Abstract
Purpose
To present the early results of pirarubicin-eluting microsphere transarterial chemoembolization (PE-TACE) for patients with unresectable hepatocellular carcinoma (HCC).
Materials and methods
We retrospectively analyzed 55 consecutive patients with HCC who received PE-TACE between April 1, 2015 and August 30, 2016. The complication rate, tumor response rate, progression-free survival (PFS), and overall survival (OS) were analyzed.
Results
Adverse events were generally mild and included abdominal pain and fever, although a major complication was reported in 1 patient (1.8%). During a median follow-up of 10.0 months (range, 3.0–24.0 months), 14 patients (25.5%) achieved a complete tumor response, 25 (45.5%) had a partial response, 9 (16.4%) showed stable disease, and 7 (12.7%) had disease progression. The 1-month overall response rate was 70.9%, and the local tumor response rate was 89.0%. The 1-month tumor response rate was 100% for Barcelona Clinic Liver Cancer (BCLC) stage A or B disease and 62.8% for BCLC stage C disease. The median PFS was 6.1 months (95% confidence interval [95%CI], 3.4–8.8 months; range, 1.0–24.0 months). The median OS was 11.0 months (95%CI, 7.1–14.9 months; range, 2.0–24.0 months). Kaplan-Meier analysis (log-rank test) found significant differences in OS between patients grouped by tumor number (P = 0.006), tumor size (P = 0.035), and Eastern Cooperative Oncology Group (ECOG) score (P = 0.005). The tumor number (1 vs. ≥2) was the only factor independently associated with OS (hazard ratio [HR], 2.867; 95%CI, 1.330–6.181; P = 0.007).
Conclusions
PE-TACE for unresectable HCC may be safe, with favorable tumor response rates and survival time, especially in patients with a single large tumor. Longer follow-up using a larger series is necessary to confirm these preliminary results.
Keywords: Hepatocellular carcinoma, Therapeutic chemoembolization, Drug-eluting chemoembolization, Microspheres, Treatment outcome
Introduction
Hepatocellular carcinoma (HCC) is a major cause of cancer-related mortality, andone of the most common and lethal malignancies worldwide.1 Over 700,000 new cases of HCC are diagnosed annually, many of which occur in China and other parts of the Asia-Pacific region.2,3 Only about 30% of patients with HCC are diagnosed at an early stage and can receive curative therapy such as surgical resection.4 For unresectable HCC, transcatheter arterial chemoembolization (TACE) is the current standard of care for patients with multinodular disease, as defined by the Barcelona Clinic Liver Cancer (BCLC) system.4, 5, 6, 7 However, the median overall survival (OS) for patients with HCC treated with TACE is only 9–18 months,8, 9, 10 highlighting the need for improvements in the techniques used to treat unresectable HCC.
Conventional TACE (cTACE) is performed by injecting a mixture of lipiodol and chemotherapeutic agent, such as doxorubicin, before using embolic materials to occlude the tumor-supplying arteries. Pirarubicin (tetrahydropyranoyl-adriamycin) is an antineoplastic cytotoxic agent that is less cardiotoxic than doxorubicin11,12 and exhibits activity against some doxorubicin-resistant cell lines.13 When used to treat hematologic malignances, intravenous infusion of pirarubicin was associated with lower incidences of alopecia, gastrointestinal toxicities, and arrhythmia than doxorubicin-based chemotherapy regimens.11
Drug-eluting chemoembolization (deTACE) has been developed to optimize TACE by increasing the concentration of chemotherapeutic drug in target lesions and allowing controlled drugrelease. According to previous studies comparing cTACE and deTACE, the peak plasma concentration (Cmax) and area under the plasma drug concentration-time curve (AUC) of doxorubicin were significantly lower for deTACE than for cTACE, indicating a favorable pharmacokinetic profile for deTACE.14,15 This was clinically reflected in the post-hoc analysis of the PRECISION V trial, which showed that deTACE (with doxorubicin) was associated with less liver, gastrointestinal, and cardiac toxicity than cTACE in patients with intermediate HCC.16 Several other clinical investigations have determined that deTACE can achieve a disease control rate of 79%–89% at 1 month post-therapy,15,17, 18, 19 and is associated with a low incidence of adverse events, most of which are mild in severity.17,20,21 Direct comparisons of deTACE and cTACE have reported either comparable outcomes20,22, 23, 24, 25 or better outcomes for deTACE in terms of survival26,27 and tumor response.27,28 Furthermore, most studies have found that the safety profiles of deTACE and cTACE are broadly similar,20,22,26 although deTACE was associated with lower hepatotoxicity,22,24,28 fewer doxorubicin-related adverse effects,28 less post-procedural abdominal pain25, and shorter hospital stays.24
A variety of drug-eluting microspheres are available29;however, most previous studies have focused on DC-Beads (Biocompatibles, Farnham, UK), whereas clinical investigations of HepaSphere (Merit Medical, South Jordan, UT, USA) are more limited.15,17,20, 21, 22 Notably, clinical data for the efficacy and safety of HepaSphere in Chinese patients with unresectable HCC are rare. In addition, no published studies have reported the use of pirarubicin-eluting microsphere TACE (PE-TACE) in patients with HCC, despite the evidence that pirarubicin may provide a better survival benefit than doxorubicin when used with cTACE.13,30,31 Therefore, the aim of our study was to retrospectively assess the clinical outcomes and safety-related data in patients with unresectable HCC treated with PE-TACE using HepaSphere. The primary endpoints were tumor response and safety profile, and the secondary endpoints were OS and progression-free survival (PFS). In addition, the prognostic factor(s) for OS were evaluated.
Materials and methods
Patient selection
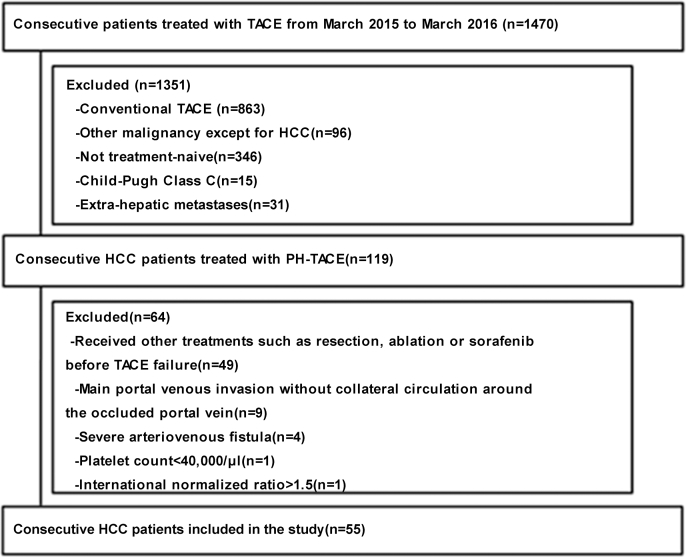
This retrospective study screened consecutive HCC patients treated with TACE in two hospitals (the Third Affiliated Hospital, Sun Yat-Sen University and Ling-nan Hospital, Sun Yat-Sen University) between April 1, 2015 and August 30, 2016. The study participants were enrolled according to predefined eligibility criteria. The institutional review board of Sun Yat-Sen University approved this study, and written informed consent was waived given the retrospective nature of the study.
