ligament was considered as the IVCD. Congenital or acquired passage narrowing may lead to iliac vein thrombosis.
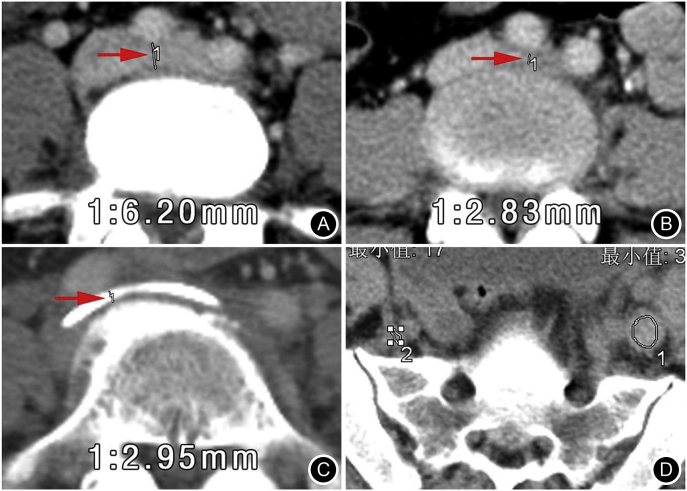
Fig. 1.
Central plane of the iliac vein channel on the axial CT images. A. Measurement of normal IVCD (arrows) in a 40-year-old female patient; B. The right iliac artery compressed the iliac vein channel anterior to the vertebral body and caused the tunnel stenosis (arrow) in 46-year-old female patient; C. The anterior iliac artery and the posterior lumbar osteophyte compressed the channel together (arrow) in 67-year-old male patient.
Causes of iliac vein compression (Fig. 1B–C): On reconstructed cross-sectional and sagittal plane CT images, the factors of iliac vein compression, such as simple iliac artery oppression or combined lumbar vertebrae degeneration changes (osteophyte, disc herniation, anterior longitudinal ligament hypertrophy, calcification, lumbar spondylolisthesis) or hematoma and abscesses surrounding it, were identified.
Density of the iliac vein thrombus: On the CT axial image of the common iliac veins, using the thrombus CT value of the left iliac vein minus the contralateral iliac vein blood CT value (Fig. 1D), the increased CT value was considered thrombus density, which reflects the hardness of the thrombus.
1.4. Interventional therapy
In accordance with previous studies,2,3,6,7 a path of procedural staged treatment was designed as follows: The first stage was catheter-directed thrombolysis (CDT) for the target vein, that is, downstream thrombolysis by puncturing the ipsilateral popliteal vein and detaining the thrombolysis catheter at the far end of the thrombus in the target vein. The CDT combined with oral anticoagulants persisted for 3 days. Patients ineffective treatment and relapse in the first stage were arranged to undergo the second stage treatment.
The second stage was mechanically thrombi-broken aspiration and balloon dilatation. This was performed by selectively stabbing the left femoral or popliteal vein, antegrade or retrograde breaking the thrombus in the iliac vein and femoral vein above the hip, or in the femoral vein and popliteal vein under the hip and sucking away the smashed thrombus before using a balloon to dilate the affected veins. And CDT combined with oral anticoagulants was maintained for 3 days after operation. Patients ineffective treatment and relapse in the second stage were arranged to undergo the third stage treatment.
The third stage was stent implantation after balloon dilatation. After interventional therapy, CDT combined with oral anticoagulants was also performed for 3 days. Oral anticoagulants were arranged for 6 months continuously after each stage treatment. Relapse patients were recalled to perform the next stage treatment.
1.5. Evaluation indicators
The suspected influencing factors for therapeutic effect were the patient’s course of disease, IVCS type, thrombus hardness, embolization length, and therapeutic regimen.
The immediate effect after interventional therapies was assessed according to venous angiography, which is divided into four grades: Grades I, II, and III represent effective treatment, while IV grade is ineffective.8
The follow-up effect classified into the following four levels9: excellent, good, and medium, which represent effective treatment, and poor, which represents an ineffective treatment. The treatment was deemed to be ineffective if recurrence was observed during the 24 month follow-up, and effective if there was no recurrence.
