Abstract
Objective
To explore the clinical significance of serum midkine (MDK) levels for the diagnosis of hepatocellular carcinoma (HCC) and evaluate the efficacy of interventional therapy.
Methods
Eighty-four patients with HCC were enrolled in this retrospective study. They received an interventional treatment. A follow-up was performed every 2 months, using magnetic resonance imaging, to determine whether the treatment should be continued. Serum alpha-fetoprotein (AFP) and MDK levels were measured at the first diagnosis and during the follow-ups, and the HCC detection rates based on the cutoff values of these two measurements were compared. The relationships between AFP and MDK and the clinical tumor characteristics and changes in APK and MDK before and after treatment were also compared using a rank sum test and χ2 test, respectively. The prognostic significance of MDK for HCC was determined through regression analysis. A two-sided P < 0.05 was considered statistically significant.
Results
MDK expression was detected in 95.24% of the cases. Subgroup analysis revealed MDK expression in 95.35%, 95.12%, 85.19%, 86.67%, and 83.33% of the AFP-positive, AFP-negative, stage A Barcelona clinic liver cancer (BCLC-A), BCLC-A/AFP-positive, and BCLC-A/AFP-negative cases, respectively. MDK expression after the interventional treatment (66.7%) was significantly lower than that before the treatment (95.2%). The mean post-treatment MDK level was 0.67 ng/mL in patients with a positive response to therapy as compared with 3.66 ng/mL in those with no positive response. All patients were followed up for 18 months, and those positive for MDK expression before the intervention were more likely to relapse than patients without MDK expression. Subgroup analysis revealed the highest recurrence rate for patients who were positive for MDK expression before and after treatment.
Conclusions
Serum MDK may serve as a powerful complement to AFP in the diagnosis of HCC. MDK measurement may improve the detection rate of BCLC-A and AFP-negative HCC. Serum MDK may help to determine the vascular invasion and poor clinical staging of HCC tumors. Patients with MDK-positive HCC before treatment may be more prone to postoperative tumor progression.
Keywords: Hepatocellular carcinoma, Midkine, Interventional treatment, Diagnosis, Efficacy evaluation
Introduction
Interventional therapy is one of the main treatment regimens for mid-to-late hepatocellular carcinoma (HCC), which is difficult to cure and tends to relapse after treatment.1 Alpha-fetoprotein (AFP) is the most widely used serum biomarker to detect HCC, However, approximately 30% of the patients with HCC show no expression of AFP.2 Thus, there is a need for a simple and an effective method to assist HCC diagnosis and assess the efficacy and prognosis of HCC treatment. Midkine (MDK), a heparin-binding growth factor, is the product of a highly conserved and developmentally regulated gene that is widely expressed in different cell types. It is strongly induced by retinoic acid in the second trimester, and is therefore named “midkine”.3 The serum level of MDK is low in normal individuals and patients with cirrhosis but rises in patients with HCC.4 Serum MDK level has been reported to be more sensitive and specific for HCC detection than AFP, and is also thought to aid in the early diagnosis of HCC.5 The role of MDK in assessing the effects of interventional therapies on patients with HCC is, however, unclear. In this study, we measured the peripheral blood level of MDK in newly diagnosed patients with HCC before and after an interventional therapy and compared it with imaging results from the same time period to determine the potential applicability of serum MDK testing in the assistive diagnosis of HCC and to investigate the prognostic effects of the intervention.
Materials and methods
Ethical approval
The study was approved by the ethics committee of Henan Cancer Hospital. All clinical practices and observations were conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from each patient before the study was conducted.
This retrospective study enrolled 84 patients with HCC, who were treated with a minimally invasive interventional therapy at Henan Cancer Hospital (Dongming Road No.127, Zhengzhou, China)between January 2016 and December 2017 (Table 1). According to the Barcelona Clinic Liver Cancer (BCLC) staging system,6 27 patients with stage A HCC underwent radiofrequency ablation, 29 patients with stage B HCC underwent transarterial chemoembolization (TACE), and 28 patients with stage C HCC were subjected to TACE combined with sorafenib. The patients were reviewed and followed-up every 2 months. The serum AFP and MDK levels were detected at the first diagnosis and at the time of the follow-up. AFP expression was detected using an electrochemiluminescence immunoassay with an AFP detection kit (Roche Diagnostic Products Co., Ltd, Shanghai, China). The MDK level was measured using a human MDK enzyme -linked immunosorbent assay (ELISA) kit (Huamei Biological Engineering Co., Ltd,Wuhan, China).7 The patients were made to undergo a chest computed tomography plain scan and an upper abdominal magnetic resonance imaging plain scan combined with a dynamic enhancement scan at the time of re-examination in order for us to investigate treatment efficacy according to the mRECIST criteria8 and determine the need for a sequential intervention based on the evaluation results. Patients with stage A and B HCC underwent radiofrequency ablation and TACE, respectively, while those with stage C HCC were subjected to TACE combined with sorafenib.