The eligibility criteria were as follows: 1) HCC diagnosis confirmed by pathology or according to the current guidelines of the American Association for the Study of Liver Diseases32; 2) unresectable/non-ablatable HCC due to tumor burden or comorbidities; 3) treatment-naïve patient receiving PE-TACE as their initial therapy; 4) no extra-hepatic metastases; 5) Eastern Cooperative Oncology Group performance status (ECOG PS) 0–2; and 6) Child-Pugh class A or B cirrhosis. The exclusion criteria were as follows: 1) Tumor invasion of the main portal vein without collateral circulation around the occluded portal vein; 2) severe arteriovenous fistula; 3) platelet count <40,000/μL; and 4) international normalized ratio >1.5.
Treatment procedures
The TACE procedures were performed by two interventional oncologists (M.S.H. and Z.R.L.) with more than 10 years of experience in interventional techniques. Arteriography was performed under local anesthesiain order to identify the accessory arteries and then the TACE procedure was performed with a 5-F RH catheter (Cook Medical, Bloomington, IN,USA) super selectively in the tumor-feeding arteries using a microcatheter (Renegade, Boston Scientific, MA, USA; Progreat, Terumo, Tokyo, Japan) as described in our previous study.33
The HepaSphere microspheres used in this study were loadable microspheres with a dry caliber of 30–60 μm that expanded to 120–240 μm after loading with pirarubicin. For drug loading, a 2.5 mg/mL solution of pirarubicin was prepared by adding 4 mL of normal saline to a vial containing 10 mg pirarubicin. The pirarubicin solution was loaded into the HepaSphere microspheres by mixing gently 5–10 times and then allowing the mixture to stand for 50 min. The median dosage of pirarubicin used in this study was 40 mg (range, 20–60 mg). After extraction of the supernatant, the pirarubicin-eluting HepaSphere microspheres were placed into a syringe, 20 mL of nonionic contrast medium (Iopromide, Bayer, Leverkusen, Germany) was added, and the microspheres and contrast medium were mixed gently until homogeneity was achieved.
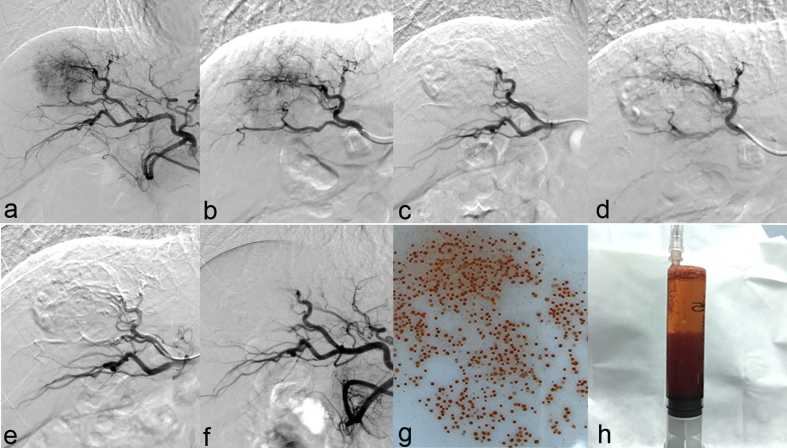
During TACE, the pirarubicin-loaded HepaSphere microspheres were injected slowly at a rate of 1 mL/min until stasis was observed. A waiting time of 5 min was used to allow the microspheres to redistribute in the feeding arteries. Angiography was performed to ensure complete devascularization of the feeding arteries, and additional microspheres were injected until stasis was achieved. If angiography revealed staining of the tumor after two vials of HepaSphere had been used, Embosphere microspheres (100–300 μm and 300–500 μm in diameter, Merit Medical Systems, South Jordan, UT, USA) were injected into the feeding arteries. Representative angiography images acquired during the injection of HepaSphere microspheres, and images showing the HepaSphere microspheres, are presented in Fig. 1.
Fig. 1.
Pirarubicin-eluting HepaSphere microspheres. (a) Angiography showed hepatocellular carcinoma in the right liver. (b) Stasis was observed after embolization. (c) Repeat angiography performed 5 min later showed recanalization of the tumor-feeding arteries. (d) Repeat angiography performed 5 min after additional embolization showed stasis of the tumor arteries. (e) Repeat angiography confirmed stasis of the tumor arteries. (f–g) Pirarubicin-eluting HepaSphere beads. The diameter of the beads used in this study was 120–240 μm.
Follow-up
The follow-up period was defined as the duration from the date of the initial treatment until death or the last visit, up to January 30, 2017. According to the standard follow-up protocol used in our centers, four-phase contrast-enhanced abdominal computed tomography or magnetic resonance images were obtained on the first, third, sixth, ninth, and twelfth month after PE-TACE for the first year and every 6 months thereafter. Repeated PE-TACE treatments were performed on demand for patients with viable tumors until TACE failure, as defined by the Liver Cancer Study Group of Japan,34 was confirmed. No other chemotherapy regimens were used during the period of PE-TACE therapy. However, if TACE failure occurred, subsequent treatment included sorafenib or best supportive care based on the clinical judgment of our multidisciplinary teams. Antiviral therapy was routinely administered to patients with hepatitis virus infection.
Safety assessment
A primary outcome of our study was safety, which included treatment-related death, major complications, and minor complications. Treatment-related death was defined as death from any cause within 30 days of each PE-TACE treatment. Adverse events were classified using Common Terminology Criteria for Adverse Events (CTCAE)version 4.0.35 Minor and major complications were defined in accordance with the Society of Interventional Radiology Classification System for Complications by Outcome36:Minor complications were classified as grade A (no therapy, no consequence) or B (no therapy, no consequence, overnight admission for observation only), and major complications were classified as grade C (required therapy, minor hospitalization <48 h), D (required major therapy, unplanned increase in level of care, prolonged hospitalization), E (permanent adverse sequelae), or F (death).
As an additional safety evaluation, the following laboratory investigations were performed (using venous blood samples) at baseline, 3 days post-TACE, and 30 days post-TACE: Albumin, alanine transaminase (ALT), aspartate transaminase (AST), cholinesterase (CHE), gamma-glutamyltransferase (GGT), platelet count (PLT), total bilirubin (TBIL), and white blood cell count (WBC).
Efficacy assessment
The efficacy outcomes measured were tumor response rates, PFS, and OS. The tumor response was evaluated 1, 3, and 6 months after the initial TACE using the modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria37; the evaluation was performed independently by two experienced radiologists (G.S.H. and W.C.). Target and non-target lesions were defined in accordance with the mRECIST criteria. The tumor response at each time point was classified as a complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). For the analysis, the tumor response in each patient was defined as the best tumor response of the three time points. The overall response rate (ORR) and disease control rate (DCR) were defined as CR + PR and CR + PR + SD, respectively. In addition, the serum alpha-fetoprotein (AFP) level was measured before (baseline) and 1month after PE-TACE. The PFS was defined as the time interval from the first PE-TACEto the date of tumor progression, death from any cause, or the last visit. The OS was defined as the time interval from the first PE-TACE to the date of death from any cause, or the last visit.
