Abstract
Objective
To investigate the safety, efficacy, and prognostic factors of hepatic arterial infusion chemotherapy (HAIC) with raltitrexed and oxaliplatin post-transarterial chemoembolization (TACE) for unresectable hepatocellular carcinoma (uHCC).
Methods
Thirty-seven patients with uHCC who received HAIC with raltitrexed and oxaliplatin post-TACE between June 2014 and December 2016 at our hospital were recruited. The primary endpoint was overall survival (OS), and secondary endpoint was progression-free survival (PFS). The overall response rate (ORR) was evaluated using the modified Response Evaluation Criteria in Solid Tumors. Toxicity was assessed according to the Common Terminology Criteria for Adverse Events (v4.0). The OS and prognostic factors were analyzed using the Kaplan–Meier method, log-rank test, and Cox regression models.
Results
Three (8.1%) patients achieved complete response, 17 (46.0%) patients achieved partial response, and the ORR was54.0%.The median OS and median PFS were 19.0 months and 12.0 months, respectively. The common toxicities included grade 3–4 increased aspartate aminotransferase levels (8/37,21.6%), grade 1–2 hyperbilirubinemia (75.7%, 28/37), nonspecific abdominal pain and fever, and grade 2–3 thrombocytopenia (18.9%, 7/37); no patients developed grade 3–4 neutropenia. Univariate analysis showed that the tumor diameter (≤50 mm, p = 0.028), Barcelona Clinic Liver Cancer (BCLC) stage (p = 0.012), hepatitis B virus DNA level (p = 0.033), and derived neutrophil-to-lymphocyte ratio (dNLR; derived neutrophils/leukocytes minus neutrophils) (p = 0.003) were predictive factors for prognosis. Multivariate analysis showed that patients with BCLC stage B disease (p = 0.029) and dNLR≤2 before therapy (p = 0.004) had better prognosis.
Conclusions
HAIC with raltitrexed and oxaliplatin post-TACE is a safe and efficacious therapy for patients with uHCC; in particular, those with BCLC stage B and dNLR≤2 have better prognosis.
Keywords: Hepatocellular carcinoma, Transcatheter arterial chemoembolization (TACE), Hepatic arterial infusion chemotherapy (HAIC), Oxaliplatin, Raltitrexed
Introduction
The mortality of hepatocellular carcinoma (HCC) is the second highest among cancers worldwide, and disease progression is the leading cause of death in patients with HCC.1 In particular, in the Asia–Pacific region, the incidence of HCC and annual mortality rates related to HCC have increased during the past several years.2
Liver resection (LR) and liver transplantation (LT) are the first-line options for curative treatment; however, due to a shortage in donor livers for LT, LR is the main therapeutic modality.3 In addition, because most patients have advanced-stage disease at the time of diagnosis, only 15% of patients with HCC can undergo surgery.4 Unfortunately, approximately 70% of patients experience recurrence within five years, and the prognosis of HCC is poor.5
Transarterial chemoembolization (TACE) is the first-line treatment for unresectable HCC (uHCC), especially for tumors with a diameter>3 cm or for multifocal lesions (>3) that do not exhibit vascular invasion or extrahepatic metastasis.6 Many reports indicate that TACE is a safe and efficacious regimen for patients with uHCC; TACE is associated with lower mortality and a shorter hospital stay than those associated with other treatment modalities.7 The drugs used in TACE are fluorouracil (5-FU), doxorubicin (DOX), and oxaliplatin8. Some reports have indicated that TACE with raltitrexed and oxaliplatinis safe and confers fewer adverse effects (AEs) in patients with uHCC. The median overall survival (mOS) and median progression-free survival (mPFS) are longer in patients receiving raltitrexed than in patients receiving 5-FU and DOX (mOS: 13.4–7.4 vs. 8.5–5.8 months; mPFS: 6.7–3.6 vs 4.6–2.6 months). Increased aspartate aminotransferase levels (78.9% vs. 81.3%) and abdominal pain (68.4% vs. 78.7%), are also lower in those receiving raltitrexed TACE than in those receiving 5-FU and DOX TACE.8,9 The efficacy of TACE for massive uHCC lesions (≥10 cm) or those with vascular invasion or widespread nodules is not satisfactory; however, hepatic arterial infusion chemotherapy (HAIC) may still be effective.10,11 For some patients with uHCC that is resistant to TACE, HAIC may be effective.12 Researchers have reported that HAIC with 5-FU, mitoxantrone, and cisplatin has significant efficacy for uHCC; the mOS is 11.3–14.0 months and mPFS is 7.0–7.7 months.13,14 Some researchers have reported that HAIC with raltitrexed and oxaliplatin for liver metastasis of colorectal cancer is associated with less toxicity, longer overall survival (OS), and longer progression-free survival (PFS) than those associated with HAIC with other drugs for uHCC. HAIC with raltitrexed and oxaliplatin remains feasible and promising, even after the failure of irinotecan and oxaliplatin for patients with liver metastasis of colorectal cancer.15,16 TACE is an effective approach for uHCC, and HAIC with raltitrexed and oxaliplatin post-TACE in patients with uHCC has been rarely reported. The present study's aim was to evaluate the safety, efficacy, and prognostic factors of HAIC with raltitrexed and oxaliplatin post-TACE in patients with uHCC.