Table 1.
Clinical and pathological characteristics of HCC patients.
| Characteristics | Group | HCC(number) |
|---|---|---|
| Sex | ||
| Male | 62 | |
| Female | 22 | |
| Age (years) | ||
| <60 | 45 | |
| ≥60 | 39 | |
| Etiology | ||
| HBV | 68 | |
| HCV | 16 | |
| Tumor number | ||
| <5 | 42 | |
| ≥5 | 42 | |
| Tumor size | ||
| <10 cm | 56 | |
| ≥10 cm | 28 | |
| Vascular invasion | ||
| no | 56 | |
| yes | 28 | |
| BCLC stage | ||
| Stage A | 27 | |
| Stage B | 29 | |
| Stage C | 28 | |
Note: BCLC, Barcelona Clinic Liver Cancer. HBV, Hepatitis B virus.
HCC, hepatocellular carcinoma. HCV, Hepatitis C virus.
Statistical analysis
Statistical analysis was performed using SPSS software package (version 18.0; SPSS Inc., Chicago, IL, USA). AFP ≥20 μg/L and MDK ≥0.654 ng/mL were considered positive values.9 For descriptive results, normally distributed data were expressed as mean ± standard deviation and non-normally distributed data were expressed as median. The following clinical tumor characteristics were analyzed: tumor number (<5 vs. ≥ 5), tumor size (<10 cm vs. ≥ 10 cm), vascular invasion (yes vs. no), and BCLC stage (A vs. B–C). The relationship of AFP and MDK levels with clinical tumor characteristics were evaluated by rank sum tests.The sensitivity of changes in the AFP and MDK levels before and after treatment and the effect of the treatment were assessed using χ2 test and rank sum test. All patients were followed-up for 18 months. Time to progression (TTP) was defined as the time interval from interventional therapy to tumor progression and was compared between the groups through a regression analysis using the log-rank (Mantel–Cox) method. All P values were two-sided, and P ≤ 0.05 was considered statistically significant.