Two radiation intervention physicians, with 20 years and 11 years’ experience in this field, evaluated the indicators, and these two physicians discussed and then decided the results.
1.6. Statistical analysis
The measurement data were expressed as mean ± standard error, and included age of onset, course of disease, thrombus density, and IVCD. The difference between the two groups was analyzed by variance. The chi-square test was used to analyze the differences between groups in terms of the iliac vein oppression position and embolization range. The amount of strain variable binary logistic regression analysis was performed for the effect of treatment on IVCS and its influencing factors. The independent variables values and variables assignments are listed in Table 1 for the logistic regression analysis. Statistical analysis was performed using SPSS 17.0 software, and significance was set at P < 0.05.
Table 1.
The independent variables of influencing factors and variables assignments.
| Assignment No. | Lower extremity swelling and pain period | Iliac vein thrombus density (Ipsilateral - contralateral) (unit) |
Types of IVCS | Embolization ranges | Therapeutic programs |
|---|---|---|---|---|---|
| 1 | ≤2 weeks | Density difference ≤ 10 units | sIVCS | Iliac vein only | Simple thrombolysis |
| 2 | >2 weeks | 10 < Density difference ≤ 20 | dIVCS | Femoral vein involved | 1 + Thrombi-broken suction and balloon dilatation |
| 3 | 20 < Density difference ≤ 30 | oIVCS | Popliteal vein involved | 1 + 2 + iliac vein stent implantation |
2. Results
2.1. General clinical characteristics and computed tomography (CT) features of iliac vein compression syndrome (IVCS)
In a total of 69 patients, there were 22 cases of sIVCS (6 males and 16 females), 33 cases of dIVCS (12 males, 21 females), and 14 cases of oIVCS (7 males and 7 females). In terms of the course of disease, 44 cases had a disease course ≤2 weeks and 25 cases had a disease course of 2 weeks–6 weeks. With regards to the thrombus density, 21 cases had a density ≤10 HU, 19 cases had a density of 10–20 HU, and 29 cases had a density of 20–30 HU. In 33 dIVCS cases, 17 had intervertebral disc anterior bulge compressing the iliac vein, 16 had vertebral osteophyte compressing the iliac vein, and 8 had inferior lumbar spondylolisthesis compressing iliac vein. Fourteen oIVCSs included 6 cases of lumbar tuberculosis, 4 cases of inflammation, and 4 cases of fracture. With regards to the embolization range, 12 cases were only in the iliac vein, 38 cases involved the popliteal vein and above, and 19 cases involved the popliteal vein and below (Table 2).
Table 2.
General clinical characteristics and computed tomography (CT) features of iliac vein compression syndrome (IVCS).
| IVCS group | Age of onset | Lower extremity swelling period (days) | Thrombus density(Hu) | IVTD (mm) | Iliac vein compression position |
Embolization ranges |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L4/5 front | L5 left front | L5 straight front | L5 right front | Iliac vein only | Femoral vein involved | Popliteal vein involved | |||||
| sIVCS | 42.3 ± 6.5 | 12.1 ± 9.2 | 14.30 ± 6.60 | 2.50 ± 0.50 | 4 | 0 | 4 | 14 | 0 | 6 | 16 |
| dIVCS | 61.5 ± 10.6 | 22.5 ± 7.6 | 18.40 ± 6.10 | 2.30 ± 0.50 | 9 | 3 | 18 | 3 | 6 | 10 | 17 |
| oIVCS | 53.1 ± 16.8 | 6.8 ± 6.7 | 11.60 ± 9.50 | 5.90 ± 2.30 | 0 | 0 | 0 | 0 | 4 | 6 | 4 |
| F(χ2) P |
11.03 <0.01 |
7.81 <0.01 |
1.96, 0.16 |
125.27 <0.01 |
19.31 <0.01a |
--, 0.03b |
|||||
Using χ2 test.
Using the Fisher exact algorithm.