Methods
Forty patients were diagnosed with uHCC based on pathological or radiological findings between December 2014 and September 2016 at our center; 3 patients were lost to follow-up, and 37 patients were included in the study. All patients satisfied the following criteria:1 Child–Pugh class A or B disease, white blood cell count>2 × 109/L, neutrophil count>1 × 109/L, and platelet count>50 × 109/L;2 total bilirubin<50 μmol/L and serum creatinine<2 mg/dL;3 unresectable tumors or tumors unsuitable for local ablation therapy;4Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; and5 no past history of treatment with raltitrexed.
The exclusion criteria were as follows:1 other histological tumors;2 unstable angina or severe heart failure;3 uncontrollable infection (grade >2 infections; infections requiring antibiotic, antifungal, or antiviral interventions; infections requiring radiological or surgical interventions; infections with life-threatening consequences; or infections requiring urgent interventions according to the Common Terminology Criteria for Adverse Events (CTCAE);4 pregnant or lactating; and5 severe allergy to contrast media or other medicines.
The chemoembolization treatments for all patients were performed using digital subtraction angiography (DSA) equipment in our hospital. DSA of the celiac, superior mesenteric, and splenic arteries was performed to evaluate the hepatic arterial anatomy and tumor blood supply.
A 2.7-F microcatheter was advanced coaxially through an external catheter to select the tumor arteries. Subsequently, 40 mg of emulsified epirubic in (EPI, Main Luck Pharmaceutical, Shenzhen, China) and lipiodol (total volume<20 ml; Lipiodol, Laboratoire Andre Guerbet, Aulnaysous-Bois, France) were injected to embolize the tumor arteries; for some large tumors, polyvinyl alcohol particles were used to embolize the tumor arteries. If there was extrahepatic parasitic blood supply to the tumor (for example, from the left gastric, right phrenic, or right adrenal artery), chemoembolization was recommended after selective catheterization via the parasitic artery. If the microcatheter tip was near the gastroduodenal or right gastric artery, microcoil embolization was performed to avoid drug flow into the arteries of the gastrointestinal tract to protect the gastrointestinal tract.
The microcatheter was externally connected to an arterial infusion pump (Model LP 2000-P2) in the ward, and oxaliplatin (85 mg/m2, Hengrui Medicine Co., Ltd., Jiangsu, China) was administered intra-arterially for 0–4 h; raltitrexed (3 mg/m2, Tianqing Pharmaceutical Co., Ltd., Nanjing, China) was administered for approximately 1 h.17 HAIC post-TACE was regularly performed every 4–6 weeks. The endpoint was death or Child–Pugh class C disease. Enhanced computed tomography (CT) or magnetic resonance imaging (MRI) and laboratory tests (blood routine examination, biochemical tests, measurement of alpha-fetoprotein levels, coagulation testing) were regularly performed before therapy; patients were followed up until death or until they were lost to follow-up. The overall response rate (ORR) was evaluated using the modified Response Evaluation Criteria in Solid Tumors (mRECIST), and AEs were evaluated using the CTCAE v4.0.
Statistical analysis
OS was calculated from the date of the first HAIC post-TACE treatment to the date of death or until the date on which the patient was considered lost to follow-up. PFS was defined from the date of the first HAIC treatment to the date of tumor progression. PFS and OS were estimated using the Kaplan–Meier method, and univariate analyses were performed using the log-rank test. Multivariate analysis was performed using Cox proportional hazards models. All analyses were performed with SPSS version 22.0 (IBM Corp., Armonk, NY, United States SPSS version 22.0), and a P-value<0.05 was considered statistically significant.
Results
Baseline characteristics of patients
Details of the characteristics of the 37 patients who were treated with HAIC with raltitrexed and oxaliplatin post-TACE are shown in Table 1. The mean age was 57.2 years (ranging from 32 to 81years). The sex ratio (female to male) was 4:33. Most patients had hepatitis B virus (HBV)-related disease (33, 89.2%), and 13 patients (33.3%) had HBV DNA levels>102 copies/mL. Twenty-seven patients (81.2%) had an ECOG status of 0, and 9 (27.3%) patients had an ECOG status of 1. Seventeen patients (45.9%) had Barcelona Clinic Liver Cancer (BCLC) stage B disease, and 20 patients (54.1%) had BCLC stage C disease. Thirty (81.1%) patients had Child–Pugh class A disease, and the mean tumor diameter was 75.3 mm. Of all the patients, 21 patients (56.8%) had one solitary lesion and 8 (21.6%) had vascular invasion. 4 patients (10.8%) had extrahepatic metastases; 3 had lung metastases, and 1 patient had adrenal and bone metastases.
Table 1.