Results
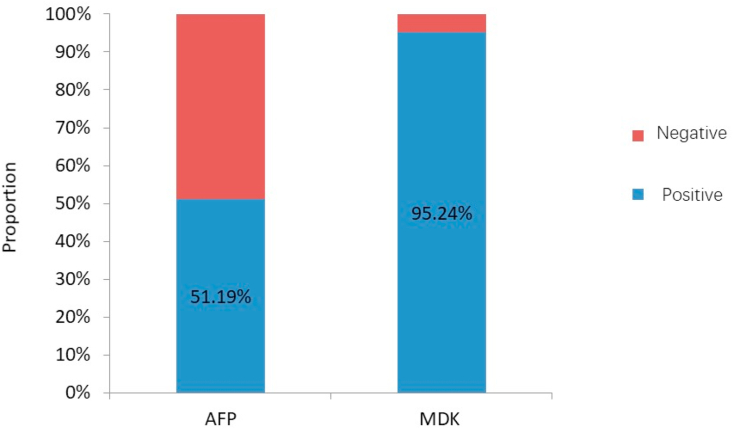
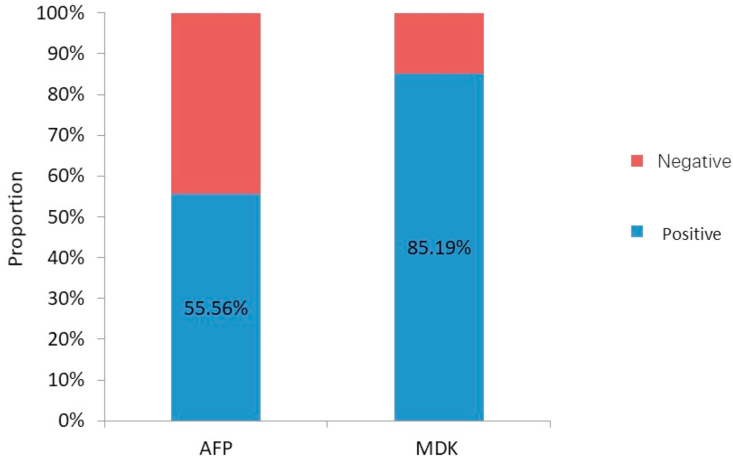
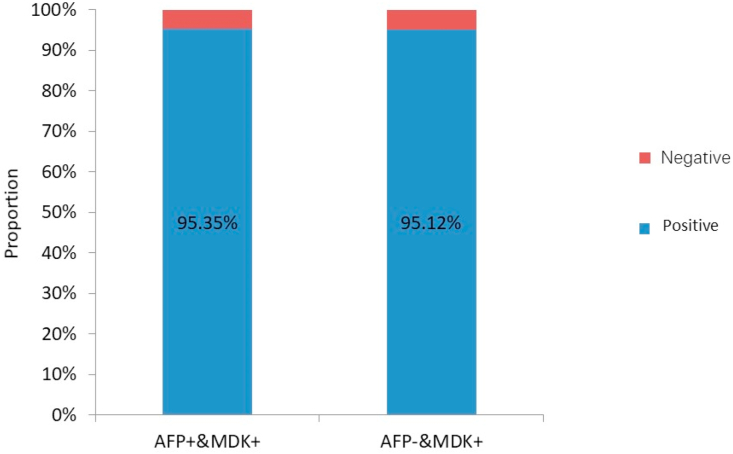
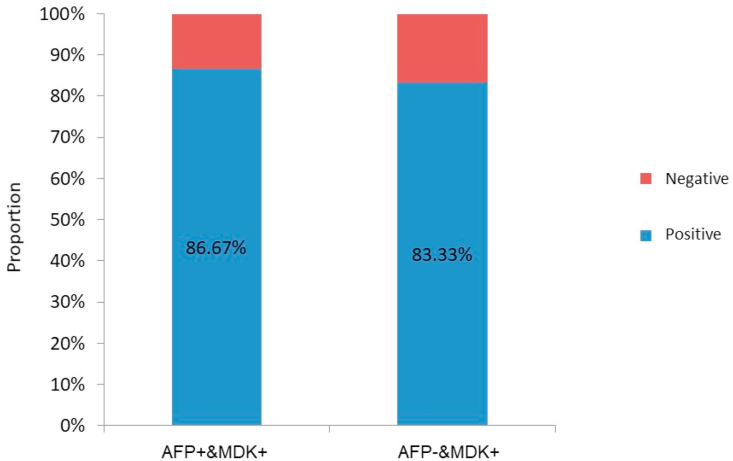
In this study, MDK and AFP expression was detected in 95.24% and 51.19% cases, respectively, before treatment (Fig. 1). The corresponding values in patients with early-stage (BCLC-A) HCC were 85.19% and 55.56%, respectively (Fig. 2). The positive expression rates of MDK were 95.35% and 95.12% among the AFP-positive and AFP-negative groups, respectively (Fig. 3), and the rates in patients with BCLC-A HCC were 86.67% and 83.33%, respectively (Fig. 4). Positive AFP expression was not significantly related to any of the clinical characteristics (Table 2); however, MDK expression was significantly associated with various clinical characteristics (Table 3).
Fig. 1.
Comparison of positive detection rates of serum AFP, MDK in patients with HCC. AFP, alpha fetoprotein; MDK, midkine; HCC, hepatocellular carcinoma.
Fig. 2.
Comparison of positive detection rates of serum AFP, MDK in patients with BCLC-A stage HCC. AFP, alpha fetoprotein; BCLC, Barcelona Clinic Liver Cancer; MDK, midkine; HCC, hepatocellular carcinoma.
Fig. 3.
Positive detection rate of serum MDK in AFP-negative HCC patients.
AFP, alpha fetoprotein; MDK, midkine; HCC, hepatocellular carcinoma.
Fig. 4.
