Abstract
Accessory renal arteries (ARAs) are common and usually originate from the abdominal aorta and the renal artery. Inferior phrenic arteries (IPAs) can also arise from the abdominal aorta or its branches. In this paper, we present the first case of a common trunk of the right ARA and right IPA arising from the thoracic artery at the level of T10, which was discovered by multidetector-row computed tomography in pretherapeutic evaluation and clearly confirmed by selective angiography. It is important to recognize this anatomical variation when performing cardiovascular and interventional radiological procedures.
Keywords: Renal artery, Inferior phrenic artery, Thoracic artery, Anatomic variation
Abbreviations: ARA, Accessory renal artery; IPA, Inferior phrenic artery; CT, Computed tomography
Introduction
Accessory renal arteries (ARAs) are the most common anatomical variations in the kidney vasculature. The reported incidence of ARAs ranges from 8.7% to 75.7%, with an average incidence of 28.2%.1 ARAs most commonly originate from the abdominal aorta and the renal artery.1 The inferior phrenic artery (IPA) usually originates at the T12 and L2 levels.2 The origin of the IPA varies; it can arise from the abdominal aorta, celiac axis, renal artery, accessory renal artery, left gastric artery, hepatic artery, superior mesenteric artery, or spermatic artery.2,3 It is clinically important to recognize anatomic variations of ARAs and IPAs.1,3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 We report here the first case of a common trunk of the right ARA and right IPA arising from the thoracic artery as identified by multidetector-row computed tomography (CT) angiography and digital subtraction angiography.
Case report
A 62-year-old man was referred to our institution to undergo bronchial artery embolization for the management of massive hemoptysis caused by bronchiectasis. He underwent multidetector-row CT angiography as part of a pre-therapeutic evaluation. CT angiography was performed with a 64-detector-row scanner in the craniocaudal direction from the base of the neck to the level of the renal arteries. A 20-gauge intravenous catheter was inserted into the antecubital vein, and 100 mL of iohexol (350 mg of iodine/mL; Omnipaque; GE Healthcare, Milwaukee, WI, USA) was injected at a rate of 4.0 mL/s, followed by 30 mL of saline solution. A region of interest was identified in the descending aorta. When the attenuation reached 100 HU, craniocaudal scanning was started 6 s later during a single breath hold.
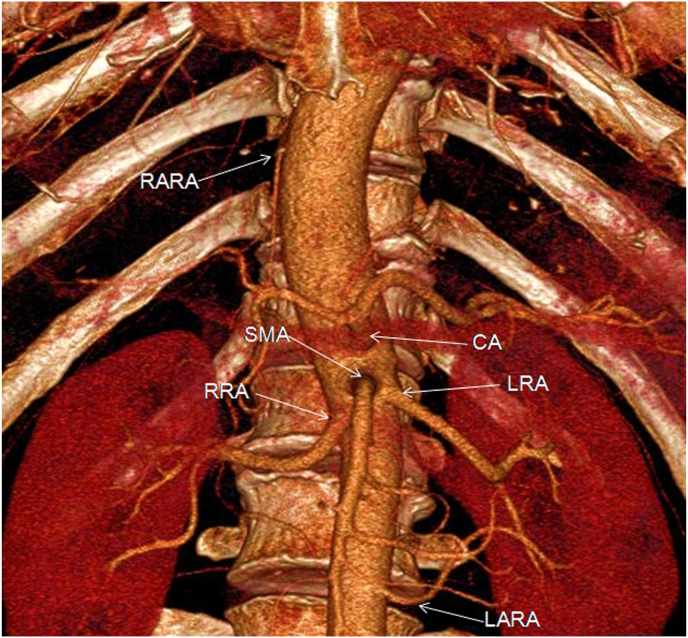
CT showed bilateral bronchiectasis, which was severe in the right middle lobe. CT angiography showed two right bronchial arteries, two left bronchial arteries, and one right internal thoracic artery that was definitely hypertrophic. The right IPA was dubiously hypertrophic. Simultaneously, CT angiography showed the common trunk of the right ARA and right IPA arising from the right lateral posterior aspect of the thoracic artery at the level of T10. The trunk arose from the point of origin of the thoracic artery located at 3.5 mm, and the angle between the trunk and the thoracic artery was 16.3°. It passed anteroinferiorly (7.3 cm) and ran along the right posterior side of the thoracic artery and right anterior side of T10 to T12, passed anteroinferiorly, penetrated the diaphragm, and finally gave rise to the right ARA and right IPA (Fig. 1). CT also revealed the structure of arteries (celiac artery, superior mesenteric artery, right renal artery, left renal artery, and left ARA) in the upper abdomen (Fig. 2).
Fig. 1.