Baseline characteristics.
| Baseline characteristics | Value |
|---|---|
| Age, years | |
| >60 | 20 (54.1%) |
| ≤60 | 17 (45.9%) |
| Sex | |
| Male | 33 (89.2%) |
| Female | 4 (10.8%) |
| Diameter (mm) | |
| <50 | 13 (35.1%) |
| 50–100 | 8 (21.6%) |
| ≥100 | 16 (43.3%) |
| Child–Pugh stage | |
| A | 30 (81.1%) |
| B | 7 (18.9%) |
| BCLC | |
| B | 17 (45.9%) |
| C | 20 (54.1%) |
| ECOG performance status | |
| 0 | 27 (73.0%) |
| 1 | 9 (24.3%) |
| 2 | 1 (2.7%) |
| Tumor nodules | |
| Solitary | 16 (43.2%) |
| Multiple | 21 (56.8%) |
| Vascular invasion | |
| With | 8 (21.6%) |
| Without | 29 (78.4%) |
| Extrahepatic metastasis | |
| With | 4 (10.8%) |
| Without | 33 (89.2%) |
| HBV infection | |
| Yes | 33 (89.2%) |
| No | 4 (10.8%) |
| HBV DNA level >2 × 103 copies/mL | |
| Yes | 13 (33.3%) |
| No | 24 (66.7%) |
| Cirrhosis | |
| With | 32 (86.5%) |
| Without | 5 (13.5%) |
| AFP | |
| ≤400 ng/ml | 27 (73.0%) |
| >400 ng/ml | 10 (27.0%) |
| dNLRa ≤2 | |
| Yes | 17 (45.9%) |
| No | 20 (54.1%) |
AFP: alpha-fetoprotein; BCLC: Barcelona Clinic Liver Cancer; ECOG: Eastern Cooperative Oncology Group; HBV: hepatitis B virus; dNLR: derived neutrophil-to-leukocyte ratio.
dNLR = leukocyte/(absolute leukocyte count - absolute neutrophil count).
Safety
There were no procedure-related deaths in the present study. Common AEs included nausea and vomiting (48.6%), grade3–4 increased aspartate aminotransferase levels (21.6%, 8/37), grade 1–2 hyperbilirubinemia (75.7%, 28/37), grade 1–2 pain (32.4%), grade 1 fever (37.8%,14/37), and grade 2–3 thrombocytopenia (18.9%,7/37). No patients developed neutropenia or other serious complications (Table 2).
Table 2.
Observed toxicity according to the CTCAE.
| Severity grade | Value | ||
|---|---|---|---|
| Hematological | |||
| Thrombocytopenia | 2–3 | 7 (18.9%) | |
| Neutropenia | 3–4 | 0 (0.0%) | |
| Non-hematological | |||
| Elevation of liver enzymes | 3–4 | 8 (21.6%) | |
| Elevation of bilirubin | 1–2 | 28 (75.7%) | |
| Nausea/vomiting | 1–2 | 18 (48.6%) | |
| Pain | 1–2 | 12 (32.4%) | |
| Fever | 1 | 14 (37.8%) | |
Toxicity was assessed according to the CTCAE v.4.0 criteria. CTCAE, Common Terminology Criteria for Adverse Events.
Survival
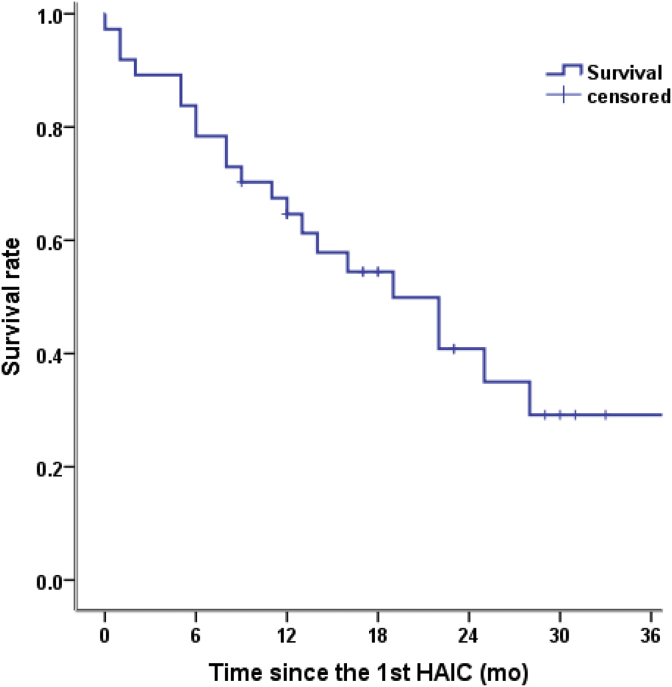
The median follow-up period was 34 months, and them OS of the 37 patients was 19.0 months (Fig. 1). The mOS of patients with BCLC stage B and BCLC stage C uHCC were 34 months and 13 months, respectively (p = 0.012). In the present study, the PFS was 12.0 months; the mPFS of patients with BCLC stage B uHCC was 17 months and that of patients with BCLC stage C uHCC was 8 months(p = 0.054). The 6-, 12-, and 24-month mOS rates were 84%, 64%, and 41%, respectively (Fig. 1). The tumor response rates were as follows: complete response (CR) was achieved in 8.1% (3/37) of patients, and partial response (PR) was achieved in 46.0% (17/37) of patients (Table 3).