Positive detection rate of serum MDK in patients with AFP-negative BCLC-A stage HCC. AFP, alpha fetoprotein; BCLC, Barcelona Clinic Liver Cancer; MDK, midkine; HCC, hepatocellular carcinoma.
Table 2.
Analysis of relationship between peripheral blood AFP and clinical parameters of tumor.
| Clinical parameters | Group | AFP(ug/l) | Z | P |
|---|---|---|---|---|
| Tumor number | ||||
| <5 | 20.85 | −0.39 | 0.69 | |
| ≥5 | 36.20 | |||
| Tumor size | ||||
| <10 cm | 27.91 | −0.41 | 0.68 | |
| ≥10 cm | 26.42 | |||
| Vascular invasion | ||||
| No | 27.17 | −0.13 | 0.89 | |
| Yes | 28.29 | |||
| Clinical stage | ||||
| BCLC-A | 27.91 | −0.01 | 0.99 | |
| BCLC B–C | 26.42 | |||
Note: AFP, alpha fetoprotein. BCLC, Barcelona Clinic Liver Cancer.
Table 3.
Analysis of relationship between peripheral blood MDK and clinical parameters of tumor.
| Clinical parameters | Group | MDK (ng/ml) | t/Z | P |
|---|---|---|---|---|
| Tumor number | ||||
| <5 | 1.25 | −4.97 | 0.00 | |
| ≥5 | 3.29 | |||
| Tumor size | ||||
| <10 cm | 1.62 | −1.98 | 0.048 | |
| ≥10 cm | 2.42 | |||
| Vascular invasion | ||||
| No | 1.44 | −3.98 | 0.00 | |
| Yes | 4.37 | |||
| Clinical stage | ||||
| BCLC-A | 1.23 | −6.50 | 0.00 | |
| BCLC-B-C | 2.90 | |||
Note: AFP, alpha fetoprotein. BCLC, Barcelona Clinic Liver Cancer.MDK,Midkine.
The expression of AFP (from 51.2% to 35.7% of the cases) and MDK (from 95.2% to 66.7% of the cases) significantly decreased after treatment (both P < 0.05) (Table 4).
Table 4.
AFP, MDK positive rate changes before and after intervention treatment.
| Biomark | Group | Before treatment(n) | positive rate (%) |
After treatment(n) | positive rate (%) |
χ2 | P |
|---|---|---|---|---|---|---|---|
| AFP | |||||||
| <20 ng/ml | 41 | 54 | |||||
| >20 ng/ml | 43 | 51.2 | 30 | 35.7 | 4.09 | 0.04 | |
| MDK | |||||||
| <0.654 ng/ml | 4 | 28 | |||||
| ≥0.654 ng/ml | 80 | 95.2 | 56 | 66.7 | 22.24 | 0.00 | |
Note: AFP, alpha fetoprotein. MDK,Midkine.
The post-treatment AFP and MDK levels were significantly lower in the patients who achieved a complete or partial response (6.73 μg/L and 0.67 ng/mL, respectively) than in those who had a stable or progressive disease (995.75 μg/L and 3.66 ng/mL, respectively) (both P < 0.05) (Table 5).
Table 5.
Serum AFP, MDK changes and efficacy comparison.
| Biomark | CR + PR group | SD + PD group | z | P |
|---|---|---|---|---|
| AFP | 6.73 μg/l | 995.75 μg/l | −4.609 | 0.000 |
| MDK | 0.67 ng/ml | 3.66 ng/ml | −6.261 | 0.000 |
Note: AFP, alpha fetoprotein. CR, complete response. MDK, Midkine. PD, progressive disease. PR, partial response. SD, stable disease.