2.2. Evaluation of factors affecting the therapeutic effect
The treatment process and results are shown in Table 3. Regression analysis showed that IVCS type (β = 4.14; Wald test P, < 0.01); before treatment lower limb swelling and pain duration (β = -5.33; Wald test, P = 0.02), iliac vein thrombus density (β = -6.46; Wald test, P = 0.01); deep vein thrombus length (β = 2.74; Wald test, P = 0.03); and the treatment regimen (β = 11.92; Wald test, P = 0.01) had significant influences on IVCS therapeutic efficacy (Fig. 2).
Table 3.
IVCS interventional therapeutic strategies and results.
| Group | First stage CDT (n = 69) |
Second stage thrombi-broken and suction, and balloon dilatation (n = 54) |
Third stage stent implantation (n = 37) |
||||
|---|---|---|---|---|---|---|---|
| Therapeutic effective |
Therapeutic ineffective | Therapeutic effective |
Therapeutic ineffective | Therapeutic effective | |||
| No recurrence | Relapse | No recurrence | Relapse | ||||
| sIVCS (n = 22) | 0 | 0 | 22 | 3 | 3 | 16 | 19 |
| dIVCS (n = 33) | 5 | 0 | 28 | 10 | 6 | 12 | 18 |
| oIVCS (n = 14) | 10 | 0 | 4 | 4 | 0 | 0 | – |
| χ2 value P-value |
27.27 <0.01a |
12.19 <0.01a |
– --b |
||||
IVCS: Iliac vein compression syndrome.
Data analysis, preliminary therapeutic effective cases included no recurrence cases and relapse cases, and relapse cases were scheduled for the second stage treatment with those who fail to respond to previous treatment.
In the third stage, sIVCS and dIVCS groups were all attained effective treatment, and oIVCS group had no case into the stage, so the third stage did not need for statistical differences test.
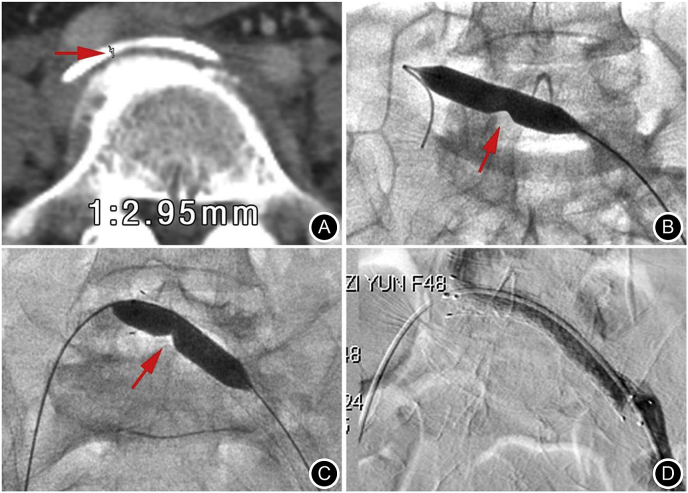
Fig. 2.
Measurement of thrombus density in the left iliac vein: ROI 1 was the central 2/3 area of the embolic left iliac vein on the axial cross-sectional CT image; the CT value was 64.29 Hu. ROI 2 at the right iliac vein was set as the control, with a CT value of 43.26 Hu. So the more difference of the bilateral CT values, the more hardness of the thrombus.
Fig. 2. 67-year-old male, CT diagnosis of dIVCS. A. Shows the vertebral anterior iliac vein channel oppressed and narrowed by the right iliac artery and the posterior of the fifth lumbar osteophyte (arrow). B. Due to ineffective iliac vein CDT, further shredded, plunger aspiration, and balloon dilatation stenosis were performed (arrow), and the embolized iliac vein became patency. C. Three-month follow-up found recurrent embolization; thus, further use of balloon dilatation-assisted implantation of a venous stent (arrow), iliac vein became patency again. D. Postoperative anticoagulant therapy for 6 months was followed up for 24 months without recurrence.