Fig. 1.
Kaplan–Meier survival curves for patients receiving HAIC with raltitrexed and oxaliplatin post-TACE. HAIC: hepatic arterial infusion chemotherapy; TACE: transarterial chemoembolization.
Table 3.
Tumor response according to the mRECIST.
| Outcome | HAIC with raltitrexed and oxaliplatin post-TACE |
|---|---|
| DCRa | 75.7%28 |
| ORRb | 54.1%20 |
| CR | 8.1%3 |
| PR | 46.0%17 |
| SD | 21.6%8 |
| PD | 24.3%9 |
mRECIST: modified Response Evaluation Criteria in Solid Tumors; DCR: disease control rate; ORR, overall response rate; CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease.
DCR was calculated as CR + PR + SD.
ORR was calculated as CR + PR.
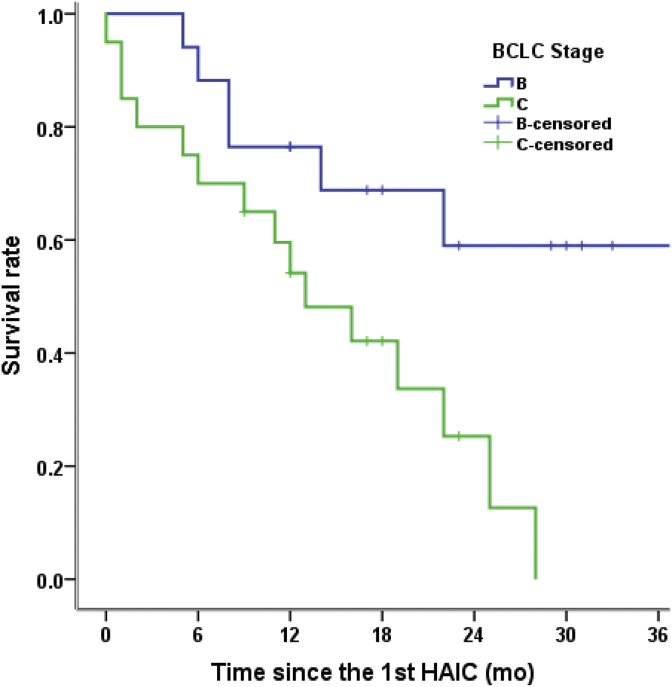
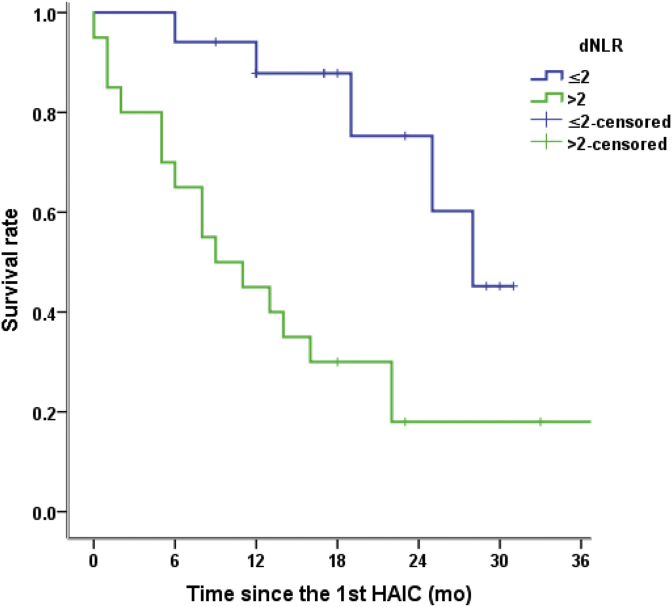
Twelve prognostic factors were analyzed univariately, and four prognostic variables were identified (Table 4). Univariate analysis showed that the tumor diameter (≤50 mm, p = 0.028), BCLC stage (p = 0.012), HBV DNA level (p = 0.033), and derived neutrophil-to-lymphocyte ratio (dNLR; derived neutrophils/leukocytes minus neutrophils) (p = 0.003) were predictive factors for prognosis. In multivariate analysis, BCLC stage (p = 0.029, Fig. 2) and adNLR≤2 (p = 0.004, Fig. 3) were significant factors for patient survival after HAIC with raltitrexed and oxaliplat in post-TACE (Fig. 3).
Table 4.