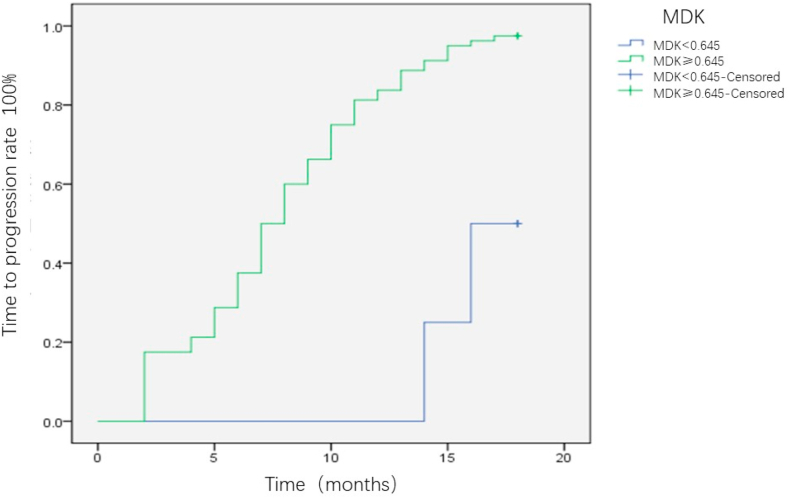
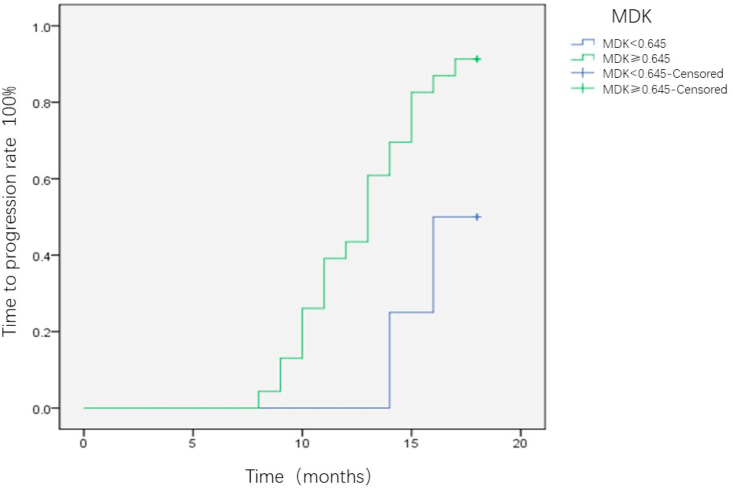
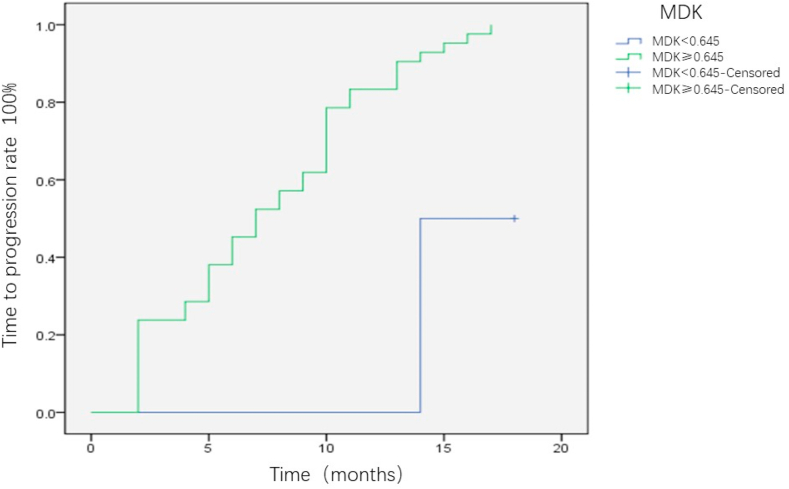
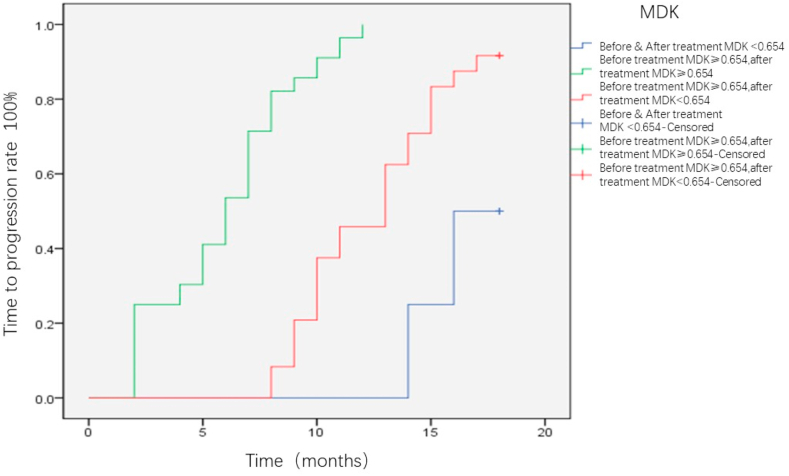
All the patients were followed-up for 18 months, and the differences in TTP were compared between the MDK-positive and MDK-negative groups. Patients with positive MDK expression before intervention were significantly more likely to relapse than those without MDK expression (P = 0.002) (Fig. 5). This trend was consistently observed in BCLC-A (P = 0.047; Fig. 6) and AFP-negative patients (P = 0.024; Fig. 7). Patients with HCC were divided into three groups as follows: Group I, MDK-negative before and after treatment; Group II, MDK-positive before but MDK-negative after treatment; and Group III, MDK-positive before and after treatment. We compared TPP among the three groups and found that the recurrence rate was the lowest in Group I followed by Group II, and that the highest value was observed for Group III (P < 0.001) (Fig. 8).