Statistical analysis
Data processing and statistical analyses were performed using SPSS 20.0 software (IBM Corp., Armonk, NY, USA). Quantitative variables were tested for normality andare presented as the mean ± standard deviation and range if normally distributed or as median (range) if non-normally distributed. For tumor response, subgroup analyses were performed based on the tumor lesion type (target or non-target, as defined by the mRECIST criteria) and BCLC stage (A, B, or C). The cumulative PFS and OS were calculated using the Kaplan-Meier method, and compared between subgroups using the log-rank test. The factors associated with thePFS and OS were identified using univariate and multivariate Cox regression models. The following parameters were enrolled in the univariate analysis: Age (<55 years vs. ≥55 years), number of tumors (1 vs. ≥2), largest tumor diameter (<100 mm vs. ≥100 mm), ECOG score (0/1 vs. 2), BCLC stage (A/B vs. C), Child-Pugh class (A vs. B), macroscopic vascular invasion (yes vs. no), AFP level (≤400 ng/mL vs. >400 ng/mL), and additional therapy with sorafenib (yes vs. no). Those parameters with P < 0.05 in the univariate analysis were included in the multivariate Cox regression analysis (using the enter method). The HRs and 95%CIs were calculated, and statistical significance was defined as P < 0.05.
Results
Study population
Among the 1470 patients screened, a total of 55 were included in the final analysis (Fig. 2). The demographic, clinical, and tumor characteristics of these 55 patients are summarized in Table 1. The study population was mostly male (85.5%), with a median age of 52.8 years (range, 32–86 years). The majority of the study participants had hepatitis B virus infection (89.1%) and were classified as ECOGPS 1 (56.4%) and Child-Pugh class A (83.6%). Twenty (36.4%) patients had 3 or more tumor lesions, 8 (14.5%) had 2 lesions, and 27 (49.1%) had only 1 lesion. The diameters of the largest tumor in each patient ranged from 8 to 160 mm, with a median size of 60.0 mm. The overall median follow-up was 10.0 months (range, 3.0–24.0 months). A total of 94 sessions of PE-TACE were performed: A single session in 56.4% of patients, 2 sessions in 25.5%, 3 sessions in 12.7%, 4 sessions in 1.8%, and 5 sessions in 6.3%. The median number of PE-TACE sessions was 1, and the mean number of vials of pirarubicin-loaded HepaSphere microspheres used per session was 1.02 (range, 1–2).
Fig. 2.
Enrolment of the study participants. HCC:Hepatocellular carcinoma, PE-TACE:Pirarubicin-eluting microsphere transcatheter arterial chemoembolization, TACE:Transcatheter arterial chemoembolization.
Table 1.
Baseline demographic and clinical characteristics of the study participants.
| Baseline characteristic | Value (n = 55) |
|---|---|
| Age (years), mean ± standard deviation (range) | 52.8 ± 12.5 (32–86) |
| Sex (male/female) | 47/8 |
| Etiology (HBV/other) | 49/6 |
| Cirrhosis (yes/no) | 45/10 |
| BCLC stage (A/B/C) | 7/5/43 |
| Child-Pugh class (A/B) | 46/9 |
| ECOG PS score (0/1/2) | 17/31/7 |
| Largest tumor diameter (mm), median (range) | 60 (8–160) |
| Number of tumors (1/2/≥3) | 27/8/20 |
| Macroscopic vascular invasion (yes/no) | 29/26 |
| Ascites (yes/no) | 6/49 |
| Serum AFP level (<20 ng/mL/≥20 ng/mL) | 17/38 |
| HBV DNA level (<104 IU/mL/≥104 IU/mL) | 31/24 |
| Additional therapy (sorafenib/best supportive care) | 10/45 |
AFP:Alpha-fetoprotein, BCLC: Barcelona Clinic Liver Cancer, ECOG: Eastern Cooperative Oncology Group, HBV:Hepatitis B virus.
Safety
The patients were hospitalized for an average of 3 days (range, 1–15 days) after PE-TACE. There were no procedure-related deaths within 1 month of PE-TACE. The complications observed after treatment are shown in Table 2. The only major complication was massive hydrothorax in one patient (1/55, 1.8%), but the symptoms subsided after percutaneous catheter drainage and albumin infusion. Post-embolization syndrome (PES) occurred as a minor complication in 80.0% of patients, and the symptoms included abdominal pain (70.9%), fever (32.7%), abdominal distention (7.3%), and nausea/vomiting (5.5%). However, if present, PES was mild in severity and self-limiting, without the requirement for any treatment.
Table 2.
Complications after treatment.
| Complication | No. | % |
|---|---|---|
| Pain | 39 | 70.9 |
| Grade A | 28 | 50.9 |
| Grade B | 11 | 20.0 |
| Fever | 18 | 32.7 |
| Grade A | 15 | 27.2 |
| Grade B | 3 | 5.5 |
| Abdominal distention | 4 | 7.3 |
| Grade A | 3 | 5.5 |
| Grade B | 1 | 1.8 |
| Nausea/vomiting | 3 | 5.5 |
| Grade A | 1 | 1.8 |
| Grade B | 2 | 3.6 |
| Ascites | 4 | 7.3 |
| Grade A | 2 | 3.6 |
| Grade B | 2 | 3.6 |
| Hydrothorax | ||
| Grade C | 1 | 1.8 |
Table 3 shows the results of laboratory investigations made before PE-TACE (baseline), 3 days post-TACE, and 30 days post-TACE. There were significant increases in markers of liver injury (AST, ALT, GGT, and TBIL) at 3 days post-TACE (all P < 0.001 vs. baseline), although these changes were asymptomatic. Notably, serum levels of AST, ALT, GGT, and TBIL were not elevated at 30 days post-TACE; indeed, the levels of the three enzymes at 30 days post-TACE were significantly lower than at baseline (P < 0.05), indicating resolution of any treatment-related hepatotoxicity.
Table 3.
Results of laboratory investigations.
| Variable | Baseline (n = 55) | 3 days post-TACE (n = 55) | P | 30 days post-TACE (n = 55) | P |
|---|---|---|---|---|---|
| ALT (U/L) | 60.6 ± 109.7 | 344.5 ± 1121.5 | <0.001 | 45.7 ± 72.7 | 0.027 |
| AST (U/L) | 55.6 ± 37.8 | 283.1 ± 368.5 | <0.001 | 46.3 ± 27.1 | 0.009 |
| Albumin (g/L) | 38.1 ± 4.6 | 36.2 ± 4.7 | <0.001 | 37.3 ± 5.1 | 0.157 |
| TBIL (μmol/L) | 27.6 ± 60.1 | 37.5 ± 58.7 | <0.001 | 29.1 ± 67.4 | 0.671 |
| GGT (g/L) | 136.2 ± 124.7 | 141.3 ± 128.0 | <0.001 | 107.1 ± 75.8 | 0.009 |
| CHE (U/L) | 5385.9 ± 2068.0 | 4782.6 ± 2124.3 | <0.001 | 4689.3 ± 2159.6 | 0.001 |
| PLT ( × 109/L) | 174.4 ± 103.3 | 153.0 ± 88.9 | <0.001 | 165.0 ± 105.2 | 0.391 |
| WBC ( × 109/L) | 6.5 ± 2.8 | 9.6 ± 6.0 | <0.001 | 5.4 ± 2.3 | <0.001 |
Data are presented as the mean ± standard deviation.