3. Discussion
IVCS can lead to serious clinical symptoms and adverse consequences that require timely and effective clinical intervention measures. Surgical treatment can achieve a higher iliac vein embolization open rate, and a study by Brazeau et al.3 found that different surgical procedures after surgery and long-term (postoperative 3–4 years) opening rate up to 54% and 83%; however, in this study, patients were subjected to highly invasive surgical techniques, and as such, a high mortality rate was to be expected. Vascular interventional therapy of IVCS has proven to be a safe and effective therapeutic method. Pre-operative anticoagulation therapy is an important part of the standard treatment for deep vein thrombus.10 Furthermore, thrombolytic therapy is recommended by the US Food and Drug Administration11; however, this treatment does not treat the reasons for the oppression and the "intravenous thorns" structure.12 Similarly, balloon dilatation therapy cannot effectively open the iliac vein cavity in IVCS.13 Moreover, the effects of these treatments were variable across different studies.6 Therefore, this study used a unified treatment path and evaluation method to analyze the individual impact factors influencing the therapeutic effect.
In this study, IVCS was divided into sIVCS, dIVCS, and oIVCS according to the CT manifestations and etiology differences. The results showed that the onset age of sIVCS was earlier than that of dIVCS, and the course of disease of lower extremity swelling and pain was shorter in dIVCS; this was associated with a more hardness in dIVCS compared to sIVCS. Since the lumbar degenerative changes compressing the iliac vein is a chronic progressive oppression, which leads to an increasing narrowing of the iliac vein channel. Although both sIVCS and dIVCS are caused by stenosis of the iliac venous channel, sIVCS is simply oppressed by the pulsating iliac artery from the anterior, while dIVCS is simultaneously oppressed by the front iliac artery and the back osteophyte, intervertebral disc, and spondylolisthesis of the vertebral body; thus, sIVCS represents a primary iliac vein channel stenosis, whereas dIVCS is a secondary iliac vein channel stenosis. Lower lumbar degenerative changes are also known to induce or promote the occurrence of dIVCS.14,15
The preferred treatment for IVCS is to place a vena cava filter and target intravascular thrombolysis and iliac venous stent. Due to hyperplasia osteophyte, spondylolisthesis of the lower lumbar vertebraes, and more hard thrombi, dIVCS needs an implantation of longer and harder stent compared to sIVCS. The primary disease symptoms of oIVCS (lumbar tuberculosis, inflammation, or fracture) are obvious, as are its complications; thus the course is shorter and the thrombi are softer. Furthermore, the iliac vein channel has no true narrow and is enlarged by abscess or hematoma, thus, its intervention therapy is simpler, and the effect is better, than that of sIVCS and dIVCS.
Regression analysis showed that IVCS types, course of disease, thrombus density, embolization range, and treatment regimen have significant effects on the outcomes. In this study, the first stage was the target intravenous thrombolytic therapy with CDT, and the second stage was the thrombi smashing and sucking away, followed by treatment with balloon dilatation. Despite this, the treatment was ineffective in 51.85% (28/54) of patients, and the treatment was effective, but with recurrence, in 16.67% (9/54) of patients (mainly those with sIVCS and dIVCS). In the clinic, these two types of patients occurred insidiously, had a longer treatment duration, and relapsed easily; thus a combination of target vein catheterization thrombolysis, thrombus smashing and sucking away, and balloon dilatation and iliac vein stent implantation was necessary. Additionally, these patients required sufficient thrombolytic drugs and an extended treatment duration, including interventional treatment and follow-up treatment. The extend of deep venous thrombus also had a significant influence on the treatment effect because long and large range embolization blocked the venous return channel. So in case of this, sucking away the deep vein thrombus, balloon dilatation and iliac vein stent implantation for reconstruction of the venous channel as early as possible is an excellent choice. This treatment regimen makes foot dorsal vein intravenous thrombolysis effectively, avoiding the long time CDT retention and its possible complication of exogenous infection.
In summary, this study demonstrates that there are significant differences in the age of onset, course of disease, thrombus hardness, and embolization extent in IVCS. These factors affect the curative effect of interventional therapy, and before the intervention, detailed image evaluation of IVCS features are important for rationalizing the treatment regimen and improving efficacy.
Funding
① Regional Development Project of Fujian Province (2019Y3007); ② Military Logistics Research Projects (CLB18J060); ③ Supporting Army Project of Zhangzhou City Government (ZZ2018KD01).