Fig. 5.
The difference of TTP between MDK positive group and MDK negative group for HCC·HCC, hepatocellular carcinoma; MDK, midkine; TTP, time to progression.
Fig. 6.
The difference of TTP between MDK positive group and MDK negative group for BCLC-A stage HCC patients. BCLC, Barcelona Clinic Liver Cancer; HCC, hepatocellular carcinoma; MDK, midkine TTP, time to progression.
Fig. 7.
The difference of TTP between MDK positive group and MDK negative group for AFP negative HCC patients. AFP, alpha fetoprotein; HCC, hepatocellular carcinoma; MDK, midkine TTP, time to progression; MDK, midkine.
Fig. 8.
Correlation analysis of MDK positive value before and after interventional treatment and patient prognosis. MDK, midkine.
Discussion
MDK is a basic heparin-binding growth factor10 that is strongly induced during the second trimester. The gene encodes important retinoic acid response products.11 Although its expression is low in adult tissues and it exhibits a restricted distribution, MDK has been shown to play an important role in carcinogenesis-related activities in solid tumors.12 However, the role of MDK in the diagnosis of HCC and the efficacy of interventional therapy are unclear.
AFP test is known to be the most widely used for the diagnosis of HCC worldwide. However, about 30% of early stage HCC can’t be detected using AFP analysis. Furthermore, serum AFP levels remain normal in 15–30% advanced HCC.13In the current study, 48.8% of the HCC patients, including those with large lesions, had normal AFP levels. Considering the use of serum MDK as a marker for the diagnosis of HCC, Vongsuvanh et al.4 found that 59.18% of the ACC-negative HCC patients had elevated MDK levels. MDK expression significantly increased the rate of detection of HCC as compared with AFP. Hodeib et al. and Omran et al.14,15 showed the superior sensitivity and specificity of MDK in the diagnosis of HCC as compared with AFP and recommended the combined use of MDK and AFP as diagnostic markers for HCC. In the present study, 95.12% of the AFP-negative patients showed positive expression of MDK, and MDK significantly increased the rate of detection of HCC. Furthermore, 85.19% of the patients with early HCC showed MDK expression, as compared with only 55.56% who were positive for AFP expression. Subgroup analysis showed that the expression of MDK was detected in 83.33% of the patients with early-stage AFP-negative HCC. These results suggest that MDK detection is a powerful supplement to AFP detection for the diagnosis of HCC, especially early-stage and AFP-negative HCC.
Here, we reported that MDK expression increased in patients with multiple nodules, vascular invasion, and mid-to-late stage HCC. This observation was consistent with the results of the study by Vongsuvanh et al.4 The high expression of MDK may be related to tumor angiogenesis.16 Huang et al. examined the interaction between MDK and progranulin, which is a secreted glycoprotein known to participate in cell cycle processes and regulate tumorigenesis and angiogenesis.17 These authors suggested the plausible relationship between MDK and progranulin and showed that MDK affected endothelial cells and exerted an angiogenic effect in HCC.18
The positive expression of serum AFP and MDK may be useful for HCC-assisted diagnosis and may reflect the tumor activity. The decrease in their expression after interventional treatment is reflective of the therapeutic effect of the treatment. Based on the positive reference values reported in the literature,19 the current study showed that serum AFP and MDK expression significantly decreased after interventional treatment. Thus, the changes in the serum AFP and MDK expression levels may reflect treatment efficacy to a certain extent.