ALT: Alanine transaminase, AST: Aspartate transaminase, CHE: Cholinesterase, GGT: Gamma-glutamyltransferase, PLT: Platelet count, TACE: Transcatheter arterial chemoembolization, TBIL: Total bilirubin, WBC: White blood cell count. The P values are for comparisons with the respective baseline value.
Efficacy
Tumor response rates
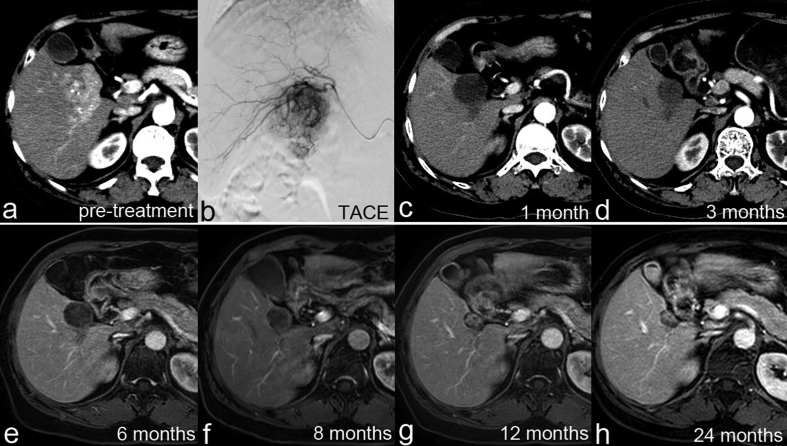
Data for the overall and local tumor response rates at 1, 3, and 6 months after initial TACE are summarized in Table 4, Table 5. According to the mRECIST criteria, 14 (25.5%) patients achieved CR (Fig. 3), 25 (45.5%) patients showed PR, 9 (16.4%) patients had SD, and 7 (12.7%) patients had PD. At 1month post-TACE, the ORR was 70.9%, the local tumor response in the target lesion was 89.1% (49/55), and the DCR was 87.3% (Table 4). The ORR and DCR were 60.4% and 66.0%, respectively, at 3 months post-TACE and 60.0% and 62.5%, respectively, at 6 months post-TACE (Table 5).
Table 4.
Tumor response according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria.
| Overall tumor response (n = 55) | Target tumor response (n = 55) | Non-target tumor response (n = 32) | Newly developed tumor (n = 4) | BCLC stage A (n = 7) | BCLC stage B (n = 5) | BCLC stage C (n = 43) | |
|---|---|---|---|---|---|---|---|
| CR | 14 (25.5%) | 15 (27.3%) | 2 (6.3%) | 6 (85.7%) | 0 | 8 (18.6%) | |
| PR | 25 (45.5%) | 34 (61.8%) | 11 (34.4%) | 1 (14.3%) | 5 (100%) | 19 (44.2%) | |
| SD | 9 (16.4%) | 5 (9.1%) | 13 (40.6%) | 0 | 0 | 9 (20.9%) | |
| PD | 7 (12.7%) | 1 (1.8%) | 6 (18.7%) | 4 (100%) | 0 | 0 | 7 (16.3%) |
| ORR | 39 (70.9%) | 49 (89.1%) | 13 (40.6%) | 7 (100%) | 5 (100%) | 27 (62.8%) | |
| DCR | 48 (87.3%) | 54 (98.2%) | 26 (81.3%) | 7 (100%) | 5 (100%) | 36 (83.7%) |
Data are presented as n (%). The tumor response for each patient was assessed as the best tumor response during the entire follow-up period. BCLC: Barcelona Clinic Liver Cancer, CR:Complete response, DCR:Disease control rate, ORR:Overall response rate, PD:Progressive disease, PR:Partial response, SD:Stable disease. The ORR was defined as CR + PR; DCR was defined as CR + PR + SD.
Table 5.
Rates of overall tumor response according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria at 1, 3, and 6 months after the first transcatheter arterial chemoembolization.
| Follow-up | CR | PR | SD | PD | ORR | DCR |
|---|---|---|---|---|---|---|
| 1 month (n = 55) | 9 (16.4%) | 30 (54.5%) | 9 (16.4%) | 7 (12.7%) | 39 (70.9%) | 48 (87.3%) |
| 3 months (n = 53a) | 8 (15.1%) | 24 (45.3%) | 3 (5.7%) | 18 (34.0%) | 32 (60.4%) | 35 (66.0%) |
| 6 months (n = 40b) | 12 (30.0%) | 12 (30.0%) | 1 (2.5%) | 15 (37.5%) | 24 (60.0%) | 25 (62.5%) |
Data are presented as n (%). CR:Complete response, DCR:Disease control rate, ORR:Overall response rate, PD:Progressive disease, PR:Partial response, SD:Stable disease. ORR was defined as CR + PR; DCR was defined as CR + PR + SD.
A total of 2 patients died before the 3-month follow-up.
A total of 15 patients died before the 6-month follow-up.
Fig. 3.
Imaging data from a patient showing a complete tumor response. (a) Contrast-enhanced computed tomography (CT) image showing hepatocellular carcinoma in the right liver. (b–h) Follow-up CT images at different time points (1.5, 4.5, 6, 8, 11, 14, and 25 months, respectively) indicated a complete tumor response to therapy.
Sub-group analysis (Table 4) showed that the 1-month ORRs for BCLC stages A, B, and C were 100%, 100%, and 62.8%, respectively. For patients with BCLC stage A disease, 85.7% achieved CR and 14.3% had PR. All patients with BCLC stage B disease showed PR. For patients with BCLC stage C disease, 18.6% had CR, 44.2% had PR, and 20.9% had SD; the ORR and DCR were 62.8% and 83.7%, respectively.
AFP level
A total of 38 (69.1%) patients had AFP-positive HCC (AFP levels ≥20 ng/mL) and 17 (30.9%) had AFP-negative HCC. Among the 38 AFP-positive patients, 9 (23.7%) exhibited an increase in AFP levels at 1 month post-TACE, whereas 29 (76.3%) showed a decrease in AFP levels at 1 month post-TACE; in 5 of the 29 cases showing a decrease, AFP fell to within the normal range. Two of the 17 patients with AFP-negative HCC (11.8%) showed an elevation of AFP to ≥20 ng/mL at 1 month post-TACE. The median AFP values before and after PE-TACE were 268 ng/mL and 79 ng/mL, respectively, indicating a clinical response to PE-TACE (P < 0.05).