Univariate and multivariate analyses of predictors of overall survival.
| Characteristic | Univariate (p*-value) | Multivariate (p*-value) | HR | 95% CI |
|---|---|---|---|---|
| Age (≤65 y) | 0.276 | |||
| Sex | 0.395 | |||
| Diameter (≤50 mm) | 0.028 | |||
| Child–Pugh stage | 0.789 | |||
| BCLC | 0.012 | 0.029 | 3.080 | 1.122–8.455 |
| ECOG performance status | 0.183 | |||
| Tumor nodules | 0.159 | |||
| Vascular invasion | 0.064 | |||
| HBV DNA level >2 × 103 copies/mL | 0.033 | |||
| Cirrhosis | 0.759 | |||
| AFP | 0.544 | |||
| dNLRa ≤2 | 0.003 | 0.004 | 0.020 | 0.064–0.599 |
AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; dNLR, derived neutrophil-to-leukocyte ratio; HR, hazard ratio; CI, confidence interval.
dNLR = leukocyte/(absolute leukocyte count - absolute neutrophil count); * < 0.05.
Fig. 2.
Kaplan–Meier survival curves for patients receiving HAIC with raltitrexed and oxaliplatin post-TACE. P-value based on a log-rank test. The BCLC stage is associated with OS (P = 0.012). HAIC: hepatic arterial infusion chemotherapy; BCLC: Barcelona Clinic Liver Cancer; OS: overall survival.
Fig. 3.
Kaplan–Meier survival curves for patients receiving HAIC with raltitrexed and oxaliplatin post-TACE. P-value based on a log-rank test. dNLR = leukocyte/(absolute leukocyte count minus absolute neutrophil count) (P = 0.003). HAIC: hepatic arterial infusion chemotherapy; TACE: transarterial chemoembolization; dNLR: derived neutrophil-to-lymphocyte ratio.
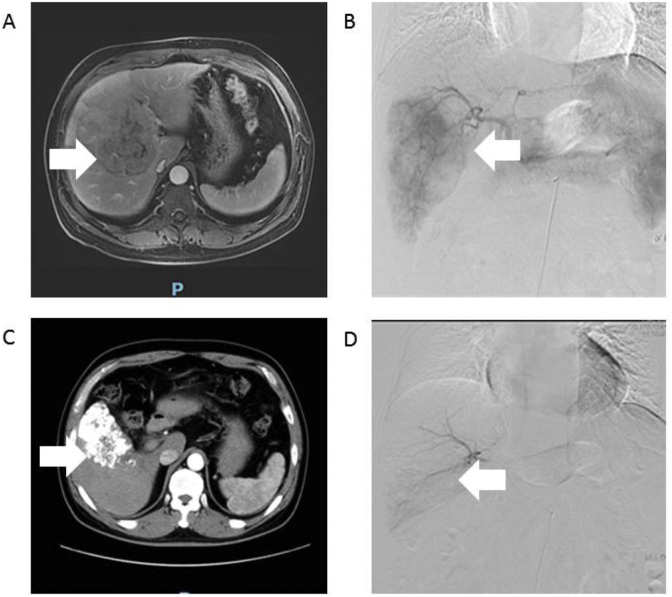
In a 53-year-old man with HCC (Case 1, Fig. 4),pre-operative MRI revealed a lesion in the liver (Fig. 4A). Angiography via the proper hepatic artery revealed a tumor stain in the liver (Fig. 4B). Post-operative CT revealed scattered deposition of lipiodol within the lesions in the liver (Fig. 4C). After 4 weeks, angiography via the proper hepatic artery revealed scattered deposition of lipiodol within the lesions in the liver (Fig. 4D).
Fig. 4.
Images of case 1. A: Pre-operative MRI revealed the lesion in the liver (as indicated by the arrow). B: Angiography via the proper hepatic artery revealed a tumor stain in the liver (as indicated by the arrow). C: Post-operative CT revealed scattered deposition of lipiodol within the lesions in the liver(as indicated by the arrow). D: After 4 weeks, angiography via the proper hepatic artery revealed scattered deposition of lipiodol within the lesions in the liver (as indicated by the arrow). MRI: magnetic resonance imaging; CT: computed tomography.
Discussion
HAIC has been used for advanced HCC in Southeast and East Asian countries and is a safe and efficacious treatment for uHCC. In the present study, HAIC with raltitrexed and oxaliplat in post-TACE were evaluated.
Raltitrexed and oxaliplatin are safe and efficient drugs for liver metastasis of colorectal cancer. The safety and efficacy of HAIC for uHCC have also been explored previously.15,16 In prior studies of efficacy and survival data for patients with uHCC who received HAIC with 5-FU, mitoxantrone, and cisplatin, the mOS ranged from 6.0 to 14.0 months, ORR ranged from 16.7% to 45.6%, and mPFS ranged from 3.6 to 7.7months; these results are unsatisfactory. In some reports, HAIC post-TACE for uHCC has been suggested to be effective and confer longer OS and PFS than those associated with TACE alone.17,18 Thus, the current study evaluated the use of HAIC with raltitrexed and oxaliplatin for uHCC post-TACE. In the present study, the mOS after HAIC post-TACE was 19.3 months; the advantages of HAIC with raltitrexed and oxaliplatin compared with HAIC with 5-FU, mitoxantrone, and cisplatin (OS: 6–14 months) are obvious. The ORR in our study was 54%, which is higher than that reported by previous studies (15.7–45.6%) that evaluated HAIC with 5-FU, mitoxantrone, and cisplatin. The mPFS in our study was 12.0 months, which is also higher than that revealed in prior studies (3.6–7.7months).19,20
The advantages of TACE with raltitrexed and oxaliplatin for uHCC compared with TACE with 5-FU, EPI, and oxaliplatin have been shown in previous studies; the OS ranged from 7.4 to 13.4 months, mPFS ranged from 3.6 to 6.7 months, and ORR (CR + PR) ranged from 2.5 to 67.2%.8,9 In the present study, the mOS was19.3 months, mPFS was 5.0 months, and ORR was 54.1%. HAIC with raltitrexed and oxaliplatin post-TACE may prolong patient survival and has a higher disease control rate than that associated with TACE alone with raltitrexed and oxaliplatin, but more data need to be collected.