Ak et al.19 assessed the value of MDK expression for the prognosis of patients with malignant pleural mesothelioma and showed that high MDK expression was closely related to the progress of patients after treatment. Yamashita et al.20 revealed the relationship between the serum MDK level and malignant tumors, chemosensitivity, and prognosis in patients with head and neck squamous cell carcinoma. Thus, serum MDK may be a useful biomarker for the detection of early-stage tumors and can assist treatment-related decision-making and prognosis prediction. In the current study, the MNK level reduced in patients who were at different stages of HCC after radiofrequency, TACE, and TACE combined with targeted therapy.
The present results showed that patients with positive MDK expression before intervention were more likely to relapse than those without MDK expression. Patients with MDK expression both before and after treatment were the most likely to relapse, followed by MDK-positive patients who became MDK-negative after treatment. In contrast, patients who lacked MDK expression throughout were the least likely to relapse. Numerous studies have investigated the link between MDK and tumor prognosis. Kim et al.21 studied the correlation between MDK and prognosis in patients with sporadic scleroderma and showed that high MDK expression level in the peripheral blood was closely related to disease progression after tumor treatment, suggestive of MDK as a predictor of tumor recurrence. Ma et al.22 also reported that increased MDK expression was associated with the poor prognosis of patients with glioma. MDK expression is related to tumor vascular invasion and metastasis. MDK can promote the formation of hepatic tumor blood vessels16 and preserve circulating tumor cells via an anti-apoptotic effect, thereby promoting tumor recurrence.23 MDK detection after therapeutic interventions can thus be used as a novel indicator of micrometastases, which cannot be detected using the conventional imaging tools, and as an indicator of post-treatment recurrence risk to achieve the goal of on-demand treatment for patients with HCC.
This study had several limitations. Firstly, this was a single-center study with a small sample size. Therefore, further multi-center studies with larger sample sizes are planned to achieve more objective results. Secondly, because all patients were from the tumor hospital, this study did not include patients with liver cirrhosis. Detection of AFP and MDK in patients with liver cirrhosis will more accurately assess the sensitivity and specificity of the two biomarkers for the diagnosis of HCC.
Conclusions
In summary, the results of this study suggest that the peripheral blood MDK level may serve as an effective biomarker to assist the diagnosis of HCC, particularly early-stage and AFP-negative HCC. MDK was significantly positively related to the number of tumors, tumor size, vascular invasion, and clinical stage of HCC. Therefore, it can be said that patients with MDK expression before treatment may be more prone to postoperative tumor progression. MDK level may thus facilitate the evaluation of the efficacy of interventional treatment and the prognostic analysis of patients with HCC.
Funding
This research was supported by Henan Provincial Medical Science and Technology Research Project (co-built by provinces and ministries) (SBGJ2008090).
Patient consent
Witten informed consent was obtained from patients for publication of these case reports and any accompanying images.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Hartke J., Johnson M., Ghabril M. The diagnosis and treatment of hepatocellular carcinoma. Semin Diagn Pathol. 2017;34:153–159. doi: 10.1053/j.semdp.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed Mohammed H.F., Roberts L.R. Should AFP (or any biomarkers) be used for HCC surveillance? Curr Hepat Rep. 2017;16:137–145. doi: 10.1007/s11901-017-0349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma M.C., Chen Y.J., Chiu T.J. Positive expression of Midkine predicts early recurrence and poor prognosis of initially resectable combined hepatocellular cholangiocarcinoma. BMC Canc. 2018;18:227. doi: 10.1186/s12885-018-4146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vongsuvanh R., van der Poorten D., Iseli T. Midkine increases diagnostic yield in AFP negative and NASH-related hepatocellular carcinoma. PloS One. 2016;11 doi: 10.1371/journal.pone.0155800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee H.M., Joh J.W., Seo S.R. Cell-surface major vault protein promotes cancer progression through harboring mesenchymal and intermediate circulating tumor cells in hepatocellular carcinomas. Sci Rep. 2017;7:13201. doi: 10.1038/s41598-017-13501-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsilimigras D.I., Bagante F., Sahara K. Prognosis after resection of Barcelona clinic liver cancer (BCLC) stage 0, A, and B hepatocellular carcinoma: a comprehensive assessment of the current BCLC classification. Ann Surg Oncol. 2019;26:3693–3700. doi: 10.1245/s10434-019-07580-9. [DOI] [PubMed] [Google Scholar]