A Multi-oblique maximum intensity projection (MIP) computed tomography (CT) shows a common trunk (black arrow) of the right accessory renal artery (ARA) and right inferior phrenic artery (IPA) arising from the thoracic artery and right renal artery (white arrow). B Transverse MIP CT shows the trunk (arrow) arising from the right lateral posterior aspect of the thoracic artery at the level of T11. C Transverse MIP CT shows the trunk penetrating through the right diaphragm (white arrows).
Fig. 2.
Three-dimensional CT angiography with maximum intensity projection of the right thoracic ARA and other major arteries in the upper abdomen. CA celiac artery, LARA left accessory renal artery, LRA left renal artery, RARA right accessory renal artery, RRA right renal artery, SMA superior mesenteric artery.
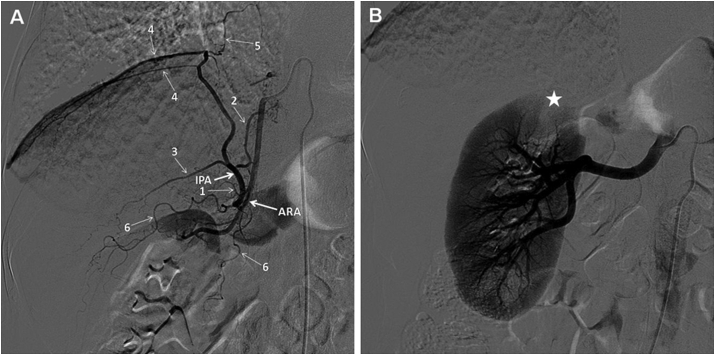
Procedural access to catheterization was performed through the right femoral artery. Embolization of the pathological bronchial arteries and the right internal thoracic artery was performed using polyvinyl alcohol particles (PVA-300; Cook Medical, Bloomington, IN, USA). After embolization of the bronchial and internal thoracic arteries, the patient underwent selective angiography of the common trunk of the right ARA and right IPA using a 5F Mickelson catheter (Cook Medical), which confirmed that the CT angiography findings of the anatomy and the right IPA were not pathological. The angiogram clearly demonstrated that the branches of the right IPA were the superior adrenal branch, inferior vena caval branch, posterior and anterior branches of the IPA, and the diaphragmatic branch (Fig. 3A). The right ARA gave rise to two renal capsule arteries and then supplied the upper pole of the kidney (Fig. 3A). With the consent of the patient (obtained before the procedure), an additional selective angiography of the right renal artery was performed and the angiography demonstrated an absence of perfusion defects in the blood supply area of the right ARA (Fig. 3B). Hemoptysis was completely brought under control with this procedure, and the patient experienced no further episodes of hemoptysis in the 11-month follow-up period.
Fig. 3.
A selective angiogram of the common trunk of the right ARA and right IPA. The branches are clearly demonstrated (1 superior adrenal branch, 2 inferior vena caval branch of the IPA, 3 posterior branch of the IPA, 4 anterior branches of the IPA, 5 diaphragmatic branch of the IPA, 6 renal capsule arteries). b Selective angiogram of the right renal artery shows the absence of any perfusion defects (star) of the upper pole of the kidney.
Discussion
The sites of origin of the renal artery, ARA, and IPA have numerous anatomical variations. Three variations similar to our case have been reported: a thoracic origin of the right renal artery, a thoracic origin of the right ARA, and a common trunk of the IPAs originating from the left ARA.4, 5, 6 A renal origin of the IPA has usually been described as involving the right side, and a thoracic origin of the renal artery or ARA has been depicted as involving the right side in all previous reports.1, 2, 3, 4, 5, 6 The anatomical variation in the present case was the thoracic origin of the common trunk of the right ARA and right IPA.
There are several possible embryological explanations for this variation. The renal arteries develop from primitive mesonephric arteries, which form a vascular net on both sides of the aorta between the C6 and L3 vertebrae, which is known as the rete arteriosum urogenitale.4 The persistence of primitive mesonephric vessels results in the development of highly anomalous renal arteries, and the right renal artery orifice is usually superior to that on the left side.4 This might be why all reported cases of the renal artery or ARA arising from the thoracic aorta are the right renal arteries. IPA develops independently of the primitive mesonephric artery.6 Therefore, our case might have involved the development of a highly anomalous right ARA combined with a right IPA connection at the renal artery.
The IPAs give rise to the anterior, posterior, superior suprarenal, and middle suprarenal branches.3 In addition, the right IPA gives rise to the inferior vena caval and diaphragmatic branches, and the left IPA gives rise to gastric, esophageal, and accessory splenic branches.3 Therefore, IPAs supply not only the diaphragm but also most other organs. Knowledge of the variations in ARAs and IPAs is very important for clinicians in the fields of interventional radiology, surgery, cardiology, nephrology, and similar medical disciplines.