There were a similar number of AEs noted in the present study compared to that in previous studies of HAIC with5-FU, mitoxantrone, and cisplatin (nausea and vomiting (60.0–71.4%), abdominal pain (68.4%), increased serum liver enzymes (59.5–78.9%), leukopenia (36.0–37.1%), neutropenia (35–39%), and thrombocytopenia (19–38.1%)).8, 9, 10,21,22 One study revealed that the rate of AEs was significantly lower in patients treated with HAIC with a combination of 5-FU, leucovorin, and oxaliplatin than in those treated with TACE among patients with massive uHCC; in particular, the liver dysfunction rate among patients in the HAIC group was significantly lower than that among patients in the TACE group (p < 0.001).10 Some studies have also demonstrated that the rates of embolization complications after HAIC were lower than those after TACE.13,20,23 Another study showed that there were no significant differences in overall AEs after raltitrexed- and oxaliplatin-based, fluorouracil- andoxaliplatin-based, and DOX- andoxaliplatin-based TACE.8 Raltitrexed- and oxaliplatin-based TACE may not increase toxicity. HAIC with raltitrexed and oxaliplatin may be safer than TACE with raltitrexed and oxaliplatin or HAIC with 5-FU, mitoxantrone, and cisplatin. In conclusion, HIAC with raltitrexed and oxaliplatin post-TACE is safe.
In previous studies, HBV DNA levels were significantly correlated with liver function exacerbation after TACE or partial hepatectomy.24,25 However, researchers have reported that high HBV DNA levels before TACE (≥2 × 103 copies/mL) reduce mOS, but do not affect hepatic failure-related mortality.26 If these patients do not undergo antiviral therapy, they have an increased risk of reactive HBV after hepatectomy or TACE.24 Asia–Pacific clinical practice guidelines have highlighted that HBV viral load is associated with recurrence after radical treatment of HCC. In one report, patients with HCC with hepatitis B surface antigen levels>250 IU/mL prior to curative resection had an increased risk of late recurrence compared with those with lower viral loads.27 Moreover, the outcome of HBV DNA levels correlated with OS was observed in the present study, and high HBV DNA levels before therapy may confer poor OS in patients with uHCC. High HBV DNA levels may induce HCC progression. Antiviral therapy for HBV may be crucial in patients with HBV-related HCC to prevent recurrence and improve survival.
Multivariate analysis revealed that a dNLR≤2 was an independent risk factor associated with OS. Many previous studies have demonstrated that the dNLR is related to immune function and inflammation. The dNLR has been shown to be associated with outcomes in various cancer entities. For instance, previous studies have shown that in patients with melanoma, those with a dNLR>3 have shorter time to progression (TTP) and OS. Another study showed that in patients with colon cancer, those with a dNLR≤3 had longer TTP than those with a dNLR >3 (p < 0.001) and that those with adNLR≤2.2 had long OS(p = 0.018).28,29 In patients with melanoma, the baseline dNLR was associated with prognosis in patients with metastatic melanoma receiving ipilimumab.30 Studies of HCC have led to similar conclusions. The dNLR is an independent prognostic factor for OS; a dNLR≥1.8 predicts poor prognosis in patients with HBV-associated HCC after TACE.31,32 In a study of patients with uHCC treated with HAIC, those with a dNLR<2.87 had longer PFS and OS.33 The limitation of all these studies is that none have used consistent values for the dNLR. The importance of the dNLR in HCC or other cancers should be further investigated.
The present study also found that BCLC stage CHCC was an independent risk factor associated with OS in the multivariate analysis. Patients with BCLC stage B HCC had longer OS than those with BCLC stage C HCC (p = 0.011). The mOS times for patients with BCLC stage B and C HCC were34 months and 13 months, respectively, and the mPFS times were12.0months and6.0 months, respectively. Currently, the BCLC stage system is recommended in many guidelines. Some studies have shown that patients with BCLC stage B HCC have obviously improved prognosis; those with substage B1 HCC have a longer OS than those with substage B2,B3, or B4 HCC (33.0 months vs. 20.8 months vs. 16.1 months vs. 22.2 months, p = 0.003).34 One study revealed that a new subclassification of BCLC stage C HCC (C0,C1,C2,C3, and C4) had significant differences(p < 0.001) when different treatment modalities were selected for patients within each substage of disease.35 The sample size in the present study was too small to classify patients with different substages of BCLC stage B or CHCC.