Survival
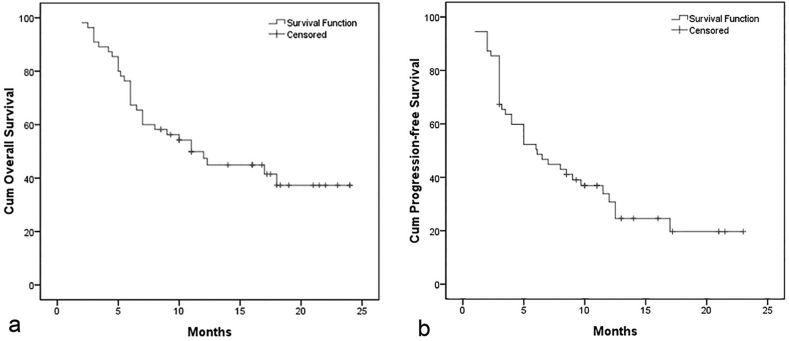
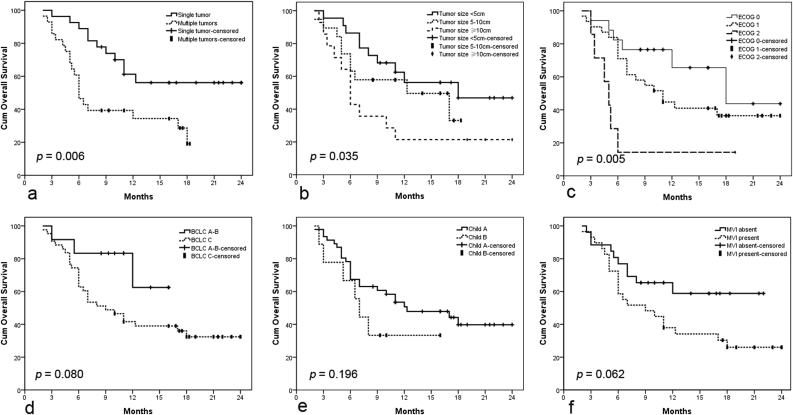
During the median follow-up period of 10.0 months (range, 3.0–24.0 months), 27 patients died and 15 patients survived; 25 patients died as a result of tumor progression, and the other 2 died of severe hepatic dysfunction. The median overall survival (OS) was 11.0 months (95%CI, 7.1–14.9 months; range, 2.0–24.0 months), with 6 month and 1 year OS rates of 75.0% and 53.1%, respectively (Fig. 4a). In univariate analysis, the tumor number(HR, 2.663; 95%CI, 1.269–5.588;P = 0.006), tumor size(HR, 1.465; 95%CI, 1.012–2.121;P = 0.035), and ECOG PS score (HR, 1.829; 95%CI, 1.158–2.888;P = 0.005) were significantly associated with OS (Table 6). However, in multivariate analysis, the tumor number (1 vs. ≥2: HR, 2.867; 95%CI, 1.330–6.181; P = 0.007) was the only factor significantly associated with OS (Table 6). Kaplan-Meier analysis (with the log-rank test) found significant differences in OS between patients grouped on the basis of tumor number (P = 0.010), tumor size (P = 0.035), and ECOG PS score (P = 0.010), but not those grouped by BCLC stage, Child-Pugh class, or presence/absence of macroscopic vascular invasion (Fig. 5).
Fig. 4.
Kaplan-Meier curves illustrating overall survival and progression-free survival in the 55 patients treated by pirarubicin-eluting microsphere transcatheter arterial chemoembolization. (a) The median overall survival was 11.0 months (95% confidence interval, 7.1–14.9 months; range, 2.0–24.0 months). The 6-month and 1-year survival rates were 75.0% and 53.1%, respectively. (b) The median progression-free survival was 6.1 months (95% confidence interval, 3.4–8.8 months; range, 1.0–24.0 months).
Table 6.
Univariate and multivariate analysis of factors associated with overall survival.
| Variable | n | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | ||
| Age | 0.912 | ||||||
| <55 years | 33 | 0.961 | 0.466–1.980 | ||||
| ≥55 years | 22 | 1 | |||||
| Tumor number | 0.006 | 0.007 | |||||
| 1 | 27 | 2.663 | 1.269–5.588 | 2.867 | 1.330–6.181 | ||
| ≥2 | 28 | 1 | 1 | ||||
| Tumor size | 0.035 | 0.094 | |||||
| <100 mm | 41 | 1.465 | 1.012–2.121 | 1.418 | 0.942–2.134 | ||
| ≥100 mm | 14 | 1 | 1 | ||||
| ECOG score | 0.005 | 0.099 | |||||
| 0–1 | 48 | 1.829 | 1.158–2.888 | 1.516 | 0.925–2.485 | ||
| 2 | 7 | 1 | 1 | ||||
| BCLC stage | 0.080 | ||||||
| A–B | 12 | 2.741 | 0.829–9.067 | ||||
| C | 43 | 1 | |||||
| Child-Pugh class | 0.196 | ||||||
| A | 46 | 1.787 | 0.721–4.429 | ||||
| B | 9 | 1 | |||||
| MVI | 0.062 | ||||||
| Absent | 26 | 2.003 | 0.940–4.267 | ||||
| Present | 29 | 1 | |||||
| AFP level | 0.352 | ||||||
| ≤400 ng/mL | 30 | 1.390 | 0.685–2.821 | ||||
| >400 ng/mL | 25 | 1 | |||||
| Additional therapy with sorafenib | 0.707–3.549 | 0.264 | |||||
| Yes | 10 | 1.584 | |||||
| No | 45 | 1 | |||||
95%CI: 95% confidence interval, AFP:Alpha fetoprotein, BCLC: Barcelona Clinic Liver Cancer, ECOG: Eastern Cooperative Oncology Group, HR:Hazard ratio,MVI:Macroscopic vascular invasion.
Fig. 5.
Kaplan-Meier analysis of factors affecting overall survival (OS). (a) Log-rank analysis of OS stratified according to tumor number (single vs. multiple, P = 0.006). (b) Log-rank analysis of OS stratified according to tumor size (<5 cm vs. 5–10 cm vs. ≥10 cm, P = 0.035). (c) Log-rank analysis of OS stratified according to ECOG score (0 vs. 1 vs. 2, P = 0.005). (d) Log-rank analysis of OS stratified according to BCLC stage (A/B vs. C, P = 0.080). (e) Log-rank analysis of OS stratified according to Child-Pugh class (A vs. B, P = 0.196). (f) Log-rank analysis of OS stratified according to macroscopic vascular invasion (absent vs. present, P = 0.062).
The median PFS was 6.1 months (95%CI, 3.4–8.8 months; range, 1.0–24.0 months; Fig. 4b). In univariate analysis, the tumor size, tumor number, macroscopic vascular invasion, BCLC stage, and ECOG PS score were associated with PFS. In multivariate analysis, the tumor number (1 vs. ≥2: HR, 2.235; 95%CI, 1.173–4.259;P = 0.014), tumor size(<5 cm vs. ≥5 cm: HR, 2.375; 95%CI, 1.187–4.750; P = 0.014), and BCLC stage (A/B vs. C: HR, 7.406; 95%CI, 1.781–30.796; P = 0.006) were independently associated with PFS.