- 7.Mashaly A.H., Anwar R., Ebrahim M.A. Diagnostic and prognostic value of talin-1 and midkine as tumor markers in hepatocellular carcinoma in Egyptian patients. Asian Pac J Cancer Prev APJCP. 2018;19:1503–1508. doi: 10.22034/APJCP.2018.19.6.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llovet J.M., Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol. 2020;72:288–306. doi: 10.1016/j.jhep.2019.09.026. [DOI] [PubMed] [Google Scholar]
- 9.Marrero J.A., Fontana R.J., Fu S. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol. 2005;42:218–224. doi: 10.1016/j.jhep.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Chiu T.J., Chen Y.J., Rau K.M. Midkine neurite growth-promoting factor 2 expression as a potential prognostic marker of adjuvant therapy in head and neck squamous cell carcinoma. Biomarkers. 2013;18:687–698. doi: 10.3109/1354750X.2013.846412. [DOI] [PubMed] [Google Scholar]
- 11.Olmeda D., Cerezo-Wallis D., Riveiro-Falkenbach E. Whole-body imaging of lymphovascular niches identifies pre-metastatic roles of midkine. Nature. 2017;546:676–680. doi: 10.1038/nature22977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vu Van D., Heberling U., Wirth M.P. Validation of the diagnostic utility of urinary midkine for the detection of bladder cancer. Oncol Lett. 2016;12:3143–3152. doi: 10.3892/ol.2016.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llovet J.M., Zucman-Rossi J., Pikarsky E. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 14.Hodeib H.,O.E.L., Selim A. Serum midkine and osteopontin levels as diagnostic biomarkers of hepatocellular carcinoma. Electron Physician. 2017;9:3492–3498. doi: 10.19082/3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Omran M.M., Farid K., Omar M.A. A combination of alpha-fetoprotein, midkine, thioredoxin and a metabolite for predicting hepatocellular carcinoma. Ann Hepatol. 2020;19:179–185. doi: 10.1016/j.aohep.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Huang H., Li J., Lu Y. Role of midkine-progranulin interaction during angiogenesis of hepatocellular carcinoma. Int J Clin Exp Pathol. 2015;8:8809–8820. [PMC free article] [PubMed] [Google Scholar]
- 17.Eguchi R., Nakano T., Wakabayashi I. Progranulin and granulin-like protein as novel VEGF-independent angiogenic factors derived from human mesothelioma cells. Oncogene. 2017;36:714–722. doi: 10.1038/onc.2016.226. [DOI] [PubMed] [Google Scholar]
- 18.Takemoto Y., Horiba M., Harada M. Midkine promotes atherosclerotic plaque formation through its pro-inflammatory, angiogenic and anti-apoptotic functions in apolipoprotein E-knockout mice. Circ J. 2017;82:19–27. doi: 10.1253/circj.CJ-17-0043. [DOI] [PubMed] [Google Scholar]
- 19.Zhu W.W., Guo J.J., Guo L. Evaluation of midkine as a diagnostic serum biomarker in hepatocellular carcinoma. Clin Canc Res. 2013;19:3944–3954. doi: 10.1158/1078-0432.CCR-12-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamashita T., Shimada H., Tanaka S. Serum midkine as a biomarker for malignancy, prognosis, and chemosensitivity in head and neck squamous cell carcinoma. Cancer Med. 2016;5:415–425. doi: 10.1002/cam4.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim H.S., Kim J., Nam K.H. Clinical significance of midkine expression in sporadic desmoid tumors. Oncol Lett. 2016;11:1677–1684. doi: 10.3892/ol.2016.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma J., Lang B., Wang X. Co-expression of midkine and pleiotrophin predicts poor survival in human glioma. J Clin Neurosci. 2014;21:1885–1890. doi: 10.1016/j.jocn.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 23.Sun B., Hu C., Yang Z. Midkine promotes hepatocellular carcinoma metastasis by elevating anoikis resistance of circulating tumor cells. Oncotarget. 2017;8:32523–32535. doi: 10.18632/oncotarget.15808. [DOI] [PMC free article] [PubMed] [Google Scholar]