In this retrospective study, the safety and effectiveness of HAIC with raltitrexed and oxaliplatin were demonstrated in patients with uHCC. The limitations of this study were the limited sample size and single-arm retrospective study design. Compared to previous reports, the outcomes were favorable. In addition, the sample size was limited; thus, some bias was difficult to avoid. More detailed data and multicenter cooperation are needed to demonstrate the advantages of HAIC with raltitrexed and oxaliplatin post-TACE in patients with uHCC.
Conclusion
HAIC with raltitrexed and oxaliplatin post-TACE is a safe and efficacious therapy in patients with uHCC, and this treatment may provide new clues for clinical practice. In the present study, BCLC stage B and dNLR≤2 were independent risk factors associated with OS.
Funding
This work was supported by the National Natural Science Foundation of China (81571781).
References
- 1.Torre L.A., Bray F., Siegel R.L. Global cancer statistics. CA Cancer J Clin. 2012;65:87–108. doi: 10.3322/caac.21262. 2015. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R., Naghavi M., Foreman K. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziogas I.A., Tsoulfas G. Advances and challenges in laparoscopic surgery in the management of hepatocellular carcinoma. World J Gastrointest Surg. 2017;9:233–245. doi: 10.4240/wjgs.v9.i12.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chedid M.F., Kruel C.R.P., Pinto M.A. Hepatocellular carcinoma: diagnosis and operative management. Arq Bras Cir Dig. 2017;30:272–278. doi: 10.1590/0102-6720201700040011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forner A., Reig M., Bruix J. Hepatocellular carcinoma. The Lancet. 2018;391:1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 6.Omata M., Cheng A.L., Kokudo N. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mumtaz K., Patel N., Modi R.M. Trends and outcomes of transarterial chemoembolization in hepatocellular carcinoma: a national survey. Hepatobiliary Pancreat Dis Int. 2017;16:624–630. doi: 10.1016/S1499-3872(17)60077-8. [DOI] [PubMed] [Google Scholar]
- 8.Zhao C., Fan L., Qi F. Raltitrexed plus oxaliplatin-based transarterial chemoembolization in patients with unresectable hepatocellular carcinoma. Anti Cancer Drugs. 2016;27:689–694. doi: 10.1097/CAD.0000000000000371. [DOI] [PubMed] [Google Scholar]
- 9.Cui W., Fan W., Zhang Q. Comparison of two transarterial chemoembolization regimens in patients with unresectable hepatocellular carcinoma: raltitrexed plus oxaliplatin versus 5-fluorouracil plus oxaliplatin. Oncotarget. 2017;8:79165–79174. doi: 10.18632/oncotarget.16298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He M.K., Le Y., Li Q.J. Hepatic artery infusion chemotherapy using mFOLFOX versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: a prospective non-randomized study. Chin J Canc. 2017;36:83. doi: 10.1186/s40880-017-0251-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J.H., Xie X.Y., Zhang L. Oxaliplatin and 5-fluorouracil hepatic infusion with lipiodolized chemoembolization in large hepatocellular carcinoma. World J Gastroenterol. 2015;21:3970–3977. doi: 10.3748/wjg.v21.i13.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirikoshi H., Yoneda M., Mawatari H. Is hepatic arterial infusion chemotherapy effective treatment for advanced hepatocellular carcinoma resistant to transarterial chemoembolization? World J Gastroenterol. 2012;18:1933–1939. doi: 10.3748/wjg.v18.i16.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woo H.Y., Bae S.H., Park J.Y. A randomized comparative study of high-dose and low-dose hepatic arterial infusion chemotherapy for intractable, advanced hepatocellular carcinoma. Cancer Chemother Pharmacol. 2010;65:373–382. doi: 10.1007/s00280-009-1126-2. [DOI] [PubMed] [Google Scholar]
- 14.Nouso K., Miyahara K., Uchida D. Effect of hepatic arterial infusion chemotherapy of 5-fluorouracil and cisplatin for advanced hepatocellular carcinoma in the Nationwide Survey of Primary Liver Cancer in Japan. Br J Canc. 2013;109:1904–1907. doi: 10.1038/bjc.2013.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo J.H., Zhang H.Y., Gao S. Hepatic artery infusion with raltitrexed or 5-fluorouracil for colorectal cancer liver metastasis. World J Gastroenterol. 2017;23:1406–1411. doi: 10.3748/wjg.v23.i8.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khouri C., Guiu B., Cercueil J.P. Raltitrexed and oxaliplatin hepatic arterial infusion for advanced colorectal cancer: a retrospective study. Anti Cancer Drugs. 2010;21:656–661. doi: 10.1097/CAD.0b013e328337d469. [DOI] [PubMed] [Google Scholar]