Discussion
To the best of our knowledge, this is the first study to use pirarubicin-eluting HepaSphere microspheres in the treatment of unresectable HCC by TACE. The tumor response rates obtained in our research suggest that PE-TACE may show clinical efficacy in patients with unresectable HCC. The 1-month ORR was 70.9% and the CR rate was 25.5%. Our results are comparable to those of previous studies using TACE with doxorubicin-eluting beads (DEB-TACE) to treat HCC, which reported ORR and CR rates as 51.6%–88.7% and 17.8–51.4%, respectively(25, 28, 38), despite differences between the studies in terms of the baseline characteristics of the study participants. Most previous investigations reporting a high tumor response rate included patients with early-or intermediate-stage HCC(28, 38). In our study, most of the enrolled patients (43/55, 78.2%) had advanced-stage HCC (BCLC stage C), and a notable finding of the subgroup analysis was that promising tumor response rates were observed in patients with BCLC stage C HCC: ACR rate of 18.6%, a PR rate of 44.2%, an ORR of 62.8%, and a DCR of 83.7%.For patients in our study with early-or immediate-stage HCC (BCLC A or B), the CR rate was 50% and the 1-month ORR and DCR were both 100%. Although direct comparisons between studies should be made with caution, our tumor response rates are similar, if not higher, to those reported in 45 patients with BCLC stage A-B HCC treated with doxorubicin-loaded HepaSphere TACE (CR rate and ORR of 17.8% and 68.9%, respectively).15 Moreover, the 1-month target tumor response was as high as 89.1% in our study. One possible reason for the apparent higher tumor response in our study than in the report of Malagari et al.15 might be our use of pirarubicin rather than doxorubicin. Pirarubicin has been reported to have a greater cytotoxic effect than doxorubicin on HepG2 and Hu-H7 human hepatoma cell lines cultured in vitro.13 Furthermore, patients with HCC treated with cTACE and pirarubicin had better survival than those treated with cTACE and doxorubicin(30). Another reason might be the use of an optimal microsphere size (120–240 μm) in our study,39 since the therapeutic action of drug-eluting microspheres seems to be related to microsphere diameter.40 Optimally sized microspheres might be able to penetrate better into the tumor vascular network and thereby exert a stronger chemoembolization effect; hence, greater tumor necrosis might be achieved for microspheres 120–240 μm in size than for larger beads (≥300 μm).40 Indeed, a previous study found that DEB-TACE using microspheres of a 100–300 μm diameter was associated with a significantly higher survival rate and lower complication rate than DEB-TACE using larger beads(300–500 μm or 500–700 μm).40 A third reason for the good tumor response rates observed in our study may be the use of a high-quality PE-TACE procedure; a specialized team of experienced interventional radiologists performed all of our PE-TACE procedures using a standard protocol and super-selective technique. Furthermore, we injected the microspheres very slowly under free flow and used repeat angiography to confirm the embolization end-point. Overall, our preliminary results suggest that pirarubicin may be a promising chemotherapeutic for use in deTACE. Nonetheless, additional prospective studies directly comparing DEB-TACE and PE-TACE are needed.
Since most of our enrolled patients (78.2%) had advanced-stage HCC, we consider the median OS of 11.0 months and 1 year OS rate of 53.1% after PE-TACE to be satisfactory. Our results using PE-TACE are similar to those obtained using sorafenib (a standard therapeutic approach), which provides a median OS of 6.5–10.7 months and a 1 year OS rate of 30.0%–44.0%.41, 42, 43 If our findings are confirmed by additional clinical research, PE-TACE might be considered as an alternative or adjunct therapy to sorafenib since it is well tolerated and would not preclude the use of sorafenib; future randomized controlled trials are warranted to explore this possibility. The OS achieved in our study using PE-TACE to treat advanced-stage HCC was similar to, or perhaps better than, that reported in retrospective analyses of DEB-TACE.39,44 Baur et al. reviewed 14 patients with advanced-stage HCC treated with DEB-TACE and found the median OS to be 9.2 months.44 Furthermore, Gorodetski et al. analyzed data from 113 patients with advanced-stage HCC and portal vein thrombosis and calculated the median OS to be 5.0 months in those treated with c-TACE (n = 95) and 3.3 months in those treated with DEB-TACE(n = 38).39
Our data suggest that it may be feasible to use PE-TACE in the management of HCC to achieve good clinical outcomes. In our study, the most common cause of tumor progression and mortality was intrahepatic progression of daughter lesions rather than target tumor progression, as demonstrated by the local tumor response rate of the target lesions. On the one hand our results indicate good control of the target tumor, but on the other hand our data suggest that it may be difficult to control intrahepatic spread due to advanced tumor stage and poor liver function reserve. This highlights the importance of identifying and treating daughter lesions. Both the univariate and multivariate analyses found that the tumor number (1 versus ≥2) was significantly associated with OS, as previously established.45, 46, 47 The univariate analysis also identified tumor size and ECOG PS score as being associated with OS, consistent with existing publications.48, 49, 50 Taken together, our data imply that the best candidate for PE-TACE is a single and large HCC (>5 cm). In addition, some guidelines suggest that complete remission of a large tumor is hard to achieve using cTACE; thus, deTACE might be a good option for these patients.44,51 The effect of PE-TACE alone was limited in patients with multiple tumors, and further research is needed in order to explore whether the combination of PE-TACE with sorafenib or ablation could provide longer survival benefits than PE-TACE alone.
Our study also revealed that PE-TACE was well tolerated, and there were no treatment-related deaths, which is consistent with other studies of HepaSphere(15, 17, 29). In the present series, there were no cases of cholecystitis, biliary damage, or abscess formation, which have been reported in previous studies of deTACE.14,19,52,53 This may be due to differences in microsphere sizes and materials, since we have found no published studies reporting an association of HepaSphere with biliary damage. Future studies should focus on direct comparisons of safety and efficacy between different microspheres in order to determine the optimal microsphere for use in deTACE procedures. The only major complication in our study was massive hydrothorax in 1 (1.8%) case; we speculate that the massive hydrothorax might have been related to severe hypoalbuminemia after TACE and a poor baseline liver function (Child-Pugh class B8). The symptoms subsided after drainage of the hydrothorax, and the patient recovered; nonetheless, we suggest that close monitoring may be needed for patients with hypoalbuminemia who are treated with deTACE. There was no chemotherapy-related or systemic toxicity after PE-TACE in our study. The most common complication after PE-TACE was PES, which included abdominal pain (70.9%), fever (32.7%), and elevation of alanine transaminase/bilirubin (47.3%). These complications were all mild and self-limiting and did not require treatment. Furthermore, the incidence of minor complications in our study was similar to the value of 60%–80% that was previously reported in patients with advanced HCC receiving cTACE or DEB-TACE.28,38,39,44 However, some studies have observed a lower rate of minor complications (8.4%–18.0%) in patients treated with deTACE(25, 28, 38, 51); this apparent discrepancy may be due to the heavier tumor burden in most of our patients (a median tumor size of 6 cm, multiple tumors in 50.9% of patients, and macroscopic vascular invasion in 52.7%).