Footnotes
Research direction: Radiology diagnosis and interventional therapy of bone and joint diseases.
References
- 1.Gil Martín A.R., Carreras Aja M., Arrieta Ardieta I. Cockett’s syndrome, May-Thurner syndrome, or iliac vein compression syndrome. Radiologia. 2014;56(5):e5–8. doi: 10.1016/j.rx.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Bækgaard N., Broholm R., Just S. Indications for stenting during thrombolysis. Phlebology. 2013;28(Suppl 1):112–116. doi: 10.1177/0268355513476818. [DOI] [PubMed] [Google Scholar]
- 3.Brazeau N.F., Harvey H.B., Pinto E.G. May-Thurner syndrome: diagnosis and management. Vasa. 2013;42(2):96–105. doi: 10.1024/0301-1526/a000252. [DOI] [PubMed] [Google Scholar]
- 4.Ou-Yang L., Lu G.M. Underlying anatomy and typing diagnosis of may-thurner syndrome and clinical significance: an observation based on CT. Spine. 2016;41(21):1284–1291. doi: 10.1097/BRS.0000000000001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narayan A., Eng J., Carmi L. Iliac vein compression as risk factor for left- versus right-sided deep venous thrombosis: case-control study. Radiology. 2012;265(3):949–957. doi: 10.1148/radiol.12111580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akimasa M., Norikazu Y., Yoshito O. Early and long-term outcomes of venous stent implantation for iliac venous stenosis after catheter-directed thrombolysis for acute deep vein thrombosis. Circulation Journal Official Journal of the Japanese Circulation Society. 2014;78:1234–1239. doi: 10.1253/circj.cj-13-1247. [DOI] [PubMed] [Google Scholar]
- 7.Ibrahim W., Al Safran Z., Hasan H. Endovascular management of may-thurner syndrome. Vasa. 2013;42(2):96–105. doi: 10.3400/avd.cr.12.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo Dingyuan, Li Honghao, Long Miao Yun. Comparison of the effects of surgical thrombectomy and drug thrombolysis on acute iliac femoral deep vein thrombosis. Chin J Gen Surg. 2010;25(11):876–879. [Google Scholar]
- 9.Zhou Yubin, Xu Ke, Xiao Liang. Interventional treatment of acute iliac - femoral vein thrombosis caused by iliac vein compression syndrome. Chin J Med Imaging Technol. 2002;18(6):553–555. [Google Scholar]
- 10.Nazir S.A., Ganeshan A., Nazir S. Endovascular treatment options in the management of lower limb deep venous Th rombosis. Cardiovasc Interv Radiol. 2009;32:861–876. doi: 10.1007/s00270-009-9662-z. [DOI] [PubMed] [Google Scholar]
- 11.Grunwald M.R., Goldberg M.J., ofmann L.V. Endovascular management of May-Thurner syndrome. Am J Roentgenol. 2004;183:1523–1524. doi: 10.2214/ajr.183.5.1831523. [DOI] [PubMed] [Google Scholar]
- 12.Mitsuoka H., Ohta T., Hayashi S. Histological study on the left common iliac vein spur. Annals of Vascular Diseases. 2014;7(3):261–265. doi: 10.3400/avd.oa.14-00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo Chuanbin, Ou Guanggan, Si Guangyan. Multislices spiral CT angiography, digital subtraction angiography and interventional therapy for iliac vein compression syndrome. Chinese Journal of Medical Imaging. 2013;21(8):576–578. [Google Scholar]
- 14.Ouyang Lin, He ping, Hu Tiansong, Lu Guangming. Underlying CT anatomy and subtype diagnosis of iliac vein compression syndrome. Chin J Radiol. 2016;50(4):274–279. [Google Scholar]
- 15.Ouyang Lin, He Ping, Xu Siding, Chen SHuibin, Zhou Manzhen. Correlations of the iliac venous tunnel ahead the lower lumbar vertebrae with sex,age and its clinical significance. Chin J Anat Clin. 2017;22(1):11–17. [Google Scholar]