- 17.Gao S., Zhang P.J., Guo J.H. Chemoembolization alone vs combined chemoembolization and hepatic arterial infusion chemotherapy in inoperable hepatocellular carcinoma patients. World J Gastroenterol. 2015;21:10443–10452. doi: 10.3748/wjg.v21.i36.10443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu L.Z., Xu S., Qian H.L. Transarterial embolization and low-dose continuous hepatic arterial infusion chemotherapy with oxaliplatin and raltitrexed for hepatocellular carcinoma with major portal vein tumor thrombus. World J Gastroenterol. 2018;24:2501–2507. doi: 10.3748/wjg.v24.i23.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melichar B., Dvorak J., Ferko A. Hepatic arterial infusion in hepatocellular carcinoma: a single center experience. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159:139–144. doi: 10.5507/bp.2014.054. [DOI] [PubMed] [Google Scholar]
- 20.Song D.S., Song M.J., Bae S.H. A comparative study between sorafenib and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. J Gastroenterol. 2015;50:445–454. doi: 10.1007/s00535-014-0978-3. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda M., Okusaka T., Sato Y. A Phase I/II trial of continuous hepatic intra-arterial infusion of 5-fluorouracil, mitoxantrone and cisplatin for advanced hepatocellular carcinoma. Jpn J Clin Oncol. 2017;47:512–519. doi: 10.1093/jjco/hyx038. [DOI] [PubMed] [Google Scholar]
- 22.Feng M., Tang C., Feng W. Hepatic artery-infusion chemotherapy improved survival of hepatocellular carcinoma after radical hepatectomy. OncoTargets Ther. 2017;10:3001–3005. doi: 10.2147/OTT.S136806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamashita T., Arai K., Sunagozaka H. Randomized, phase II study comparing interferon combined with hepatic arterial infusion of fluorouracil plus cisplatin and fluorouracil alone in patients with advanced hepatocellular carcinoma. Oncology. 2011;81:281–290. doi: 10.1159/000334439. [DOI] [PubMed] [Google Scholar]
- 24.Lao X.M., Luo G., Ye L.T. Effects of antiviral therapy on hepatitis B virus reactivation and liver function after resection or chemoembolization for hepatocellular carcinoma. Liver Int. 2013;33:595–604. doi: 10.1111/liv.12112. [DOI] [PubMed] [Google Scholar]
- 25.Huang G., Lai E.C., Lau W.Y. Posthepatectomy HBV reactivation in hepatitis B-related hepatocellular carcinoma influences postoperative survival in patients with preoperative low HBV-DNA levels. Ann Surg. 2013;257:490–505. doi: 10.1097/SLA.0b013e318262b218. [DOI] [PubMed] [Google Scholar]
- 26.Yu S.J., Lee J.H., Jang E.S. Hepatocellular carcinoma: high hepatitis B viral load and mortality in patients treated with transarterial chemoembolization. Radiology. 2013;267:638–647. doi: 10.1148/radiol.13121498. [DOI] [PubMed] [Google Scholar]
- 27.Qu L.S., Liu J.X., Zhu J. Risk factors for prognosis of hepatocellular carcinoma after curative resection in patients with low hepatitis B viral load. Ann Hepatol. 2017;16:412–420. doi: 10.5604/16652681.1235484. [DOI] [PubMed] [Google Scholar]
- 28.Absenger G., Szkandera J., Pichler M. A derived neutrophil to lymphocyte ratio predicts clinical outcome in stage II and III colon cancer patients. Br J Canc. 2013;109 doi: 10.1038/bjc.2013.346. 395-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mezquita L., Auclin E., Ferrara R. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018;4:351–357. doi: 10.1001/jamaoncol.2017.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrucci P.F., Ascierto P.A., Pigozzo J. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol. 2016;27:732–738. doi: 10.1093/annonc/mdw016. [DOI] [PubMed] [Google Scholar]
- 31.Liu C., Jia B.S., Zou B.W. Neutrophil-to-lymphocyte and aspartate-to-alanine aminotransferase ratios predict hepatocellular carcinoma prognosis after transarterial embolization. Medicine (Baltim) 2017;96 doi: 10.1097/MD.0000000000008512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou D., Liang J., Xu L.I. Derived neutrophil to lymphocyte ratio predicts prognosis for patients with HBV-associated hepatocellular carcinoma following transarterial chemoembolization. Oncol Lett. 2016;11:2987–2994. doi: 10.3892/ol.2016.4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terashima T., Yamashita T., Iida N. Blood neutrophil to lymphocyte ratio as a predictor in patients with advanced hepatocellular carcinoma treated with hepatic arterial infusion chemotherapy. Hepatol Res. 2015;45:949–959. doi: 10.1111/hepr.12436. [DOI] [PubMed] [Google Scholar]
- 34.Biolato M., Gallusi G., Iavarone M. Prognostic ability of BCLC-B subclassification in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Ann Hepatol. 2018;17:110–118. doi: 10.5604/01.3001.0010.7542. [DOI] [PubMed] [Google Scholar]
- 35.Jun C.H., Yoon J.H., Cho E. Barcelona clinic liver cancer-stage C hepatocellular carcinoma: a novel approach to subclassification and treatment. Medicine (Baltim) 2017;96 doi: 10.1097/MD.0000000000006745. [DOI] [PMC free article] [PubMed] [Google Scholar]