There are a few potential limitations of our study. Firstly, this was a single-center, retrospective study that enrolled patients with different stages of HCC and with diverse baseline characteristics. Secondly, the sample size was small and the follow-up time was relatively short. Thus, our study maybe subject to selection and/or reporting bias, and longer follow-up on larger series is mandatory to confirm these preliminary results. Finally, our study lacked a control group, for example patients treated with c-TACE or sorafenib. Further research is needed to extend our observations.
Conclusions
Our findings provide preliminary evidence that PE-TACE may be a feasible and safe treatment for unresectable HCC that confers a good tumor response rate and long survival time, especially in patients with a single and large (>5 cm) HCC.
Author contribution
Ming-sheng Huang: Study supervision, Study concept and design, Administrative, technical, or material support.
Zheng-ran Li: Study concept and design.
Ming-jun Bai: Acquisition of data, Statistical analysis.
Tao Pan: Analysis and interpretation of data, Drafting of the manuscript, Statistical analysis.
Jun-wei Chen: Analysis and interpretation of data, Critical revision of the manuscript for intellectual content.
Chu-ren Zhou: Drafting of the manuscript, Critical revision of the manuscript for intellectual content.
Zhao-lin Zeng: Critical revision of the manuscript for intellectual content. Duo Zhu: Critical revision of the manuscript for intellectual content.
Chun Wu: Critical revision of the manuscript for intellectual content. Chu-ren Zhou: Critical revision of the manuscript for intellectual content.
Ming-an Li: Critical revision of the manuscript for intellectual content.
Zai-bo Jiang: Critical revision of the manuscript for intellectual content, Administrative, technical, or material support.
Contributor Information
Zhengran Li, Email: andyreede@msn.com.
Mingsheng Huang, Email: huangmsh@mail.sysu.edu.cn.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics. Ca - Cancer J Clin. 2015;65(2015):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J., Gores G.J., Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844–855. doi: 10.1136/gutjnl-2013-306627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venook A.P., Papandreou C., Furuse J. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. The Oncologist. 2010;15(Suppl 4):5–13. doi: 10.1634/theoncologist.2010-S4-05. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J., Reig M., Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150:835–853. doi: 10.1053/j.gastro.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 5.Omata M., Lesmana L.A., Tateishi R. Asian Pacific association for the study of the liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439–474. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kudo M., Izumi N., Kokudo N. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice guidelines proposed by the Japan society of hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339–364. doi: 10.1159/000327577. [DOI] [PubMed] [Google Scholar]
- 7.Choi J.Y. Treatment algorithm for intermediate and advanced stage hepatocellular carcinoma: Korea. Oncology. 2011;81(Suppl 1):141–147. doi: 10.1159/000333277. [DOI] [PubMed] [Google Scholar]
- 8.Muhammad A., Dhamija M., Vidyarthi G. Comparative effectiveness of traditional chemoembolization with or without sorafenib for hepatocellular carcinoma. World J Hepatol. 2013;5:364–371. doi: 10.4254/wjh.v5.i7.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao X., Yan D., Zeng H. Concurrent sorafenib therapy extends the interval to subsequent TACE for patients with unresectable hepatocellular carcinoma. J Surg Oncol. 2016;113:672–677. doi: 10.1002/jso.24215. [DOI] [PubMed] [Google Scholar]
- 10.Bettinger D., Spode R., Glaser N. Survival benefit of transarterial chemoembolization in patients with metastatic hepatocellular carcinoma: a single center experience. BMC Gastroenterol. 2017;17:98. doi: 10.1186/s12876-017-0656-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhai L., Guo C., Cao Y. Long-term results of pirarubicin versus doxorubicin in combination chemotherapy for aggressive non-Hodgkin's lymphoma: single center, 15-year experience. Int J Hematol. 2010;91:78–86. doi: 10.1007/s12185-009-0461-8. [DOI] [PubMed] [Google Scholar]
- 12.Niitsu N., Umeda M. Response and adverse drug reactions to combination chemotherapy in elderly patients with aggressive non-Hodgkin's lymphoma: comparison of CHOP, COP-BLAM, COP-BLAM III, and THP-COPBLM. Eur J Haematol. 1999;63:337–344. doi: 10.1111/j.1600-0609.1999.tb01137.x. [DOI] [PubMed] [Google Scholar]
- 13.Favoulet P., Cercueil J.P., Faure P. Increased cytotoxicity and stability of Lipiodol-pirarubicin emulsion compared to classical doxorubicin-Lipiodol: potential advantage for chemoembolization of unresectable hepatocellular carcinoma. Anti Cancer Drugs. 2001;12:801–806. doi: 10.1097/00001813-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Varela M., Real M.I., Burrel M. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–481. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 15.Malagari K., Pomoni M., Moschouris H. Chemoembolization of hepatocellular carcinoma with HepaSphere 30-60 mum. Safety and efficacy study. Cardiovasc Interv Radiol. 2014;37:165–175. doi: 10.1007/s00270-013-0777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogl T.J., Lammer J., Lencioni R. Liver, gastrointestinal, and cardiac toxicity in intermediate hepatocellular carcinoma treated with PRECISION TACE with drug-eluting beads: results from the PRECISION V randomized trial. AJR Am J Roentgenol. 2011;197:W562–W570. doi: 10.2214/AJR.10.4379. [DOI] [PubMed] [Google Scholar]
- 17.Sun J.H., Zhou G.H., Zhang Y.L. Chemoembolization of liver cancer with drug-loading microsphere 50-100mum. Oncotarget. 2017;8:5392–5399. doi: 10.18632/oncotarget.14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aliberti C., Carandina R., Sarti D. Chemoembolization adopting polyethylene glycol drug-eluting embolics loaded with doxorubicin for the treatment of hepatocellular carcinoma. AJR Am J Roentgenol. 2017;209:430–434. doi: 10.2214/AJR.16.17477. [DOI] [PubMed] [Google Scholar]
- 19.Sattler T., Bredt C., Surwald S. Efficacy and safety of drug eluting bead TACE with microspheres <150 mum for the treatment of hepatocellular carcinoma. Anticancer Res. 2018;38:1025–1032. doi: 10.21873/anticanres.12318. [DOI] [PubMed] [Google Scholar]
- 20.Duan F., Wang E.Q., Lam M.G. Superselective chemoembolization of HCC: comparison of short-term safety and efficacy between drug-eluting LC beads, Quadra Spheres, and conventional ethiodized oil emulsion. Radiology. 2016;278:612–621. doi: 10.1148/radiol.2015141417. [DOI] [PubMed] [Google Scholar]