Knowledge of this variation is important for cardiovascular and interventional radiologic procedures. IPA is the most common extrahepatic collateral blood supply for hepatocellular carcinoma.3 The IPA is an important nonbronchial systemic artery in endovascular embolization for the treatment of hemoptysis.7 Bleeding from the right IPA can occur after liver transplantation or pheochromocytoma rupture of the adrenal gland.8,9 The IPA also serves as a feeding artery during transarterial infusion for lung cancer.10 Knowledge of renal arterial anatomy is important in aortic stent-grafting and the management of renovascular hypertension, renal trauma, and renal cancer.1,11, 12, 13 Furthermore, the ARA was recently reported to be an important artery in catheter-based renal sympathetic denervation.14
Although a common trunk of the right ARA and right IPA arising from the thoracic artery represents an extremely rare variation in vascular anatomy, its clinical importance cannot be underestimated.
Patient consent
Written informed consent was obtained from patients for publication of this case reports and any accompanying images.
Declaration of competing interest
The author declare that they have no conflict of interests to this work. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Contributor Information
Lingling Li, Email: lilingling323@163.com.
Bing Jie, Email: jbshh@163.com.
Dong Yu, Email: yudong_mail@126.com.
Xu Ma, Email: walyj823@163.com.
Sen Jiang, Email: jasfly77@vip.163.com.
References
- 1.Satyapal K.S., Haffejee A.A., Singh B. Additional renal arteries: incidence and morphometry. Surg Radiol Anat. 2001;23:33–38. doi: 10.1007/s00276-001-0033-y. [DOI] [PubMed] [Google Scholar]
- 2.Greig H.W., Anson B.J., Coleman S.S. The inferior phrenic artery; types of origin in 850 body-halves and diaphragmatic relationship. Q Bull Northwest Univ Med Sch. 1951;25:345–350. [PMC free article] [PubMed] [Google Scholar]
- 3.Gwon D.I., Ko G.Y., Yoon H.K. Inferior phrenic artery: anatomy, variations, pathologic conditions, and interventional management. Radiographics. 2007;27:687–705. doi: 10.1148/rg.273065036. [DOI] [PubMed] [Google Scholar]
- 4.Ichikawa T., Iino M., Koizumi J. A case of right renal artery originating from the thoracic aorta. Jpn J Radiol. 2014;32:716–720. doi: 10.1007/s11604-014-0360-7. [DOI] [PubMed] [Google Scholar]
- 5.Talović E., Voljevica A. An unusual renal accessory artery originating from the thoracic aorta and its potential clinical implications. Acta Med Acad. 2013;42:80–82. doi: 10.5644/ama2006-124.74. [DOI] [PubMed] [Google Scholar]
- 6.Miclaus G.D., Matusz P., Loukas M. Rare case of the trunk of the inferior phrenic arteries originating from a common stem with a superior additional left renal artery from the abdominal aorta. Clin Anat. 2012;25:979–982. doi: 10.1002/ca.22161. [DOI] [PubMed] [Google Scholar]
- 7.Yoon W., Kim J.K., Kim Y.H. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics. 2002;22:1395–1409. doi: 10.1148/rg.226015180. [DOI] [PubMed] [Google Scholar]
- 8.Mizobata Y., Yokota J., Yajima Y. Two cases of blunt hepatic injury with active bleeding from the right inferior phrenic artery. J Trauma. 2002;48:1153–1155. doi: 10.1097/00005373-200006000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Edo N., Yamamoto T., Takahashi S. Optimizing hemodynamics with transcatheter arterial embolization in adrenal pheochromocytoma rupture. Intern Med. 2018;57:1873–1878. doi: 10.2169/internalmedicine.9907-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakanishi M., Yoshida Y., Natazuka T. Prospective study of transarterial infusion of docetaxel and cisplatin to treat nonsmall-cell lung cancer in patients contraindicated for standard chemotherapy. Lung Canc. 2012;77:353–358. doi: 10.1016/j.lungcan.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Dorffner R., Thurnher S., Prokesch R. Spiral CT during selective accessory renal artery angiography: assessment of vascular territory before aortic stent-grafting. Cardiovasc Intervent Radiol. 1998;21:179–182. doi: 10.1007/s002709900239. [DOI] [PubMed] [Google Scholar]
- 12.Sommer C.M., Stampfl U., Bellemann N. Patients with life-threatening arterial renal hemorrhage: CT angiography and catheterangiography with subsequent superselective embolization. Cardiovasc Intervent Radiol. 2010;33:498–508. doi: 10.1007/s00270-009-9787-0. [DOI] [PubMed] [Google Scholar]
- 13.Karalli A., Ghaffarpour R., Axelsson R. Transarterial chemoembolization of renal cell carcinoma: a prospective controlled trial. J Vasc Intervent Radiol. 2017;28:1664–1672. doi: 10.1016/j.jvir.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Ewen S., Ukena C., Lüscher T.F. Anatomical and procedural determinants of catheter-based renal denervation. Cardiovasc Revascularization Med. 2016;17:474–479. doi: 10.1016/j.carrev.2016.08.004. [DOI] [PubMed] [Google Scholar]