- 21.Odisio B.C., Ashton A., Yan Y. Transarterial hepatic chemoembolization with 70-150 microm drug-eluting beads: assessment of clinical safety and liver toxicity profile. J Vasc Interv Radiol. 2015;26:965–971. doi: 10.1016/j.jvir.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kucukay F., Badem S., Karan A. A single-center retrospective comparison of doxorubicin-loaded HepaSphere transarterial chemoembolization with conventional transarterial chemoembolization for patients with unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2015;26:1622–1629. doi: 10.1016/j.jvir.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Kloeckner R., Weinmann A., Prinz F. Conventional transarterial chemoembolization versus drug-eluting bead transarterial chemoembolization for the treatment of hepatocellular carcinoma. BMC Canc. 2015;15:465. doi: 10.1186/s12885-015-1480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arabi M., BenMousa A., Bzeizi K. Doxorubicin-loaded drug-eluting beads versus conventional transarterial chemoembolization for nonresectable hepatocellular carcinoma. Saudi J Gastroenterol. 2015;21:175–180. doi: 10.4103/1319-3767.157571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golfieri R., Giampalma E., Renzulli M. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Canc. 2014;111:255–2264. doi: 10.1038/bjc.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiggermann P., Sieron D., Brosche C. Transarterial Chemoembolization of Child-A hepatocellular carcinoma: drug-eluting bead TACE (DEB TACE) vs. TACE with cisplatin/lipiodol (cTACE) Med Sci Monit. 2011;17:CR189–195. doi: 10.12659/MSM.881714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang K., Zhou Q., Wang R. Doxorubicin-eluting beads versus conventional transarterial chemoembolization for the treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29:920–925. doi: 10.1111/jgh.12439. [DOI] [PubMed] [Google Scholar]
- 28.Lammer J., Malagari K., Vogl T. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Interv Radiol. 2010;33:41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Baere T., Plotkin S., Yu R. An in vitro evaluation of four types of drug-eluting microspheres loaded with doxorubicin. J Vasc Interv Radiol. 2016;27:1425–1431. doi: 10.1016/j.jvir.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Ueno K., Miyazono N., Inoue H. Transcatheter arterial chemoembolization therapy using iodized oil for patients with unresectable hepatocellular carcinoma: evaluation of three kinds of regimens and analysis of prognostic factors. Cancer. 2000;88:1574–1581. [PubMed] [Google Scholar]
- 31.Liu S., Zhang Y., Zhao G. Complete remission of diffuse hepatocellular carcinoma in a young adult after GSP-TACE: a case report. World J Surg Oncol. 2014;12:300. doi: 10.1186/1477-7819-12-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forner A., Llovet J.M., Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 33.Zhu K., Chen J., Lai L. Hepatocellular carcinoma with portal vein tumor thrombus: treatment with transarterial chemoembolization combined with sorafenib--a retrospective controlled study. Radiology. 2014;272:284–293. doi: 10.1148/radiol.14131946. [DOI] [PubMed] [Google Scholar]
- 34.Kudo M., Matsui O., Izumi N. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver Cancer. 2014;3:458–468. doi: 10.1159/000343875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.U.S. Department Of Health And Human Services NIoH, National Cancer Institute. Common Terminology criteria for adverse events (CTCAE) version 4.0. Available at: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf.
- 36.Sacks D., McClenny T.E., Cardella J.F. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9 Pt 2):S199–S202. doi: 10.1097/01.rvi.0000094584.83406.3e. [DOI] [PubMed] [Google Scholar]
- 37.Lencioni R., Llovet J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 38.Sacco R., Bargellini I., Bertini M. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2011;22:1545–1552. doi: 10.1016/j.jvir.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Gorodetski B., Chapiro J., Schernthaner R. Advanced-stage hepatocellular carcinoma with portal vein thrombosis: conventional versus drug-eluting beads transcatheter arterial chemoembolization. Eur Radiol. 2017;27:526–535. doi: 10.1007/s00330-016-4445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prajapati H.J., Xing M., Spivey J.R. Survival, efficacy, and safety of small versus large doxorubicin drug-eluting beads TACE chemoembolization in patients with unresectable HCC. AJR Am J Roentgenol. 2014;203:W706–W714. doi: 10.2214/AJR.13.12308. [DOI] [PubMed] [Google Scholar]
- 41.Yang M., Fang Z., Yan Z. Transarterial chemoembolisation (TACE) combined with endovascular implantation of an iodine-125 seed strand for the treatment of hepatocellular carcinoma with portal vein tumour thrombosis versus TACE alone: a two-arm, randomised clinical trial. J Cancer Res Clin Oncol. 2014;140:211–219. doi: 10.1007/s00432-013-1568-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng A.L., Kang Y.K., Chen Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 43.Llovet J.M., Ricci S., Mazzaferro V. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 44.Baur J., Ritter C.O., Germer C.T. Transarterial chemoembolization with drug-eluting beads versus conventional transarterial chemoembolization in locally advanced hepatocellular carcinoma. Hepat Med. 2016;8:69–74. doi: 10.2147/HMER.S105395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogasawara S., Chiba T., Ooka Y. A prognostic score for patients with intermediate-stage hepatocellular carcinoma treated with transarterial chemoembolization. PLoS One. 2015;10 doi: 10.1371/journal.pone.0125244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu K.T., Wang C.C., Lu L.G. Hepatocellular carcinoma: clinical study of long-term survival and choice of treatment modalities. World J Gastroenterol. 2013;19:3649–3657. doi: 10.3748/wjg.v19.i23.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao J., Li G., Lin S. Prognostic factors of hepatocellular carcinoma patients treated by transarterial chemoembolization. Int J Clin Exp Pathol. 2014;7:1114–1123. [PMC free article] [PubMed] [Google Scholar]
- 48.Tandon P., Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int. 2009;29:502–510. doi: 10.1111/j.1478-3231.2008.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gomaa A.I., Hashim M.S., Waked I. Comparing staging systems for predicting prognosis and survival in patients with hepatocellular carcinoma in Egypt. PLoS One. 2014;9 doi: 10.1371/journal.pone.0090929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalva S.P., Pectasides M., Liu R., al net. Safety and effectiveness of chemoembolization with drug-eluting beads for advanced-stage hepatocellular carcinoma. Cardiovasc Interv Radiol. 2014;37:381–387. doi: 10.1007/s00270-013-0654-7. [DOI] [PubMed] [Google Scholar]
- 51.Grosso M., Vignali C., Quaretti P. Transarterial chemoembolization for hepatocellular carcinoma with drug-eluting microspheres: preliminary results from an Italian multicentre study. Cardiovasc Interv Radiol. 2008;31:1141–1149. doi: 10.1007/s00270-008-9409-2. [DOI] [PubMed] [Google Scholar]
- 52.Lim E.J., Spanger M., Lubel J.S. Gallbladder perforation following transarterial chemoembolisation; a rare but serious complication. Frontline Gastroenterol. 2013;4:135–137. doi: 10.1136/flgastro-2012-100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nawawi O., Hazman M., Abdullah B. Transarterial embolisation of hepatocellular carcinoma with doxorubicin-eluting beads: single centre early experience. Biomed Imaging Interv J. 2010;6:e7. doi: 10.2349/biij.6.1.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]